Abstract
Aim
The aim of this study is to evaluate the effects of the synbiotic Bifidobacterium lactis B94 plus inulin addition to the standard triple therapy on Helicobacter pylori (H. pylori) infection eradication rates.
Methods
Children aged 6–16 years who had biopsy proven H. pylori infection were randomly classified into two groups. The first group received the standard triple therapy consisting of amoxicillin + clarithromycin + omeprazole. The second group was treated with the standard triple therapy and Bifidobacterium lactis B94 (5 × 109 CFU/dose) plus inulin (900 mg) for 14 days, concurrently. Eradication was determined by 14C-urea breath test 4–6 weeks after therapy discontinuation.
Results
From a total of 69 H. pylori infected children (F/M = 36/33; mean ± SD = 11.2 ± 3.0 years), eradication was achieved in 20/34 participants in the standard therapy group and 27/35 participants in the synbiotic group. The eradication rates were not significantly different between the standard therapy and the synbiotic groups [intent-to-treat, 58.8% and 77.1%, resp., p = 0.16; per-protocol, 64.5% and 81.8%, resp., p = 0.19]. There was no difference between the groups in terms of symptom relief (p = 0.193). The reported side effects were ignorable.
Conclusion
Considering the eradication rates, synbiotic addition to therapy showed no superiority over the standard triple therapy conducted alone. This trial is registered with NCT03165253.
1. Introduction
Helicobacter pylori (H. pylori) infection is one of the most common chronic bacterial diseases worldwide with an estimated prevalence of more than 50% in adults [1, 2]. Studies in Turkish patients reported that 70–80% of adults and 30–56% of children are infected with H. pylori [3–5]. In Western populations, H. pylori prevalence is low in children; however, it is still a problem as immigrants from countries where H. pylori is endemic constitute a risk group and a reservoir for the infection [6]. If left untreated, it can cause chronic gastritis, peptic ulcer, gastric adenocarcinomas [7], and mucosa-associated lymphoid tissue (MALT) lymphomas [8]. Fortunately, H. pylori associated gastric cancer has not been reported in pediatric age group, but MALT lymphomas have been described in a few H. pylori infected children [9–11]. In an adult study, early eradication of H. pylori has been associated with a sixfold reduction in the recurrence of peptic ulcers as well as a twofold to threefold reduction in the risk of gastric carcinoma [12]. Since H. pylori is mostly acquired in childhood, it is important to eradicate the infection in children as well. As stated in Kyoto global consensus report on H. pylori gastritis, “the maximum benefit of H. pylori eradication is obtained if it is done while the mucosal damage has not progressed beyond the nonatrophic stage” [13]. Further advantages of eradication of the infection in adolescents and young adults are also stated as reduction or prevention of the transmission of the infection to others and especially to their children [13].
Current guidelines recommend eradication of H. pylori infection in symptomatic children especially with peptic ulcer disease and with a family history of gastric carcinoma [14, 15]. The standard triple therapy that consists of a proton pump inhibitor and a combination of two antimicrobial agents, amoxicillin and either clarithromycin or an imidazole, for at least 7–14 days, is the main regimen used in both children and adults [14, 16] However, the success rate of this regimen declined from 80 to 96% in the previous years to 60–70% today [17–20]. Sequential therapy or bismuth salts based triple therapies are also mentioned as first-line treatments [14]. With so many different options but far from ideal eradication rates, it is hard to say which treatment is the best for children. Therefore, alternative therapies are still being searched.
Adjuvant treatment with probiotics, prebiotics, and/or synbiotics may be promising options. Probiotics are defined as “living microorganisms that are beneficial to the health of the host when consumed at adequate amounts” [21]. Prebiotics are indigestible foods that enhance the growth or activity of beneficial probiotic bacteria in the gut [22]. Synbiotics are the combination of probiotics and prebiotics, which can work synergistically. In vitro studies demonstrated inhibitory activity of certain probiotic strains against H. pylori bacteria [23, 24]. Furthermore, there are randomized controlled trials (RCT) demonstrating favorable outcomes of probiotic addition to H. pylori eradication treatments [25–28]. They are believed to increase patient compliance by reducing the side effects such as antibiotic-associated diarrhea [26, 28–30]. Most studies in this area are mainly designed by using a single probiotic strain or a combination of strains. To our knowledge, there are currently a few studies assessing the effects of synbiotics on H. pylori eradication [30–32].
