Summary
Background
Praziquantel has been the drug of choice for schistosomiasis control for more than 40 years, yet surprisingly, the optimal dose for children younger than 4 years is not known. We aimed to assess the efficacy and safety of escalating praziquantel dosages in preschool-aged children (PSAC).
Methods
We did a randomised controlled, parallel-group, single-blind, dose-ranging, phase 2 trial in PSAC (2–5 years) and school-aged children (SAC; aged 6–15 years) as a comparator group in southern Côte d'Ivoire. Children were randomly assigned (1:1:1:1) to 20 mg/kg, 40 mg/kg, or 60 mg/kg praziquantel or placebo. Participants, investigators, and laboratory technicians were masked to group assignment, while the investigator providing treatment was aware of the treatment group. The primary objective was to estimate the nature of the dose–response relation in terms of cure rate using the Kato Katz technique. Dose–response curves were estimated using Emax models. Available case analysis was done including all participants with primary endpoint data. This trial is registered with International Standard Randomised Controlled Trial, number ISRCTN15280205.
Findings
Between Nov 11, 2014, and Feb 18, 2015, 660 PSAC and 225 SAC were assessed for eligibility; of whom 161 (24%) PSAC and 180 (80%) SAC had a detectable Schistosoma mansoni infection. 161 PSAC were randomly allocated of whom 154 received treatment: 42 were assigned to 20 mg/kg praziquantel, of whom 40 received treatment; 38 were assigned to 40 mg/kg praziquantel, of whom 38 received treatment; 41 were assigned to 60 mg/kg praziquantel, of whom 39 received treatment; and 40 were assigned to placebo, of whom 37 received placebo. 180 SAC were randomly allocated of whom 177 received treatment: 49 were assigned to 20 mg/kg praziquantel, of whom 47 received treatment; 46 were assigned to 40 mg/kg praziquantel, of whom 46 received treatment; 42 were assigned to 60 mg/kg praziquantel, of whom 42 received treatment; and 43 were assigned to placebo, of whom 43 received treatment. Follow-up (available-case) data were available for 143 PSAC and 174 SAC. In PSAC, the 20 mg/kg dose resulted in cure in 23 children (62%; 95% CI 44·8–77·5), 40 mg/kg in 26 children (72%; 54·8–85·8), 60 mg/kg in 25 children (71%; 53·7–85·4), and placebo in 13 children (37%; 21·5–55·1). In SAC, the 20 mg/kg dose resulted in cure in 14 children (30%; 95% CI 17·7–45·8), 40 mg/kg in 31 children (69%; 53·4–81·8), 60 mg/kg in 34 children (83%; 67·9–92·8), and placebo in five children (12%; 4·0–25·6). For both age groups, the number of adverse events was similar among the three praziquantel treatment groups, with fewer adverse events observed in the placebo groups. The most common adverse events in PSAC were diarrhoea (11 [9%] of 124) and stomach ache (ten [8%]) and in SAC were diarrhoea (50 [28%] of 177), stomach ache (66 [37%]), and vomiting (26 [15%]) 3 h post treatment. No serious adverse events were reported.
Interpretation
Praziquantel shows a flat dose-response and overall lower efficacy in PSAC compared with in SAC. In the absence of treatment alternatives, a single dose of praziquantel of 40 mg/kg, recommended by the WHO for S mansoni infections in SAC can be endorsed for PSAC in preventive chemotherapy programmes.
Funding
European Research Council.
Introduction
Schistosomiasis is a major public health problem in many parts of the developing world, especially in sub-Saharan Africa. The disease is caused by blood flukes (trematode worms) of the genus Schistosoma, with Schistosoma haematobium, Schistosoma japonicum, and Schistosoma mansoni triggering most infections.1, 2, 3, 4, 5 Indeed, more than 200 million people are infected globally, with about half of them suffering from morbid sequelae, including haematuria, dysuria, nutritional deficiencies, anaemia, hepatic granulomas leading to (severe) peri-portal fibrosis and consequent portal hypertension, and delayed physical and cognitive development.6, 7 Praziquantel is the drug of choice for treatment of infections with all Schistosoma species in the framework of preventive chemotherapy programmes.8 While school-aged children (SAC) are the main target population for treatment, it is becoming increasingly clear that younger children (<6 years) are also affected by schistosomiasis and suffer from morbidity.9, 10, 11, 12 Hence, in 2010, WHO recommended inclusion of preschool-aged children (PSAC) in large scale treatment programmes. In the absence of an appropriate paediatric formulation, which is currently under development, broken or crushed praziquantel tablets have been recommended.13 Indeed, the efficacy and safety of crushed praziquantel tablets in infants have been assessed in several endemic settings mainly throughout Africa.11, 14, 15, 16, 17
Research in context.
Evidence before this study
We searched PubMed for studies published before Dec 1, 2016, using the search terms “praziquantel”, “schistosomiasis”, “dose finding”, “school-aged children”, and “preschool-aged children”. Our search identified numerous articles on the use of 40 mg/kg praziquantel in school-aged children, which concluded that this regimen is efficacious and safe. We noted from the year 2000 onwards, there was an increase of studies elucidating the efficacy and safety of crushed praziquantel (40 mg/kg dosage) in preschool-aged children (PSAC). Although this treatment regimen was deemed efficacious and safe, the nature of the dose-related effect has not been studied in both age groups.
Added value of this study
The results of our randomised controlled, parallel-group, single-blind, dose-ranging, phase 2 trial study show increasing cure rates and egg reduction rates (ERRs) for school-aged children (SAC) with escalating dosages of praziquantel, while a dose–response relation could not be observed in PSAC using the diagnostic of choice, the Kato-Katz method. The Emax model predicted an ERR of 99% at 65 mg/kg in SAC and an ERR of 95% at 50 mg/kg in PSAC, while the ERR of 99% was out of the observed range. Adverse events were mild and transient and included stomach ache, cough, diarrhoea, and vomiting.
Implications of all the available evidence
Our dose finding study supports the widely used dose 40 mg/kg of praziquantel for schistosomiasis morbidity control in SAC. Based on our results this dose can also be recommended for PSAC. Drug discovery efforts should be strengthened to have safe and effective alternative treatment options for schistosomiasis available in a timely manner.
Despite the above-mentioned studies, surprisingly, the effective dose for children younger than 4 years is not known. At the moment, praziquantel is widely used off-label at a standard dose of 40 mg/kg to treat PSAC,18, 19 because this is the recommended dose used for SAC and adults. However, a simple extrapolation of adult praziquantel dosages to children is very uncertain in view of the maturational differences in absorption, metabolism, and elimination.
In more detail, the oral bioavailability of drugs might vary in paediatric and adult populations due to differences in gastric pH and emptying time, intestinal transit time, immaturity of secretion, and the activity of bile and pancreatic fluid. Drug distribution in children and adults differs due to changes in membrane permeability, plasma protein binding, and total body water. Finally, the immaturity of enzyme systems (cytochrome P450), glomerular filtration, renal tubular secretion, and tubular reabsorption in children account for a different excretion of drugs in the paediatric population compared with in adults.20, 21 Despite this, thorough, quality, dose-finding clinical studies using praziquantel have not been done in PSAC to date.
We aimed to determine the nature of the dose–response of praziquantel in PSAC infected with S mansoni to determine the dose of praziquantel that shows an efficacy comparable to the standard dose of 40 mg/kg in SAC in an area where S mansoni is endemic.22 Our findings, along with concurrent investigations pertaining to the pharmacokinetics of praziquantel in SAC and PSAC (Kovač and colleagues, under preparation) might be pivotal to further optimise the control of schistosomiasis.
