Abstract
Fibrosis in multiple organs, including the liver, kidney, and lung, often occurs secondary to environmental exposure. Asbestos exposure is one important environmental cause of lung fibrosis. The mechanisms that mediate fibrosis is not fully understood, although mitochondrial oxidative stress in alveolar macrophages is critical for fibrosis development. Mitochondrial Ca2+ levels can be associated with production of reactive oxygen species. Here, we show that patients with asbestosis have higher levels of mitochondrial Ca2+ compared with normal patients. The mitochondrial calcium uniporter (MCU) is a highly selective ion channel that transports Ca2+ into the mitochondrial matrix to modulate metabolism. Asbestos exposure increased mitochondrial Ca2+ influx in alveolar macrophages from wild-type, but not MCU+/−, mice. MCU expression polarized macrophages to a profibrotic phenotype after exposure to asbestos, and the profibrotic polarization was regulated by MCU-mediated ATP production. Profibrotic polarization was abrogated when MCU was absent or its activity was blocked. Of more importance, mice that were deficient in MCU were protected from pulmonary fibrosis. Regulation of mitochondrial Ca2+ suggests that MCU may play a pivotal role in the development of fibrosis and could potentially be a therapeutic target for pulmonary fibrosis.—Gu, L., Larson-Casey, J. L., Carter, A. B. Macrophages utilize the mitochondrial calcium uniporter for profibrotic polarization.
Keywords: MCU, asbestos, reactive oxygen species, pulmonary fibrosis, ATP
Pulmonary fibrosis is characterized by aberrant collagen deposition during progressive wound healing. Asbestosis is a prototypical form of pulmonary fibrosis. There are no accepted therapies for asbestosis, and recently approved antifibrotic therapies have limited efficacy. Macrophages are critical for the progression of liver, kidney, and lung fibrosis (1–3). Moreover, mitochondrial oxidative stress and mitochondrial turnover in alveolar macrophages are directly linked to pulmonary fibrosis (4–6); however, the molecular mechanisms that modulate mitochondrial dynamics is not known.
The mitochondrial calcium uniporter (MCU) is a highly selective ion channel with the primary function of transferring cytosolic and endoplasmic reticulum Ca2+ into the mitochondrial matrix (7). MCU function is dependent on 2 steps. First, cytosolic Ca2+ is transported through the permeable voltage-dependent anion channel—the most abundant protein in the outer mitochondrial membrane (8). Second, Ca2+ is further transported through the inner mitochondrial membrane into the mitochondrial matrix via MCU activity (8–10).
There is increasing evidence that MCU regulates a wide spectrum of biologic processes, including cellular metabolism, cell viability, and reactive oxygen species (ROS) production. Studies show that mitochondrial Ca2+ influx contributes to increased generation of ROS in mitochondria (11, 12). Generation of ROS plays a critical role in lung injury and subsequent fibrosis because the abrogation of mitochondrial ROS attenuates the development of pulmonary fibrosis in mice (6, 13, 14). In addition to serving as an important site of ROS production, mitochondria are the dominant site for ATP synthesis during oxidative phosphorylation. Ca2+ influx facilitates ATP synthesis by enhancing mitochondrial oxidative phosphorylation (15, 16).
Macrophages can polarize to either classically activated macrophages or alternatively activated macrophages (AAMs) on the basis of the response to different stimuli. Classically activated macrophages present a proinflammatory phenotype and are associated with inflammation, acute injury, and infection (17–19). AAMs are involved in many pathologic processes, including parasitic infection, tumorigenesis, and end organ fibrosis (13, 20, 21); however, ROS that are derived from the mitochondria mediate profibrotic polarization of AAMs that may modulate the development of pulmonary fibrosis (2, 22–24). Furthermore, studies demonstrate that Cu,Zn-SOD, Akt1, macrophage receptor with collagenous structure, and Rac1 are closed linked to profibrotic polarization (4, 5, 22, 25); however, the role of MCU in profibrotic polarization is not known.
In this study, we hypothesized that the alveolar macrophage, MCU, is required for pulmonary fibrosis as it polarizes macrophages to a profibrotic phenotype. We demonstrate that MCU-mediated mitochondrial Ca2+ influx, as well as ROS and ATP production, modulate macrophage alternative activation to a profibrotic phenotype. Understanding the modulation of MCU and the regulation of mitochondrial calcium concentration ([Ca2+]mt) may provide an important therapeutic target for preventing the development and/or progression of pulmonary fibrosis.
MATERIALS AND METHODS
Human participants
The human subjects review board of the University of Alabama at Birmingham approved the protocol of obtaining alveolar macrophages from normal participants and patients with asbestosis. Normal participants had to meet the following criteria: 1) age between 18 and 55 yr; 2) no history of cardiopulmonary disease or other chronic disease; 3) no prescription or nonprescription medication except oral contraceptives; 4) no recent or current evidence of infection; and 5) lifetime nonsmoker. Patients with asbestosis had to meet the following criteria: 1) forced vital capacity and diffusion capacity of the lung for CO had to be at least 50% predicted; 2) current nonsmoker; 3) no recent or current evidence of infection; 4) evidence of restrictive physiology on pulmonary function tests; and 5) interstitial fibrosis on chest computed tomography scan. Fiber optic bronchoscopy with bronchoalveolar lavage (BAL) was performed after participants received intramuscular atropine (0.6 mg) and local anesthesia. Three subsegments of the lung were lavaged with five 20-ml aliquots of normal saline, and the first aliquot in each was discarded. The percentage of alveolar macrophages was determined by Wright-Giemsa stain and varied from 90 to 98%.
Mice
Wild-type (WT) and MCU+/− mice were littermates and were confirmed by genotyping using tail DNA. MCU+/− mice were derived by crossing MCU−/− with WT C57BL/6 mice. MCU−/− mice were a generous gift from Dr. Mark E. Anderson (Johns Hopkins University, Baltimore, MD, USA). Mice were 6–12 wk old, and equal numbers of male and female mice were selected for exposures. All protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Cell culture and asbestos
Human THP-1 monocyte and mouse MH-S alveolar macrophage cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI 1640 that was supplemented with 10% fetal bovine serum and penicillin/streptomycin. All in vitro experiments were performed in RPMI with 0.5% serum supplement. Libby amphibole asbestos (a generous gift from Dr. Stephen H. Gavett, Environmental Protection Agency, Washington, D.C., USA) or chrysotile asbestos [North American Insulation Manufacturers Association (NAIMA) Fiber Repository, Alexandria, VA, USA] were used in these studies.
