Abstract
Lipodystrophy is a rare disorder characterized by complete or partial loss of adipose tissue. Patients with lipodystrophy exhibit hypertriglyceridemia, severe insulin resistance, type 2 diabetes, and nonalcoholic steatohepatitis (NASH). Efforts to ameliorate NASH in lipodystrophies with pharmacologic agents have met with limited success. We examined whether a controlled-release mitochondrial protonophore (CRMP) that produces mild liver-targeted mitochondrial uncoupling could decrease hypertriglyceridemia and reverse NASH and diabetes in a mouse model (fatless AZIP/F-1 mice) of severe lipodystrophy and diabetes. After 4 wk of oral CRMP (2 mg/kg body weight per day) or vehicle treatment, mice underwent hyperinsulinemic-euglycemic clamps combined with radiolabeled glucose to assess liver and muscle insulin responsiveness and tissue lipid measurements. CRMP treatment reversed hypertriglyceridemia and insulin resistance in liver and skeletal muscle. Reversal of insulin resistance could be attributed to reductions in diacylglycerol content and reduced PKC-ε and PKC-θ activity in liver and muscle respectively. CRMP treatment also reversed NASH as reflected by reductions in plasma aspartate aminotransferase and alanine aminotransferase concentrations; hepatic steatosis; and hepatic expression of IL-1α, -β, -2, -4, -6, -10, -12, CD69, and caspase 3 and attenuated activation of the IRE-1α branch of the unfolded protein response. Taken together, these results provide proof of concept for the development of liver-targeted mitochondrial uncoupling agents as a potential novel therapy for lipodystrophy-associated hypertriglyceridemia, NASH and diabetes.—Abulizi, A., Perry, R. J., Camporez, J. P. G., Jurczak, M. J., Petersen, K. F., Aspichueta, P., Shulman, G. I. A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice.
Keywords: NAFLD, NASH, type 2 diabetes, insulin resistance, mitochondrial uncoupling
Lipodystrophies are a heterogenous group of adipose tissue disorders characterized by selective loss of subcutaneous and visceral fat and, as a consequence, leptin deficiency, and hyperphagia. The disease is associated with hypertriglyceridemia, nonalcoholic fatty liver disease (NAFLD), steatohepatitis (NASH), severe insulin resistance, and type 2 diabetes (1–5). Leptin replacement therapy is effective at reversing NAFLD and diabetes in patients with severe, generalized lipodystrophy, an effect that can mostly be attributed to reductions in hyperphagia (6, 7). In contrast, partial forms of lipodystrophy share these metabolic abnormalities, but because circulating leptin levels are proportionately higher, even reaching normal values in some patients (8), leptin replacement therapy in these patients is not effective in improving fasting and postprandial hyperglycemia, insulin resistance, and hemoglobin 1Ac levels (9, 10). Recently, we developed an orally available, controlled-release, liver-targeted mitochondrial protonophore (CRMP) and showed that this agent reduced hepatic steatosis and reversed diabetes in rodent models of type 2 diabetes (11). To examine whether CRMP might also be effective in reversing hypertriglyceridemia, insulin resistance, diabetes, and NAFLD/NASH in lipodystrophy, we treated AZIP/F-1 fatless mice, which express a transgene encoding a dominant-negative protein that inhibits the transcriptional regulation of adipogenesis, thus preventing fat tissue development, with CRMP for 4 wk followed by comprehensive metabolic phenotyping. Similar to humans with lipodystrophy, AZIP/F-1 mice display hyperlipidemia, NASH, and diabetes (3, 12).
MATERIALS AND METHODS
Animal care
All experimental procedures were approved by and conducted in accordance with the Institutional Animal Care and Use Committee guidelines of Yale University School of Medicine. Regular chow-fed male fatless AZIP/F-1 hemizygous mice on the C57BL6 background were randomly divided into 2 groups. They were treated daily with CRMP (2 mg/kg) mixed in a small amount (0.2 ∼ 0.3 g) of peanut butter or with peanut butter alone for 4 wk. All mice consumed the peanut butter with or without CRMP within 2 min.
Metabolic cage studies
Lean body mass was assessed by 1H-magnetic resonance spectroscopy (Bruker BioSpin, Billerica, MA, USA). A Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH, USA) was used to evaluate O2 consumption, CO2 production, energy expenditure, activity, food consumption, and drinking.
