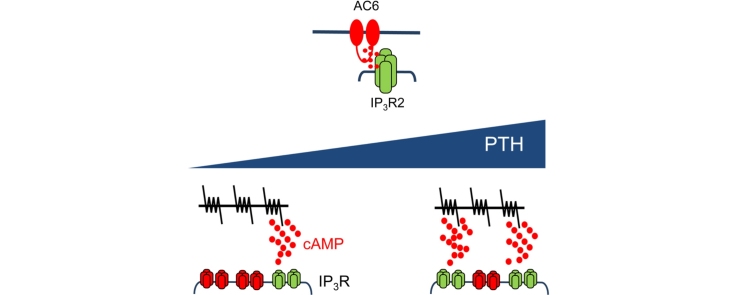
Graphical abstract
Abbreviations: AC, adenylyl cyclase; EPAC, exchange protein activated by cAMP; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; IRAG, IP3R-associated cGMP kinase substrate; IRBIT, IP3R-binding protein released by IP3; PKA, protein kinase A (cAMP-dependent protein kinase); PKG, protein kinase G (cGMP-dependent protein kinase); PLC, phospholipase C; Po, single-channel open probability; PTH, parathyroid hormone
Keywords: Ca2+ stores, Cyclic AMP, IP3 receptor, Protein kinase A, Parathyroid hormone, Signalling junctions
Highlights
-
•
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are modulated by cAMP.
-
•
Phosphorylation by cAMP-dependent protein kinase (PKA) potentiates IP3-evoked Ca2+ release through IP3R1 and IP3R2.
-
•
Delivery of cAMP to IP3R2s within signalling junctions directly potentiates their responses to IP3.
Abstract
Ca2+ and cAMP are ubiquitous intracellular messengers and interactions between them are commonplace. Here the effects of cAMP on inositol 1,4,5-trisphosphate receptors (IP3Rs) are briefly reviewed. All three subtypes of IP3R are phosphorylated by cAMP-dependent protein kinase (PKA). This potentiates IP3-evoked Ca2+ release through IP3R1 and IP3R2, but probably has little effect on IP3R3. In addition, cAMP can directly sensitize all three IP3R subtypes to IP3. The high concentrations of cAMP required for this PKA-independent modulation of IP3Rs is delivered to them within signalling junctions that include type 6 adenylyl cyclase and IP3R2.
1. Introduction
Cyclic AMP and Ca2+ are ubiquitous intracellular messengers used by all eukaryotic cells from plants and animals to coordinate their behaviours in response to both extracellular signals and intracellular activity [1], [2], [3]. These messengers create a signalling ‘bottleneck’ through which many extracellular signals funnel to regulate diverse cellular responses. The capacity of a rather limited repertoire of intracellular messengers to selectively regulate cellular activities depends in large part on the spatial organization of the messengers within the cell, the time frames over which they are delivered, and interactions between messengers. The latter often endows signalling pathways with capacities to function as coincidence detectors: conveying signals onward only when several conditions are met [4]. As might be expected of the prototypical intracellular messengers, analyses of the interactions between cAMP and Ca2+ have a long history [5], [6] that has revealed interactions at many levels. Ca2+, for example, regulates formation and degradation of cAMP [2], [7], and cAMP can regulate both the channels that allow Ca2+ to flow into the cytosol and the Ca2+ pumps that extrude it [8], [9].