Methods
Study design and participants
We did a randomised controlled, parallel-group, single-blind, dose-ranging, phase 2 trial was done in five villages located in the health district of Azaguié, southern Côte d'Ivoire. A detailed census was done in November, 2014, which generated lists of PSAC and SAC, including their name, age, and sex. PSAC (aged 2–5 years) and SAC (6-15 years) were enrolled. While the age of SAC was assessed based on their birth certificate at school level, the age of PSAC was confirmed by three potential sources: birth certificate, child's health card—where the date of birth is mentioned, and the verbal statement of the mother in case the two previously mentioned documents were unavailable. The village census identified 141, 167, 139, and 290 PSAC (2–5 years) and 231 SAC (6–15 years). All PSAC (n=737) and SAC registered during the census were invited to participate to the baseline survey.
Ethical approval for the study was obtained by the National Ethics Committee of the Ministry of Health in Côte d'Ivoire (CNER, reference number 037/MSLS/CNER-dkn) and the Ethical Committee of Northwestern and Central Switzerland (EKNZ; reference numner 162/2014).
Community meetings were held to explain the purpose, procedures, potential risks, and benefits of the study. Written informed consent was obtained from parents or legal guardians of participants. SAC were invited to give their assent by writing their name and ticking the following sentence, “I agree to participate in this study” on the assent form. Parents or legal guardians were well informed on the fact that the participation was voluntary hence, they could withdraw their children from the study at any time with no further obligations.
Children were assessed for the presence of an S mansoni infection. S mansoni-positive children were eligible to participate in this trial. A clinical examination and an oral medical history by active questioning were obtained from all eligible children. Of note, mothers or guardians of the PSAC were asked about the medical history on behalf of their children. Children were excluded and treated with a standard dose of 40 mg/kg praziquantel if they had taken an antimalarial or anthelminthic drug in the past 4 weeks or had any systematic illness—namely, clinical malaria (presence of fever plus positive rapid malaria diagnostic test [ICT Malaria Plasmodium falciparum (HRPII), Cape Town, South Africa] according to the national guidelines) or hepatosplenic schistosomiasis. To determine the presence of hepatosplenic schistosomiasis, the extension of the left liver lobe beneath the sternum was measured in centimetres from the mid-sternal line and the extension of the right liver lobe beneath the rib cage was measured in centimetres from the right mid-clavicular line. At the end of the study, 3 months after the inclusion of the first participant, all children enrolled in the study were offered albendazole (400 mg) and praziquantel (40 mg/kg) for the treatment of helminth infections.
Randomisation and masking
PSAC and SAC with a parasitologically confirmed S mansoni infection were stratified by light, moderate, or heavy baseline infection intensities and randomly assigned (1:1:1:1) to placebo or 20 mg/kg, 40 mg/kg, or 60 mg/kg praziquantel using computer-generated stratified block randomisation codes provided by an independent statistician (stratified by two infection intensity strata; block size of eight). Children and laboratory technicians undertaking the diagnostics were masked, while the investigator delivering the treatment was aware of the treatment assignments. Masking was maintained throughout the trial until data cut off. Randomisation codes were released after the database was unlocked.
Procedures
During the baseline survey, at school level, children were provided with plastic containers labelled with unique identification numbers (IDs) and asked to deliver a fresh stool and urine sample. For the PSAC, mothers or guardians were given the plastic containers labelled with a unique ID and they were asked to obtain a fresh stool and urine sample of their child. From each participating child, two stool samples over two consecutive days and a single urine sample were collected. Stool and urine samples were transferred to a nearby laboratory in Azaguié town and examined on the day of collection. For the diagnosis of S mansoni, stool samples were each subjected to duplicate Kato-Katz thick smears (standard template of 41·7 mg).23 Eggs of soil-transmitted helminths—ie, Ascaris lumbricoides, hookworm, and Trichuris trichiura—were also assessed and recorded for each parasite species separately. A subsequent independent quality control of sample results (about 10%) was done. In brief, the result from each slide among the 10% slides is considered correct if the following tolerance margin is not exceeded between the reading of two laboratory technicians: (1) for counts of 100 eggs or less, the difference between technicians' egg counts must not be greater than 10 eggs; and (2) for counts of 100 eggs or more the difference between technicians' egg counts must not be greater than 20 eggs. In case of discrepancy between the results of quality control and the initial reading, all the slides were read once again by the senior technician. Urine samples were subjected to the urine filtration technique for the diagnosis of S haematobium using the same quality control process. A commercially available Point-of-Care Circulating Cathodic Antigen (POC-CCA) cassette test (batch number: 34066; Rapid Medical Diagnostics, Pretoria, South Africa) for the diagnosis of S mansoni was applied on urine samples from the first day of samples collection. The POC-CCA tests were done and read as described elsewhere.24 In addition, a finger prick blood sample was taken, and thick and thin blood smears were prepared for the diagnosis of Plasmodium species. Blood smears were stained with Giemsa and examined under a microscope using 100 x oil immersion. The Plasmodium density was counted against 200 leucocytes, assuming 8000 leucocytes per μL of blood. If less than 10 Plasmodium were found, the reading was continued up to 500 leucocytes. All slides were double-checked by a second laboratory technician and only considered negative if no Plasmodium were detected in 100 x oil immersion field by the two independent microscopists. In addition, a rapid malaria diagnostic test was used and the haemoglobin value measured using a calibrated HaemoCue device (HaemoCue 301 system, Ängelholm, Sweden) according to the manufacturer's instructions. Weight was measured in kg using the domestic HAMSON bathroom weighing scale (Graduation increments of 0·1 kg) and height was measured using a common builder's measuring tape.
To assess treatment efficacy, another two stool samples and one urine sample were collected between 21 days and 25 days post-treatment for the follow-up and subjected to the same diagnostic approaches applied at the baseline survey.
Procedures
After the baseline screening and clinical examination, eligible participants were treated. Before treatment, each participant received breakfast. The breakfast comprised of an equally sized piece of buttered baguette for each child. Praziquantel tablets (600 mg Cesol, kindly provided by Merck (Darmstadt, Germany) or placebo tablets (Fagron, Germany) were given according to the calculated dose per kg of bodyweight in half tablet increments (eg, 1·3 tablets calculated = 1·5 tablets given or 1·2 tablets calculated = 1 tablet given). For PSAC, tablets were crushed using a mortar and pestle and dissolved in a small volume of syrup-flavoured water. SAC and the mothers or guardians of PSAC were interviewed 3 h, 24 h, 48 h, and 72 h after treatment about the occurrence of adverse events and mitigating drugs were provided if necessary. An adverse event was defined as any unfavourable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal or investigational product, whether or not related to the treatment. All adverse event intensities were judged by the study physician, following guidelines by the European Medicine Agency and were graded as mild, moderate, severe, or intolerable.
Outcomes
Our primary outcome was the cure rate (the percentage of egg-positive children at baseline who became egg-negative after treatment) resulting from different doses of praziquantel based on the Kato-Katz method. The secondary outcomes were egg reduction rate (ERR) and the safety of different doses of praziquantel.
Statistical analysis
Simulations showed that with 36 children enrolled in each of the four treatment study groups (0 mg/kg, 20 mg/kg, 40 mg/kg, and 60 mg/kg), the dose response prediction model should have a median precision (one half length of the 95% CIs) of 10% points, assuming associated cure rates of 2·8%, 50%, 75%,25 and 90%, respectively. The suggested sample size is also in line with the recommendations from Klingenberg in 2009.26 To account for losses in the follow-up, the sample size was increased to 40 children in each study group.
Data were double entered into an Excel spreadsheet, transferred into EpiInfo version 3.5.2 (Centers for Disease Control and Prevention; Atlanta, GA, USA) and cross-checked.