Glucose tolerance test
After withholding food for 6 h, mice were injected intraperitoneally with 1 g/kg dextrose. Plasma glucose after the injection was measured enzymatically on the YSI 2700 SELECT Biochemistry Analyzer (YSI Life Sciences, Yellow Springs, OH, USA), and plasma insulin was measured by radioimmunoassay.
Hyperinsulinemic-euglycemic clamp studies
Hyperinsulinemic-euglycemic clamps were performed in chronically catheterized awake mice as previously described (13). A jugular venous catheter was implanted 6 to 7 d before the studies were performed. To assess basal whole-body glucose turnover, [3-3H]-glucose (HPLC purified) (PerkinElmer Life Sciences, Waltham, MA, USA) was infused at a rate of 0.05 μCi/min for 120 min into the jugular catheter after withholding food overnight. After the basal period, hyperinsulinemic-euglycemic clamps were conducted for 140 min with a 4-min primed infusion of insulin [10 mU/(kg-min)] and [3-3H]-glucose (0.24 μCi/min), followed by a continuous [4.5 mU/(kg-min)] infusion of human insulin (Novolin; Novo Nordisk, Bagsværd, Denmark) and [3-3H]-glucose (0.1 μCi/min), and a variable infusion of 20% dextrose. A 10-μCi bolus of 2-deoxy-d-[1-14C]glucose (PerkinElmer) was injected after 85 min to determine insulin-stimulated tissue glucose uptake. Plasma samples were obtained from the tip of the tail at 0, 25, 45, 65, 80, 90, 100, 110, 120, 130, and 140 min. The tail cut was made at least 2 h before the first blood sample was taken to allow for acclimatization, according to standard operating procedures. Mice received an intravenous artificial plasma solution (115 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2-6H2O, 1.2 mM NaH2PO4-H2O, 1.2 mM Na2SO4, 2.5 mM CaCl-2H2O, 25 mM NaHCO3, and 4% BSA, pH 7.4) at a rate of 4.2 μl/min during the insulin-stimulated period of the clamp to compensate for volume loss secondary to blood sampling. At the end of the clamps, mice were anesthetized with sodium pentobarbital injection (150 mg/kg), and all tissues taken were snap frozen in liquid nitrogen and stored at −80°C for subsequent use.
Liver, skeletal muscle, and plasma lipid measurements
Plasma triglyceride (TAG) concentrations were measured using a DCL TG (Diagnostic Chemicals Ltd. TAG) reagent (Diagnostic Chemicals, Charlottetown, PEI, Canada) Tissue TAGs were extracted using the method of Bligh and Dyer (14) and measured using a DCL TG reagent. For diacylglycerol (DAG) extraction, livers were homogenized in a buffer solution (20 mM Tris-HCl, 1 mM EDTA, 0.25 mM EGTA, 250 mM sucrose, and 2 mM phenylmethylsulfonyl fluoride) containing a protease inhibitor mixture (Roche, Indianapolis, IN, USA), and samples were centrifuged at 100,000 g for 1 h. The supernatants containing the cytosolic fraction were collected. DAG levels were then measured by liquid chromatography tandem mass spectrometry as previously described (15). Total cytosolic DAG content is expressed as the sum of individual species. Ceramide was measured as previously described (16). All lipid measurements were done from tissues of animals after a 6-h food withdrawal.
Immunoblotting analysis
Tissues were homogenized in lysis buffer supplemented with protease inhibitor cocktail (Roche) for protein isolation. Proteins from homogenized liver tissue (30 μg of protein extracts) were electrophoretically separated by 4–12% SDS-PAGE (Invitrogen, Carlsbad, CA, USA) and then transferred to PVDF membranes (Millipore, Bedford, MA, USA) using a semidry transfer cell (Bio-Rad, Hercules, CA, USA) for 120 min. After blockade of nonspecific sites with 5% nonfat dry milk, Tris-buffered saline, and Tween 20 (10 mM Tris, 100 mM NaCl, and 0.1% Tween 20) solution, membranes were incubated overnight at 4°C with antibodies to protein kinase C (PKC)-ε (BD Transduction Laboratories, Lexington, KY, USA), PKC-θ (BD Transduction Laboratories), or CD69 (Novus Biotech, Littleton, CO, USA). For endoplasmic reticulum (ER) stress markers, after blockade with 5% bovine serum albumin Tris-buffered saline and Tween 20 (10 mM Tris, 100 mM NaCl, and 0.1% Tween 20) solution, membranes were incubated overnight at 4°C with antibodies against IRE1α (Cell Signaling Technology, Danvers, MA, USA), p-IRE1-α (Abcam, Cambridge, United Kingdom), eiF2-α (Santa Cruz Biotechnology, Dallas, TX, USA), p-eiF2-α (Cell Signaling Technology), KDEL (GRP78 and -94) (Enzo Life Science, Farmingdale, NY, USA), and GAPDH (Cell Signaling Technology). After washing with Tris-buffered saline and Tween 20, membranes were incubated with peroxidase-conjugated anti-rabbit and anti-mouse antibody. Membranes were thoroughly washed, and immune complexes were detected using an enhanced luminol chemiluminescence system (Thermo Fisher Scientific, Rockford, IL, USA) and subjected to photographic films. Signals on the immunoblot were quantified by optical densitometry (Scion Image Software, Frederick, MD, USA).