A ubiquitous pathway from extracellular stimuli to cytosolic Ca2+ signals is provided by receptors that stimulate phospholipase C (PLC), production of inositol 1,4,5-trisphosphate (IP3) and thereby Ca2+ release through IP3 receptors (IP3R) [10]. Cyclic AMP also modulates this pathway by, for example, regulating PLC [11] and the coupling of receptors to PLC [12]. However, in this short review, I focus on just one level of interaction, that between cAMP and IP3Rs [13], [14]. IP3R subunits are encoded by three genes in vertebrates. The three large, closely related subunits assemble into homo- and hetero-tetrameric structures, which form large-conductance Ca2+-permeable channels within intracellular membranes, primarily those of the endoplasmic reticulum [10]. Opening of the central pore is initiated by binding of IP3 to all four IP3R subunits [15], which evokes conformational changes within the N-terminal domains of the IP3R [16]. These conformational changes are proposed to facilitate binding of Ca2+, which then triggers opening of the pore. Hence, the IP3R is itself a coincidence detector, responding only when provided with both cytosolic IP3 and Ca2+. High-resolution structures of the N-terminal region of an IP3R with and without IP3 bound [16], and cryo-electron microscopy reconstructions of the entire IP3R in a closed state [17] have begun to reveal the workings of the IP3R machinery. However, the mechanisms linking IP3 binding to channel gating are not yet fully resolved. While IP3 and Ca2+ are the essential regulators of IP3R gating, many additional signals modulate IP3R behaviour [18]. My focus on cAMP therefore provides only a rather restricted view of the capacity of IP3Rs to integrate information provided by different signalling pathways.
2. Regulation of IP3Rs by PKA
Cyclic AMP-dependent protein kinase (protein kinase A, PKA), exchange proteins activated by cAMP (EPACs), cyclic nucleotide-activated cation channels (CNGs), and some cyclic nucleotide phosphodiesterases (PDEs) are the major targets of cAMP in mammals. At least some of these targets regulate IP3-evoked Ca2+ signalling. PKA, for example, stimulates Ca2+ uptake into the sarcoplasmic reticulum, and EPACs through the small G protein rap2B stimulate PLCε [11]. However, only PKA has been convincingly shown to interact directly with IP3Rs. The three IP3R subtypes are closely related, but each has a distinctive distribution of PKA phosphorylation sites. The many effects of cAMP within Ca2+ signalling pathways were sources of some confusion in the pioneering studies of IP3R phosphorylation [19], but the consensus now is that PKA-mediated phosphorylation of IP3R1 and IP3R2 enhances their activity, while the functional significance of such phosphorylation for IP3R3 is less clear [14], [20].
Two residues (S1589 and S1755) within the central cytosolic domain of IP3R1 are phosphorylated by PKA, and their replacement by non-phosphorylatable alanine residues confirms that they are the only sites [21]. Phosphorylation of IP3R1 by PKA or introduction of phosphomimetic residues (S1589E/S1755E) do not themselves open the channel, but they increase the open probability (Po) of channels activated by IP3. The increased Po results from shortening of the gaps between bursts of channel openings and an increase in the duration of the bursts, with no obvious effect on IP3 binding or the sensitivity to Ca2+ regulation [22]. Hence, phosphorylation of IP3R1 by PKA improves the coupling of IP3 and Ca2+ binding to channel gating by both stabilizing the bursting state of the IP3R and destabilizing a prolonged closed state. An alternative splice site (S2, residues 1693–1732), which encodes 40 residues and is removed from non-neuronal IP3R1, abuts the second phosphorylation site (S1755). For the neuronal S2+ form of IP3R1, S1755 entirely mediates the effects of PKA, while in the peripheral S2− form both residues (S1589 and S1755) must be phosphorylated for PKA to enhance IP3-evoked Ca2+ release [23]. Effective phosphorylation and dephosphorylation of IP3R1 are facilitated by tethering of PKA to IP3R1 by AKAP9 (A-kinase-anchoring protein 9) [24] and of the protein phosphatase, PP1α, by IRBIT [25], AKAP9 or directly to the C-terminal tail of IP3R1 [26].
The consensus sequences for PKA and cGMP-dependent protein kinase (PKG) are similar, such that some residues (e.g. S1755 in IP3R1S2+) are phosphorylated by either kinase. Yet in native tissues PKG and PKA often exert opposing effects on IP3-evoked Ca2+ release. The difference may, at least in part, be due to expression of IRAG (IP3R-associated cGMP kinase substrate), which blocks phosphorylation of IP3R1 by PKA, and IRAG phosphorylated by PKG inhibits IP3R [27]. Hence, IRAG diverts PKG from the PKA-phosphorylation sites and imposes its own inhibition. PKA also modulates the interaction of IP3R1 with its endogenous antagonist, IRBIT (IP3R-binding protein released by IP3), apparently decreasing the affinity for IRBIT so that IP3 more effectively competes for occupancy of their shared binding site on the IP3R [28]. Hence in secretory epithelia, receptors that stimulate formation of cAMP and IP3 synergistically stimulate release of IRBIT from IP3Rs, and IRBIT then directly stimulates two of the ion transporters that sustain fluid transport [28].