Data were analysed with Stata version 13 (Stata Corp; College Station, TX, USA). Participants who had at least one stool sample examined each with duplicate Kato-Katz thick smears at follow-up, were present at treatment day, and not excluded due to a medical condition were included in the final analysis (available case analysis). Imputation of missing data with treatment failure or success was assessed in an intention-to-treat analysis.
Eggs per gram of stool (EPG) were assessed by adding up the egg counts from the duplicate or quadruplicate Kato-Katz thick smears and multiplying this number by a factor of 12 or six, respectively. We classified infection intensity as light (<100 EPG), moderate (100–399 EPG), or heavy (>400 EPG).27
Geometric mean egg counts were calculated as:
And the corresponding ERR 100 as:
A bootstrap resampling method with 5000 replicates was used to calculate 95% CIs for ERRs.
We used an Emax model as a primary model to predict the dose–response curves in terms of cure rates and ERRs. The analysis was done with the DoseFinding package (version 0·9–14) of the statistical software environment R (version 3.3.0). Emax model was predicted in two stages. For binary data, first cure rates and their covariances were estimated on logit scale via logistic regression. In the second stage, doses and estimated cure rates were fitted to the non-linear Emax model to determine the basal effect (treatment effect at dose 0), the asymptotic maximum effect, and ED50 (the dose resulting in 0·5 * Emax). We converted estimates on logit scale to probabilities in the cure rate related dose-response figures.
Role of funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
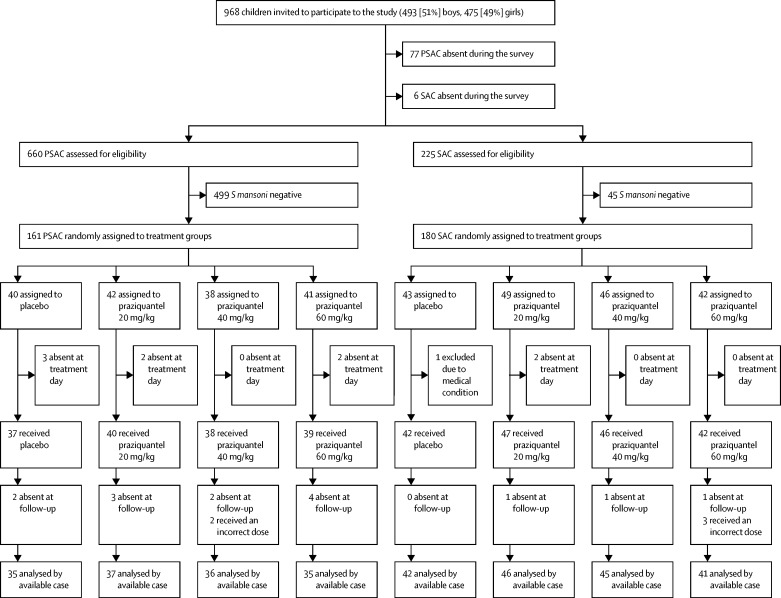
Between Nov 11, 2014, and Feb 18, 2015, 968 children were invited to participate in the study. 660 PSAC and 225 SAC participated in the baseline survey (figure 1). Of these, 161 (24%) PSAC and 180 (80%) SAC had a detectable S mansoni infection and were randomly assigned to treatment groups. In the PSAC group, 42 were assigned to 20 mg/kg praziquantel, of whom 40 received treatment; 38 were assigned to 40 mg/kg praziquantel, of whom 38 received treatment; 41 were assigned to 60 mg/kg praziquantel, of whom 39 received treatment; and 40 were assigned to placebo, of whom 37 received placebo. In the SAC group, 49 were assigned to 20 mg/kg praziquantel, of whom 47 received treatment; 46 were assigned to 40 mg/kg praziquantel, of whom 46 received treatment; 42 were assigned to 60 mg/kg praziquantel, of whom 42 treatment; and 43 were assigned to placebo, of whom 43 received treatment.
Figure 1.
Trial profile
PSAC=preschool-aged children. SAC=school-aged children. S mansoni=Schistosomiasis mansoni.
Follow-up (available-case) data were available for 143 PSAC and 174 SAC (figure 1). Of note, 11 PSAC and six SAC provided only a single stool sample at follow-up.
For all treated children, demographic and parasitological baseline data are shown in table 1. The median age, weight, height, and sex of PSAC and SAC were balanced among the treatment groups. At pre-treatment, the geometric mean EPG of faeces ranged between 24·7 and 39·4 in the PSAC treatment groups, and between 71·7 and 84·1 in the SAC treatment groups (table 2). 129 (80%) of PSAC harboured a light S mansoni infection versus 93 (52%) of SAC, while about half (87 [49%]) of the SAC had moderate or heavy infection intensities versus 32 (20%) of PSAC. More than half of the SAC were co-infected with P falciparum (112 [62%]), while PSAC harboured fewer Plasmodium co-infections (53 [33%]). Co-infections with T trichiura were common in the study setting, with 56 (35%) PSAC and 97 (54%) SAC co-infected. By contrast, co-infections with Ascaris, hookworm, and S haematobium were rare, and ranged between 0% and 11% in the different treatment groups. Haemoglobin values were in the normal range with median values between 10·5 and 11·3 in PSAC and between 11·4 and 12·0 in SAC (table 1).
Table 1.
Baseline characteristics of treatment groups
|
Preschool-aged children |
School-aged children |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=40) | Praziquantel 20 mg/kg (n=42) | Praziquantel 40 mg/kg (n=38) | Praziquantel 60 mg/kg (n=41) | Total (n=161) | Placebo (n=43) | Praziquantel 20 mg/kg (n=49) | Praziquantel 40 mg/kg (n=46) | Praziquantel 60 mg/kg (n=42) | Total (n=180) | ||
| Age, years | 4 (2–5) | 4 (2–5) | 4 (2–5) | 4 (2–5) | 4 (2–5) | 8 (6–14) | 9 (6–12) | 9 (6–13) | 9 (6–12) | 9 (6–13) | |
| Weight, kg | 14 (8–18) | 14 (8–18) | 14 (11–18) | 13 (10–18) | 14 (9–18) | 23 (15–46) | 23 (17–40) | 23 (15–41) | 24 (16–40) | 23 (16–43) | |
| Height, cm | 100 (83–105) | 95 (77–108) | 97 (84–110) | 95 (81–105) | 96 (80–108) | 123 (108–164) | 128 (106–152) | 126 (106–152) | 128 (107–147) | 127 (106–156) | |
| Haemoglobin (g/dL) | 10·5 (10·3–11·2)* | 10·9 (10·3–11·6)† | 11·3 (10·2–12·0)‡ | 10·8 (9·9–11·8)§ | 10·8 (10·1–11·6)¶ | 11·6 (10·6–12·6) | 11·4 (10·8–12·4) | 12·0 (11·3–12·5) | 11·6 (10·9–12·9) | 11·7 (10·9–12·5) | |
| Sex | |||||||||||
| Girls | 17 (43%) | 21 (50%) | 22 (58%) | 20 (49%) | 80 (50%) | 19 (44%) | 26 (53%) | 21 (46%) | 25 (60%) | 91 (51%) | |
| Boys | 23 (58%) | 21 (50%) | 16 (42%) | 21 (51%) | 81 (50%) | 24 (56%) | 23 (47%) | 25 (54%) | 17 (40%) | 89 (49%) | |
| Infection intensity | |||||||||||
| Light | 30 (75%) | 34 (81%) | 32 (84%) | 33 (81%) | 129 (80%) | 23 (54%) | 26 (53%) | 23 (50%) | 21 (50%) | 93 (52%) | |
| Moderate | 8 (20%) | 6 (14%) | 6 (16%) | 7 (17%) | 27 (17%) | 15 (35%) | 16 (33%) | 16 (35%) | 12 (29%) | 59 (33%) | |
| Heavy | 2 (5%) | 2 (5%) | 0 | 1 (2%) | 5 (3%) | 5 (12%) | 7 (14%) | 7 (15%) | 9 (21%) | 28 (16%) | |
| Co-infections | |||||||||||
| Schistosoma haematobium | 1 (3%) | 0 | 1 (3%) | 3 (7%) | 5 (3%) | 3 (7%) | 3 (6%) | 3 (7%) | 2 (5%) | 11 (6%) | |
| Ascaris lumbricoides | 5 (13%) | 1 (2%) | 0 | 3 (7%) | 9 (6%) | 0 | 3 (6%) | 5 (11%) | 1 (2%) | 9 (5%) | |
| Trichuris trichiura | 15 (38%) | 15 (36%) | 12 (32%) | 14 (34%) | 56 (35%) | 23 (54%) | 24 (49%) | 27 (59%) | 23 (55%) | 97 (54%) | |
| Hookworm | 0 | 1 (2%) | 1 (3%) | 0 | 2 (1%) | 4 (9%) | 3 (6%) | 5 (11%) | 2 (5%) | 14 (8%) | |
| Plasmodium falciparum (based on thin or thick smear) | 10 (25%) | 17 (41%) | 13 (34%) | 13 (32%) | 53 (33%) | 24 (56%) | 33 (67%) | 28 (61%) | 27 (64%) | 112 (62%) | |
| Plasmodium falciparum(based on RDT) | 10 (25%) | 14 (33%) | 11 (26%) | 13 (32%) | 48 (30%) | 24 (57%) | 32 (67%) | 27 (57%) | 25 (58%) | 108 (61%) | |
Data are median (IQR) or n (%). RDT=rapid diagnostic test.
