Bianco and colleagues discuss key milestones in the development and use of mesenchymal stem cells. The authors also review future prospects for the use of these cells in the clinic.
Abstract
At the turn of a decade of intensive wishful thinking, “mesenchymal stem cells” are changing their profile, while retaining their charm. As hopes to turn bone into brain or vice versa seem on the wane, we learn (1) that the archetypal “mesenchymal stem cell,” the skeletal stem cell found in the bone marrow, can be directly identified as a specialized type of mural cell/pericyte, found in the wall of sinusoids and long known as adventitial reticular cells; (2) that bone marrow skeletal stem cells are also defined by expression of CD146, and can self-renew in vivo, while giving rise to skeletal tissues, and therefore earn consideration as bona fide stem cells; (3) that a broader class of microvascular mural cells endowed with clonogenicity and progenitor properties may exist in other tissues, although their true potency needs to be firmly established by stringent assays and thorough comparisons across tissues; (4) that bone marrow skeletal stem cells display unique angiopoietic and hematopoietic niche-related functions, consisting in their ability to transfer the hematopoietic microenvironment and to guide the assembly of microvascular networks, which seem to define their inherent biology; and (5) that use of skeletal stem cells as disease models, and as models of high-risk strategies for cell and gene therapy specifically in incurable skeletal diseases, may provide new challenges for the next decade, and perhaps reward for medicine in the one that follows.
A Seminal Experiment
A single seminal experiment (Tavassoli and Crosby, 1968) marks the birth date of two areas or research that were to become the focus of increasing interest up to the present day—the field of the hematopoietic microenvironment/niche (Schofield, 1978) and the field of “mesenchymal stem cells” (Caplan, 1991). The experiment was designed to address the question concerning whether hematopoiesis could be transplanted to extraosseous sites; that is, to prove or dispel the idea that a local microenvironment would be crucial for the establishment and maintenance of hematopoiesis. The answer provided by nature was, at a glance, cryptic and unrelated to the question being asked—there is osteogenic potential in bone. Indeed, heterotopic transplantation of boneless fragments of postnatal marrow resulted, first and foremost, in the generation of an ectopic “ossicle,” that is, a structure mimicking closely the architectural layout and histological structure of a miniature bone. The ossicle formed in the classical experiment performed by Tavassoli and Crosby (1968) consisted of a shell of bone encasing a marrow cavity within which hematopoietic cells lodged, along with blood vessels and marrow adipocytes, as well as sparse bone trabeculae.
A Second Type of Stem Cell in the Bone Marrow
Although there was a noted antecedent in the nineteenth century (Goujon, 1869), and even though the link between bone and hematopoiesis was to remain poorly understood for decades thereafter, this experiment indicated that there was osteogenic potential in the soft, jelly-like bone marrow of postnatal mammals. Through the work of Alexander Friedenstein and coworkers (Friedenstein et al., 1966, 1970, 1974, 1987; Friedenstein, 1976, 1990), this potential was later ascribed to (1) cells of nonhematopoietic nature and lineage, and therefore belonging to the broadly defined “stroma” of the bone marrow; and (2) to single stromal cells, the colony-forming unit-fibroblast (CFU-F), the clonal progeny of which were shown to be able to give rise to multiple tissues found within a skeletal segment—bone, cartilage, adipocytes, fibroblasts, and myelosupportive stroma. Of note, it was this multiplicity of differentiative potency of the progeny of a single cell that gave rise to the idea that a second type of stem cell, nonhematopoietic in nature, could be found within the bone marrow stroma. The idea, formulated and disseminated by Friedenstein and Owen (Owen and Friedenstein, 1988; Friedenstein, 1990), was later reformulated and to a significant degree distorted, into the current concept of a “mesenchymal stem cell” (Caplan, 1991).
The Second Life of a Second Stem Cell
Friedenstein's idea was based on studies of the bone marrow stroma. Hence, the putative, newly recognized stem cell would be rightly labeled, as it were, a “stromal stem cell” (Owen and Friedenstein, 1988). The actual experimental data disclosed the ability of this stem cell to give rise to skeletal tissues, but not to any other tissue, hence the name “osteogenic stem cell” was also adopted (Friedenstein, 1990), and later resonated in the currently proposed term, “skeletal stem cell” (Bianco and Robey, 2004; Bianco et al., 2006). Although the “mesenchymal stem cells” of Pittenger and colleagues (1999) were indeed the same osteogenic, stromal, or skeletal stem cells as of Friedenstein and Owen, and others, the use of the alternative term, “mesenchymal,” proposed by Caplan (1991) and widely adopted, conveyed a concept of the nature and potency of the putative stem cells quite departed from Friedenstein's original concept. Conceptually, two major characteristics mark the distinction of “MSCs” from osteogenic, stromal, or skeletal stem cells. Whereas the latter are found in the bone marrow stroma, the former are thought to be found almost everywhere (heart, liver, synovium, muscle, placenta, pancreas, cord blood, etc.; reviewed in Bianco et al., 2008). Whereas the latter give rise to skeletal tissues, the former are thought to be endowed with a much broader measure of multipotency (reviewed in Bianco et al., 2008). Not restricted to skeletal tissues, the potency of the “mesenchymal stem cell” would be extended to nonskeletal mesodermal derivatives, such as heart, endothelial cells, and muscle (Fig. 1). Essentially all mesodermal derivatives could be traced to a common ancestor, and this common ancestor could be found in postnatal organisms. Seductive as it sounds, this hypothesis, which has never been conclusively proven experimentally (unlike the concept of a common postnatal progenitor of skeletal tissues), is difficult to reconcile with some fundamental tenets of developmental biology, such as the segregation of myogenic and osteogenic potency in somites, or the lack of myogenic potential in lateral mesoderm. Once regions of mesoderm are specified, a common progenitor of mesodermal derivatives is no longer found, not even in prenatal life. In addition, bone (the first recognized differentiated product of the pursued postnatal “stromal” nonhematopoietic stem cell), does not have a common ancestor in prenatal life; axial and limb skeletal components derive from different specifications of mesoderm, and many craniofacial bones derive from neuroectoderm. At about the same time when skeletal stem cells were being rediscovered and renamed as “mesenchymal stem cells,” potently suggestive reports hit the scientific arena and the lay public, fueled by the first evidence that pluripotent embryonic human cells (considered equivalent to murine embryonic stem [ES] cells) could be isolated and maintained in culture. Many did not resist the temptation to see the newly discovered “MSCs” as much more than mesodermal, and thus, able to generate even ectodermal (neurons) and endodermal (liver) derivatives, implying (at least) that they would provide an “ethically neutral” alternative to human ES cell research and clinical use. Of note, these suggestions were never supported by direct and reproducible experimental evidence.