Long before the discovery IP3Rs, synergistic stimulation of a Ca2+-sensitive K+ channel by α1-adrenoceptors (which stimulate PLC) and β-adrenoceptors (which stimulate formation of cAMP) in hepatocytes suggested that cAMP might enhance receptor-mediated Ca2+ release from intracellular stores [29]. Subsequent studies confirmed that PKA stimulates phosphorylation of hepatic IP3Rs [30] and potentiates IP3-evoked Ca2+ release [31], [32]. IP3R2, the major IP3R subtype in hepatocytes, is phosphorylated by PKA at a single residue (Ser937), although others suggest that IP3R2 is a rather poor substrate for PKA [20]. Ser937 is unique to IP3R2, but the functional consequences of the phosphorylation appear similar to those seen with IP3R1, namely enhanced bursts of IP3R gating [33]. Additional effects of PKA, including an increase in IP3 binding affinity [30] and recruitment of IP3Rs into functional Ca2+ stores [32], may also contribute to the effects of PKA on IP3R2 in intact cells.
The effects of PKA on IP3R3 have been least explored. In intact cells, IP3R3 is phosphorylated by PKA at three sites (S916, S934, S1832) that are unique to IP3R3, with S934 being the most extensively phosphorylated [34]. But, at least in cells expressing only IP3R3, PKA has no effect on IP3-evoked Ca2+ release triggered by cell-surface receptors [34]. Whether the phosphorylation affects other aspects of IP3R3 behaviour remain to be determined.
3. Direct regulation of IP3Rs by cAMP
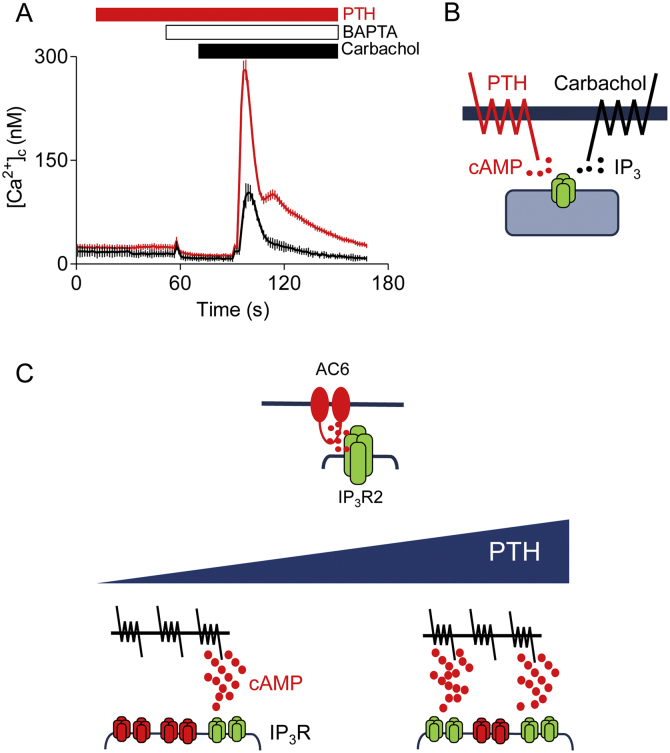
In HEK-293 cells stably expressing human type 1 receptors for parathyroid hormone (PTH), PTH stimulates formation of cAMP, but it does not alone evoke an increase in cytosolic Ca2+ concentration ([Ca2+]c). However, PTH potentiates the increase in [Ca2+]c evoked by receptors that stimulate PLC, the endogenous muscarinic M3 receptors of HEK-293 cells, for example, which can be activated by carbachol (Fig. 1A). This effect of PTH is mimicked by stimulation of endogenous prostanoid receptors or β-adrenoceptors, by direct activation of adenylyl cyclase with forskolin or by addition of a membrane-permeant analog of cAMP, 8-Br-cAMP. The non-additive effects of maximally effective concentrations of PTH and 8-Br-cAMP confirm that the effect of PTH on carbachol-evoked Ca2+ signals is entirely mediated by cAMP (Fig. 1B) [35], [36]. Responses to other PLC-coupled receptors are also potentiated by PTH, and the enhanced responses are not associated with increased production of IP3 [35], [37]. Furthermore, cAMP also potentiates the Ca2+ signals evoked by a membrane-permeant form of IP3 (IP3-BM) [38]. These results, demonstrating that cAMP acts downstream