Markers of liver apoptosis and inflammation
Caspase-3 and inflammatory cytokines were measured in liver homogenates by ELISA (MyBioSource, San Diego, CA, USA and Qiagen, Hilden, Germany) and were normalized to total protein content measured by Bradford assay.
Histology studies
Liver samples were prepared and stained with Oil Red O by the Yale Research Histology core and analyzed as described in Kleiner et al. (17).
Statistical analysis
All data are expressed as means ± sem. Results were assessed using 2-tailed, unpaired Student’s t test (Prism 5; GraphPad, La Jolla, CA, USA). A value of P < 0.05 was considered significant.
RESULTS
Energy expenditure and food intake
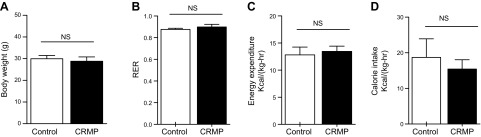
To examine the effect of CRMP on whole-body energy metabolism in AZIP/F-1 mice, we performed metabolic cage studies after 4 wk of treatment. In agreement with previous observations in wild-type C57BL/6J mice (11), short-term treatment with CRMP did not alter body weight in AZIP/F-1 mice compared with control mice. Consistent with this observation, there were also no observed differences between groups in respiratory quotient, energy expenditure, and feeding (Fig. 1).
Figure 1.
Daily CRMP treatment did not alter whole-body energy balance in mice. A) Body weight. B) Respiratory exchange ratio (RER). C) Energy expenditure throughout the day. D) Food intake. Data are means ± sem (n = 6 per group). Statistical significance was determined by Student’s t test. NS, not significant.
CRMP improved glucose tolerance and whole-body insulin sensitivity
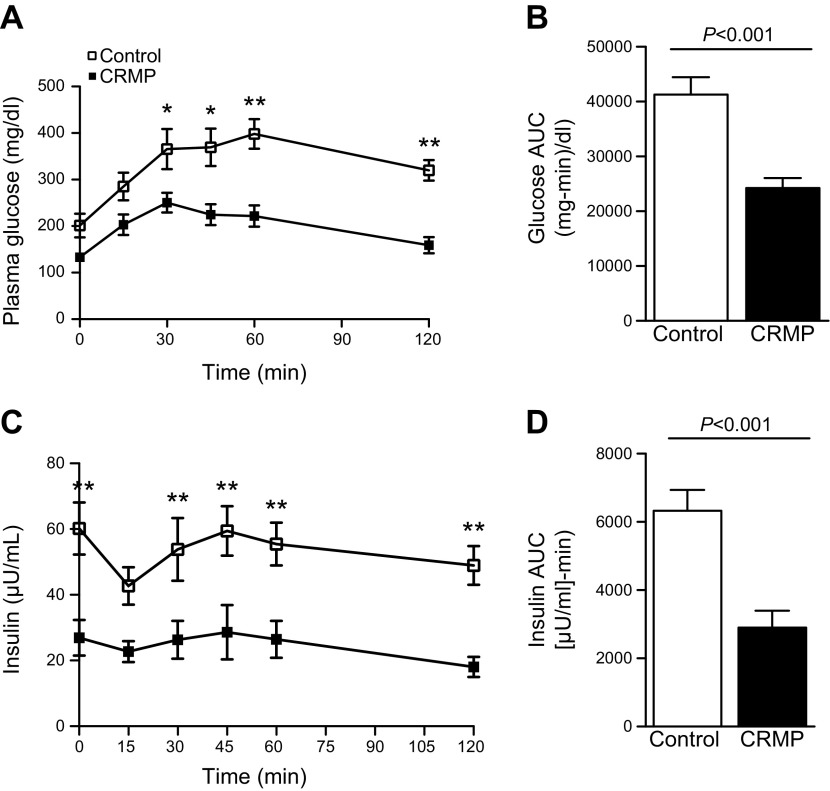
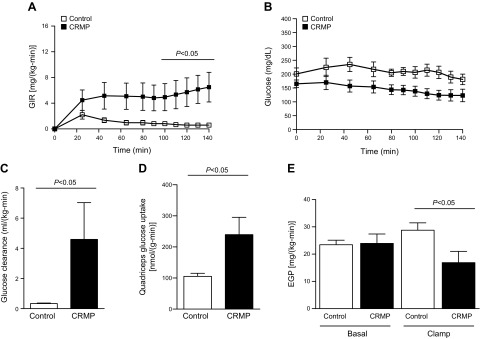
At the end of 4 wk of treatment, plasma glucose measurements from ad libitum fed mice confirmed severe hyperglycemia in AZIP/F-1 control mice. CRMP-treated mice were much more glucose tolerant, as reflected by 30–40% reductions in plasma glucose and insulin concentrations at each time point during an intraperitoneal glucose tolerance test (Fig. 2). CRMP-treated mice also manifested increased whole-body insulin responsiveness as reflected by the increased glucose infusion rate required to maintain euglycemia during a hyperinsulinemic clamp (Fig. 