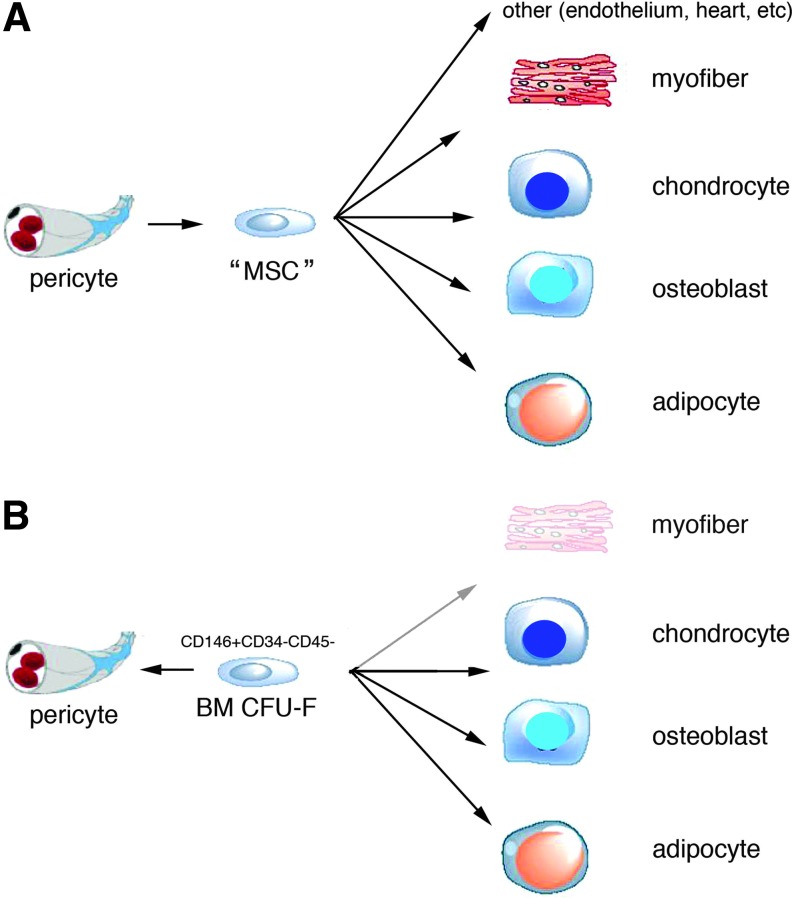
FIG. 1.
The two alternative concepts of mesenchymal stem cells (MSCs) and bone marrow skeletal stem cells. (A) The dominant view of MSCs holds that they are able to differentiate into more cell types than those found within a skeletal segment (bone, cartilage, fibrous tissue, fat, and myelosupportive stroma), to include striated muscle, other nonskeletal mesoderm derivatives, and possibly ecto- and mesoderm derivatives. In addition, MSCs as commonly viewed are not found only in the bone marrow stroma, but in virtually every tissue. (B) Skeletal stem cells are found only in the bone marrow stroma, and are able to give rise, without exposure to reprogramming cues (e.g., 5-azacytodine and BMPs) and in vivo, to skeletal tissues (bone, cartilage, fat, myelosupportive stroma, and fibrous tissue) but not to skeletal muscle, other mesoderm-derived tissues, and nonmesodermally derived tissues. It is likely that local committed progenitors also exist in other tissues, such as myogenic microvascular cells in skeletal muscle, with restricted and tissue-specific differentiation potential. The two views also diverge in the relationship of tissue progenitors to mural cells/pericytes. In the MSC view, pericytes from any tissue give rise to MSCs with identical potency. In the alternative view, it is preexisting local committed progenitors that are recruited to a mural cell fate, as is shown to happen in vivo for bone marrow skeletal stem cells (Sacchetti et al., 2007). This again postulates diversity rather than equivalence of pericytes across tissues (Bianco et al., 2008).
How Should MSCs Be Defined?
Stem cells, in general, are defined by self-renewal and potency. Self-renewal and potency, in turn, are defined by assays. Assays (usually in vivo assays) are typically a popularized version of seminal experiments, which become fixed into a widely reproduced benchmark routine. Much like hematopoietic reconstitution became a defining property and assay of hematopoietic stem cells, the seminal experiment of Tavassoli and Crosby (heterotopic skeletogenesis) gave rise to the defining assay of “MSCs”; that is, the heterotopic transplantation of clonal or nonclonal cell strains to test their ability to form skeletal tissues. Many variations on this theme are available in the literature, or were used over time and in different laboratories. However, the specific significance and limitations of these assays are regularly overlooked in the vast literature on “MSCs.” These assays generate histology-proven tissues, the donor origin of which can be directly proven. They generate mature tissue in vivo, not in plastic. They probe the native potency of a cell strain, in the absence of artificial cues. If conducted with clonal cell populations (i.e., cell strains generated by a single cell), they prove multipotency of the original single cell. In contrast, the widespread use of “in vitro” assays in lieu of the defining in vivo assays is one of the most important sources of confusion, or at least controversy and disagreement, as to the nature, identity, and potency of “MSCs.” In vitro mineralization assays conducted in the presence of a concentration of phosphate that no living cell ever sees in vivo, and assessed by histochemical or physical assessment of mineral deposition regardless of its potential dystrophic nature, cannot define osteogenic potential, for any cell, with any stringency. Adipogenesis assays conducted in the presence of agonists and regulators of relevant transcription factors do not equate to spontaneous development of adipocytes in vivo. Heterotypic fusion of test cells with myogenic cells such as C2C12, or marginal expression of myogenic markers after exposure to demethylating agents such as 5-azacytidine, do not prove myogenesis. Shape changes induced in any fibroblastic population by agonists, stimulators, or mediators of the cAMP signaling, known since the dawn of bone cell biology (Peck et al., 1964), as reflecting transient disruption of F-actin, do not represent neural differentiation, even if associated with up-regulation of a few neural markers detected by RT-PCR. In brief, stringency of assays is a must to claim any kind of potency, and in vitro assays are not stringent enough. Therefore, whereas a bulk of evidence widely reproduced in multiple laboratories demonstrates the genuine skeletogenic potential of bone marrow-derived stromal progenitors, evidence in support of the idea of a virtually ubiquitous, broadly multipotent postnatal progenitor of mesodermal and nonmesodermal derivatives is not equally as strong, as it is largely based on nonstringent assays. Importantly, when in vitro and in vivo assays are conducted on the same test cell population, it is obvious that the results yielded by the two kinds of assays may or may not converge (Krebsbach et al., 1999; Satomura et al., 2000). Hence, it is impossible to predict in vivo bone formation on the basis of in vitro mineralization, in vivo adipogenesis on the basis of in vitro adipogenesis, and so on. Myogenic differentiation is an even more critical issue. Cell fusion with inherently myogenic cells can notoriously reprogram almost any kind of cell type (including monocytes and lymphoid cells [Blau et al., 1983, 1985; Chiu and Blau, 1984]) to express myogenic genes, and MyoD1 is sufficient to do so in a number of embryonic fibroblastic cell lines (Murray, 1993), otherwise nonmyogenic and sometimes even tumorigenic in vivo. Hence, neither coculture with myogenic cells, nor direct injection into muscle where local myoblasts are, of course, available to fuse with grafted cells, represents an assay that is stringent enough to claim the myogenic potential of any cell type. To date, satellite cells, the resident stem cells of skeletal muscle, remain the only cell type endowed with the proven and reproduced ability to generate myotubes in the absence of exogenous myoblasts (Sherwood et al., 2004).