of IP3, are important because cAMP can, through EPACs, stimulate PLCε [11]. However, the effects of PTH are neither mimicked by EPAC-selective analogs of cAMP [36] nor blocked by an EPAC antagonist [39]. The enhanced IP3 −evoked increases in [Ca2+]c are not due inhibition of Ca2+ extrusion from the cytosol by cAMP [38]. Furthermore, cAMP potentiates IP3-evoked Ca2+ release in permeabilized cells [40], and it enhances IP3-gated channel activity in nuclear patch-clamp recordings of IP3R [40]. These results, where cAMP potentiates the activation of IP3R by IP3, seem consistent with the many reports suggesting that phosphorylation of IP3R1 and IP3R2 by PKA enhances responses to IP3 (see preceding section). However, several lines of evidence demonstrate that this is not a sufficient explanation:
-
1.
When PTH-evoked protein phosphorylation is blocked by inhibition of either PKA activity (using H89) or the association of PKA with A-kinase-anchoring proteins (AKAPs, using a membrane-permeant form of an uncoupling protein, ht-89), there is no effect on the ability of any concentration of PTH to potentiate the Ca2+ signals evoked by carbachol [36], [38], [39]. Others have also suggested that potentiation of carbachol-evoked Ca2+ signals by β2-adrenoceptors is insensitive to inhibition of PKA in HEK-293 cells [41]. Similar results were reported for rat osteoblasts, where potentiation of ATP-evoked Ca2+ signals by PTH was unaffected by inhibition of PKA [42].
-
2.
In permeabilized HEK-293 cells, the catalytic subunit of PKA causes minimal phosphorylation of IP3R and a barely detectable increase in the sensitivity of the Ca2+ stores to IP3, while cAMP and 8-Br-cAMP cause substantial increases in IP3 sensitivity [36].
-
3.
The concentrations of 8-Br-cAMP (in intact cells) and of cAMP (in permeabilized cells) needed to sensitize IP3Rs to IP3 are much higher than those required to activate PKA [36].
-
4.
In DT40 cells expressing single IP3R subtypes, high concentrations of cAMP potentiate IP3-evoked Ca2+ release through IP3R1, IP3R2 and IP3R3 [40]. This does not align with the consensus that PKA increases the sensitivity of only IP3R1 and IP3R2 [14].
-
5.
In permeabilized DT40 cells expressing IP3R2, cAMP potentiates the Ca2+ release evoked by IP3, and the effect of cAMP is unaffected by addition of either H89 (to inhibit PKA) or the catalytic subunit of PKA.
-
6.
In nuclear patch-clamp recordings from IP3R2 expressed in DT40 cells and stimulated with IP3, cAMP increases channel activity in the absence of ATP [40], confirming that protein phosphorylation is not required.
Fig. 1.
Regulation of IP3Rs by cAMP signalling junctions.