Real-time quantitative PCR
Total RNA was isolated by using Trizol reagent (Sigma-Aldrich, St. Louis, MO, USA) and reverse transcribed by using iScript reverse transcription kit (Bio-Rad, Hercules, CA, USA). Expression of mRNA was determined by real-time quantitative PCR using the SYBR Green kit (Bio-Rad). The primer sets, mouse TGF-β1, collagen 1a1, TNF-α, arginase1, IL-10, IL-1β, iNOS, and FIZZ1, and human IL-10 and TGF-β1 have been described (13, 22, 24–26). The primer set for mouse VEGF-A was as follows: (forward) 5′-TATTCAGCGGACTCACCAGC-3′; (reverse) 5′-CTGGGACCACTTGGCATGG-3′. Data were calculated by using the ΔΔCt method. mRNA measurements were normalized to β-actin (mouse) or hypoxanthine-guanine phosphoribosyltransferase (human) and expressed in arbitrary units.
Plasmids and transfections
The plasmids, pFIV-CMV-MCUWT and pFIV-CMV-MCUDN, were generous gifts from Dr. Mark E. Anderson. Cells were transfected with X-treme Gene 9 Transfection Reagent (Sigma-Aldrich) according to the manufacturer's protocol.
Small interfering RNA
Macrophages were transfected with MCU or Rieske small interfering RNA duplexes (Integrated DNA Technologies, Coralville, IA, USA) by using Dharmafect 2 (human), Dharmafect 4 (mouse), or Duo (GE Dharmacon, Pittsburgh, PA, USA) according to the manufacturer's protocol.
[Ca2+]mt quantitation
To visualize mitochondrial Ca2+ by confocal microscopy, macrophages were stained with MitoTracker Green (50 nM, 20 min; Thermo Fisher Scientific, Waltham, MA, USA) and Rhod-2, AM (4 µM, 20 min; Thermo Fisher Scientific). Cells were washed 3 times and subjected to imaging. To quantitate [Ca2+]mt by using a calcium assay kit (Cayman Chemical, Ann Arbor, MI, USA), Ca2+ standards or isolated mitochondria were loaded onto a 96-well plate. After 5 min incubation with the working detector reagent, the plate was read at an absorbance of 575 nm. The linear algebraic formula generated from [Ca2+] standards was used to determine Ca2+ concentrations.
Mitochondrial ROS detection
H2O2 production was measured by parahydroxyphenylacetic acid assay fluorometrically, as previously described (27). MitoSox mitochondrial superoxide indicator (Thermo Fisher Scientific) was used to detect mitochondrial superoxide anion according to manufacturer protocol. Equal numbers of cells were subjected to fluorescent reading (excitation, 510 nm; emission, 580 nm).
Mitochondrial ATP and ATP synthase activity measurement
Mitochondrial ATP was quantitated by CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. In brief, mitochondria were isolated from cells and resuspended in mitochondrial buffer that contained 2% of trichloroacetic acid. Equal amounts of mitochondria were subjected to luminescence reading in the SpectraMax M2 plate reader (Molecular Devices, Sunnyvale, CA, USA). Mitochondrial ATP synthase activity was measured by using the ATP Synthase Enzyme Activity Microplate Assay Kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s protocol. Equal amounts of mitochondria were loaded onto the provided plate and incubated at room temperature. Wells were washed twice and incubated at room temperature with the provided lipid mix. Reagent mix was added and the plate was read at OD 340 nm and expressed as ΔOD340/min.
Mitochondrial membrane potential measurement
To visualize mitochondrial membrane potential (∆ψm) by confocal microscopy, mice alveolar macrophages were stained with Mito Tracker Red (250 nM; Thermo Fisher Scientific) and JC-1 probe (10 µg/ml; Thermo Fisher Scientific) for 20 min at 37°C. Cells were washed 3 times and subjected to imaging. To quantitate ∆ψm, equal amounts of cells were labeled with JC-1 probe in HBSS buffer for 20 min at 37°C. Fluorescence was measured by using a SpectraMax M2 plate reader (monomer: excitation, 514 nm; emission, 525 nm; and aggregate: excitation, 585 nm; emission, 595 nm). Carbonyl cyanide m-chlorophenylhydrazone was used as a positive control.
Oxygen consumption rate determination
Oxygen consumption rate (OCR) was determined by using a Seahorse XF24 bioanalyzer (Seahorse Bioscience, Billerica, MA, USA). In brief, alveolar macrophages from mice (WT and MCU+/−) were placed (1 × 105 cells/well) in an XF24 cell culture microplate (Seahorse Bioscience) for 30 min. The plate was subjected to OCR measurement in the XF24 extracellular flux analyzer (Seahorse Bioscience) with sequential additions of the following compounds: oligomycin (0.25 µg/ml), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (0.5 μM), and antimycin A/rotenone (4/1 μM).
ELISA
Active TGF-β1, TNF-α, and Ym1 in BAL fluid from WT and MCU+/− mice or conditioned media were measured by using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Hydroxyproline assay
Lung tissue homogenates were dried to a stable weight and acid-hydrolyzed with 6N hydrochloric acid for 24 h at 112°C. Hydroxyproline concentration was normalized to dry weight of the lung, as previously described (28).
Immunoblot analysis
Primary Abs used in this study were as follows: MCU (D2Z3B) rabbit mAb (14997; Cell Signaling Technologies, Danvers, MA, USA), anti-UQCRFS1 Ab 5A5 (ab14746; Abcam), voltage-dependent anion channel Ab (4866; Cell Signaling Technologies), and mAb anti–β-actin (A5441; Sigma-Aldrich).
Indirect fluorescence assay detecting CD206
Cells were fixed with 4% paraformaldehyde in PBS for 45 min at room temperature, followed by permeabilization for 3 min. Cells were then incubated with DPBS containing 10% BSA for 30 min. Cells were incubated with rat anti-mouse CD206(MMR)-FITC (1:200 in DPBS containing 2% BSA, 141703; BioLegend, San Diego, CA, USA) and counterstained with DAPI. Nikon A1 Confocal was utilized to take fluorescent pictures. The level of CD206 was quantitated using ImageJ software (National Institutes of Health, Bethesda, MD, USA) according to the software manual. Results were expressed as fluorescence per square centimeter.
Statistical analyses
All data are expressed as means ± sem. Normal distribution was analyzed to determine whether data met the assumptions of the statistical test. Statistical analyses were performed with either an unpaired Student’s t test or 1-way ANOVA with Tukey’s post hoc test, with P < 0.05 considered significant.
RESULTS
MCU expression and [Ca2+]mt are increased in fibrotic macrophages
Because MCU functions to regulate [Ca2+]mt, we investigated whether asbestos induced Ca2+ influx into macrophage mitochondria. Alveolar macrophages had mitochondrial Ca2+ influx after asbestos exposure (Fig. 1A). Quantification revealed that asbestos exposure triggered an increase in [Ca2+]mt that was further significantly increased with MCUWT overexpression (Fig. 1B). In contrast, [Ca2+]mt in cells that expressed dominant negative MCU (MCUDN) was significantly reduced to levels similar to that in unexposed cells. Similar findings were found by silencing MCU in macrophages (Fig. 1C).