In our study, we aimed to investigate the effects of the synbiotic Bifidobacterium lactis (B. lactis) B94 plus inulin addition to standard triple therapy in terms of eradication rates, relief of symptoms, and patient compliance.
2. Materials and Methods
The study was conducted between June 2011 and June 2012 in the pediatric gastroenterology clinic of Bulent Ecevit University Faculty of Medicine, Zonguldak, Turkey. The study population consisted of 69 children aged 6–16 years who were investigated by a standard esophagogastroduodenoscopy (EGD) for gastrointestinal symptoms, those suggesting an organic disease such as chronic abdominal pain, unexplained nausea and/or vomiting, severe regurgitation, gastrointestinal bleeding, unexplained weight loss, or chronic diarrhea. Written informed consent was obtained from the parents. The study was approved by the Scientific Research Ethics Committee of the Bulent Ecevit University (Protocol number, 2011-10-08/03).
Patients who were treated for H. pylori infection previously, who used an antimicrobial agent, bismuth, a nonsteroid anti-inflammatory drug, or any form of gastric acid suppressor during the eight weeks prior the EGD, or who had history of major gastrointestinal surgery, chronic renal, or hepatic disease and who were known to have drug allergy were excluded from the study.
The patients underwent a standard EGD and biopsies from stomach and duodenum were taken at least two per site. Endoscopic findings such as antral hyperemia, antral nodularity, and peptic ulcer were noted. The biopsy specimens were evaluated by the same pathologist at the Department of Medical Pathology, Bulent Ecevit University Faculty of Medicine. Children who were found to have H. pylori infection proven by histopathological examination were randomly assigned to two groups in a double blind manner using a prepared randomization list. Each patient was given a number. Patients in the standard triple therapy group were treated with amoxicillin 50 mg/kg/d and clarithromycin 15 mg/kg/d twice daily for 14 days and omeprazole (OMP) 1 mg/kg/d once daily for a month. The synbiotic group received the same standard triple therapy and Bifidobacterium lactis B94 (5 × 109 CFU/dose) plus 900 mg of inulin (Maflor sachet, Mamsel, Turkey) given a single dose for 14 days concurrently. Required amount of drugs for both treatment groups was prescribed. A detailed chart explaining the usage of drugs and a form to record the changes in symptoms and the side effects related to treatment (nausea, vomiting, diarrhea, and abdominal or epigastric pain) were given to the patients and/or their parents. The patients were called for follow-up 4–6 weeks after the cessation of OMP treatment. Then, they were assessed for relief of symptoms and 14C-urea breath test (14C-UBT) was scheduled. Negative 14C-UBT was considered as eradication.
3. Statistical Analysis
Statistical analysis was performed with SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Distribution of data was determined by Shapiro-Wilk test. Continuous variables were expressed as mean ± std. deviation, categorical variables as frequency and percent. Continuous variables were compared with the independent sample t-test or Mann–Whitney U test for two groups. Categorical variables were compared using Pearson's Chi-square test or Fisher Exact Chi-square test. The eradication rates were determined using both the intent-to-treat (ITT) and per-protocol (PP) analyses. p value of less than 0.05 was considered statistically significant for all tests.
4. Results
Sixty-nine patients with H. pylori infection were randomly assigned to the standard therapy group (n = 34) and synbiotic group (n = 35) (Figure 1). The demographic characteristics of patients were similar between the groups (Table 1). The most frequent complaint was abdominal pain in both groups (87% in the standard therapy group and 97% in the synbiotic group, p = 0.19). It was mainly described as epigastric pain by 40.7% of patients in the standard therapy group and 43.8% of patients in the synbiotic group (p = 0.09). The second frequent complaint was nausea in 64.5% and 60.6% of patients in the standard therapy and synbiotic groups, respectively (p = 0.9). A total of 5 children dropped out the study, since they did not present to the follow-up (Table 2). All the other patients completed the treatment as prescribed. Patients were reassessed at follow-up 4–6 six weeks after the end of OMP treatment.