(A) Populations of fluo4-loaded HEK-293 cells stably expressing type 1 receptors for PTH were stimulated with PTH (100 nM, red line) and carbachol (20 μM, both lines) as indicated after addition of BAPTA to chelate extracellular Ca2+. Results show that PTH does not alone evoke an increase in [Ca2+]c, but it potentiates the Ca2+ signal evoked by carbachol. Similar results are shown in Refs. [36], [39]. (B) In HEK-293 cells, PTH through heterologously expressed type 1 PTH receptors stimulates adenylyl cyclase and so formation of cAMP. Carbachol stimulates endogenous M3 muscarinic receptors, which activate PLC and thereby formation of IP3 and release of Ca2+ from the ER through IP3Rs. The potentiation of carbachol-evoked Ca2+ signals by PTH is entirely mediated by cAMP, which enhances IP3-evoked Ca2+ release from IP3Rs. (C) IP3R2 and AC6 are selectively associated at cAMP signalling junctions. Within these junctions, cAMP is delivered from AC to IP3Rs at concentrations far greater than needed to maximally sensitize the associated IP3Rs. This allows each junction to function as a digital ‘on-off’ switch, it ensures that cAMP-mediated signalling operates with a considerable safety margin, and it allows rapid activation of the associated IP3Rs (as cAMP is locally delivered at high concentration) and rapid de-activation (as diffusion of cAMP form the junction reduces its concentration to below that needed for sensitizing IP3Rs). Since each signalling junction operates as an ‘on-off’ switch, the concentration-dependent effects of PTH are proposed to be due to recruitment of junctions, rather than to graded activity within individual junctions.
These observations suggest that cAMP can regulate IP3R activity via both PKA and by mechanisms that do not require activation of either PKA or EPACs. The observations are intriguing because they suggest an effect of cAMP that is not mediated by any of its conventional targets. Our results indicate that while cAMP alone cannot activate IP3Rs and nor does cAMP affect IP3 binding to IP3Rs [40], it does enhance the effectiveness with which the essential co-agonists, IP3 and Ca2+, stimulate channel opening. The mechanisms underlying these non-canonical actions of cAMP are not yet resolved. In light of evidence that the effects of cAMP are preserved in isolated nuclei and permeabilized cells [36], [40], it seems likely that binding of cAMP to a low-affinity site within either the IP3R itself or a tightly associated protein mediates this allosteric regulation of IP3Rs by cAMP.
4. Signalling to IP3Rs at cAMP junctions
Despite compelling evidence that cAMP entirely mediates the potentiating effects of PTH on IP3-evoked Ca2+ signals [36], there are some puzzling features of the signalling pathway that initially led us to a different conclusion. Firstly, the direct effects of cAMP on IP3Rs require much higher concentrations of cAMP than are needed for activation of PKA or EPACs, and probably much higher than the average concentrations achieved in stimulated cells [36]. Secondly, although many different stimuli evoke cAMP formation and potentiation of carbachol-evoked Ca2+ signals, the relationship between their effects on cAMP and Ca2+ are entirely different for different stimuli. For example, for concentrations of PTH and isoproterenol (which activates β-adrenoceptors) that cause similar submaximal potentiation of Ca2+ signals, PTH evokes a more than 10-fold greater increase in intracellular cAMP concentration than does isopreterenol [36]. This immediately suggests that the cAMP that regulates IP3R activity cannot be uniformly distributed in the cytosol. Thirdly, and more troublesome, are the many manipulations of cAMP formation that fail to affect the carbachol-evoked Ca2+ signals. Hence substantial inhibition of cAMP formation by either low-affinity inhibitors of adenylyl cyclase (AC, ∼90% inhibition in Ref. [38] and ∼70% in Ref. [36]) or siRNA-mediated knockdown of AC3 (the major subtype in HEK-293 cells), or an enhancement of cAMP accumulation after inhibition of cyclic nucleotide phosphodiesterase [36], [38], [39] had no effect on the ability of any concentration of PTH to potentiate carbachol-evoked Ca2+ signals. This initially led us to conclude that the effects of PTH were not mediated by cAMP [38], but we had then to revise that conclusion in light of evidence that cAMP does mediate the effects of PTH.