27 participants.
34 participants.
30 participants.
33 participants.
124 participants.
Table 2.
Available case analysis of cure and egg reduction rates of 20 mg/kg, 40 mg/kg, and 60 mg/kg praziquantel versus placebo against intestinal schistosomiasis in PSAC and SAC based on Kato-Katz and POC-CCA
|
Preschool-aged children (PSAC) |
School-aged children (SAC) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Praziquantel 20 mg/kg | Praziquantel 40 mg/kg | Praziquantel 60 mg/kg | Placebo | Praziquantel 20 mg/kg | Praziquantel 40 mg/kg | Praziquantel 60 mg/kg | ||
| Kato-Katz | |||||||||
| Infected children before treatment | 35 | 37 | 36 | 35 | 42 | 46 | 45 | 41 | |
| Cured children after treatment | 13 (37·1%; 21·5–55·1) | 23 (62·2%;44·8–77·5) | 26 (72·2%;54·8–85·8) | 25 (71·4%;53·7–85·4) | 5 (11·9%;4·0–25·6) | 14 (30·4%;17·7–45·8) | 31 (68·9%;53·4–81·8) | 34 (82·9%;67·9–92·8) | |
| Cured children by infection intensity† | |||||||||
| Low | 11/27 | 19/27 | 23/30 | 21/26 | 5/24 | 11/25 | 16/17 | 19/20 | |
| Moderate | 2/6 | 4/8 | 3/6 | 3/8 | 0/14 | 3/16 | 10/15 | 9/10 | |
| Heavy | 0/2 | 0/2 | 0/0 | 1/1 | 0/4 | 0/5 | 5/13 | 6/11 | |
| Geometric mean EPG | |||||||||
| Before treatment | 40·0 | 31·7 | 24·7 | 39·4 | 71·7 | 76·5 | 84·1 | 80·2 | |
| After treatment | 7·9 | 2·9 | 1·3 | 1·7 | 31·5 | 12·1 | 1·4 | 0·7 | |
| Egg reduction rate | 80·1% (66·3–88·9) | 90·7% (82·0–95·7) | 94·8% (89·1–98·0) | 95·8% (90·2–98·5) | 56·0% (36·9–69·7) | 84·2% (70·9–91·5) | 98·3% (96·7–99·3) | 99·1% (97·9–99·8) | |
| Arithmetic mean EPG | |||||||||
| Before treatment | 112·3 | 140·1 | 56·5 | 87·6 | 179·6 | 193·7 | 220·3 | 292 | |
| After treatment | 45·6 | 26·8 | 9·0 | 16·1 | 132·9 | 80·2 | 7·3 | 29·4 | |
| Egg reduction rate | 59·4% (21·6–76·3) | 80·9% (56·4–88·6) | 84·1% (61·9–94·6) | 81·6% (57·1–94·6) | 26·0% (0–58·5) | 58·6% (24·1–75·9) | 96·7% (93·2–98·0) | 89·9% (61·9–99·5) | |
| POC-CCA*(with “trace” as positive) | |||||||||
| Infected children before treatment | 28 | 26 | 25 | 24 | 37 | 42 | 39 | 40 | |
| Cured children after treatment | 6 (21·4%; 8·3–40·9) | 4 (15·4%; 4·4–34·9) | 8 (32·0%; 14·9–53·5) | 8 (33·3%; 15·6–55·3) | 4 (10·8%; 3·0–25·4) | 8 (19·0%; 8·6–34·1) | 15 (38·5%; 23·4–55·4) | 22 (55·0%; 38·5–70·7) | |
| POC-CCA (with “trace'” as negative) | |||||||||
| Infected children before treatment | 24 | 23 | 21 | 23 | 33 | 38 | 35 | 35 | |
| Cured children after treatment | 4 (16·7%; 4·7–37·4) | 5 (21·7%; 7·5–43·7) | 7 (33·3%; 14·6–57·0) | 12 (52·2%; 30·6–73·2) | 4 (12·1%; 3·4–28·2) | 11 (28·9%; 15·4–45·9) | 16 (45·7%; 28·9–63·3) | 23 (65·7%; 47·8–80·9) | |
Data are n, n (%), n/N, or n (%; 95% CI). EPG=eggs per gram. POC-CCA=Point-of-Care Circulating Cathodic Antigen cassette test.
POC-CCA tests were applied on the first urine sample collected from each participant on the first day of collection. Among PSAC and SAC, respectively (seven vs five) in placebo, (11 vs four) in 20 mg/kg, (11 vs six) in 40 mg/kg, and (11 vs one) in 60 mg/kg did not provide urine samples on the first day of urine collection
Schistosoma mansoni infection intensity was stratified into low (1–99 EPG), moderate (100–399 EPG), and heavy (≥400 EPG) infection.