Colony-Forming Units-Fibroblast
One of the features that originally defined the “mesenchymal stem cells” found in the bone marrow stroma, even before they were renamed as such, was their obvious ability to generate colonies on plating single cells at low density (Friedenstein et al., 1970). Given the overall fibroblastic appearance of the colonies, and by analogy with terminology used in experimental hematology for hematopoietic progenitors, the colony-initiating cell came to be known as the colony-forming unit-fibroblastic (reviewed in Bianco et al., 2008). Clonogenicity has remained a major, and for some, the defining characteristic of bone marrow skeletal (“mesenchymal”) stem cells. Here again, attention must be paid to conditions under which clonogenicity is assayed, in order to avoid misinterpretation leading to hasty conclusions as to the “MSC” nature of a given test cell population. When cells are plated at high densities, colonies form that are not clonal in nature, and therefore do not reflect clonogenicity of single cells. Hence, enumerating colonies with cell populations plated at high density is a somewhat pointless exercise. This is particularly relevant when tissue sources other than bone marrow, or cells isolated by immunoselection, are being tested. In both cases, in the absence of nonadherent hematopoietic cells, cell density equates the density of adherent cells, hence it cannot exceed 1.6 cells/cm2 in order to remain within a bona fide clonal range. Clonogenicity means, in essence, the ability of a single cell to initiate density-insensitive growth (Sherley et al., 1995; Sherley, 2002), a property not shared by all cells in a tissue, and rather ascribed to a defined subset of cells. It is for this reason that clonogenicity is regarded as a token of a progenitor's nature. It is among the clonogenic subset of bone marrow stromal cells that assayable multipotent progenitors are found, and for this reason, CFU-F enumeration, when done at proper initial cell densities, can be regarded as a rough estimation of the frequency of progenitors within a given bone marrow sample. One quite significant implication of the diverse ability of stromal cells to grow in culture is that early-passage cell strains will display divergent characteristics if established through clonal primary cultures (i.e., by expanding one or more single colonies) or through bulk explant cultures (Sacchetti et al., 2007). In the latter case, short-lived cells that would not be able to grow at clonal density would significantly contribute to the composition of the resulting cell strain.
Ways of purifying/enriching CFU-Fs have long been available. A number of surface epitopes can be exploited for immunoselection; for example, the time-honored STRO-1 (Simmons and Torok-Storb, 1991) is the first and still one of the most efficient, but by no means the only one (e.g., Barry et al., 1999; Deschaseaux and Charbord, 2000). It has been largely overlooked, however, that for all intents and purposes, “prospective” isolation of CFU-Fs (i.e., of a population of cells enriched in progenitor/stem cells) through immunoselection, and conventional isolation by plastic adherence at clonal density, actually coincide with one another. The importance of prospective isolation in the case of “MSCs” is somewhat diminished by the circumstance that virtually all assays available do involve culturing after isolation, rather than direct in vivo transplantation of isolated and uncultured cells. This is due to the low numbers of prospectively isolatable cells in humans, and the much higher numbers of cells needed for either assay or translational use. Nonetheless, pursuit of ways to prospectively isolate “MSCs” is justified in view of future developments in the way they can be assayed, and in defining their biological nature, independent of their more differentiated progeny.
Notoriously, not all CFU-Fs are equal in terms of growth and differentiation potential (reviewed in Bianco et al., 2008). This “heterogeneity” of CFU-Fs should not be confused with the more popular “heterogeneity” in the composition of nonclonal cultured strains that are commonly referred to as “MSCs.” The latter is simply a special case of the inherent, unavoidable heterogeneity of any nontransformed, cultured cell strain, in which asymmetric kinetics, random differentiation, and senescence occur by definition (Sherley et al., 1995; Sherley, 2002). The idea that any nontransformed cell strain can be “expanded,” leading to the generation of a uniform progeny of identical cells, collides with fundamental tenets of cell biology. On the other hand, the “heterogeneity” of CFU-Fs per se is obvious, inherent to the very notion of clonogenic cell and clone, and has become common wisdom, yet it is largely overstated as a token of “imperfect purity” of a putative progenitor population. As a token of such overstatement, one should simply consider that only 21% of “purified” murine hematopoietic stem cells (HSCs) reconstitute hematopoiesis long term (Osawa et al., 1996), and that ∼25% of the disappointingly “heterogeneous” CFU-Fs (or MSCs) generate a complete ossicle on heterotopic transplantation (Friedenstein et al., 1970, 1987; Kuznetsov et al., 1997; Sacchetti et al., 2007). That is to say, assuming formation of heterotopic ossicles as the defining assay of skeletogenesis by “MSCs,” much like hematopoietic reconstitution is the defining assay of HSCs, “MSCs” isolated as human bone marrow CFU-Fs would be about as pure as murine HSCs, or vice versa, murine HSCs are about as heterogeneous as human bone marrow CFU-Fs. Clearly, variability intrinsic to specific experimental conditions and inherent hurdles thereof, cannot be easily dissected out from inherent variability of the test population.