It is easy to envisage how the effects of a maximal concentration of PTH might be unaffected by even very substantial inhibition of AC if there are ‘spare receptors’, such that maximal activation of the receptors can provide more cAMP than needed to cause maximal activation of IP3Rs. However, that argument cannot be employed to explain the lack of effect of AC inhibitors on the Ca2+ responses evoked by submaximal concentrations of PTH. We therefore proposed that the ‘spare’ signalling capacity might exist within subcellular compartments or ‘signalling junctions’. We suggest that cAMP is delivered to IP3Rs locally at concentrations substantially greater than required to cause maximal sensitization of the associated IP3Rs (Fig. 1C). The concentration-dependent effects of PTH, we suggest, come from recruitment of these hyperactive signalling junctions, rather than from graded activity within individual junctions. Each signalling junction is, in effect, an ‘on-off’ switch with a considerable safety margin because once activated it delivers more cAMP than needed to fully sensitize the associated IP3Rs. Our scheme neatly accounts for both the inconsistent relationship between cAMP and response for different stimuli (because different stimuli operate with different safety margins) and it provides a mechanism that would allow IP3Rs to be exposed to high concentrations of cAMP. It also accommodates the results showing that even manipulations of cAMP concentration fail to effect the Ca2+ signals evoked by PTH (because the large safety margin protects the signalling pathway from even substantial perturbations of cAMP).
The involvement of signalling junctions is supported by additional evidence [36]. Notably, there is a selective association between AC6 (which accounts for only 5% of AC in HEK-293 cells) and IP3R2 in HEK-293 cells, consistent with targeted delivery of cAMP from AC to IP3R. Loss of IP3R2 (using siRNA) selectively attenuates the potentiation of carbachol-evoked Ca2+ signals by PTH. Global inhibition of AC activity by low-affinity inhibitors reduces PTH-evoked cAMP formation without affecting Ca2+ signals, whereas the converse occurs when expression of AC6 is reduced. Loss of AC6 has no perceptible effect on cAMP levels, but it attenuates the potentiation of carbachol-evoked Ca2+ signals by PTH. The rationale, we suggest, is that all signalling junctions feel the effect of the low-affinity inhibitors, which thereby reduces the cAMP delivered within junctions but not sufficiently to obliterate the safety margin, whereas removing AC6 from individual junctions (each perhaps containing only a single AC) will incapacitate that junction. Finally, in cells with diminished expression of αs, which couples receptors to AC, the safety margin is reduced such that further inhibition of AC (using the low-affinity AC inhibitors) does reduce the ability of PTH to potentiate carbachol-evoked Ca2+ signals [36].
In conclusion, there are at least two routes through which cAMP can directly modulate IP3R gating. Phosphorylation of IP3R1 and IP3R2 by PKA increases the effectiveness with which IP3 and Ca2+ evoke bursts of channel openings. In addition, binding of cAMP to a low-affinity site that seems to be closely associated with the IP3R can also increase the apparent efficacy of IP3 and Ca2+ in gating each IP3R subtype. The need for high concentrations of cAMP for this direct action demands local delivery of cAMP to IP3Rs, and that has so far been shown to occur for only IP3R2 [36]. The low-affinity of IP3Rs for cAMP effectively insulates them from global changes in cytosolic cAMP concentration and allows them to respond only to cAMP delivered to them within signalling junctions (Fig. 1C). Because each active junction delivers cAMP at a super-saturating concentration to associated IP3Rs, the junction behaves as a robust digital switch that can rapidly respond to changes in extracellular stimulus intensity. The cAMP is delivered rapidly and at a high concentration driving rapid association of cAMP with IP3Rs, and as soon as the AC stimulus is removed the focal concentration of cAMP dissipates by diffusion, rapidly terminating the effects of cAMP on IP3Rs.
Acknowledgements
Supported by the Wellcome Trust (101844) and Biotechnology and Biological Sciences Research Council UK (BB/L000075/1).