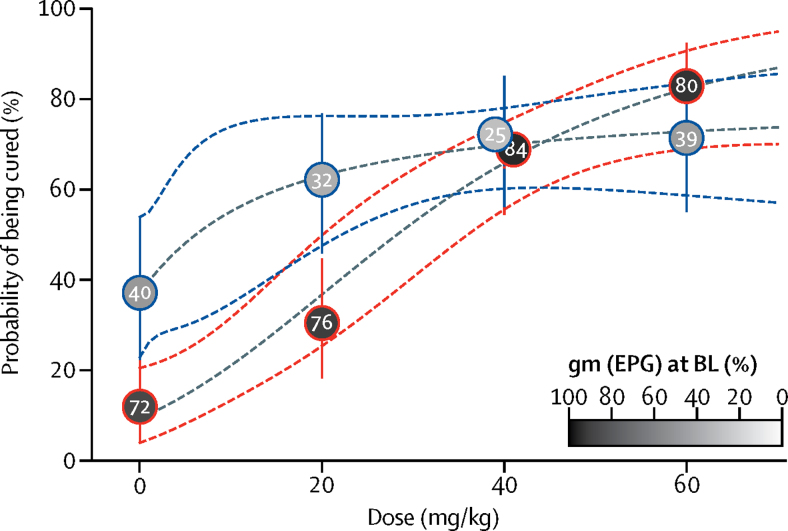
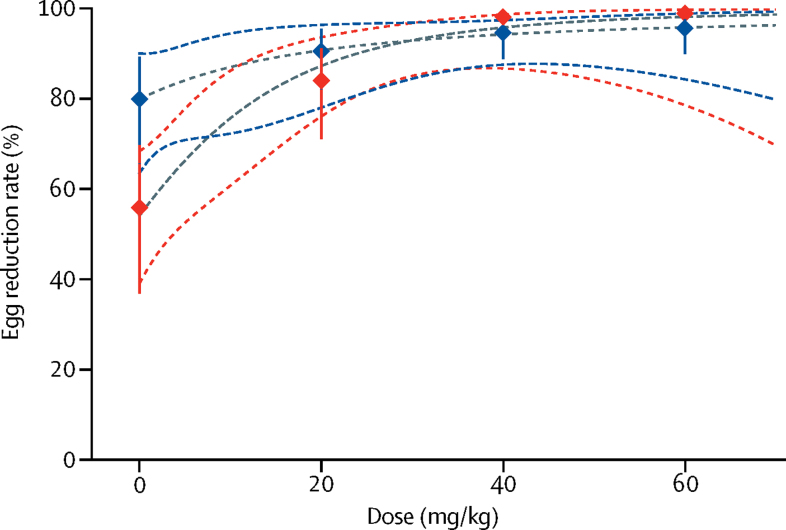
In PSAC, based on the Kato-Katz technique, both cure rates and ERRs increased only incrementally starting at 20 mg/kg. In more detail, cure rate and ERR (geometric mean) for the 20 mg/kg group were 62·1% (95% CI 44·8–77·5) and 90·7% (82·0–95·7), respectively. In the 40 mg/kg and 60 mg/kg treatment groups, similar cure rates (72·2% [95% CI 54·8–85·8] and 71·4% [53·7–85·4], respectively) were observed (table 2; figure 2). The corresponding ERRs (geometric mean) were 94·8% (95% CI 89·1–98·0) and 95·8% (90·2–98·5), respectively. PSAC in the placebo group, S mansoni eggs were not detected in a proportion of 37·1% (95% CI 21·5–55·1) of children with a corresponding ERR of 80·1% (66·3–88·9). The Emax model predicted that in PSAC, an ERR of 99% is out of the observed range, but an ERR of 95% is estimated at 50 mg/kg (figure 3). ERRs based on arithmetic means are shown in table 2.
Figure 2.
Cure rates in PSAC (blue lines) and SAC (red lines)
Circles show observed cure rates with 95% CIs (vertical lines). Numbers and colour code in the circles show geometric mean infection intensities at baseline (BL). Dashed lines represent the estimated dose–response curve and corresponding 95% confidence bands predicted by the Emax models. Epg=eggs per gram of stool. PSAC=preschool-aged children. SAC=school-aged children.
Figure 3.
Egg reduction rates in PSAC (blue lines) and SAC (red lines)
Diamonds show observed cure rates with 95% CIs (vertical lines). Dashed lines represent the estimated dose response curve and corresponding 95% confidence bands predicted by the Emax model. PSAC=preschool-aged children. SAC=school-aged children.
The observed cure rates in SAC increased with escalating dosages (table 2). The 20 mg/kg dose resulted in cure in 14 (30·4%; 95% CI 17·7–45·8) children, the 40 mg/kg resulted in cure of 31 (68·9%; 53·4–81·8) children, and the 60 mg/kg dose resulted in cure of 34 (82·9%; 67·9–92·8) children based on the Kato-Katz method. Egg reduction rates (geometric mean) were moderate for the 20 mg/kg dose (84·2% [95% CI 70·9–91·5]) and high for the 40 mg/kg and 60 mg/kg treatment groups (98·3% [96·7–99·3]) and 99·1% [97·9–99·8], respectively. In the SAC in the placebo group, we observed a cure rate of 11·9% (95% CI 4·8–25·6) and an ERR of 56·0% (36·9–69·7). ERRs based on arithmetic means are shown in table 2. The Emax model predicted an ERR of 99·0% at 65 mg/kg in SAC (figure 3).
Cure rates in PSAC were lower based on the POC-CCA cassette test compared with the Kato-Katz technique. Considering “traces” as a positive result revealed a slightly higher cure rate at 40 mg/kg and 60 mg/kg (32·0% [95% CI 14·9–53·5]) and (33·3% [15·6–55·3]) compared with 20 mg/kg (15·4% [4·4–34·9]) and placebo (21·1% [8·3–40·9]). Considering “traces” as a negative result yielded cure rates from 21·7% (95% CI 7·5–43·7; 20 mg/kg) to 52·2% (30·6–73·2; 60 mg/kg). The POC-CCA confirmed the dose–response in SAC (table 2). Considering “traces” as a positive result revealed cure rates ranging from 19·0–55·0% for 20–60 mg/kg praziquantel and a cure rate of 10·8 in placebo treated SAC. Considering “traces” as a negative result yielded cure rates from 12·1% (placebo) to 28·9% (20 mg/kg) to 65·7% (60 mg/kg).
Imputation of missing data with treatment failure or success in the intention-to-treat analysis did not change the observed outcomes (appendix p 3). Although our study was not powered to detect differences in sex, our study did not show an effect of sex on the efficacy of praziquantel (data not shown).
Three children in the 60 mg/kg treatment group were wrongly dosed: one received a dose of 16·8 mg/kg (PSAC), one a dose of 74·5 mg/kg (PSAC), and one a dose of 36·1 mg/kg (SAC). Two PSAC in the 40 mg/kg treatment group were treated with 60·8 mg/kg and 26·3 mg/kg, respectively (figure 1). Actual doses given in PSAC were 15·2–37·5 mg/kg (20 mg/kg), 24·4–60·8 (40 mg/kg), and 36·1–72·0 mg/kg (60 mg/kg). SAC doses ranged from 13·5–28·7 mg/kg (20 mg/kg dose), 33·1–49·5 mg/kg (40 mg/kg), and 51·4–74·5 (60 mg/kg). The Emax model based on actual doses is shown in the appendix (p 4). The Emax model based on actual doses also showed a flat dose response for PSAC, whereas the response for SAC showed increasing efficacy at escalating dosages.
In an assessment of the cure rates for each S mansoni infection intensity category, we found that cure rates decreased as the infection intensity increased (table 2) in both age groups (PSAC or SAC) and at all praziquantel doses (appendix p 5).
Table 3 shows the number of treated children with adverse events and their dynamics over time. Adverse events data were available for 124 PSAC and 177 SAC. In the PSAC group, 30 children were absent (placebo [n=9], 20 mg/kg [n=5], 40 mg/kg [n=9], and 60 mg/kg [n=7]) following treatment and were not assessed for adverse events. Before treatment, overall 78 (63%) of 124 PSAC and 104 (59%) of 177 SAC reported mild symptoms. The recorded number of complaints was similar among treatment groups, with stomach ache and coughing most frequently reported. At 3 h post treatment, PSAC reported fewer symptoms compared with pre-treatment. In SAC there were slightly more reported episodes compared with pre-treatment at this timepoint. Overall, 29 (23%) of 124 PSAC and 124 (70%) of 177 SAC reported adverse events 3 h post treatment. Most adverse events were mild (29 [91%] of 32 episodes in PSAC and 124 [98%] of 126 episodes in SAC). No serious adverse events were reported. For both age groups, the number of adverse events was similar among the three praziquantel treatment groups, with fewer adverse events observed in the placebo groups. The most common adverse events in PSAC 3 h post treatment were diarrhoea (11 [9%] of 124) and stomach ache (ten [8%] of 124). Adverse events commonly observed in SAC were stomach ache (66 [37%] of 177), diarrhoea (50 [28%] of 177), and headache (27 [15%] of 177; table 3).