Self-Renewal
Use of clonal cell strains in in vivo transplantation experiments has previously proved that a single bone marrow-derived CFU-F can be a multipotent skeletal progenitor. However, evidence for self-renewal of bone marrow skeletal progenitors, or for any other entity alluded to as “MSCs,” has long been missing, and was actually left as one of the major unsolved questions in the original work of Friedenstein. As a result, the question has remained open whether bone marrow-derived “MSCs” can indeed be seen as stem cells, or should rather be called simply “mesenchymal stromal cells,” in the lack of evidence of self-renewal as a defining feature of “stemness” (Horwitz et al., 2005; Dominici et al., 2006). Literally the renewal of self, self-renewal is commonly mistaken for extensive proliferation in culture, leading to the pursuit of extraordinary numbers of population doublings as a purported proof of stemness for a variety of putative stem cells. In fact, the paradigm of self-renewal and the model assay to assess it are provided by seminal experiments focusing on HSCs. Serial reconstitution of hematopoiesis on serial transplantation of single, phenotype-defined putative stem cells establishes the ability of HSCs to self-renew (Morrison and Weissman, 1994). Likewise, efforts in the direction of proving self-renewal (and therefore stemness) of “MSCs” should borrow the same fundamental principle as that guiding the proof of self-renewal of HSCs experimentally. This was made possible specifically by the definition of a defining (albeit operationally defining, temporarily defining) minimal surface phenotype (Spangrude et al., 1988). This, in particular, has been missing for “MSCs,” and actually the importance of a phenotype of uncultured putative “MSCs” was diluted into the seriously confounding pursuit of a surface phenotype of cultured “MSCs.” Surface antigens are regulated in culture in unpredictable ways, and none of the in vitro-expressed epitopes that are commonly seen as defining “MSCs” in culture are actually suitable to clearly distinguish cultures of “MSCs” from cultures of common fibroblasts. Even though a number of markers have long been available for isolating and enriching to near-purity clonogenic stromal progenitors from bone marrow, most antibodies recognizing those markers would fall short of a major fundamental characteristic; that is, their suitability for correlative in vivo/ex vivo studies. It has now been recognized that clonogenic stromal cells (CFU-Fs) explanted ex vivo, and adventitial reticular cells of sinusoids, as seen in the intact bone marrow in vivo, express the same markers, indicating that clonogenic progenitors are found in the wall of sinusoids, where they reside at the abluminal surface of endothelial cells (Sacchetti et al., 2007). Once explanted, grown in culture, and retransplanted, these cells generate bone, adipocytes, and hematopoiesis-supporting stroma, and self-renew into adventitial reticular cells at the wall of sinusoids in the heterotopic ossicle. Furthermore, they self-renew into CFU-Fs that can be secondarily passaged at least once. Here again, borrowing the experimental paradigm and approach from the best known success in the history of stem cell purification (murine HSCs) pays, but again raises the eyebrow of the purist. Can human “MSCs” be serially transplanted, like murine HSCs? The answer is a definite maybe. However, one fundamental fact, again, prevents overly strict comparisons or equations between the two systems. Purified murine HSCs are neither cultured, nor expanded, nor in any way induced to proliferate ex vivo before transplantation, whereas human “MSCs” are. Hence, unless one can provide a rigorous assessment of self-renewal over a defined number of population doublings, it cannot be stated whether “MSCs” are shorter-lived (less extensively self-renewing) than murine HSCs. Serial transplantation may not necessarily be required to claim self-renewal in general, beyond the boundaries of hematopoiesis. In addition, whereas self-renewal seems a reasonable attribute of any bona fide stem cell, by no means does it seem reasonable to expect an identical ability to self-renew for stem cells of tissues that by themselves are vastly different in self-renewal. Whereas the epidermis turns over in its entirety once every 30 days, the whole skeleton is turned over, in its entirety, only three to five times in an adult life span. The respective stem cells should not be expected to be identical to one another in terms of self-renewal ability, if the tissues where they belong are not.
Hematopoiesis and Angiopoiesis
In a way, demonstrating that bone marrow “MSCs” can self-renew in vivo coincided with demonstrating that they are able to establish the heterotopic microenvironment at heterotopic sites. Explanted as adventitial reticular cells, skeletal stem cells can generate, besides differentiated skeletal tissues such as bone and adipocytes, adventitial reticular cells. Explanted as stromal cells residing amidst hematopoietic cells, as part of the hematopoietic microenvironment, transplanted skeletal stem cells self-renew into cells that establish the hematopoietic microenvironment. Explanted as CFU-Fs with a defined surface phenotype, transplanted skeletal stem cells self-renew into secondarily passaged CFU-Fs with the same phenotype. This suggests that bone marrow-derived “MSCs” (also known as skeletal stem cells, adventitial reticular cells, and CD146-expressing CFU-Fs) are capable of genuine self-renewal in vivo and can for this reason be regarded not just as multipotent progenitors of differentiated skeletal phenotypes (which has long been known) but as bona fide stem cells.
Besides coinciding with the most obvious phenomenon, that is, the ability of bone marrow stromal cells to self-renew as stromal cells, the ability of bone marrow “MSCs” to transfer the hematopoietic microenvironment to heterotopic sites is highly relevant to two open questions. First, it provides evidence for a functional role not of mature osteoblasts, as widely maintained (Calvi et al., 2003; Zhang et al., 2003; Moore and Lemischka, 2006), but of osteoprogenitors, in establishing the hematopoietic “niche.” Second, it highlights a set of properties as yet only vaguely defined, which may indeed posit the specific biological function of these cells. Skeletal stem cells can guide and organize the formation of vascular networks in vitro and in vivo (Sacchetti et al., 2007; Au et al., 2008; Melero-Martin et al., 2008), and this function is a prerequisite for the establishment of the hematopoietic microenvironment. This function is quite distinct from either angiogenesis or vasculogenesis, in that it neither entails the growth of new vessels from preexisting ones, nor the de novo differentiation of endothelial cells from mesenchyme. Therefore, to mark the distinction of this process from both angiogenesis and vasculogenesis, we suggest that this function is best referred to as “angiopoiesis.” On coculture (Sacchetti et al., 2007) or cotransplantation (Au et al., 2008; Melero-Martin et al., 2008) with endothelial cells, “MSCs” can direct their assembly into vascular networks outside the context of the formation of heterotopic ossicles. In addition, bone marrow skeletal stem cells express all genes hitherto regarded as functionally crucial in the homing, support, or differentiation of hematopoietic progenitors (Sacchetti et al., 2007). In particular, they are potent producers of CXCL12/stromal cell-derived factor-1, which defines murine CXCL12-abundant reticular (CAR) cells (Sugiyama et al., 2006), and angiopoietin-1, the ligand of the tyrosine receptor kinase, Tie-2, expressed in endothelial cells and HSCs. Angiopoietin-1 is produced by embryonic pericytes and mesenchymal cells (Suri et al., 1996), and contributes to keeping HSCs and endothelial cells in a quiescent state, barring proliferation and apoptosis (Arai et al., 2004). Stated in a simplistic way that echoes traditional views of the hematopoietic microenvironment, bone marrow “MSCs” (skeletal stem cells) exert a “trophic” and “regulatory” function on cells of hematopoietic (and immune, indeed, as B cells are formed in the bone marrow) lineage, and on endothelial cells. Current interest in similar effects exerted by “MSCs” on nonhematopoietic cell types should not be oblivious to this fundamental fact, nor to the notion that at the tissue level, part of any trophic effect may indeed have to do with the organization of local vascular networks subsequent to tissue damage. Here, the new focus on novel properties of “MSCs” circles back to their original function in the bone marrow, that is, to support hematopoiesis and to stabilize blood vessels.