References
- 1.Gehring C. Adenyl cyclases and cAMP in plant signaling—past and present. Cell Commun. Signal. 2010;8:15–19. doi: 10.1186/1478-811X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper D.M., Tabbasum V.G. Adenylate cyclase-centred microdomains. Biochem. J. 2014;462:199–213. doi: 10.1042/BJ20140560. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M.J. Unlocking the secrets of cell signaling. Annu. Rev. Physiol. 2005;67:1–21. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- 4.Bray D. Protein molecules as computational elements in living cells. Nature. 1995;376:307–312. doi: 10.1038/376307a0. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen H. Cell communciation, calcium ion, and cyclic adenosine monophosphate. Science. 1970;170:404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- 6.Berridge M.J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv. Cyclic Nucl. Prot. Phos. Res. 1975;6:1–98. [PubMed] [Google Scholar]
- 7.Omori K., Kotera J. Overview of PDEs and their regulation. Circ. Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 8.Vandecaetsbeek I., Vangheluwe P., Raeymaekers L., Wuytack F., Vanoevelen J. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb. Perspect. Biol. 2012:a004184. doi: 10.1101/cshperspect.a004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2012;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor C.W., Tovey S.C. IP3 receptors: toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2012;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M., Evellin S., Weernink P.A.O., vom Dorp F., Rehmann H., Lomasney J.W. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 12.Morgan S.J., Deshpande D.A., Tiegs B.C., Misior A.M., Yan H., Hershfeld A.V. b-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J. Biol. Chem. 2014;289:23065–23074. doi: 10.1074/jbc.M114.557652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderheyden V., Devogelaere B., Missiaen L., De Smedt H., Bultynck G., Parys J.B. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betzenhauser M.J., Yule D.I. Regulation of inositol 1,4,5-trisphosphate receptors by phosphorylation and adenine nucleotides. Curr. Top. Membr. 2010;66:273–298. doi: 10.1016/S1063-5823(10)66012-7. [DOI] [PubMed] [Google Scholar]
- 15.Alzayady K.J., Wang L., Chandrasekhar R., Wagner L.E., 2nd, Van Petegem F., Yule D.I. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal. 2016;9:ra35. doi: 10.1126/scisignal.aad6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo M.-D., Velamakanni S., Ishiyama N., Stathopulos P.B., Rossi A.M., Khan S.A. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan G., Baker M.L., Wang Z., Baker M.R., Sinyagovskiy P.A., Chiu W. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature. 2015;527:336–341. doi: 10.1038/nature15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prole D.L., Taylor C.W. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J. Physiol. 2016;594:2849–2866. doi: 10.1113/JP271139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supattapone S., Danoff S.K., Theibert A., Joseph S.K., Steiner J., Snyder S.H. Cyclic AMP-dependent phosphorylation of brain inositol trisphosphate receptor decreases its release of calcium. Proc. Natl. Acad. Sci. U. S. A. 1988;85:8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soulsby M.D., Wojcikiewicz R.J. Calcium mobilization via type III inositol 1,4,5-trisphosphate receptors is not altered by PKA-mediated phosphorylation of serines 916, 934, and 1832. Cell Calcium. 2007;42:261–270. doi: 10.1016/j.ceca.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soulsby M.D., Alzayady K., Xu Q., Wojcikiewicz R.J. The contribution of serine residues 1588 and 1755 to phosphorylation of the type I inositol 1,4,5-trisphosphate receptor by PKA and PKG. FEBS Lett. 2004;557:181–184. doi: 10.1016/s0014-5793(03)01487-x. [DOI] [PubMed] [Google Scholar]
- 22.Wagner L.E., 2nd, Joseph S.K., Yule D.I. Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J. Physiol. 2008;586:3577–3596. doi: 10.1113/jphysiol.2008.152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner L.E., Li W.-H., Yule D.L. Phosphorylation of type 1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases. J. Biol. Chem. 2003;278:45811–45817. doi: 10.1074/jbc.M306270200. [DOI] [PubMed] [Google Scholar]
- 24.Tu H., Tang T.-S., Wang Z., Bezprozvanny I. Association of type 1 inositol 1,4,5-trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A. J. Biol. Chem. 2004;279:19375–19382. doi: 10.1074/jbc.M313476200. [DOI] [PubMed] [Google Scholar]