3A). Notably, control AZIP/F-1 mice required very little exogenous glucose during the hyperinsulinemic clamp, and their plasma glucose levels remained close to approximately 200 mg/dl (Fig. 3B), reflecting severe whole-body insulin resistance. In contrast, the CRMP-treated AZIP/F-1 mice responded to insulin during the hyperinsulinemic clamp and required significantly more exogenous glucose to maintain euglycemia, despite being clamped at a lower plasma glucose concentration of approximately 150 mg/dl (Fig. 3B). The glucose clearance rate during the clamp was significantly increased in the CRMP-treated group, reflecting increase muscle insulin responsiveness (Fig. 3C). To confirm this, insulin-stimulated tissue-specific 2-deoxyglucose uptake was quantified at the end of the clamp, and it was greatly increased in mice treated with CRMP (Fig. 3D). Additionally, insulin-mediated suppression of endogenous glucose production was significantly increased in CRMP-treated mice, indicating improved hepatic insulin responsiveness (Fig. 3E). These results demonstrate improvements in both hepatic and skeletal muscle insulin sensitivity in CRMP-treated AZIP/F-1 lipodystrophic mice.
Figure 2.
CRMP improved glucose tolerance and whole-body insulin sensitivity. A, B) Plasma glucose concentrations (A) and area under the curve (AUC) (B) during intraperitoneal glucose tolerance test. C, D) Plasma insulin concentrations (C) and AUC (D) during intraperitoneal glucose tolerance test. Mice were unfed for 6 h before the glucose tolerance test and euglycemic clamp study. Data are means ± sem (n = 7–8 per group). *P < 0.05, **P < 0.01 by 2-tailed, unpaired Student’s t test.
Figure 3.
CRMP improved whole-body insulin sensitivity. A, B) Glucose infusion rate (GIR) (A) and plasma glucose concentrations (B) during the hyperinsulinemic clamp. C) Glucose clearance. D) Insulin-stimulated glucose uptake in quadriceps. E) Hepatic glucose production. Food was withheld from mice for 6 h before the glucose tolerance test and hyperinsulinemic clamp study. Data are represented as means ± sem. Statistical significance determined by Student’s t test (n = 7–8 per group). EGP, endogenous glucose production.