Figure 1.
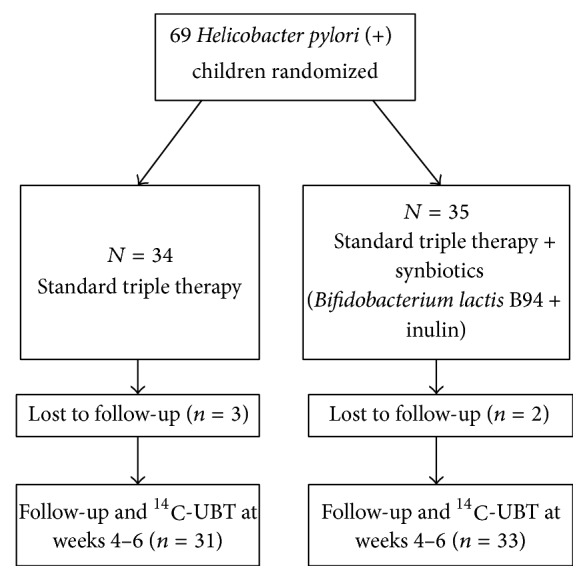
Flow diagram of the patients' progression through the study. 14C-UBT: carbon-14 labelled urea breath test.
Table 1.
Demographic characteristics and endoscopic findings of study groups.
| Standard therapy |
Synbiotic |
||
|---|---|---|---|
| Patient characteristics | n = 34 | n = 35 | p |
|
| |||
| Age (year) | 11.2 ± 3.1 | 11.2 ± 2.9 | 0.97 |
| Sex (female/male) | 0.88 | 1.35 | 0.55 |
| Height (cm) | 145.5 ± 3.0 | 147.2 ± 3.3 | 0.71 |
| Weight (kg) | 40.9 ± 3.0 | 42.5 ± 2.5 | 0.39 |
|
| |||
| Endoscopic findings | n = 31 | n = 33 | p |
|
| |||
| Antral hyperemia | 26 (83.9%) | 28 (84.8%) | 0.99 |
| Antral nodularity | 4 (12.9%) | 4 (12.1%) | |
| Antral ulcer | 1 (3.2 %) | 1 (3%) | |
| Normal duodenum | 22 (71%) | 27 (81.8%) | 0.69 |
| Duodenitis | 5 (16.1%) | 4 (12.1%) | |
| Duodenal ulcer | 4 (12.9%) | 2 (6.1%) | |
Table 2.
The eradication success in study groups.
| Status | Standard therapy (n) | Synbiotic (n) |
|---|---|---|
| Eradication | 20 | 27 |
| Failed eradication | 11 | 6 |
| Missing data | 3 | 2 |
|
| ||
| Total | 34 | 35 |
None of the patients in each group experienced a side effect that would require termination of the treatment. Only one patient complained of increased abdominal pain in the standard therapy group, and one patient had a new onset of diarrhea during the antibiotherapy in the synbiotic group. In the standard therapy group, there was no change of symptoms in 4 patients (12.9%), while a slight relief of symptoms in 11 patients (35.5%) and a marked relief of symptoms in 16 patients (51.6%) were observed. In the synbiotic group, no change of symptoms was reported by 1 patient (3%), whereas a slight relief of symptoms and a marked relief of symptoms were reported by 7 patients (21.2%) and by 25 patients (75.8%), respectively. Although, the symptoms were much more improved in the synbiotic group, the difference was not statistically significant between the groups (p = 0.09).
H. pylori eradication was confirmed by 14C-UBT. The eradication rates in both the synbiotic and the standard therapy groups were evaluated using ITT and PP analyses (Table 3). Eradication rates did not differ significantly between the groups (p = 0.16 and p = 0.19, by ITT and PP analyses, resp.).