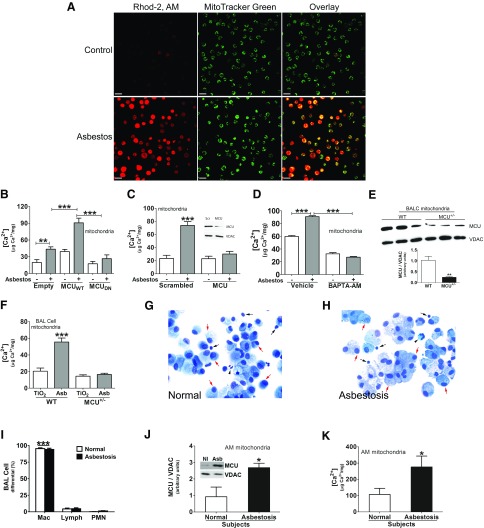
Figure 1.
MCU and [Ca2+]mt are increased in asbestosis alveolar macrophages. A) Mitochondrial Ca2+ in BAL cells from WT mice was visualized by confocal microscopy. Cells were stained with MitoTracker Green and Rhod-2, AM. Scale bars, 20 µm. B) MH-S cells were transfected with empty, MCUWT, or MCUDN plasmid and exposed to asbestos for 30 min. [Ca2+]mt was measured in macrophage mitochondria (n = 6). C) MH-S cells were transfected with scrambled (Scr) or MCU small interfering RNA (siRNA) and exposed to asbestos. [Ca2+]mt was measured in isolated mitochondria (n = 7). D) MH-S cells were transfected with MCUWT, treated with BAPTA-AM (5 μM, overnight), and exposed to asbestos. [Ca2+]mt was measured in isolated mitochondria (n = 4). E) Immunoblot analysis of MCU in BAL cell mitochondria from WT or MCU+/− mice. MCU levels were quantitated to voltage-dependent anion channel (VDAC; n = 3). F) WT and MCU+/− mice were intratracheally exposed to 125 μg TiO2 or asbestos (Asb). Alveolar macrophages were obtained by BAL after 21 d. [Ca2+]mt was measured (n = 3). G, H) Cell differential from BAL of normal participants (G) and patients with asbestosis (H) was determined by Wright-Giemsa stain. Macrophages, red arrow; lymphocytes, black arrow; neutrophils, black arrowhead. I) Statistical quantitation of cell differential (n = 6). J) Immunoblot analysis of MCU in alveolar macrophages from human participants. MCU densitometry was calculated to the mitochondrial housekeeping protein VDAC (n = 4). K) [Ca2+]mt was measured in mitochondria of alveolar macrophages from human participants (n = 5). AM, alveolar macrophage; lymph, lymphocyte; macro, macrophage; NI, normal subject; PMN, polymorphonuclear leukocyte. One-way ANOVA with Tukey’s post hoc comparison (B–D, F, I). Two-tailed Student’s t test (E, J, K). *P < 0.05; **P < 0.01; ***P ≤ 0.001.
Our previous data show that asbestos exposure increases cytosolic Ca2+ content in macrophages (29). To determine whether cytosolic Ca2+ was the source of mitochondrial Ca2+, we chelated Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl ester) (BAPTA-AM). Cytosolic Ca2+ chelation by BAPTA-AM reduced [Ca2+]mt below control levels in macrophages (Fig. 1D).
Similar findings were observed in vivo. MCU expression level was significantly lower in BAL cell mitochondria of MCU+/− mice compared with WT mice (Fig. 1E). BAL cells from asbestos-exposed WT mice had a significant increase in [Ca2+]mt compared with TiO2-exposed mice (Fig. 1F). [Ca2+]mt in alveolar macrophage mitochondria from MCU+/− mice was comparable to the level observed in TiO2-exposed WT mice.
On the basis of these in vivo observations, we evaluated the role of MCU in human participants. Alveolar macrophages were the predominant cells in BAL fluid from normal participants (Fig. 1G) and patients with asbestosis (Fig. 1H). Quantification of multiple participants revealed that alveolar macrophages represented >90% of the cells in BAL fluid (Fig. 1I). Mitochondria of alveolar macrophages from patients with asbestosis showed a greater amount of MCU expression compared with normal participants (Fig. 1J). The difference in MCU expression resulted in greater [Ca2+]mt in asbestosis alveolar macrophages (Fig. 1K). Taken together, these observations suggest that regulation of alveolar macrophage mitochondrial Ca2+ is associated with fibrosis.
Mitochondrial ROS production in macrophages requires MCU
To determine whether the change in [Ca2+]mt was associated with an alteration in ∆ψm, alveolar macrophages were obtained from mice that were exposed to TiO2 or asbestos. WT alveolar macrophages had a loss in ∆ψm, which was not seen in MCU+/− alveolar macrophages from mice that were exposed to asbestos (Fig. 2A). Quantification revealed that the ∆ψm was significantly reduced in asbestos-exposed WT macrophages, unlike in MCU+/− macrophages (Fig. 2B).
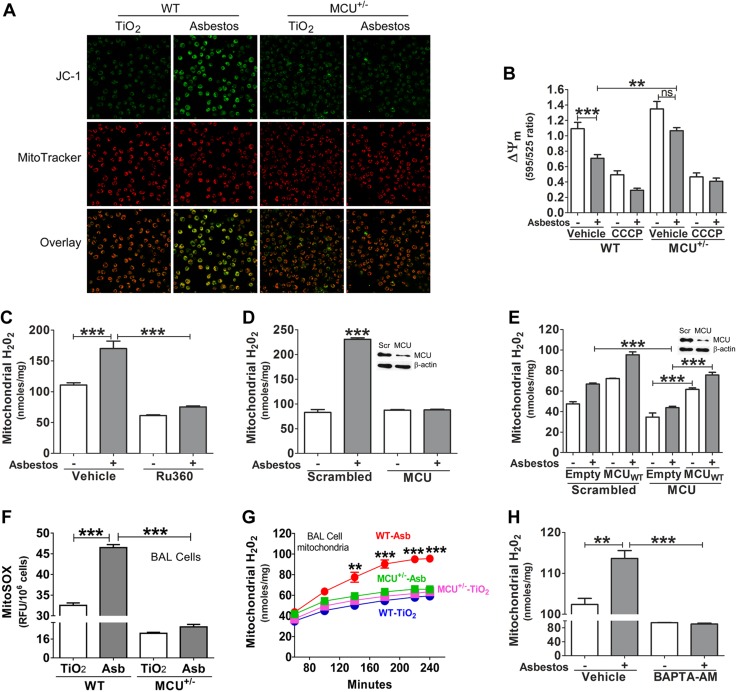
Figure 2.