CRMP reduced liver and skeletal muscle triacylglycerol and DAG content and PKC-ε (liver) and PKC-θ (muscle) activation
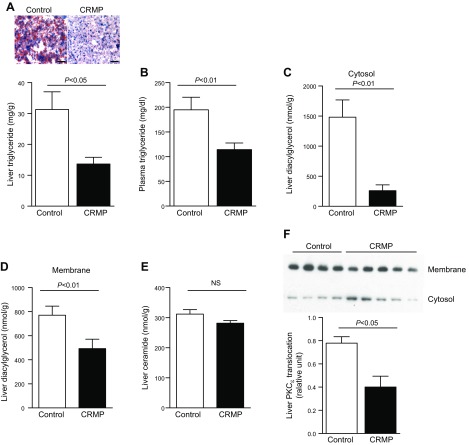
Insulin resistance in AZIP-/F-1 mice is closely associated with increased ectopic lipid content in liver and skeletal muscle (18). Increased tissue TAG, and more importantly tissue DAG levels, are strongly associated with tissue-specific insulin resistance in human subjects (19) and in rodent models of type 2 diabetes (20). Thus, we examined whether the improvements in hepatic and skeletal muscle insulin sensitivity by CRMP treatment were associated with reductions of tissue DAG as well as ceramides, which have also been implicated in insulin resistance (21, 22). CRMP-treated mice showed 50% lower liver TAG content (Fig. 4A) and ∼40% reduced plasma TAG concentrations (Fig. 4B). CRMP-treated mice also displayed reductions in hepatic DAG content in both cytosolic and membrane fractions compared with control mice (Fig. 4C, D). In contrast, despite the marked improvement in hepatic insulin responsiveness, hepatic ceramide levels in liver were not changed by CRMP treatment (Fig. 4E). Increased hepatic DAG content has been shown to promote hepatic insulin resistance through activation of PKC-ε (23, 24), which in turn promotes phosphorylation of the insulin receptor kinase threonine 1150, which leads to reductions in insulin receptor kinase activity (25). Consistent with these findings, we found that CRMP-induced reductions in hepatic DAG content and hepatic insulin resistance were associated with marked reductions in PKC-ε activity, as reflected by reduced hepatic PKC-ε translocation from cytosol to plasma membrane compared with vehicle treated mice (Fig. 4F).
Figure 4.
CRMP treatment reduced hepatic triglyceride and DAG content but did not alter hepatic ceramide content. A) Liver triglyceride (TAG) content and liver histology. Scale bars, 100 μm. B) Plasma TAG content. C, D) Liver cytosol (C) and membrane (D) DAG content. E) Liver ceramide content. F) PKC-ε translocation in liver. Data are represented as means ± sem (n = 8 per group). Statistical significance determined by Student’s t test.
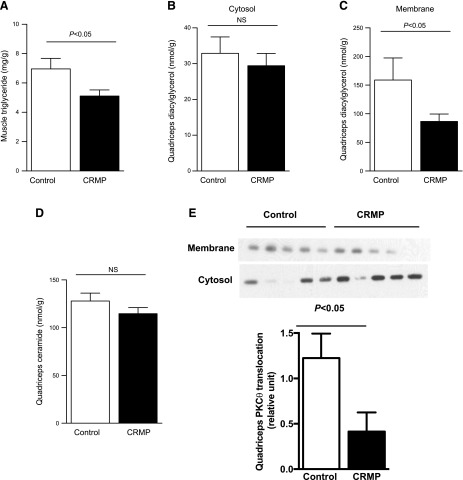
Next, we analyzed whether the improvements in skeletal muscle insulin sensitivity were also associated with reduced muscle lipid content and found that muscle TAG content was reduced 30–40% in the CRMP-treated mice (Fig. 5A). Similarly, DAG levels in muscle membrane fractions were decreased by 50% in the CRMP-treated group, whereas there was no difference in the cytosolic fraction (Fig. 5B, C). In contrast, ceramide levels in muscle were not changed by CRMP treatment despite a marked improvement in muscle insulin responsiveness (Fig. 5D). Consistent with changes in skeletal muscle insulin sensitivity and DAG levels, CRMP-treated mice showed reduced PKC-θ activation in skeletal muscle (Fig. 5E).
Figure 5.
CRMP treatment reduced muscle triglyceride and DAG content but did not alter muscle ceramide content. A) Quadriceps TAG content. B, C) Quadriceps cytosol (B) and membrane (C) DAG content. D) Quadriceps ceramide content. E) PKC-θ translocation in muscle. Data are represented as means ± sem (n = 8 per group). Statistical significance determined by Student’s t test.