Table 3.
The eradication rates in study groups.
| Analyses | Standard therapy | Synbiotic | p |
|---|---|---|---|
| ITT | 56.8% (20/34) |
77.1% (27/35) |
0.16 |
| PP | 64.5% (20/31) |
81.8% (27/33) |
0.19 |
5. Discussion
In recent years the efficacy of H. pylori eradication treatments has fallen below an acceptable level of 80%, mainly due to antimicrobial resistance and inadequate patient compliance [16]. As no new drug has been developed for H. pylori eradication, clinical trials have focused on different combinations of known drugs and adjuvant therapy. Probiotics are thought to be beneficial in H. pylori infection in two ways: (1) some strains may have a direct effect on the pathogen and inhibit its growth. (2) Some strains may reduce antibiotic-associated side effects and improve compliance to therapy. Moreover, pre- and probiotic supplementation may have immunomodulatory effects on the hosts as experimental studies demonstrated their anti-inflammatory effects in chronic inflammatory diseases like asthma, topical allergies, and inflammatory bowel disease [33, 34].
In vitro studies and animal models have demonstrated inhibitory effects of several probiotic species especially Lactobacilli and Bifidobacterium on H. pylori colonization and growth [23, 24]. This effect is achieved by the production of organic acids, autolysins, mucin, and bacteriocins and/or by the binding of some specific strains to the same glycolipid receptors as H. pylori [23, 24, 35–38]. The preclinical studies have supported the possibility of using probiotics in the treatment of H. pylori infections in clinical settings.
Several studies evaluated the effects of probiotics as monotherapy in asymptomatic children infected with H. pylori [31, 39]. Gotteland et al. demonstrated that children receiving Saccharomyces boulardii (S. boulardii) and inulin (a synbiotic) had a significant decrease in 13C-UBTs [31]. In 12% of the participants, the infection was even eradicated by the regular intake of this synbiotic [31]. Similarly, Cruchet et al. treated H. pylori infected asymptomatic children for one month with live Lactobacillus johnsonii La 1 and showed significant changes [39]. In summary, specific probiotic strains as mentioned above probably lower the bacterial load in the gastric mucosa but not completely eradicate the bacteria. Considering that a certain percentage of the patients had achieved eradication even with monotherapy, we have planned the current study assuming that we would get better results by combining a synbiotic with standard therapy.
To date, several systematic reviews and meta-analyses have been published about the effects of probiotic supplementation to standard treatment regimens of H. pylori infection in both children and adults [40, 41]. The systematic review and meta-analysis by Szajewska et al. evaluated the effects of S. boulardii supplementation to eradication therapy [40]. Eleven RCTs involving 2200 patients were included in the review. The eradication rates were found to be significantly higher (80% versus 71%) in participants taking S. boulardii. Also, overall adverse effects were lower in the probiotic group compared to the control group, and particularly the risk of diarrhea and nausea was reduced [40]. The recent meta-analysis by Lau et al. included thirty RCTs involving more than 4000 patients, both children and adults [41]. They examined the impact of probiotic supplementation on standard triple therapy and concluded that the addition of probiotics increased eradication rates (p < 0.001). Moreover, the risk of diarrhea, nausea, vomiting, and epigastric pain was also found to be reduced. In conclusion, generally the authors suggested that the eradication rates improved, and the frequency of adverse effects was reduced. However, the results should be interpreted cautiously, since collecting data on different strains in meta-analysis may be misleading.