MCU is required for mitochondrial ROS production in macrophages. A) ∆ψm in BAL cells from mice visualized by confocal microscopy using MitoTracker Red and JC-1 as indicators. B) ∆ψm was quantified with JC-1 dye on a multimode plate reader (n = 8). C, D) THP-1 cells were treated with vehicle or Ru360 (10 μm, 30 min; C) or transfected with scrambled (Scr) or MCU small interfering RNA (siRNA; D) and then exposed to asbestos for 30 min. H2O2 production was measured in isolated mitochondria by pHPA assay (n = 5). E) THP-1 cells were transfected with MCU siRNA, followed by empty or MCUWT transfection. Cells were exposed to asbestos (Asb) for 30 min. Mitochondrial H2O2 were quantitated by pHPA assay (n = 5). WT and MCU+/− mice were intratracheally exposed to 125 μg TiO2 or asbestos. Alveolar macrophages were obtained by BAL after 21 d. F, G) Mitochondrial superoxide (n = 7; F) and H2O2 generation (n = 3; G) from alveolar macrophages were measured by MitoSOX and pHPA assay, separately. H) THP-1 cells were transfected to overexpress MCUWT and were cultured in the presence of vehicle or BAPTA-AM overnight. Cells were exposed to asbestos for 30 min, and H2O2 was measured by pHPA assay (n = 3). CCCP, carbonyl cyanide m-chlorophenyl hydrazone; pHPA, parahydroxyphenylacetic acid ns, not significant. One-way ANOVA with Tukey’s comparison was applied in all assays, except panel A. **P < 0.01; ***P ≤ 0.001.
Because alveolar macrophages from patients with pulmonary fibrosis have greater mitochondrial H2O2 generation compared with normal participants (4, 5, 13), we determined whether MCU was necessary for mitochondrial ROS production in asbestos-exposed macrophages. Asbestos exposure induced a significant generation of mitochondrial H2O2 compared with unexposed vehicle control (Fig. 2C). This increase was inhibited in macrophages that were treated with the MCU inhibitor, Ru360. MCU silencing by small interfering RNA revealed similar results (Fig. 2D). We determined whether this effect on mitochondrial H2O2 generation was directly a result of MCU. Overexpression of MCUWT rescued mitochondrial H2O2 to control levels in cells with MCU silenced, which suggested that MCU, in part, is responsible for mitochondrial ROS generation (Fig. 2E).
In vivo, alveolar macrophages from asbestos-exposed WT mice had a robust increase in mitochondrial superoxide, which was reduced below control levels in MCU+/− mice (Fig. 2F). In addition, alveolar macrophages from asbestos-exposed WT mice had a significant increase in mitochondrial H2O2, whereas H2O2 generation in asbestos-exposed MCU+/− mice was near the level observed in TiO2-exposed mice (Fig. 2G). We determined whether mitochondrial Ca2+ influx directly regulated mitochondrial ROS generation. BAPTA-AM significantly decreased macrophage mitochondrial H2O2 generation below vehicle control level (Fig. 2H). In aggregate, these data suggest that MCU contributes to asbestos-mediated mitochondrial ROS generation in macrophages.
MCU triggers alveolar macrophage profibrotic polarization
A predominance of alternatively activated, profibrotic macrophages in the lung is implicated in the development of pulmonary fibrosis (4, 22, 24). Because mitochondrial ROS modulates macrophage phenotype, we questioned whether MCU had a role in the profibrotic polarization of macrophages. Alveolar macrophages represented the majority of cells in BAL from asbestos-exposed WT and MCU+/− mice (Fig. 3A). Quantification revealed that alveolar macrophages comprised >90% of cells in BAL (Fig. 3B). BAL cells from TiO2-exposed WT and MCU+/− mice showed a cell differential that was similar to asbestos-exposed mice (data not shown).
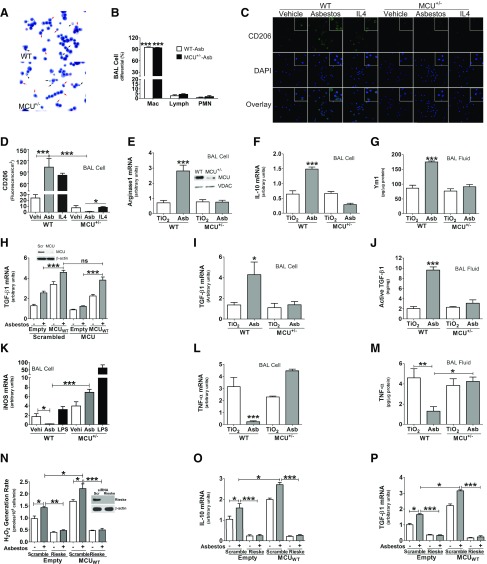
Figure 3.
MCU triggers alveolar macrophage profibrotic polarization. A) BAL cell differential from asbestos (Asb)-exposed WT or MCU mice was determined by Wright-Giemsa staining. Macrophages, red arrow; lymphocytes, black arrow; neutrophils, black arrowhead. B) Statistical quantitation of cell differential (n = 6). C) BAL cells were exposed to vehicle, asbestos, or mouse IL-4 (20 ng/ml, positive control) overnight. Cells were then fixed and stained to measure CD206 by confocal (magnification, ×60; n = 5). D) CD206 fluorescence/cm2 was quantitated by ImageJ (n = 5). WT and MCU+/− mice were exposed to TiO2 or asbestos for 21 d. Total RNA was prepared in alveolar macrophages. E, F) Quantitative real-time PCR for arginase1 (E) and IL-10 mRNA (F) was performed and normalized to β-actin (n = 6). Inset: MCU immunoblot analysis in macrophage mitochondria. G) Ym1 was quantitated in BAL fluid with ELISA (n = 6). H) MH-S cells were transfected with MCU small interfering RNA (siRNA), followed by empty or MCUWT transfection. Cells were exposed to asbestos for 4 h. TGF-β1 mRNA was determined and normalized to β-actin (n = 6). MCU immunoblot analysis in macrophages (inset). WT and MCU+/− mice were exposed to TiO2 or asbestos for 21 d. Alveolar macrophages and BAL fluid were obtained by BAL. I, J) Real-time quantitative PCR was performed for TGF-β1 mRNA in alveolar macrophages (n = 6) (I) and active TGF-β1 was measured by ELISA in BAL fluid (n = 7; J). K) BAL cells were exposed to vehicle, asbestos, or LPS (1 µg/ml, positive control) for 4 h. Total RNA was extracted to determine the iNOS mRNA level by real-time quantitative PCR (n = 6). L) Mice were exposed to TiO2 or asbestos for 21 d. Total RNA was extracted from BAL cells to determine TNF-α mRNA levels (n = 6). M) TNF-α protein level was determined in BAL fluid with ELISA (n = 6). N) THP-1 cells were cotransfected with scrambled (Scr) or Rieske siRNA combined with MCUWT plasmid. Cells were exposed to asbestos for 30 min. pHPA was performed to quantitate mitochondrial H2O2 production. P, Q) Real-time quantitative PCR was performed for IL-10 mRNA (n = 5; P) and TGF-β1 mRNA (n = 5; Q). Lymph, lymphocyte; mac, macrophage; ns, not significant; PMN, polymorphonuclear leukocyte. One-way ANOVA with Tukey’s post hoc comparison for all assays. *P < 0.05; **P < 0.01; ***P ≤ 0.001.