CRMP ameliorates liver inflammation
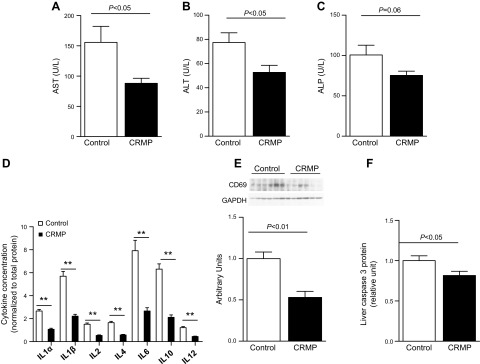
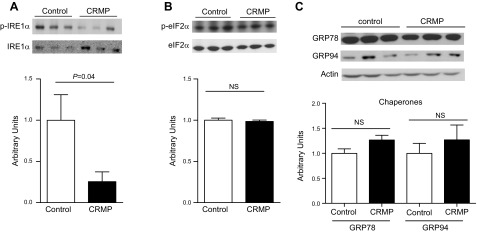
To investigate whether CRMP induced reductions in liver TAG concentrations and whether improvements in hepatic insulin sensitivity were associated with reductions in liver inflammation, we measured plasma transaminase levels in CRMP-treated and control AZIP/F-1 mice. Alanine aminotransferase and aspartate aminotransferase levels in plasma were significantly decreased in CRMP-treated mice, and there was a strong tendency for alkaline phosphatase to be decreased compare with controls (Fig. 6A–C). Consistent with these reductions in plasma transaminases, CRMP-treated mice displayed reductions in hepatic expression of several pro- and anti-inflammatory cytokines, including IL-1α, -1β, -2, -4, -6, -10, and -12 (Fig. 6D). Additionally, CRMP reduced hepatic expression of CD69 and caspase 3 protein levels, which are markers of apoptosis (Fig. 6E, F). ER stress has been demonstrated to trigger activation of NF-κB, which is a key promoter of inflammation (26). ER stress has also been linked with impairments in glucose homeostasis (27), and it is activated in patients with NAFLD (28). Thus, we analyzed whether CRMP could reduce the activation of the unfolded protein response pathways. CRMP treatment ameliorated activation of the IRE1-α branch of the unfolded protein response in AZIP/F-1 mice (Fig. 7A), whereas it did not alter phosphorylation of eIF2-α (Fig. 7B), GRP78, or -94 protein levels (Fig. 7C).
Figure 6.
CRMP treatment ameliorates liver inflammation and markers of apoptosis. A–C) Plasma aspartate aminotransferase (AST) (A), alanine aminotransferase (ALT) (B), and alkaline phosphatase (ALP) (C) concentrations. D–F) Hepatic protein expression of several proinflammatory and anti-inflammatory markers (D), CD69 (E), and caspase 3 (F). Data are means ± sem (n = 6–8 per group). Statistical significance was determined by Student’s t test. **P < 0.01.
Figure 7.
CRMP ameliorates activation of the IRE1-α branch of the unfolded protein response. A) Phosphorylation of IRE1-α. B) Phosphorylation of eIF2-α. C) GRP78 and GRP94 levels. Data are means ± sem (n = 6 per group). Statistical significance determined by Student’s t test. NS, not significant.
DISCUSSION
Although leptin replacement therapy has been shown to be effective in treating NAFLD/NASH and insulin resistance in patients with severe lipodystrophy (4, 6, 7), it is not effective in reversing these conditions in patients with partial lipodystrophy (8). Furthermore, although thiazolidinediones and lipid-lowering agents (statins and fibrates) have been commonly used in some forms of lipodystrophy, their effect on liver disease progression is uncertain (29). Therefore, there is a great unmet need for new therapies to treat NAFLD and NASH associated with partial lipodystrophy.
Here we show that a novel liver-targeted mitochondrial protonophore, CRMP, reverses hypertriglyceridemia, diabetes, and liver inflammation in an A-ZIP mouse model of severe lipodystrophy. Consistent with these findings of CRMP to improve the metabolic health of the liver in A-ZIP lipodystrophic mice, we also found that CRMP-treated mice had lower liver caspase 3 and CD69 protein levels, key molecules in the control of apoptosis (30). In addition, concentrations of a panel of 7 cytokines with pro- and anti-inflammatory functions in the liver were also reduced. Several studies have linked ER dysfunction to inflammation (26) and NAFLD (28). CRMP ameliorated activation of the IRE1-α branch of the unfolded protein response, whereas it had no effect over the PERK branch, as the phosphorylation of eIF2-α showed. The IRE1-α pathway triggers activation of JNK, which induces the expression of many inflammatory genes (31), including NF-κB. Thus, the inactivation of the IRE1-α pathway could play a role in the anti-inflammatory effects of CRMP. Taken together, these data show the potential efficacy of liver-targeted mitochondrial protonophores as a novel therapy for lipodystrophy-associated NAFLD/NASH.