Table 3.
Main type of clinical symptoms before treatment and adverse events 3 h and 24 h after praziquantel administration in SAC (n=177) and PSAC (n=124)
|
Preschool-aged children (PSAC) |
School-aged children (SAC) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=27*) | Praziquantel 20 mg/kg (n=35*) | Praziquantel 40 mg/kg (n=29*) | Praziquantel 60 mg/kg (n=33*) | Overall (n=124) | Placebo (n=42) | Praziquantel 20 mg/kg (n=47) | Praziquantel 40 mg/kg (n=46) | Praziquantel 60 mg/kg (n=42) | Overall (n=177) | |
| Before treatment | ||||||||||
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mild | 19 (70%) | 21 (60%) | 17 (59%) | 21 (64%) | 78 (63%) | 22 (52%) | 29 (62%) | 28 (61%) | 25 (60%) | 104 (59%) |
| None | 8 (30%) | 14 (40%) | 12 (41%) | 12 (36%) | 46 (37%) | 20 (48%) | 18 (38%) | 18 (39%) | 17 (40%) | 73 (41%) |
| Stomach ache | 3 (11%) | 3 (9%) | 8 (28%) | 4 (12%) | 18 (15%) | 12 (29%) | 6 (13%) | 10 (22%) | 8 (19%) | 36 (20%) |
| Cough | 9 (33%) | 11 (31%) | 10 (35%) | 6 (18%) | 36 (2%9) | 10 (24%) | 22 (47%) | 20 (43%) | 14 (33%) | 66 (37%) |
| Diarrhoea | 6 (22%) | 6 (17%) | 4 (14%) | 5 (15%) | 21 (17%) | 4 (10%) | 2 (4%) | 7 (15%) | 0 | 13 (7%) |
| Headache | 0 | 2 (6%) | 0 | 1 (3%) | 3 (2%) | 5 (12%) | 5 (11%) | 1 (2%) | 6 (14%) | 17 (10%) |
| Vomiting | 2 (7%) | 0 | 3 (10%) | 1 (3%) | 6 (5%) | 2 (5%) | 2 (4%) | 1 (2%) | 2 (5%) | 7 (4%) |
| Itching | 2 (7%) | 2 (6%) | 2 (7%) | 4 (12%) | 10 (8%) | 1 (2%) | 3 (6%) | 2 (4%) | 1 (2%) | 7 (4%) |
| Fever | 3 (11%) | 7 (20%) | 7 (24%) | 11 (33%) | 28 (23%) | 0 | 0 | 1 (2%) | 2 (5%) | 3 (2%) |
| 3 h post treatment | ||||||||||
| Moderate | 0 | 1 (3%) | 2 (7%) | 0 | 3 (2%) | 1 (2%) | 0 | 0 | 1 (2%) | 2 (1%) |
| Mild | 3 (11%) | 7 (20%) | 8 (28%) | 11 (33%) | 29 (23%) | 20 (48%) | 36 (77%) | 36 (78%) | 32 (76%) | 124 (70%) |
| None | 24 (89%) | 27 (77%) | 19 (66%) | 22 (67%) | 92 (74%) | 21 (50%) | 11 (23%) | 10 (22%) | 9 (21%) | 51 (29%) |
| Stomach ache | 0 | 1 (3%) | 5 (17%) | 4 (12%) | 10 (8%) | 11 (26%) | 22 (47%) | 17 (37%) | 16 (38%) | 66 (37%) |
| Cough | 0 | 2 (6%) | 2 (7%) | 2 (6%) | 6 (5%) | 3 (7%) | 8 (17%) | 4 (9%) | 9 (21%) | 24 (14%) |
| Diarrhoea | 1 (4%) | 5 (14%) | 2 (7%) | 3 (9%) | 11 (9%) | 5 (12%) | 12 (26%) | 17 (37%) | 16 (38%) | 50 (28%) |
| Headache | 1 (4%) | 1 (3%) | 1 (3%) | 0 | 3 (2%) | 4 (10%) | 7 (15%) | 5 (11%) | 11 (26%) | 27 (15%) |
| Vomiting | 0 | 1 (3%) | 1 (3%) | 2 (6%) | 4 (3%) | 2 (5%) | 7 (15%) | 9 (20%) | 8 (19%) | 26 (15%) |
| Itching | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 3 (6%) | 2 (4%) | 1 (2%) | 7 (4%) |
| Fever | 1 (4%) | 1 (3%) | 0 | 0 | 2 (2%) | 2 (5%) | 2 (4%) | 0 | 1 (2%) | 5 (3%) |
| 24 h post treatment | ||||||||||
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mild | 4 (15%) | 5 (14%) | 1 (3%) | 6 (18%) | 16 (13%) | 16 (38%) | 17 (36%) | 18 (39%) | 16 (38%) | 67 (38%) |
| None | 23 (85%) | 30 (86%) | 28 (97%) | 27 (82%) | 108 (87%) | 26 (62%) | 30 (64%) | 28 (61%) | 26 (62%) | 110 (62%) |
| Stomach ache | 0 | 0 | 0 | 0 | 0 | 7 (17%) | 9 (19%) | 4 (9%) | 8 (19%) | 28 (16%) |
| Cough | 0 | 0 | 0 | 2 (6%) | 0 | 5 (12%) | 5 (11%) | 5 (11%) | 5 (12%) | 20 (11%) |
| Diarrhoea | 0 | 5 (14%) | 0 | 1 (3%) | 6 (5%) | 5 (12%) | 4 (9%) | 3 (7%) | 6 (14%) | 18 (10%) |
| Headache | 1 (4%) | 0 | 1 (3%) | 1 (3%) | 3 (2%) | 2 (5%) | 2 (4%) | 3 (7%) | 4 (10%) | 11 (6%) |
| Vomiting | 2 (7%) | 1 (3%) | 0 | 1 (3%) | 4 (3%) | 2 (5%) | 2 (4%) | 3 (7%) | 4 (10%) | 11 (6%) |
| Itching | 0 | 0 | 0 | 1 (3%) | 0 | 4 (10%) | 3 (6%) | 3 (7%) | 3 (7%) | 13 (7%) |
| Fever | 1 (4%) | 0 | 0 | 1 (3%) | 2 (2%) | 1 (2%) | 0 | 0 | 0 | 1 (1%) |
Data are n (%).
30 kids were absent (placebo [n=9], 20 mg/kg [n=5], 40 mg/kg [n=9], and 60 mg/kg [n=7]) following treatment and were not assessed for adverse events.
24 h post-treatment, 16 episodes (13%) were noted by PSAC and 67 (38%) episodes by SAC. All reported adverse events were mild. No difference in the number of adverse events was noted among the different treatment groups and placebo group. Diarrhoea (six [5%] of 124) and vomiting (four [3%] of 124) were the two most common symptoms in PSAC 24 h post-treatment. Stomach ache (28 [16%] of 177), cough (20 [11%] of 177), and diarrhoea (18 [10%] of 177) were most frequently observed in SAC. Adverse events observed 48 h and 72 h post treatment are presented in the appendix p (6).