Asbestos exposure increased the number of CD206+ cells in WT BAL macrophages, similar to IL-4 stimulation, which was used as a positive control (Fig. 3C). In contrast, MCU+/− macrophages had essentially no positive cells with asbestos exposure or IL-4 stimulation. Quantification showed that CD206 expression in MCU+/− macrophages was significantly less than in vehicle control (Fig. 3D). Arginase1 (Fig. 3E) and IL-10 (Fig. 3F) gene expression, as well as Ym1 protein, (Fig. 3G), were increased in asbestos-exposed WT mice, whereas all of these markers were significantly reduced to control levels in MCU+/− mice. In vitro, asbestos exposure also potentiated FIZZ1 (Supplemental Fig. S1A) and VEGF-A (Supplemental Fig. S1B) gene expression in WT BAL macrophages, whereas MCU-deficient macrophages significantly decreased expression even with IL-4 stimulation.
Because profibrotic alveolar macrophages produce TGF-β1, and alveolar macrophages are a critical source of TGF-β1 in the lung (4), we investigated whether MCU modulated TGF-β1 gene expression. Asbestos-exposed macrophages that expressed an empty vector had an increase in TGF-β1 gene expression, which was further increased with MCUWT overexpression (Fig. 3H). TGF-β1 gene expression was significantly reduced when MCU was silenced, whereas MCUWT overexpression recovered TGF-β1 mRNA expression to greater levels than those observed in control cells, which suggested that MCU is necessary for TGF-β1 expression in macrophages.
These findings were recapitulated in vivo. Asbestos-exposed WT mice had a 4-fold increase in TGF-β1 gene expression, whereas no difference was detected in TiO2- or asbestos-exposed MCU+/− mice (Fig. 3I). Reduced TGF-β1 gene expression in MCU+/− mice corresponded with less active TGF-β1 in BAL fluid compared with WT mice (Fig. 3J).
In contrast to TGF-β1 expression, the classically activated genes in macrophages were up-regulated in MCU+/− mice. Specifically, iNOS (Fig. 3K) and TNF-α (Fig. 3L and Supplemental Fig. S1C) gene expression in BAL cells and TNF-α protein expression in BAL fluid (Fig. 3M) were significantly decreased in alveolar macrophages from asbestos-exposed WT mice, whereas alveolar macrophages from MCU+/− mice had a significant increase in the expression of these antifibrotic genes. These observations suggest that MCU has a critical role in modulating the macrophage phenotype.
To determine whether MCU-mediated mitochondrial ROS had a direct role in macrophage profibrotic polarization, we silenced Rieske, the iron-sulfur protein in complex III. Exposure to asbestos induced mitochondrial H2O2 production, which was further increased by MCUWT overexpression (Fig. 3N). Silencing Rieske reduced H2O2 generation significantly below that of control levels. Expression of profibrotic genes, IL-10 (Fig. 3O) and TGF-β1 (Fig. 3P), mirrored mitochondrial ROS generation. These observations suggest that MCU contributes to the profibrotic polarization of macrophages by modulating mitochondrial ROS.
Macrophage mitochondrial ATP synthesis requires MCU
Because energy production is essential for macrophage function and mitochondria are the primary site for energy production (30), we determined whether MCU contributed to mitochondrial ATP production. Asbestos-exposed macrophages had a significant increase in ATP in isolated mitochondria, and silencing MCU significantly reduced ATP levels below those of control levels (Fig. 4A). Overexpression of MCUWT enhanced ATP production compared with cells that expressed an empty vector, whereas ATP was significantly reduced in cells that expressed the MCUDN (Fig. 4B).
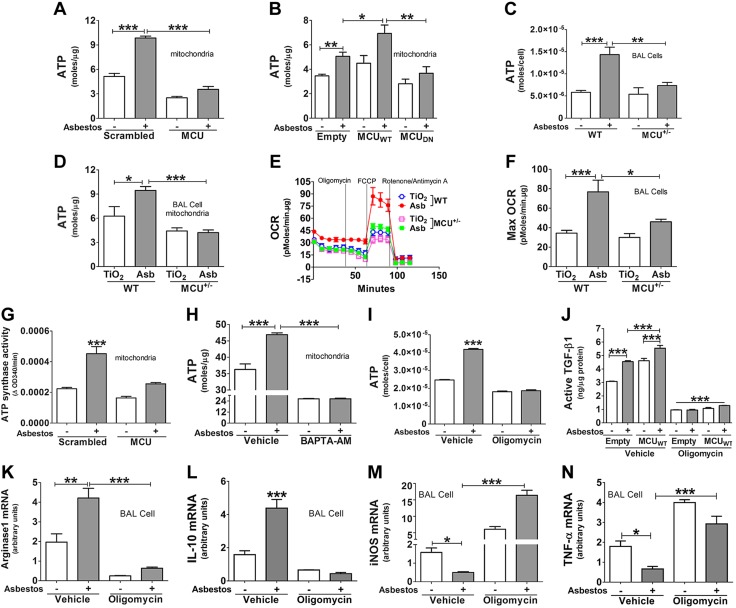
Figure 4.
MCU modulates oxygen consumption and ATP production in profibrotic macrophages. A, B) MH-S cells were transfected with scrambled (Scr) or MCU small interfering RNA (siRNA; n = 5; A) or empty, MCUWT, or MCUDN plasmids (n = 4; B). ATP was measured in isolated mitochondria. C) Alveolar macrophages from WT and MCU+/− mice were exposed to asbestos ex vivo. ATP was measured in cells (n = 5). D) Mice were intratracheally exposed to TiO2 or asbestos (Asb). At d 21, ATP was measured in isolated alveolar macrophage mitochondria (n = 6) or OCR was measured by using a Seahorse XF24 bioanalyzer (E; n = 5). F) Maximum OCR was determined from panel E. G) THP-1 were transfected with scrambled or MCU siRNA and exposed to asbestos for 30 min. ATP synthase activity was measured in isolated mitochondria (n = 4). H) THP-1 cells were transfected with MCUWT and treated BAPTA-AM (5 μM, overnight). Cells were exposed to asbestos, and ATP was measured in mitochondria (n = 4). I) MH-S cells were treated with vehicle or oligomycin (10 µg/ml, 1 h), followed by asbestos exposure for 30 min. Cellular ATP was measured (n = 8). J) MH-S cells were transfected with either empty vector or MCUWT and were pretreated with vehicle or oligomycin (10 μg/ml, 1 h) before asbestos exposure for overnight. Conditioned medium was collected for ELISA quantitating active TGF-β1 (n = 6). BAL cells were treated with oligomycin (10 µg/ml, 1 h), followed by asbestos exposure for 4 h. K–N) Total RNA was extracted to determine arginase1 (K) and IL-10 (L) as well as iNOS (M) and TNF-α (n = 4; N). One-way ANOVA with Tukey’s post hoc comparison for all assays, except panel E. *P < 0.05; **P < 0.01; ***P ≤ 0.001.