In our study, we evaluated the effects of a synbiotic “B. lactis B94 and inulin” addition to standard triple therapy in symptomatic children. Although, the eradication rate seemed to be higher in the synbiotic group, the difference was not statistically significant (ITT, p = 0.16, and PP, p = 0.19). To our knowledge, there are only two reports that used the same synbiotic treatment in H. pylori eradication [30, 32]. Similar to our results, Islek et al. could not find a positive effect of this symbiotic treatment on eradication rates in H. pylori infected children [30]. However, there was a notable decrease on the occurrence of side effects in the synbiotic group which was interpreted as an indirect positive effect on eradication success [30]. On the other hand, the study by Çekin et al. revealed significantly higher H. pylori eradication rates with sequential treatment and B. lactis B94 [32]. Furthermore, they have demonstrated lower diarrhea rates and higher treatment compliance in patients receiving B. lactis B94 [32]. Inconsistent with these studies, the patients in our study did not report any side effects that would require discontinuation of therapy. Another recent study by Shafagi et al. also investigated the effectiveness of a multistrain synbiotic combination in adult patients taking quadruple therapy [42]. The eradication rates were higher in the synbiotic group based on ITT analysis (92.1% versus 63.2%, p < 0.05) [42]. No significant difference was noted between the groups in terms of side effects. However, a considerable number of patients (14/38) were lost to follow-up in the placebo group, mostly due to noncompliance [42]. The results of this study also showed that compliance is a key factor in eradication success. Indeed, compliance to therapy was not a major problem in our study as none of the patients in both groups had an intolerable side effect. The improvement in dyspeptic symptoms was higher in the synbiotic group, although not statistically significant. However, we did not use an objective symptom scale for this evaluation.
The limitations of our study can be listed as follows: (1) H. pylori culture and antibiotic susceptibility tests were not done. (2) Bifidobacterium colonization in the feces was not evaluated. (3) The sample size was relatively small.
6. Conclusion
The results of our study demonstrated that the addition of B. lactis B94 (5 × 109 CFU/dose) plus inulin once daily to standard triple therapy showed no superiority on the eradication rates compared to the standard triple therapy given alone. There are currently a limited number of RCTs in pediatric age group conducted with a diversity of probiotic strains, and the results are conflicting with each other. Thus, it is hard to conclude which probiotic/synbiotic is effective in H. pylori eradication, at which doses and duration, and with which antimicrobial regimen. Further large-scale RCTs are needed to clarify these points.
Acknowledgments
This paper has been proofread by Fatma Tanrıverdi-Köksal, a member of the Bulent Ecevit University Article Proofreading and Editing Office.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Gonca Handan Ustundag and Halime Altuntas designed the research study, collected and analyzed the data, and wrote the manuscript. Yasemin Dilek Soysal helped in manuscript writing and reviewed the data analysis. Furuzan Kokturk contributed to study design and performed the statistical analysis of the data. All authors approved the final version of the manuscript.
References
- 1.Suerbaum S., Michetti P. Helicobacter pylori infection. The New England Journal of Medicine. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Brown L. M. Helicobacter pylori: epidemiology and routes of transmission. Epidemiologic Reviews. 2000;22(2):283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 3.Us D., Hasçelik G. Seroprevalence of Helicobacter pylori infection in an asymptomatic Turkish population. Journal of Infection. 1998;37(2):148–150. doi: 10.1016/S0163-4453(98)80169-2. [DOI] [PubMed] [Google Scholar]
- 4.Gurakan F., Kocak N., Yuce A. Helicobacter pylori serology in childhood. TheTurkish Journal of Pediatrics. 1996;38(3):329–334. [PubMed] [Google Scholar]
- 5.Özen A., Ertem D., Pehlivanoglu E. Natural history and symptomatology of Helicobacter pylori in childhood and factors determining the epidemiology of infection. Journal of Pediatric Gastroenterology and Nutrition. 2006;42(4):398–404. doi: 10.1097/01.mpg.0000215307.48169.7b. [DOI] [PubMed] [Google Scholar]