Pericytes
The observation that the archetypal “MSC” in the human bone marrow coincides with MCAM (CD146)-expressing, sinusoidal adventitial cells (mural cells, pericytes; Sacchetti et al., 2007) in bone marrow identifies adventitial reticular cells (Westen and Bainton, 1979) as the in situ counterpart of explanted CFU-Fs and skeletal progenitors. A number of previous in situ observations had suggested that adventitial reticular cells, or a subset thereof, could indeed correspond to explanted clonogenic stromal progenitors or bone marrow stromal stem cells (Bianco and Boyde, 1993). For example, adipogenesis proceeds from adventitial reticular cells in human and rodent bone marrow (Weiss, 1976; Bianco et al., 1988). Given their anatomical position, antigenic profile, and transcriptome, adventitial reticular cells can be seen as a local modulation of a vaguely defined cell type referred to, in all other tissues, as pericytes or mural cells (Hirschi and D'Amore, 1996, 1997; Jain, 2003; Jain and Booth, 2003), made different in bone marrow by the different anatomy, size, and blood flow velocity that characterize sinusoids compared with capillaries. On the basis of a number of classical observations suggesting that pericytes in a variety of tissues could represent tissue progenitors (Díaz-Flores et al., 1990, 1991a,b, 1992), it was also suggested earlier that bone marrow stromal stem cells could represent a local subset of a broader class of microvascular progenitors, identified as pericytes in non-bone marrow tissues (Bianco and Gehron Robey, 2000). Indeed, in skeletal muscle, myogenic progenitors can be found associated with the wall of microvessels (Dellavalle et al., 2007); much like in embryonic development, myogenic progenitors are associated with the wall of the dorsal aorta (De Angelis et al., 1999). Interestingly, transplantation of quail dorsal aorta cells into the developing chick wing leads to the generation of chimeric blood vessels (Minasi et al., 2002). These vessels branch progressively, while retaining a mural coat of donor cells, up to the distalmost ramifications well within target tissues. Here, donor cells are found that are indistinguishable from pericytes in morphology and position.
The identification of markers suited to isolate vascular wall cells from tissues, such as CD146, CD105, and others, is now making it possible to test directly the differentiation properties and putative progenitor nature of microvascular cells in human tissues. Use of terms originally operationally employed to denote murine embryonic cells (“mesoangioblasts”; Minasi et al., 2002) should not be extrapolated to postnatal tissues (Sampaolesi et al., 2003) and least of all to nonmurine tissues (Sampaolesi et al., 2006), unless rigorous proof of identity of the denoted cell type, and protocols for their isolation, can be obtained and detailed, respectively. Likewise, pending definitive proof by rigorous assays, the hastily drawn conclusion that all pericytes from all tissues are “MSCs” (Caplan, 2008; Crisan et al., 2008; da Silva Meirelles et al., 2008) should neither be entertained nor disseminated. Pericytes are not a lineage in and of themselves. Their nature and developmental origin are unclear, and their equivalence across different developmental stages or postnatal tissues, or species, is far from elucidated. Whereas it is clear that mural cells exist in all tissues, and that cells with clonogenic ability and generic “mesenchymal” habit (CFU-Fs) can be explanted from probably all tissues (Bianco et al., 2008), there is no rigorous or convincing proof that progenitors with identical potency exist across diverse mesoderm derivatives. Alternative views have been proposed, such as the diversity of microvascular wall-associated progenitors in diverse tissues, as a result of local recruitment to a mural cell fate of local, precommitted, tissue-specific progenitors (Bianco et al., 2008). This view would explain, for example, why bone marrow-derived “MSCs” bear the hallmark of skeletogenic commitment, such as the constitutive expression of the master gene of skeletogenesis, Runx2 (Satomura et al., 2000).
Therapy beyond Tissue Engineering
For more than a decade, the notion that MSCs would represent a handy variety of easily accessible, easily cultured stem cells with wondrous differentiation abilities has fueled the search for translational, applicative uses of these cells that have spanned the entire range of incurable human disease in all organ systems from brain to heart, from muscle to liver. The common thread to most of these approaches was the assumption that MSCs would differentiate into a number of nonskeletal cell types. At this time, this view is on the wane, as convincing evidence for unorthodox differentiation of “MSCs” has not been confirmed or reproduced. Conversely, the original view that (bone marrow) “MSCs” would serve as tools for skeletal repair or reconstruction has persisted, backed as it is by solid evidence for the true osteogenic potential in vivo of bone and bone marrow-derived “MSCs.” Whereas the clinical impact and commercial dimension of this application alone would be significant and sufficient to satisfy gigantic expectations, the hopes generated in a decade of promises would be rather dismally downplayed if this were to be the only ultimate outcome of the huge global effort in the past decade.
Indeed, the very notion of stem cells promises more than surgical application or engineering of tissues. It also promises more than the newly cheered “trophic” or “immunomodulatory effects” (reviewed in Caplan and Dennis, 2006; Le Blanc and Ringden, 2007; Prockop, 2007), which seem to have replaced hopes for making neurons out of bone or blood. These novel effects, in turn, seem to be destined to be replaced, at least to a large extent, by the proper effects of the proper chemical mediators once emergence from the sea of serendipity puts the latter, not cells, at center stage and under the dissecting microscope. The notion of stem cells promises to reveal facets of disease mechanisms, and approaches to therapy, that would not be conceivable otherwise. It promises to elucidate how lineage and differentiation intertwine with disease and organ damage (as is the case, e.g., in hematology). It promises to place before the investigator miniature in vivo (in-stem cell) models of molecular mechanisms. Curiously, the use of stem cells as disease models is rapidly gaining popularity in the embryonic stem cell field, but was in fact originally highlighted first in skeletal stem cells (Bianco et al., 1998). Transplantation of genetically abnormal skeletal stem cells (bone marrow “MSCs”) has been used to generate in vivo replicas of human and murine genetically abnormal bone (Holmbeck et al., 1999; Riminucci et al., 2001). Likewise, their use in in vitro studies has provided simple and informative disease models, and revealed even major aspects of disease pathophysiology or skeletal physiology. The notion that the skeleton functions as a hormonal regulator of renal handling of phosphate (Riminucci et al., 2003), for example, emanates from the study of skeletal stem cells from fibrous dysplasia (FD) of bone. Fibrous dysplasia (OMIM #174800) is a crippling, occasionally lethal genetic disease of the skeleton, caused by activating missense mutations in of the gene encoding the α subunit of the stimulatory G protein, Gs (reviewed in Riminucci et al., 2006). In FD, analysis of in vivo and ex vivo properties and behavior of skeletal stem cells has revealed important dynamics of disease pathogenesis. For example, the negative impact of the causative mutation on the viability of skeletal stem cells (Kuznetsov et al., 2008), or the occurrence of mechanisms of allelic selection reflecting on clinical variability and yet unrelated to parental imprinting (Michienzi et al., 2007), would not have been revealed if not by looking at the properties of single clonogenic skeletal progenitors. Of note, some of these mechanisms evoke conceivable modes of intervention that would see stem cells within their in vivo location as targets of pharmacological intervention (Riminucci et al., 2006), rather than as transplantable items.