To determine whether ATP production in macrophages was relevant biologically in primary cells, ATP was measured in alveolar macrophages from mice exposed ex vivo. ATP production was significantly elevated in asbestos-exposed WT macrophages, whereas no change was seen in MCU+/− cells (Fig. 4C). Similar findings were seen in vivo. Alveolar macrophages from asbestos-exposed WT mice had a significant increase in mitochondrial ATP compared to alveolar macrophages from TiO2-exposed WT mice, while ATP in cells from the asbestos-exposed MCU+/− mice were similar to that in macrophages from TiO2-exposed WT mice (Fig. 4D).
Profibrotic macrophages rely on oxidative phosphorylation for energy metabolism, and increased mitochondrial Ca2+ augments oxidative phosphorylation (31, 32). Because oxidative phosphorylation is coupled with increased oxygen consumption, we asked whether MCU modulated OCR in asbestos-exposed mice. OCR in alveolar macrophages from asbestos-exposed WT mice was significantly increased compared to the OCR in macrophages from the TiO2-exposed WT mice and the MCU+/− mice exposed to either TiO2 or asbestos (Fig. 4E, F). These data suggest that MCU is required for oxygen consumption in profibrotic macrophages.
Because ATP synthase (complex V) has a direct role in mitochondrial ATP synthesis, enzyme activity was measured in isolated mitochondria. Asbestos induced significantly higher complex V activity, while silencing MCU reduced activity to control levels (Fig. 4G). The requirement of MCU-mediated mitochondrial Ca2+ influx for ATP production was verified by chelating cytoplasmic Ca2+ with BAPTA-AM, which reduced mitochondrial ATP content below control levels (Fig. 4H).
To determine whether mitochondrial ATP production promotes macrophage profibrotic polarization, cells were treated with oligomycin, which specifically inhibits ATP synthase activity. Oligomycin significantly reduced ATP levels in asbestos-exposed macrophages (Fig. 4I). Asbestos-exposed macrophages that were treated with vehicle had a significant increase in active TGF-β1 in conditioned media, and MCUWT overexpression further increased active TGF-β1 (Fig. 4J). Inhibition of ATP synthesis with oligomycin markedly reduced active TGF-β1 below levels in empty control cells. Other profibrotic genes, arginase1 (Fig. 4K) and IL-10 (Fig. 4L), were regulated in a similar manner by oligomycin. In contrast, the antifibrotic markers, iNOS (Fig. 4M) and TNF-α (Fig. 4N), were significantly increased by oligomycin. In aggregate, these data suggest that macrophage MCU directly regulates mitochondrial energy production, which is required for the profibrotic polarization of alveolar macrophages.
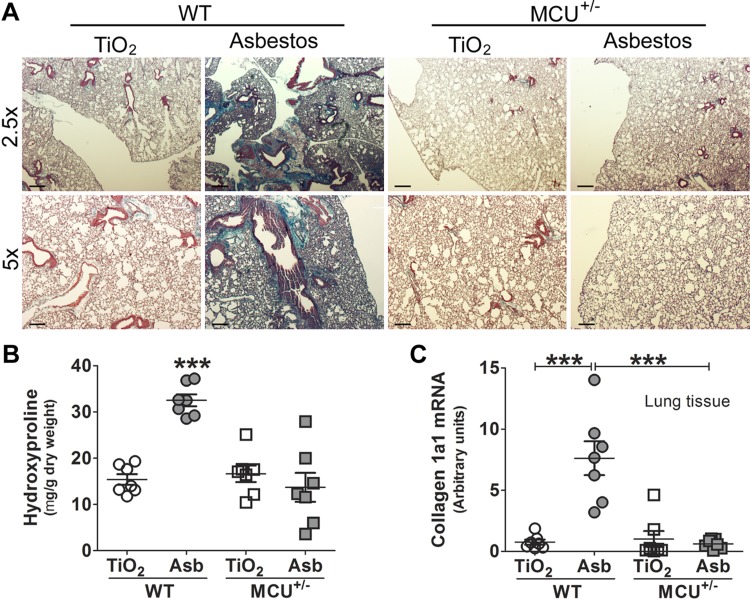
MCU+/− mice are protected from asbestos-induced pulmonary fibrosis
Because macrophages from MCU+/− mice had attenuation of profibrotic polarization, we hypothesized that MCU is necessary for the development of pulmonary fibrosis. MCU deficiency had no effect on the lung parenchyma in TiO2-exposed mice (Fig. 5A). There was dense collagen deposition and distortion of the lung architecture in asbestos-exposed WT mice, whereas MCU+/− mice had essentially normal lung architecture without collagen accumulation. Histologic findings were confirmed biochemically by hydroxyproline assay (Fig. 5B). Collagen 1a1 mRNA was also increased in lungs from asbestos-exposed WT mice (Fig. 5C). These data suggest that MCU and [Ca2+]mt are linked to fibrotic repair.
Figure 5.
MCU+/− mice are protected from asbestos (Asb)-induced pulmonary fibrosis. A) Mice were exposed to TiO2 or asbestos. At d 21, lungs were subjected to Masson’s trichrome stain. Representative micrographs from 7 mice per condition are shown. Scale bars, 500 µm at ×2.5 and 200 µm at ×5. B, C) Lung collagen content was measured by hydroxyproline assay (n = 7; B) and collagen 1a1 mRNA levels (n = 7; C). One-way ANOVA with Tukey’s post hoc comparison (B, C). ***P ≤ 0.001.