Discussion
In the absence of a paediatric formulation of praziquantel, the accurate management of young children with schistosomiasis at point-of-care and at community levels may be challenging. Yet, by focusing treatment in preventive chemotherapy programmes on the school-aged population, children of preschool age are neglected, thus preventing them from benefiting from treatment given to their older peers, and hence creating a potential health inequity. If children as young as age 2 years can be infected (as shown in ours and previous studies),12, 22 but must wait until school age to be treated, they are probably already facing consequences of the chronic infection at a young age, which might carry on into older age. At the moment, praziquantel is widely used off-label at a standard dose of 40 mg/kg to treat PSAC, because this is the dose used for SAC and adults.25, 28, 29, 30 However, the effective dose for children younger than 4 years is unknown. Although the efficacy and safety of praziquantel has been intensively assessed in SAC,28, 29, 30 and more recently in PSAC,31, 32, 33, 34 surprisingly, well designed dose–response relation studies are lacking in both age groups.
Our randomised controlled trial, assessing the efficacy and safety of 20 mg/kg, 40 mg/kg, and 60 mg/kg praziquantel in PSAC and SAC to uncover the nature of the dose-related effect, aimed to fill this gap. We used baseline and follow-up surveys at a reasonably sensitive diagnostic approach aiming for four Kato-Katz thick smears for the diagnosis of S mansoni in all study participants. Additionally, a urine POC-CCA cassette test was used for diagnosis of S mansoni.
In SAC, an increasing efficacy was noted with escalating doses of praziquantel. The highest cure rate and ERR (geometric mean) were observed with 60 mg/kg praziquantel. The Emax model predicted an ERR of 99·0% at 65 mg/kg in SAC (figure 3). 60 mg/kg showed a slightly better performance than did 40 mg/kg praziquantel in terms of cure rate and ERR; however, the overlapping confidence intervals suggest that both doses perform similarly. Our findings are in line with a recent meta-analysis, which reported a cure rate of 74·6% (95% CI 68·3–80·6) using 40 mg/kg praziquantel to treat S mansoni infections in SAC, and a significant relation between the cure rates and dose in the treatment of S mansoni.27 In addition, a multicentre study comparing the efficacy of 40 mg/kg versus 60 mg/kg praziquantel concluded that the higher dose offers no significant efficacy advantage over the standard dose.28
We did not identify a no-effect dose range in PSAC. Moderate cure rate and ERR were observed with 20 mg/kg (cure rate of 62·1%; ERR of 90·7%), which slightly increased when 40 mg/kg (cure rate of 72·2%; ERR of 94·8%) or 60 mg/kg (cure rate of 71·4%; ERR of 95·8%) were given. The Emax model predicted that in PSAC an ERR of 99% is out of the observed range. If we calculate arithmetic means, then ERRs in PSAC ranged from 80·9–84·1%. Hence, cure rates and ERRs were lower at 40 mg/kg and 60 mg/kg praziquantel in PSAC compared with in SAC (regardless of diagnostic tool). Notably, recent WHO Standard Operating Procedures suggested a reference drug efficacy value of 90% or higher based on arithmetic means for treating S mansoni infections with praziquantel.35 All doses investigated in PSAC—namely, 20 mg/kg, 40 mg/kg, and 60 mg/kg—would therefore not fulfil the criteria of clinical efficacy. Enzymatic processes and the immune system have been well documented to be age-dependent and might affect the absorption, distribution, metabolism, and elimination of drugs in young children.18, 20, 21, 36 Because SAC were treated side-by-side with the same praziquantel dosages, in the same ecological setting (villages were all within a radius of 5 km), using the same diagnostic tests, and administering the same food item, confounding factors can be ruled out. However, crushing the tablets might have altered the bioavailability and pharmacokinetic properties of praziquantel and might therefore play a part in the difference in efficacy noted in PSAC.
The efficacy observed for 40 mg/kg in PSAC corresponds with results reported by the above-mentioned meta-analysis,27 which calculated a cure rate of 69% and an ERR of 85·6% for this praziquantel dose in PSAC. A recent study in a small cohort in Ugandan children showed higher cure rates and ERRs with 60 mg/kg praziquantel (ERR 91%, cure rate 82%) versus 40 mg/kg (ERR 82%, cure rate 70%),34 however two-thirds of the examined children were school-aged and hence no real conclusion on the efficacy of praziquantel on young children could be drawn.
One limitation of our study was that, based on the tablet formulation used in the current study (600 mg praziquantel tablets; Cesol), only half tablets increments (ie, 300 mg) could be given. Using tablets, which could have been divided in four parts (ie, Distocide) could have allowed us to dose slightly more accurately. As proposed recently by Olliaro and colleagues in 2013,31 we strongly recommend that when working with PSAC, a formulation that can be divided into four parts (to give 150 mg increments) be used, particularly in children weighing less than 10 kg as long as a novel paediatric formulation based on oral dispersible tablets is under development.
Another limitation of our study is a high proportion of PSAC treated with placebo showing no eggs at the follow-up using the Kato-Katz technique. The Kato-Katz method has been well documented to be less sensitive, especially in populations with low infection intensity such as PSAC or in communities having received treatment. In our study, most PSAC had a light infection intensity. 45 PSAC (about 14%) had only one single egg found in all four Kato-Katz thick smears taken together at baseline. In 2015, Siqueira and colleagues37 assessed parasitological and molecular techniques for the diagnosis and assessment of cure in individuals infected with S mansoni harbouring a light infection and concluded that an increased number of Kato-Katz slides or a test with higher sensitivity is required for participants with a very low parasite load situation, such as after therapeutic interventions. Our study therefore included the sensitive POC-CCA cassette test as an alternative diagnostic instrument. As expected, cure rates were significantly lower using the POC-CCA compared with the Kato-Katz, but across all treatment groups, due to the higher sensitivity of this device.38 However, this finding does not only point to treatment failures of chronic S mansoni infections. Indeed, praziquantel is largely refractory against young developing stages of the worms,14 and hence antigens might be present in the urine of young children due to acute infections contributing to the low cure rates observed with POC-CCA in this study.
The nature of the dose–response relation based on POC-CCA were similar to the one observed with Kato-Katz in SAC. Regardless of whether traces were considered as positive or negative, a dose–response relation was observed in SAC with the highest efficacy observed for SAC at 60 mg/kg (55·0 and 65·7% cure rate, respectively). In PSAC, similar cure rates were observed at 40 mg/kg and 60 mg/kg considering traces as positive (32·0% and 33·3%), which were higher than the ones recorded for 20 mg/kg (cure rate of 15·4%). However, when considering traces as negative, increasing cure rates were observed with increasing dosages in this group (21·7–52·2%). Of note, debate is still ongoing about the correct interpretation to give to POC-CCA “trace” scores in the diagnosis of S mansoni,39 hence the Kato-Katz diagnosis remains the diagnostic of choice at the moment, also for regulators.
We found that praziquantel at the investigated doses, was well tolerated by SAC and PSAC with only mild adverse events observed over time. Almost all adverse events disappeared within the 24 h following praziquantel administration. Our findings are in line with previous studies assessing safety of praziquantel in SAC28, 29, 30 and PSAC.31, 32, 33 Several issues are worth highlighting. First, fewer adverse events were noted in the placebo group compared with the praziquantel groups for both age groups. Second, no statistical difference was observed in the number of adverse events between treatment groups. Praziquantel shows good tolerability even at the highest dosage of 60 mg/kg. Third, although the frequency of adverse events peaked at 3 h post treatment in SAC; in PSAC, fewer clinical symptoms were reported 3 h post treatment compared with symptoms before treatment. However, we note that mothers were acting on behalf of their children in the statement of adverse events which could strongly influence this result.
In conclusion, praziquantel shows lower efficacies in PSAC compared with in SAC with none of the doses achieving a satisfactory ERR based on WHO guidelines34 and moderate cure rates observed at all doses. However, in the absence of alternative treatments, a single dose of praziquantel (40 mg/kg), as recommended by WHO can be recommended for both S mansoni infections in PSAC and SAC in preventive chemotherapy programmes.