Beyond disease modeling, and the conception of innovative drug targets, skeletal stem cells are natural subjects of attention in genetic diseases of the skeleton, and in fibrous dysplasia in particular (Fig. 2). Here, cell therapy and gene therapy are almost inevitably the lodestone of wishful thinking. Success of these approaches in hematology enforces the wish, and at times weakens the thinking. For example, direct borrowing of strategies for transplantation of “MSCs” (Horwitz et al., 1999, 2001) from those successful with HSCs may lead to disappointing outcomes, and additional unfulfilled hopes. Efficient ways to (1) eradicate a diseased physical substrate in the target tissue, and (2) systemically target “MSCs” to the skeleton, still represent the two major unsolved hurdles in the way of systemic cell or gene therapy of genetic skeletal diseases. On the other hand, a hasty desire for translation should not undermine the pursuit of these strategies. It took no less than one century for the notion of hematopoiesis as a bone marrow-centered event to evolve into the exploitation of bone marrow cells for treating hematological disease, or simply to recognize that infusion in the blood stream, not per os administration as was done at the end of nineteenth century, was the way to proceed. Pursuit of these strategies generates, in fact, knowledge that becomes exploitable in unexpected ways over time, and our lack of know-how concerning specific facets of the medical problem should not prevent attempts to tackle problems that current technologies allow to be tackled efficiently. Gene correction in “MSCs” is, no doubt, one of these areas. At this time, work in this field remains within the zone of proof-of-principle, but even so, it does provide advances and insight. Osteogenesis imperfecta (Chamberlain et al., 2004, 2008) and fibrous dysplasia have been the focus of attention in this area. We have shown, for example, that a dominant gain-of-function point mutation in a ubiquitously expressed, indispensable gene such as GNAS (encoding the α-subunit of adenylate cyclase stimulatory G protein), can be effectively and efficiently targeted, in skeletal stem cells, using a combination of RNA interference and lentiviral transduction technologies (Piersanti et al., 2006, 2010). Reversal of the fundamental disease phenotype at the cellular level, and even reversal of some specific changes in the stem cell behavior of skeletal stem cells carrying the disease gene, can be accomplished in this way (Piersanti et al., 2010). Translation to proper in vivo models is underway.
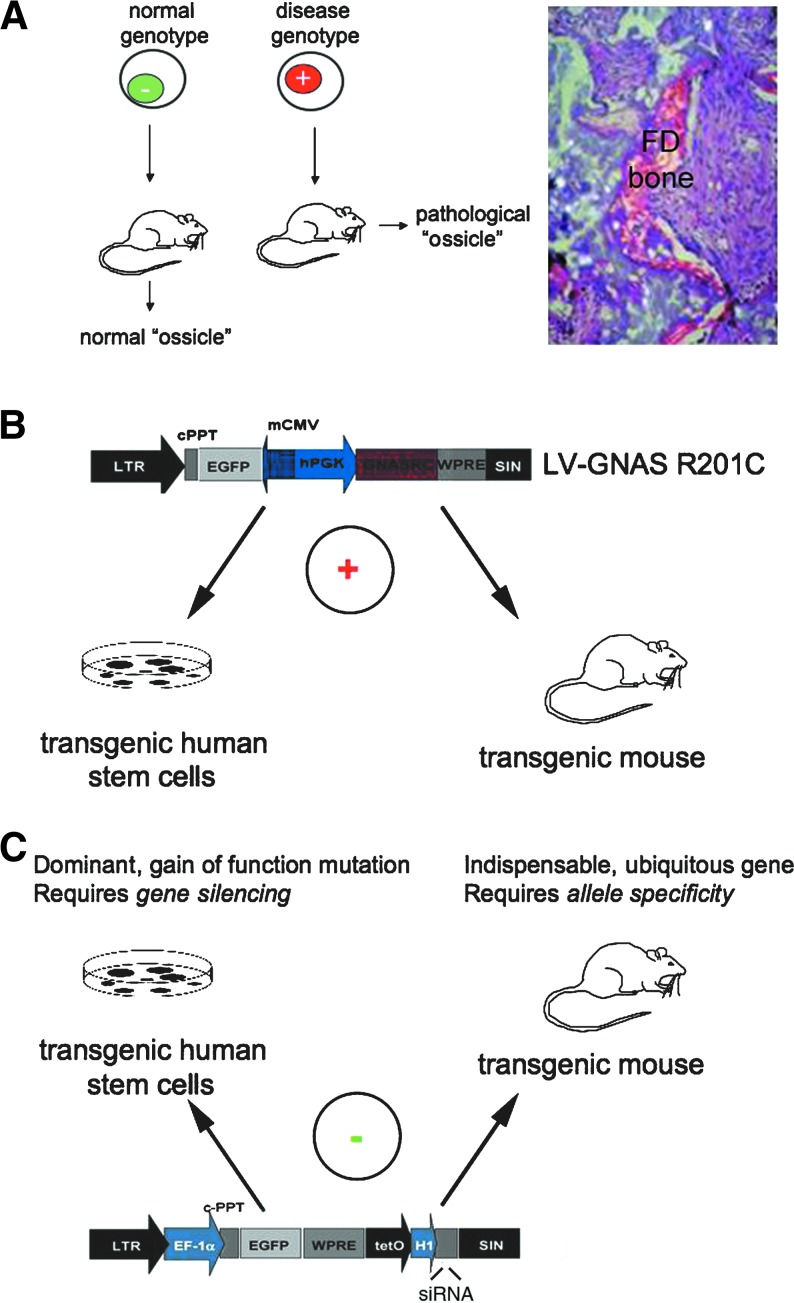
FIG. 2.