DISCUSSION
The dynamic functions of mitochondria are associated with multiple end-organ diseases. Ca2+ influx into the mitochondria is critical in the regulation of mitochondria-mediated diseases. MCU mediates mitochondrial Ca2+ influx, which suggests the potential participation of MCU in mitochondria-mediated diseases. The fundamental role of MCU has been highlighted in several studies. In the heart, MCU is necessary for physiologic heart rate acceleration, and its inhibition reduced inappropriate acceleration and impaired mechanical performance with pacing (33, 34). In skeletal muscle, MCU inhibition leads to muscle atrophy, and its overexpression leads to skeletal muscle hypertrophy (35). Although MCU expression in the brain is low, it is involved in early brain injury after subarachnoid hemorrhage (36, 37). In the lung, Ca2+ homeostasis is altered in cystic fibrosis (38), but the function of MCU in cystic fibrosis has not been investigated. Synchronized Ca2+ waves can act as intercommunications between sessile alveolar macrophages and alveolar epithelial cells to modulate immunologic responses to inhaled pathogens (39); however, the link between MCU and pulmonary fibrosis is not known. Our observations demonstrate that patients with pulmonary fibrosis have increased mitochondrial Ca2+ in alveolar macrophages and that MCU deficiency protects mice from pulmonary fibrosis.
Previous studies have demonstrated that after lung injury, the polarization of macrophages to a profibrotic phenotype contributes to the development of pulmonary fibrosis (2, 22, 24). Macrophage-derived TGF-β1 contributes to pulmonary fibrosis because mice that harbor a deletion of TGF-β1 in macrophages are protected from bleomycin-induced lung injury and fibrosis (4). Several studies have shown that TGF-β1 induces Ca2+ influx into the cytoplasm in multiple cell types. These studies suggest that TGF-β1 mediates Ca2+ signaling, which results in alteration in the actin cytoskeleton (40), cell adhesion (41), and apoptosis (42), depending on the stimulus and cell type. Regulation of TGF-β1 expression by Ca2+ has not been reported. Our prior observations have shown that cytosolic ROS in macrophages is not altered by asbestos exposure (13); however, the data in the current study suggest the importance of MCU-mediated mitochondrial ROS and ATP production in modulating the expression of macrophage-derived TGF-β1.
Profibrotic macrophages utilize oxidative phosphorylation that is associated with increased OCR (43). Although MCU has been linked to glycolysis in pancreatic β-cells (30), MCUDN mice have OCR that is similar to WT mice under normal conditions secondary to alterations in cytoplasmic Ca2+ levels (34). Previous studies have shown increased levels of ATP in the BAL fluid of patients with lung fibrosis compared with normal participants (44). Our study showed that MCU up-regulates mitochondrial ATP production by enhancing macrophage oxygen consumption. This finding is in line with a report that showed that skeletal muscle requires increased mitochondrial oxygen consumption for ATP production (45).
The alternative activation of macrophages requires energy production via the sensor AMPK (43). To our knowledge, no study has linked mitochondrial ATP production to the profibrotic polarization of macrophages. Our observations reveal that inhibition of mitochondrial ATP production with oligomycin abrogated profibrotic polarization and enhanced antifibrotic gene expression. This was also observed in MCU-deficient mice, as these mice exhibited a lower level of mitochondrial ATP and had macrophages with an antifibrotic phenotype. These observations suggest the importance of mitochondrial ATP for profibrotic macrophage polarization and pulmonary fibrosis. Understanding the modulation of MCU and regulation of mitochondrial Ca2+ may provide an important therapeutic target for humans with fibrosis.
ACKNOWLEDGMENTS
This work was supported, in whole or in part, by the U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences (Grant 2R01-ES015981-10). This work was also supported by a Merit Review from the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development Grant 1BX001135-04). OCR assays were supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases (Grant P30-DK079626). The authors thank the Diabetes Research Center Bio-Analytical Redox Biology Core, Douglas R. Moellering, and Praveen Vayalill (both from the University of Alabama at Birmingham) for technical assistance on the Seahorse assay.
Glossary
- ∆ψm
mitochondrial membrane potential
- AAM
alternatively activated macrophage
- BAL
bronchoalveolar lavage
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl ester)
- [Ca2+]mt
mitochondrial calcium concentration
- MCU
mitochondrial calcium uniporter
- MCUDN
dominate negative MCU
- MCUWT
wild-type MCU
- OCR
oxygen consumption rate
- ROS
reactive oxygen species
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. Gu and A. B. Carter developed the hypothesis and designed the experiments; L. Gu and J. L. Larson-Casey performed the experiments; L. Gu, J. L. Larson-Casey and A. B. Carter analyzed the data; and L. and Gu and A. B. Carter wrote the paper.
REFERENCES
- 1.Duffield J. S., Forbes S. J., Constandinou C. M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J. P. (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redente E. F., Keith R. C., Janssen W., Henson P. M., Ortiz L. A., Downey G. P., Bratton D. L., Riches D. W. (2014) Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am. J. Respir. Cell Mol. Biol. 50, 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwata Y., Boström E. A., Menke J., Rabacal W. A., Morel L., Wada T., Kelley V. R. (2012) Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J. Immunol. 188, 4568–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson-Casey J. L., Deshane J. S., Ryan A. J., Thannickal V. J., Carter A. B. (2016) Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44, 582–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborn-Heaford H. L., Murthy S., Gu L., Larson-Casey J. L., Ryan A. J., Shi L., Glogauer M., Neighbors J. D., Hohl R., Brent Carter A. (2015) Targeting the isoprenoid pathway to abrogate progression of pulmonary fibrosis. Free Radic. Biol. Med. 86, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborn-Heaford H. L., Ryan A. J., Murthy S., Racila A. M., He C., Sieren J. C., Spitz D. R., Carter A. B. (2012) Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J. Biol. Chem. 287, 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirichok Y., Krapivinsky G., Clapham D. E. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 [DOI] [PubMed] [Google Scholar]
- 8.Patron M., Raffaello A., Granatiero V., Tosatto A., Merli G., De Stefani D., Wright L., Pallafacchina G., Terrin A., Mammucari C., Rizzuto R. (2013) The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J. Biol. Chem. 288, 10750–10758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallilankaraman K., Doonan P., Cárdenas C., Chandramoorthy H. C., Müller M., Miller R., Hoffman N. E., Gandhirajan R. K., Molgó J., Birnbaum M. J., Rothberg B. S., Mak D. O., Foskett J. K., Madesh M. (2012) MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151, 630–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patron M., Checchetto V., Raffaello A., Teardo E., Vecellio Reane D., Mantoan M., Granatiero V., Szabò I., De Stefani D., Rizzuto R. (2014) MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell 53, 726–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson M. J., Månsson R., Morota S., Uchino H., Kallur T., Sumi T., Ishii N., Shimazu M., Keep M. F., Jegorov A., Elmér E. (2008) Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 45, 284–294 [DOI] [PubMed] [Google Scholar]