- 25.Devogelaere B., Beullens M., Sammels E., Derua R., Waelkens E., van Lint J. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem. J. 2007;407:303–311. doi: 10.1042/BJ20070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang T.-S., Tu H., Wang Z., Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase A and protein phosphatase 1a. J. Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masuda W., Betzenhauser M.J., Yule D.I. InsP3R-associated cGMP kinase substrate determines inositol 1,4,5-trisphosphate receptor susceptibility to phosphoregulation by cyclic nucleotide-dependent kinases. J. Biol. Chem. 2010;285:37927–37938. doi: 10.1074/jbc.M110.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S., Shcheynikov N., Hong J.H., Zheng C., Suh S.H., Kawaai K. Irbit mediates synergy between Ca2+ and cAMP signaling pathways during epithelial transport in mice. Gastroenterology. 2013;145:232–241. doi: 10.1053/j.gastro.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkinson D.H., Koller K. Interactions between the effects of a- and b-adrenoceptor agonists and adenine nucleotides on the membrane potential of cells in guinea-pig liver slices. Br. J. Pharmacol. 1977;59:163–175. doi: 10.1111/j.1476-5381.1977.tb06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph S.K., Ryan S.V. Phosphorylation of the inositol trisphosphate receptor in isolated rat hepatocytes. J. Biol. Chem. 1993;268:23059–23065. [PubMed] [Google Scholar]
- 31.Burgess G.M., Bird G.S.J., Obie J.F., Putney J.W., Jr. The mechanism for synergisms between phospholipase C- and adenylylcyclase-linked hormones in liver. J. Biol. Chem. 1991;266:4772–4781. [PubMed] [Google Scholar]
- 32.Hajnóczky G., Gao E., Nomura T., Hoek J.B., Thomas A.P. Multiple mechanisms by which protein kinase A potentiates inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized hepatocytes. Biochem. J. 1993;293:413–422. doi: 10.1042/bj2930413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Betzenhauser M.J., Fike J.L., Wagner L.E., 2nd, Yule D.I. Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. J. Biol. Chem. 2009;284:25116–25125. doi: 10.1074/jbc.M109.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soulsby M.D., Wojcikiewicz R.J. The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. Biochem. J. 2005;392:493–497. doi: 10.1042/BJ20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short A.D., Taylor C.W. Parathyroid hormone controls the size of the intracellular Ca2+ stores available to receptors linked to inositol trisphosphate formation. J. Biol. Chem. 2000;275:1807–1813. doi: 10.1074/jbc.275.3.1807. [DOI] [PubMed] [Google Scholar]
- 36.Tovey S.C., Dedos S.G., Taylor E.J.A., Church J.E., Taylor C.W. Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates a direct sensitization of IP3 receptors by cAMP. J. Cell Biol. 2008;183:297–311. doi: 10.1083/jcb.200803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tovey S.C., Taylor C.W. Cyclic AMP directs inositol (1,4,5)-trisphosphate-evoked Ca2+ signalling to different intracellular Ca2+ stores. J. Cell Sci. 2013;126:2305–2313. doi: 10.1242/jcs.126144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovey S.C., Goraya T.A., Taylor C.W. Parathyroid hormone increases the sensitivity of inositol trisphosphate receptors by a mechanism that is independent of cyclic AMP. Br. J. Pharmacol. 2003;138:81–90. doi: 10.1038/sj.bjp.0705011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meena A., Tovey S.C., Taylor C.W. Sustained signalling by PTH modulates IP3 accumulation and IP3 receptors via cyclic AMP junctions. J. Cell Sci. 2015;128:408–420. doi: 10.1242/jcs.163071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tovey S.C., Dedos S.G., Rahman T., Taylor E.J.A., Pantazaka E., Taylor C.W. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J. Biol. Chem. 2010;285:12979–12989. doi: 10.1074/jbc.M109.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurian N., Hall C.J., Wilkinson G.F., Sullivan M., Tobin A.B., Willars G.B. Full and partial agonists of muscarinic M3 receptors reveal single and oscillatory Ca2+ responses by b2-adrenoceptors. J. Pharmacol. Exp. Ther. 2009;330:502–512. doi: 10.1124/jpet.109.153619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckley K.A., Wagstaff S.C., McKay G., Hipskind R.A., Bilbe G., Gallagher J.A. Parathyroid hormone potentiates nucleotide-induced [Ca2+]i release in rat osteoblasts independently of Gq activation or cyclic monophosphate accumulation. J. Biol. Chem. 2001;276:9565–9571. doi: 10.1074/jbc.M005672200. [DOI] [PubMed] [Google Scholar]