Acknowledgments
Acknowledgements
This study was funded by the European Research Council (ERC-2013-CoG 614739-A_HERO). We thank all participating children and their parents; the mothers and guardians of the PSAC from the study of all five villages and the teachers from Azaguié Makouguié, where the SAC originated, for their support; and the European Research Council for financial support.
Contributors
JTC, JH, and JKe designed the study. JTC, GP, KDS, JKe, and JKo implemented the study. JTC, GP, JH, and JKe analysed and interpreted the data. JTC and JKe wrote the first draft of the report. GP, KDS, and JH revised the report. All authors read and approved the final version of the report.
Declaration of interest
We declare no competing interests.
Supplementary Material
References
- 1.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 4.Utzinger J, Raso G, Brooker S. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859–1874. doi: 10.1017/S0031182009991600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jukes MC, Nokes CA, Alcock KJ. Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava A, Jukes M, Lambo J. Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food Nutr Bull. 2003;24:332–342. doi: 10.1177/156482650302400403. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. World Health Organization; Geneva: 2006. [Google Scholar]
- 9.Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol. 2007;23:83–86. doi: 10.1016/j.pt.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29:197–205. doi: 10.1016/j.pt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulibaly JT, N'Gbesso YK, Knopp S, Keiser J, N'Goran EK, Utzinger J. Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis. 2012;6:e1917. doi: 10.1371/journal.pntd.0001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulibaly JT, N'Gbesso YK, N'Guessan NA, Winkler MS, Utzinger J, N'Goran EK. Epidemiology of schistosomiasis in two high-risk communities of south Cote d'Ivoire with particular emphasis on pre-school-aged children. Am J Trop Med Hyg. 2013;89:32–41. doi: 10.4269/ajtmh.12-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Report of a meeting to review the results of studies on the treatment of schistosomiasis in pre-school-age children. World Health Organization; Geneva: 2011. September 13–14, 2010. [Google Scholar]
- 14.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 15.Navaratnam AM, Sousa-Figueiredo JC, Stothard JR, Kabatereine NB, Fenwick A, Mutumba-Nakalembe MJ. Efficacy of praziquantel syrup versus crushed praziquantel tablets in the treatment of intestinal schistosomiasis in Ugandan preschool children, with observation on compliance and safety. Trans R Soc Trop Med Hyg. 2012;106:400–407. doi: 10.1016/j.trstmh.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Namwanje H, Kabatereine NB, Olsen A. The acceptability and safety of praziquantel alone and in combination with mebendazole in the treatment of Schistosoma mansoni and soil-transmitted helminthiasis in children aged 1-4 years in Uganda. Parasitology. 2011;138:1586–1592. doi: 10.1017/S0031182011000138. [DOI] [PubMed] [Google Scholar]
- 17.Garba A, Barkire N, Djibo A. Schistosomiasis in infants and preschool-aged children: Infection in a single Schistosoma haematobium and a mixed S. haematobium-S. mansoni foci of Niger. Acta Trop. 2010;115:212–219. doi: 10.1016/j.actatropica.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Keiser J, Ingram K, Utzinger J. Antiparasitic drugs for paediatrics: systematic review, formulations, pharmacokinetics, safety, efficacy and implications for control. Parasitology. 2011;138:1620–1632. doi: 10.1017/S0031182011000023. [DOI] [PubMed] [Google Scholar]
- 19.Stothard JR, Sousa-Figueiredo JC, Betson MA. Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in Africa infants and preschool-aged children. Parasitology. 2011;138:1593–1606. doi: 10.1017/S0031182011001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3:53–72. doi: 10.3390/pharmaceutics3010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coulibaly JT, Fürst T, Silue KD. Intestinal parasitic infections in schoolchildren in different settings of Côte d'Ivoire: effect of diagnostic approach and implications for control. Parasit Vectors. 2012;5:135. doi: 10.1186/1756-3305-5-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 24.Coulibaly JT, Knopp S, N'Guessan NA. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS Negl Trop Dis. 2011;5:e1384. doi: 10.1371/journal.pntd.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olliaro PL, Vaillant MT, Belizario VJ. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl Trop Dis. 2011;5:e1165. doi: 10.1371/journal.pntd.0001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingenberg B. Proof of concept and dose estimation with binary responses under model uncertainty. Stat Med. 2009;28:274–292. doi: 10.1002/sim.3477. [DOI] [PubMed] [Google Scholar]
- 27.WHO Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 28.Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8:e3286. doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raso G, N'Goran EK, Toty A. Efficacy and side effects of praziquantel against Schistosoma mansoni in a community of western Côte d'Ivoire. Trans R Soc Trop Med Hyg. 2004;98:18–27. doi: 10.1016/s0035-9203(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 30.N'Goran EK, Gnaka HN, Tanner M, Utzinger J. Efficacy and side-effects of two praziquantel treatments against Schistosoma haematobium infection, among schoolchildren from Côte d'Ivoire. Ann Trop Med Parasitol. 2003;97:37–51. doi: 10.1179/000349803125002553. [DOI] [PubMed] [Google Scholar]
- 31.Olliaro PL, Vaillant M, Hayes DJ, Montresor A, Chitsulo L. Practical dosing of praziquantel for schistosomiasis in preschool-aged children. Trop Med Int Health. 2013;18:1085–1089. doi: 10.1111/tmi.12152. [DOI] [PubMed] [Google Scholar]
- 32.Sousa-Figueiredo JC, Betson M, Atuhaire A. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl Trop Dis. 2012;6:e1864. doi: 10.1371/journal.pntd.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nalugwa A, Nuwaha F, Tukahebwa EM, Olsen A. Single versus double dose praziquantel comparison on efficacy and Schistosoma mansoni re-infection in preschool-age children in Uganda: a randomized controlled trial. PLoS Negl Trop Dis. 2015;9:e0003796. doi: 10.1371/journal.pntd.0003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bustinduy AL, Waterhouse D, de Sousa-Figueiredo JC. Population pharmacokinetics and pharmacodynamics of praziquantel in Ugandan children with intestinal schistosomiasis: higher dosages are required for maximal efficacy. MBio. 2016;7:e00227–e00316. doi: 10.1128/mBio.00227-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO . Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. World Health Organization; Geneva: 2013. [Google Scholar]
- 36.Mangoni AA, Jansen PA, Jackson SH. Under-representation of older adults in pharmacokinetic and pharmacodynamic studies: a solvable problem? Expert Rev Clin Pharmacol. 2013;6:35–39. doi: 10.1586/ecp.12.75. [DOI] [PubMed] [Google Scholar]
- 37.Siqueira LM, Gomes LI, Oliveira E. Evaluation of parasitological and molecular techniques for the diagnosis and assessment of cure of schistosomiasis mansoni in a low transmission area. Mem Inst Oswaldo Cruz. 2015;110:209–214. doi: 10.1590/0074-02760140375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Clercq D, Sacko M, Vercruysse J. Circulating anodic and cathodic antigen in serum and urine of mixed Schistosoma haematobium and S. mansoni infections in Office du Niger, Mali. Trop Med Int Health. 1997;2:680–685. doi: 10.1046/j.1365-3156.1997.d01-354.x. [DOI] [PubMed] [Google Scholar]
- 39.Navaratnam AM, Mutumba-Nakalembe MJ, Stothard JR, Kabatereine NB, Fenwick A, Sousa-Figueiredo JC. Notes on the use of urine-CCA dipsticks for detection of intestinal schistosomiasis in preschool children. Trans R Soc Trop Med Hyg. 2012;106:619–622. doi: 10.1016/j.trstmh.2012.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.