- 6.Den Hollander W. J., Holster I. L., Van Gilst B., et al. Intergenerational reduction in Helicobacter pylori prevalence is similar between different ethnic groups living in a Western city. Gut. 2015;64(8):1200–1208. doi: 10.1136/gutjnl-2014-307689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsonnet J., Friedman G. D., Vandersteen D. P., et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England Journal of Medicine. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 8.Eidt S., Stolte M., Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin's lymphomas. Journal of Clinical Pathology. 1994;47(5):436–439. doi: 10.1136/jcp.47.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moschovi M., Menegas D., Stefanaki K., Van-Vliet Constantinidou C., Tzortzatou-Stathopoulou F. Primary gastric Burkitt lymphoma in childhood: Associated with Helicobacter pylori? Medical and Pediatric Oncology. 2003;41(5):444–447. doi: 10.1002/mpo.10319. [DOI] [PubMed] [Google Scholar]
- 10.Kurugoglu S., Mihmanli I., Celkan T., Aki H., Aksoy H., Korman U. Radiological features in paediatric primary gastric MALT lymphoma and association with Helicobacter pylori. Pediatric Radiology. 2002;32(2):82–87. doi: 10.1007/s00247-001-0598-y. [DOI] [PubMed] [Google Scholar]
- 11.Al Furaikh S. S. Remission of high-grade B-cell lymphoma in a pediatric patient following Helicobacter pylori eradication. Pediatrics International. 2011;53(1):105–107. doi: 10.1111/j.1442-200X.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 12.Hung I. F. N., Wong B. C. Y. Assessing the risks and benefits of treating Helicobacter pylori infection. Therapeutic Advances in Gastroenterology. 2009;2(3):141–147. doi: 10.1177/1756283X08100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugano K., Tack J., Kuipers E. J., et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koletzko S., Jones N. L., Goodman K. J., et al. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. Journal of Pediatric Gastroenterology and Nutrition. 2011;53(2):230–243. doi: 10.1097/mpg.0b013e3182227e90. [DOI] [PubMed] [Google Scholar]
- 15.Drumm B., Koletzko S., Oderda G. Helicobacter pylori infection in children: A consensus statement. Journal of Pediatric Gastroenterology and Nutrition. 2000;30(2):207–213. doi: 10.1097/00005176-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Malfertheiner P., Megraud F., O'Morain C. A., et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence consensus report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 17.Vilaichone R.-K., Mahachai V., Graham D. Y. Helicobacter pylori Diagnosis and Management. Gastroenterology Clinics of North America. 2006;35(2):229–247. doi: 10.1016/j.gtc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Kadayifci A., Buyukhatipoglu H., Savas M. C., Simsek I. Eradication of Helicobacter pylori with triple therapy: an epidemiologic analysis of trends in Turkey over 10 years. Clinical Therapeutics. 2006;28(11):1960–1966. doi: 10.1016/j.clinthera.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Oderda G., Shcherbakov P., Bontems P., et al. Results from the Pediatric European Register for Treatment of Helicobacter pylori (PERTH) Helicobacter. 2007;12(2):150–156. doi: 10.1111/j.1523-5378.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 20.Kutluk G., Tutar E., Bayrak A., et al. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication in children: Any advantage in clarithromycin-resistant strains? European Journal of Gastroenterology and Hepatology. 2014;26(11):1202–1208. doi: 10.1097/MEG.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 21.Hill C., Guarner F., Reid G., et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 22.Roberfroid M. Prebiotics: the concept revisited. The Journal of Nutrition. 2007;137(3) supplement 2:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia S. J., Kochar N., Abraham P., Nair N. G., Mehta A. P. Lactobacillius acidophilus inhibits growth of Campylobacter pylori in vitro. Journal of Clinical Microbiology. 1989;27(10):2328–2330. doi: 10.1128/jcm.27.10.2328-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorca G. L., Wadström T., Font de Valdez G., Ljungh Å. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Current Microbiology. 2001;42(1):39–44. doi: 10.1007/s002840010175. [DOI] [PubMed] [Google Scholar]