- 12.Kovac S., Domijan A. M., Walker M. C., Abramov A. Y. (2014) Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 5, e1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He C., Murthy S., McCormick M. L., Spitz D. R., Ryan A. J., Carter A. B. (2011) Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J. Biol. Chem. 286, 15597–15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T. R., Horowitz J. C., Pennathur S., Martinez F. J., Thannickal V. J. (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 15, 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T., O’Rourke B. (2008) Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ. Res. 103, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths E. J., Rutter G. A. (2009) Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta 1787, 1324–1333 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 [DOI] [PubMed] [Google Scholar]
- 18.Karlmark K. R., Weiskirchen R., Zimmermann H. W., Gassler N., Ginhoux F., Weber C., Merad M., Luedde T., Trautwein C., Tacke F. (2009) Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 [DOI] [PubMed] [Google Scholar]
- 19.Shaughnessy L. M., Swanson J. A. (2007) The role of the activated macrophage in clearing Listeria monocytogenes infection. Front. Biosci. 12, 2683–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh T., Takeuchi O., Vandenbon A., Yasuda K., Tanaka Y., Kumagai Y., Miyake T., Matsushita K., Okazaki T., Saitoh T., Honma K., Matsuyama T., Yui K., Tsujimura T., Standley D. M., Nakanishi K., Nakai K., Akira S. (2010) The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 [DOI] [PubMed] [Google Scholar]
- 21.Kawamura K., Komohara Y., Takaishi K., Katabuchi H., Takeya M. (2009) Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol. Int. 59, 300–305 [DOI] [PubMed] [Google Scholar]
- 22.Murthy S., Larson-Casey J. L., Ryan A. J., He C., Kobzik L., Carter A. B. (2015) Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 29, 3527–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stahl M., Schupp J., Jäger B., Schmid M., Zissel G., Müller-Quernheim J., Prasse A. (2013) Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS One 8, e81382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C., Ryan A. J., Murthy S., Carter A. B. (2013) Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J. Biol. Chem. 288, 20745–20757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C., Larson-Casey J. L., Gu L., Ryan A. J., Murthy S., Carter A. B. (2016) Cu,Zn- superoxide dismutase-mediated redox regulation of Jumonji domain containing 3 modulates macrophage polarization and pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 55, 58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson-Casey J. L., Murthy S., Ryan A. J., Carter A. B. (2014) Modulation of the mevalonate pathway by akt regulates macrophage survival and development of pulmonary fibrosis. J. Biol. Chem. 289, 36204–36219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy S., Ryan A., He C., Mallampalli R. K., Carter A. B. (2010) Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 285, 25062–25073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy S., Adamcakova-Dodd A., Perry S. S., Tephly L. A., Keller R. M., Metwali N., Meyerholz D. K., Wang Y., Glogauer M., Thorne P. S., Carter A. B. (2009) Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L846–L855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan A. J., Larson-Casey J. L., He C., Murthy S., Carter A. B. (2014) Asbestos-induced disruption of calcium homeostasis induces endoplasmic reticulum stress in macrophages. J. Biol. Chem. 289, 33391–33403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarasov A. I., Griffiths E. J., Rutter G. A. (2012) Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 52, 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glancy B., Willis W. T., Chess D. J., Balaban R. S. (2013) Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52, 2793–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Prados J. C., Través P. G., Cuenca J., Rico D., Aragonés J., Martín-Sanz P., Cascante M., Boscá L. (2010) Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 [DOI] [PubMed] [Google Scholar]
- 33.Wu Y., Rasmussen T. P., Koval O. M., Joiner M. L., Hall D. D., Chen B., Luczak E. D., Wang Q., Rokita A. G., Wehrens X. H., Song L. S., Anderson M. E. (2015) The mitochondrial uniporter controls fight or flight heart rate increases. Nat. Commun. 6, 6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen T. P., Wu Y., Joiner M. L., Koval O. M., Wilson N. R., Luczak E. D., Wang Q., Chen B., Gao Z., Zhu Z., Wagner B. A., Soto J., McCormick M. L., Kutschke W., Weiss R. M., Yu L., Boudreau R. L., Abel E. D., Zhan F., Spitz D. R., Buettner G. R., Song L. S., Zingman L. V., Anderson M. E. (2015) Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc. Natl. Acad. Sci. USA 112, 9129–9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammucari C., Gherardi G., Zamparo I., Raffaello A., Boncompagni S., Chemello F., Cagnin S., Braga A., Zanin S., Pallafacchina G., Zentilin L., Sandri M., De Stefani D., Protasi F., Lanfranchi G., Rizzuto R. (2015) The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 10, 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan H., Zhang D., Hao S., Li K., Hang C. H. (2015) Role of mitochondrial calcium uniporter in early brain injury after experimental subarachnoid hemorrhage. Mol. Neurobiol. 52, 1637–1647 [DOI] [PubMed] [Google Scholar]
- 38.Rimessi A., Bezzerri V., Patergnani S., Marchi S., Cabrini G., Pinton P. (2015) Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 6, 6201 [DOI] [PubMed] [Google Scholar]
- 39.Westphalen K., Gusarova G. A., Islam M. N., Subramanian M., Cohen T. S., Prince A. S., Bhattacharya J. (2014) Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506, 503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGowan T. A., Madesh M., Zhu Y., Wang L., Russo M., Deelman L., Henning R., Joseph S., Hajnoczky G., Sharma K. (2002) TGF-beta-induced Ca2+ influx involves the type III IP3 receptor and regulates actin cytoskeleton. Am. J. Physiol. Renal Physiol. 282, F910–F920 [DOI] [PubMed] [Google Scholar]
- 41.Nesti L. J., Caterson E. J., Li W. J., Chang R., McCann T. D., Hoek J. B., Tuan R. S. (2007) TGF-beta1 calcium signaling in osteoblasts. J. Cell. Biochem. 101, 348–359 [DOI] [PubMed] [Google Scholar]
- 42.Gizatullina Z. Z., Grapengiesser E., Shabalina I. G., Nedergaard J., Heldin C. H., Aspenström P. (2003) Effect of transforming growth factor-beta on calcium homeostasis in prostate carcinoma cells. Biochem. Biophys. Res. Commun. 304, 643–649 [DOI] [PubMed] [Google Scholar]
- 43.Ouimet M., Ediriweera H. N., Gundra U. M., Sheedy F. J., Ramkhelawon B., Hutchison S. B., Rinehold K., van Solingen C., Fullerton M. D., Cecchini K., Rayner K. J., Steinberg G. R., Zamore P. D., Fisher E. A., Loke P., Moore K. J. (2015) MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Invest. 125, 4334–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., Kanellopoulos J., Quesniaux V. F., Marchand-Adam S., Crestani B., Ryffel B., Couillin I. (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 182, 774–783 [DOI] [PubMed] [Google Scholar]
- 45.O’Hagan K. A., Cocchiglia S., Zhdanov A. V., Tambuwala M. M., Cummins E. P., Monfared M., Agbor T. A., Garvey J. F., Papkovsky D. B., Taylor C. T., Allan B. B. (2009) PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc. Natl. Acad. Sci. USA 106, 2188–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]