Use of skeletal stem cells for modeling fibrous dysplasia (FD) and its genetic correction. (A) Isolation of mutated skeletal stem cells from FD bone marrow followed by heterotopic transplantation in immunocompromised mice generates miniature replicas of abnormal human FD bone in vivo in the mouse. This approach borrows the principle of heterotopic transplantation of normal skeletal progenitors to create normal “ossicles,” and has provided insights into the role of skeletal stem cells for generating bone lesions in FD (Bianco et al., 1998). (B) Use of lentiviral vectors for transfer of the FD disease gene (GNAS R201C) into normal human skeletal stem cells creates an ample source of mutated progenitors for experimental work, and reveals, specifically, the early responses of stem cells to mutated Gsα, including adaptive responses that can be exploited for designing pharmacological intervention (Piersanti et al., 2010). The same vectors used to create human transgenic stem cells can then be used to create murine models of disease (P. Bianco, unpublished data). (C) Genetic correction of FD requires specific silencing of the mutated Gsα allele, which carries a point mutation. This is feasible with lentivirally encoded RNA-interfering sequences. In this way, the fundamental cellular phenotype (excess production of cAMP) and certain abnormalities in the differentiation properties of mutated skeletal progenitors (loss of adipogenic potential) can be reverted in mutated skeletal stem cells. This establishes proof of principle for a model of RNA interference-based gene therapy in FD.
Acknowledgments
The authors' work mentioned in this article was funded by Telethon GP09227, Fondazione Roma, and Ministry for University Research of Italy.
Author Disclosure Statement
The authors state that they have no conflicts of interest.
References
- Arai F. Hirao A. Ohmura M. Sato H. Matsuoka S. Takubo K. Ito K. Koh G.Y. Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Au P. Tam J. Fukumura D. Jain R.K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F.P. Boynton R.E. Haynesworth S. Murphy J.M. Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem. Biophys. Res. Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- Bianco P. Boyde A. Confocal images of marrow stromal (Westen-Bainton) cells. Histochemistry. 1993;100:93–99. doi: 10.1007/BF00572894. [DOI] [PubMed] [Google Scholar]
- Bianco P. Gehron Robey P. Marrow stromal stem cells. J. Clin. Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P. Robey P.G. Skeletal stem cells. In: Lanza R.P., editor. In Handbook of Adult and Fetal Stem Cells. Academic Press; San Diego, CA: 2004. pp. 415–424. [Google Scholar]
- Bianco P. Costantini M. Dearden L.C. Bonucci E. Alkaline phosphatase positive precursors of adipocytes in the human bone marrow. Br. J. Haematol. 1988;68:401–403. doi: 10.1111/j.1365-2141.1988.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Bianco P. Kuznetsov S.A. Riminucci M. Fisher L.W. Spiegel A.M. Robey P.G. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsα-mutated skeletal progenitor cells. J. Clin. Invest. 1998;101:1737–1744. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P. Kuznetsov S.A. Riminucci M. Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117–148. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- Bianco P. Robey P.G. Simmons P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H.M. Chiu C.P. Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blau H.M. Chiu C.P. Pavlath G.K. Webster C. Muscle gene expression in heterokaryons. Adv. Exp. Med. Biol. 1985;182:231–247. doi: 10.1007/978-1-4684-4907-5_20. [DOI] [PubMed] [Google Scholar]
- Calvi L.M. Adams G.B. Weibrecht K.W. Weber J.M. Olson D.P. Knight M.C. Martin R.P. Schipani E. Divieti P. Bringhurst F.R. Milner L.A. Kronenberg H.M. Scadden D.T. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. Schwarze U. Wang P. Hirata R. Hankenson K. Pace J. Underwood R. Song K. Sussman M. Byers P. Russell D. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- Chamberlain J.R. Deyle D.R. Schwarze U. Wang P. Hirata R.K. Li Y. Byers P.H. Russell D.W. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol. Ther. 2008;16:187–193. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.P. Blau H.M. Reprogramming cell differentiation in the absence of DNA synthesis. Cell. 1984;37:879–887. doi: 10.1016/0092-8674(84)90423-9. [DOI] [PubMed] [Google Scholar]
- Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Buhring H.J. Giacobino J.P. Lazzari L. Huard J. Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L. Caplan A.I. Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- De Angelis L. Berghella L. Coletta M. Lattanzi L. Zanchi M. Cusella-De Angelis M.G. Ponzetto C. Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J. Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A. Sampaolesi M. Tonlorenzi R. Tagliafico E. Sacchetti B. Perani L. Innocenzi A. Galvez B.G. Messina G. Morosetti R. Li S. Belicchi M. Peretti G. Chamberlain J.S. Wright W.E. Torrente Y. Ferrari S. Bianco P. Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Deschaseaux F. Charbord P. Human marrow stromal precursors are α1 integrin subunit-positive. J. Cell Physiol. 2000;184:319–325. doi: 10.1002/1097-4652(200009)184:3<319::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L. Martin Herrera A.I. Garcia Montelongo R. Gutiérrez Garcia R. Role of pericytes and endothelial cells in tissue repair and related pathological processes. J. Cutan. Pathol. 1990;17:191–192. doi: 10.1111/j.1600-0560.1990.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L. Gutiérrez R. Gonzalez P. Varela H. Inducible perivascular cells contribute to the neochondrogenesis in grafted perichondrium. Anat. Rec. 1991a;229:1–8. doi: 10.1002/ar.1092290102. [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L. Gutiérrez R. Varela H. Rancel N. Valladares F. Microvascular pericytes: A review of their morphological and functional characteristics. Histol. Histopathol. 1991b;6:269–286. [PubMed] [Google Scholar]
- Díaz-Flores L. Gutiérrez R. Lopez-Alonso A. Gonzalez R. Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin. Orthop. Relat. Res. 1992;275:280–286. [PubMed] [Google Scholar]
- Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Friedenstein A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Friedenstein A.J. Osteogenic stem cells in bone marrow. In: Heersche J.N.M., editor; Kanis J.A., editor. In Bone and Mineral Research. Elsevier; New York: 1990. [Google Scholar]
- Friedenstein A.J. Piatetzky-Shapiro I.I. Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- Friedenstein A.J. Chailakhjan R.K. Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Friedenstein A.J. Chailakhyan R.K. Latsinik N.V. Panasyuk A.F. Keiliss-Borok I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Friedenstein A.J. Chailakhyan R.K. Gerasimov U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Goujon E. Recherches experimentales sur les proprietes physiologiques de la moelle des os. J. de L'Anat. et de La Physiol. 1869;6:399–412. [Google Scholar]