- 25.Sýkora J., Valečková K., Amlerová J., et al. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. Journal of Clinical Gastroenterology. 2005;39(8):692–698. doi: 10.1097/01.mcg.0000173855.77191.44. [DOI] [PubMed] [Google Scholar]
- 26.Hurduc V., Plesca D., Dragomir D., Sajin M., Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatrica, International Journal of Paediatrics. 2009;98(1):127–131. doi: 10.1111/j.1651-2227.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 27.Tolone S., Pellino V., Vitaliti G., Lanzafame A., Tolone C. Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Italian Journal of Pediatrics. 2012;38(1, article no. 63) doi: 10.1186/1824-7288-38-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad K., Fatemeh F., Mehri N., Mayram S. Probiotics for the treatment of pediatric Helicobacter pylori infection: a randomized double blind clinical trial. Iranian Journal of Pediatrics. 2013;23(1):79–84. [PMC free article] [PubMed] [Google Scholar]
- 29.Lionetti E., Miniello V. L., Castellaneta S. P., et al. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Alimentary Pharmacology and Therapeutics. 2006;24(10):1461–1468. doi: 10.1111/j.1365-2036.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- 30.Islek A., Sayar E., Yilmaz A., Artan R. Bifidobacterium lactis B94 plus inulin for treatment of Helicobacter pylori infection in children: does it increase eradication rate and patient compliance? Acta Gastro-Enterologica Belgica. 2015;78(3):282–286. [PubMed] [Google Scholar]
- 31.Gotteland M., Poliak L., Cruchet S., Brunser O. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatrica, International Journal of Paediatrics. 2005;94(12):1747–1751. doi: 10.1080/08035250500252120. [DOI] [PubMed] [Google Scholar]
- 32.Çekin A. H., Şahintürk Y., Akbay Harmandar F., Uyar S., Yolcular B. O., Çekin Y. Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance. Turkish Journal of Gastroenterology. 2017;28:3–11. doi: 10.5152/tjg.2016.0278. [DOI] [PubMed] [Google Scholar]
- 33.Peppelenbosch M. P., Ferreira C. V. Immunology of pre- and probiotic supplementation. British Journal of Nutrition. 2009;101(1):2–4. doi: 10.1017/S0007114508020746. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar A., Mandal S. Bifidobacteria—Insight into clinical outcomes and mechanisms of its probiotic action. Microbiological Research. 2016;192:159–171. doi: 10.1016/j.micres.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Hütt P., Shchepetova J., Lõivukene K., Kullisaar T., Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. Journal of Applied Microbiology. 2006;100(6):1324–1332. doi: 10.1111/j.1365-2672.2006.02857.x. [DOI] [PubMed] [Google Scholar]
- 36.Gomi A., Harima-Mizusawa N., Shibahara-Sone H., Kano M., Miyazaki K., Ishikawa F. Effect of Bifidobacterium bifidum BF-1 on gastric protection and mucin production in an acute gastric injury rat model. Journal of Dairy Science. 2013;96(2):832–837. doi: 10.3168/jds.2012-5950. [DOI] [PubMed] [Google Scholar]
- 37.Mukai T., Asasaka T., Sato E., Mori K., Matsumoto M., Ohori H. Inhibition of binding of Helicobacter pylorito the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunology and Medical Microbiology. 2002;32(2):105–110. doi: 10.1111/j.1574-695X.2002.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 38.Gotteland M., Brunser O., Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Alimentary Pharmacology and Therapeutics. 2006;23(8):1077–1086. doi: 10.1111/j.1365-2036.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 39.Cruchet S., Obregon M. C., Salazar G., Diaz E., Gotteland M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19(9):716–721. doi: 10.1016/s0899-9007(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 40.Szajewska H., Horvath A., Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics. 2015;41(12):1237–1245. doi: 10.1111/apt.13214. [DOI] [PubMed] [Google Scholar]
- 41.Lau C. S. M., Ward A., Chamberlain R. S. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: A meta-analysis. Infection and Drug Resistance. 2016;9:275–289. doi: 10.2147/IDR.S117886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafaghi A., Pourkazemi A., Khosravani M., et al. The Effect of probiotic plus prebiotic supplementation on the tolerance and efficacy of Helicobacter pylori eradication quadruple therapy: a randomized prospective double blind controlled trial. Middle East Journal of Digestive Diseases. 2016;8(3):179–188. doi: 10.15171/mejdd.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]