CRMP-induced reductions in ectopic lipid deposition in liver and skeletal muscle were associated with lower plasma glucose concentrations and a marked improvement in glucose tolerance associated with lower plasma insulin concentrations, reflecting improved whole-body insulin sensitivity. Consistent with this improved glucose tolerance, CRMP also markedly increased whole-body insulin responsiveness, as reflected by an increased glucose infusion rate during a hyperinsulinemic clamp. This increase in whole-body insulin sensitivity could be attributed to improvements in both hepatic and skeletal muscle insulin sensitivity and was strongly associated with reductions in liver and skeletal muscle TAG and DAG content. Interestingly, these CRMP-induced reductions in liver and muscle TAG and DAG content were totally dissociated from any changes in liver or muscle ceramide content, thus dissociating tissue ceramide content from potentially mediating changes in liver and muscle insulin sensitivity in these animals. Consistent with CRMP-induced reductions in liver and skeletal muscle DAG content, we observed CRMP-induced reductions in PKC-ε and PKC-θ activation in liver and skeletal muscle protein, respectively. These findings are consistent with previous studies demonstrating a key role for DAG–PKC-ε activation in mediating hepatic insulin resistance (23–25, 32) and DAG activation of PKC-θ in mediating muscle insulin resistance (15, 16, 19). We have previously demonstrated that the mitochondrial uncoupling effect of CRMP treatment was confined to liver and that the improved insulin sensitivity in skeletal muscle could be attributed to marked reductions of very LDL production, resulting in reduced TAG export to skeletal muscle (11). Consistent with these previous observations, we observed ∼50% reductions in plasma triglyceride concentrations in CRMP-treated A-ZIP lipodystrophic mice.
In summary, here we show that CRMP can safely reverse hypertriglyceridemia, insulin resistance, diabetes, and NASH in a mouse model of severe lipodystrophy. Taken together, these data demonstrate the potential utility of CRMP and other liver-targeted mitochondrial uncoupling agents as a novel therapeutic approach for the treatment of lipodystrophy-associated NASH and type 2 diabetes.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01 DK40936), and the Harrington Discovery Institute (Grant DK099402; to M.J.J.). The authors thank Dr. Jeffrey Friedman (Rockerfeller University, New York, NY, USA) for kindly providing the AZIP/F-1 lipodystrophic mice, and Ali Nasiri and Gina Butrico (both from the Yale University School of Medicine) for their skilled technical assistance. The authors declare no conflicts of interest.
Glossary
- CRMP
controlled-release mitochondrial protonophore
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TAG
triglyceride
AUTHOR CONTRIBUTIONS
Abulizi, R. J. Perry, M. J. Jurczak, and G. I. Shulman designed the project and experimental protocols; A. Abulizi, R. J. Perry, P. Aspichueta, and M. J. Jurczak performed the experiments and contributed to data analysis; J. P. G. Camporez and K. F. Petersen contributed to discussions; and A. Abulizi, P. Aspichueta, and G. I. Shulman wrote the manuscript with input from all the other authors.
REFERENCES
- 1.Garg A. (2000) Lipodystrophies. Am. J. Med. 108, 143–152 [DOI] [PubMed] [Google Scholar]
- 2.Savage D. B. (2009) Mouse models of inherited lipodystrophy. Dis. Model. Mech. 2, 554–562 [DOI] [PubMed] [Google Scholar]
- 3.Moitra J., Mason M. M., Olive M., Krylov D., Gavrilova O., Marcus-Samuels B., Feigenbaum L., Lee E., Aoyama T., Eckhaus M., Reitman M. L., Vinson C. (1998) Life without white fat: a transgenic mouse. Genes Dev. 12, 3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javor E. D., Ghany M. G., Cochran E. K., Oral E. A., DePaoli A. M., Premkumar A., Kleiner D. E., Gorden P. (2005) Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41, 753–760 [DOI] [PubMed] [Google Scholar]
- 5.Safar Zadeh E., Lungu A. O., Cochran E. K., Brown R. J., Ghany M. G., Heller T., Kleiner D. E., Gorden P. (2013) The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J. Hepatol. 59, 131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oral E. A., Simha V., Ruiz E., Andewelt A., Premkumar A., Snell P., Wagner A. J., DePaoli A. M., Reitman M. L., Taylor S. I., Gorden P., Garg A. (2002) Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346, 570–578 [DOI] [PubMed] [Google Scholar]
- 7.Petersen K. F., Oral E. A., Dufour S., Befroy D., Ariyan C., Yu C., Cline G. W., DePaoli A. M., Taylor S. I., Gorden P., Shulman G. I. (2002) Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109, 1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque W. A., Shimomura I., Matsuzawa Y., Garg A. (2002) Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 87, 2395 [DOI] [PubMed] [Google Scholar]