- Hirschi K.K. D'Amore P.A. Pericytes in the microvasculature. Cardiovasc. Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Hirschi K.K. D'Amore P.A. Control of angiogenesis by the pericyte: Molecular mechanisms and significance. EXS. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- Holmbeck K. Bianco P. Caterina J. Yamada S. Kromer M. Kuznetsov S.A. Mankani M. Robey P.G. Poole A.R. Pidoux I. Ward J.M. Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Horwitz E.M. Prockop D.J. Fitzpatrick L.A. Koo W.W. Gordon P.L. Neel M. Sussman M. Orchard P. Marx J.C. Pyeritz R.E. Brenner M.K. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz E.M. Prockop D.J. Gordon P.L. Koo W.W. Fitzpatrick L.A. Neel M.D. McCarville M.E. Orchard P.J. Pyeritz R.E. Brenner M.K. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Horwitz E.M. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini F.C. Deans R.J. Krause D.S. Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Jain R.K. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jain R.K. Booth M.F. What brings pericytes to tumor vessels? J. Clin. Invest. 2003;112:1134–1136. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebsbach P.H. Kuznetsov S.A. Bianco P. Robey P.G. Bone marrow stromal cells: Characterization and clinical application. Crit. Rev. Oral Biol. Med. 1999;10:165–181. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S.A. Krebsbach P.H. Satomura K. Kerr J. Riminucci M. Benayahu D. Robey P.G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J. Bone Miner. Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S.A. Cherman N. Riminucci M. Collins M.T. Robey P.G. Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J. Bone Miner. Res. 2008;23:1731–1740. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K. Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- Melero-Martin J.M. De Obaldia M.E. Kang S.Y. Khan Z.A. Yuan L. Oettgen P. Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michienzi S. Cherman N. Holmbeck K. Funari A. Collins M.T. Bianco P. Robey P.G. Riminucci M. GNAS transcripts in skeletal progenitors: Evidence for random asymmetric allelic expression of Gsα. Hum. Mol. Genet. 2007;16:1921–1930. doi: 10.1093/hmg/ddm139. [DOI] [PubMed] [Google Scholar]
- Minasi M.G. Riminucci M. De Angelis L. Borello U. Berarducci B. Innocenzi A. Caprioli A. Sirabella D. Baiocchi M. De Maria R. Boratto R. Jaffredo T. Broccoli V. Bianco P. Cossu G. The meso-angioblast: A multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Moore K.A. Lemischka I.R. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Morrison S.J. Weissman I.L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Murray S.S. Induction of Myo D expression in NIH 3T3 cells produces a differentiated myocyte phenotype without passing through a determination-like state. In Vitro Cell Dev. Biol. Anim. 1993;29A:446–448. [PubMed] [Google Scholar]
- Osawa M. Hanada K. Hamada H. Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Owen M. Friedenstein A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found. Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Peck W.A. Birge S.J., Jr. Fedak S.A. Bone cells: Biochemical and biological studies after enzymatic isolation. Science. 1964;146:1476–1477. doi: 10.1126/science.146.3650.1476. [DOI] [PubMed] [Google Scholar]
- Piersanti S. Sacchetti B. Funari A. Di Cesare S. Bonci D. Cherubini G. Peschle C. Riminucci M. Bianco P. Saggio I. Lentiviral transduction of human postnatal skeletal (stromal, mesenchymal) stem cells: In vivo transplantation and gene silencing. Calcif. Tissue Int. 2006;78:372–384. doi: 10.1007/s00223-006-0001-y. [DOI] [PubMed] [Google Scholar]
- Piersanti S. Remoli C. Saggio I. Funari A. Michienzi S. Sacchetti B. Robey P.G. Riminucci M. Bianco P. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J. Bone Miner. Res. 2010;25:1103–1116. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop D.J. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin. Pharmacol. Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- Riminucci M. Collins M.T. Corsi A. Boyde A. Murphey M.D. Wientroub S. Kuznetsov S.A. Cherman N. Robey P.G. Bianco P. Gnathodiaphyseal dysplasia: A syndrome of fibro-osseous lesions of jawbones, bone fragility, and long bone bowing. J. Bone Miner. Res. 2001;16:1710–1718. doi: 10.1359/jbmr.2001.16.9.1710. [DOI] [PubMed] [Google Scholar]
- Riminucci M. Collins M.T. Fedarko N.S. Cherman N. Corsi A. White K.E. Waguespack S. Gupta A. Hannon T. Econs M.J. Bianco P. Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riminucci M. Saggio I. Robey P.G. Bianco P. Fibrous dysplasia as a stem cell disease. J. Bone Miner. Res. 2006;21(Suppl. 2):P125–P131. doi: 10.1359/jbmr.06s224. [DOI] [PubMed] [Google Scholar]
- Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I. Tagliafico E. Ferrari S. Robey P.G. Riminucci M. Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M. Torrente Y. Innocenzi A. Tonlorenzi R. D'antona G. Pellegrino M.A. Barresi R. Bresolin N. De Angelis M.G. Campbell K.P. Bottinelli R. Cossu G. Cell therapy of α-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M. Blot S. D'antona G. Granger N. Tonlorenzi R. Innocenzi A. Mognol P. Thibaud J.L. Galvez B.G. Barthelemy I. Perani L. Mantero S. Guttinger M. Pansarasa O. Rinaldi C. Cusella De Angelis M.G. Torrente Y. Bordignon C. Bottinelli R. Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Satomura K. Krebsbach P. Bianco P. Gehron Robey P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J. Cell Biochem. 2000;78:391–403. [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Sherley J.L. Asymmetric cell kinetics genes: The key to expansion of adult stem cells in culture. Sci. World J. 2002;2:1906–1921. doi: 10.1100/tsw.2002.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley J.L. Stadler P.B. Johnson D.R. Expression of the wild-type p53 antioncogene induces guanine nucleotide-dependent stem cell division kinetics. Proc. Natl. Acad. Sci. U.S.A. 1995;92:136–140. doi: 10.1073/pnas.92.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood R.I. Christensen J.L. Conboy I.M. Conboy M.J. Rando T.A. Weissman I.L. Wagers A.J. Isolation of adult mouse myogenic progenitors: Functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Simmons P.J. Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Spangrude G.J. Heimfeld S. Weissman I.L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sugiyama T. Kohara H. Noda M. Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Suri C. Jones P.F. Patan S. Bartunkova S. Maisonpierre P.C. Davis S. Sato T.N. Yancopoulos G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tavassoli M. Crosby W.H. Transplantation of marrow to extramedullary sites. Science. 1968;161:54–56. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- Weiss L. The hematopoietic microenvironment of the bone marrow: An ultrastructural study of the stroma in rats. Anat. Rec. 1976;186:161–184. doi: 10.1002/ar.1091860204. [DOI] [PubMed] [Google Scholar]
- Westen H. Bainton D.F. Association of alkaline-phosphatase-positive reticulum cells in bone marrow with granulocytic precursors. J. Exp. Med. 1979;150:919–937. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Niu C. Ye L. Huang H. He X. Tong W.G. Ross J. Haug J. Johnson T. Feng J.Q. Harris S. Wiedemann L.M. Mishina Y. Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]