- 9.Park J. Y., Javor E. D., Cochran E. K., DePaoli A. M., Gorden P. (2007) Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism 56, 508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simha V., Subramanyam L., Szczepaniak L., Quittner C., Adams-Huet B., Snell P., Garg A. (2012) Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J. Clin. Endocrinol. Metab. 97, 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry R. J., Zhang D., Zhang X. M., Boyer J. L., Shulman G. I. (2015) Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavrilova O., Leon L. R., Marcus-Samuels B., Mason M. M., Castle A. L., Refetoff S., Vinson C., Reitman M. L. (1999) Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 96, 14623–14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurczak M. J., Lee A. H., Jornayvaz F. R., Lee H. Y., Birkenfeld A. L., Guigni B. A., Kahn M., Samuel V. T., Glimcher L. H., Shulman G. I. (2012) Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J. Biol. Chem. 287, 2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 15.Griffin M. E., Marcucci M. J., Cline G. W., Bell K., Barucci N., Lee D., Goodyear L. J., Kraegen E. W., White M. F., Shulman G. I. (1999) Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48, 1270–1274 [DOI] [PubMed] [Google Scholar]
- 16.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., Atcheson B., White M. F., Kraegen E. W., Shulman G. I. (2002) Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236 [DOI] [PubMed] [Google Scholar]
- 17.Kleiner D. E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., Ferrell L. D., Liu Y. C., Torbenson M. S., Unalp-Arida A., Yeh M., McCullough A. J., Sanyal A. J.; Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 [DOI] [PubMed] [Google Scholar]
- 18.Kim J. K., Gavrilova O., Chen Y., Reitman M. L., Shulman G. I. (2000) Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 275, 8456–8460 [DOI] [PubMed] [Google Scholar]
- 19.Szendroedi J., Yoshimura T., Phielix E., Koliaki C., Marcucci M., Zhang D., Jelenik T., Müller J., Herder C., Nowotny P., Shulman G. I., Roden M. (2014) Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 111, 9597–9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel V. T., Petersen K. F., Shulman G. I. (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland W. L., Miller R. A., Wang Z. V., Sun K., Barth B. M., Bui H. H., Davis K. E., Bikman B. T., Halberg N., Rutkowski J. M., Wade M. R., Tenorio V. M., Kuo M. S., Brozinick J. T., Zhang B. B., Birnbaum M. J., Summers S. A., Scherer P. E. (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez J. A., Summers S. A. (2012) A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 [DOI] [PubMed] [Google Scholar]
- 23.Samuel V. T., Liu Z. X., Qu X., Elder B. D., Bilz S., Befroy D., Romanelli A. J., Shulman G. I. (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 [DOI] [PubMed] [Google Scholar]
- 24.Samuel V. T., Liu Z. X., Wang A., Beddow S. A., Geisler J. G., Kahn M., Zhang X. M., Monia B. P., Bhanot S., Shulman G. I. (2007) Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117, 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen M. C., Madiraju A. K., Gassaway B. M., Marcel M., Nasiri A. R., Butrico G., Marcucci M. J., Zhang D., Abulizi A., Zhang X. M., Philbrick W., Hubbard S. R., Jurczak M. J., Samuel V. T., Rinehart J., Shulman G. I. (2016) Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 126, 4361–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K., Kaufman R. J. (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 28.Puri P., Mirshahi F., Cheung O., Natarajan R., Maher J. W., Kellum J. M., Sanyal A. J. (2008) Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576 [DOI] [PubMed] [Google Scholar]
- 29.Fiorenza C. G., Chou S. H., Mantzoros C. S. (2011) Lipodystrophy: pathophysiology and advances in treatment. Nat. Rev. Endocrinol. 7, 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Rabia M. W., Blaylock M. G., Sexton D. W., Walsh G. M. (2004) Membrane receptor-mediated apoptosis and caspase activation in the differentiated EoL-1 eosinophilic cell line. J. Leukoc. Biol. 75, 1045–1055 [DOI] [PubMed] [Google Scholar]
- 31.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., Ron D. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 [DOI] [PubMed] [Google Scholar]
- 32.Kumashiro N., Erion D. M., Zhang D., Kahn M., Beddow S. A., Chu X., Still C. D., Gerhard G. S., Han X., Dziura J., Petersen K. F., Samuel V. T., Shulman G. I. (2011) Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 108, 16381–16385 [DOI] [PMC free article] [PubMed] [Google Scholar]