Abstract
Nerves are a notable feature of the tumor microenvironment in some epithelial tumors, but their role in the malignant progression of pancreatic ductal adenocarcinoma (PDAC) is uncertain. Here we identify dense innervation in the microenvironment of precancerous pancreatic lesions, known as pancreatic intraepithelial neoplasms (PanIN), and describe a unique subpopulation of neuroendocrine PanIN cells that express the neuropeptide substance P (SP) receptor Neurokinin 1-R (NK1-R). Using organoid culture, we demonstrated that sensory neurons promoted the proliferation of PanIN organoids via SP-NK1-R signaling and Stat3 activation. Nerve-responsive neuroendocrine cells exerted trophic influences and potentiated global PanIN organoid growth. Sensory denervation of a genetically engineered mouse model of PDAC led to loss of Stat3 activation, a decrease in the neoplastic neuroendocrine cell population, and impaired PanIN progression to tumor. Overall, our data provide evidence that nerves of the PanIN microenvironment promote oncogenesis, likely via direct signaling to neoplastic neuroendocrine cells capable of trophic influences. These findings identify neuroepithelial crosstalk as a potential novel target in PDAC treatment.
Keywords: Pancreatic Ductal Adenocarcinoma (PDAC), Pancreatic Intraepithelial Neoplasia (PanIN), Tumor Microenvironment, Sensory Nerves and Axons, Substance P (SP), Neurokinin 1 Receptor (NK1-R), Chromogranin A (CgA), Endocrine and Neuroendocrine Differentiation, Organoid, Sensory Ablation and Denervation
Introduction
Neuronal influences on tumorigenesis have been described in prostate (1) and gastric cancers (2) but their role in pancreatic ductal adenocarcinoma (PDAC) remains unclear. PDAC has a tropism for nerves and is associated with high rates of perineural invasion that portends a worse prognosis (3). Reciprocal molecular signaling between PDAC and nerves appears to drive perineural invasion (4,5). In turn, human PDAC appears to cause global neural remodeling as tumors show increased neural hypertrophy (3). Whether nerves are selectively present in the neoplastic microenvironment and can drive tumorigenesis of PDAC or its precursor lesion, pancreatic intraepithelial neoplasia (PanIN), is unknown.
The pancreas is richly innervated by intrinsic, autonomic and sensory nerves. Visceral sensory afferent nerves project from dorsal root ganglia (DRG) and vagal nodose ganglia (NG) neurons (6) and have both afferent (sensory) and efferent (tissue targeting) functions (for review see Ref. (7)). Unmyelinated afferents, C and Aδ fibers, express the transient receptor potential vanilloid type 1 (TRPV1) channel that mediates the release of inflammatory neuropeptides such as substance P (SP) (8). SP binding to its target G protein-coupled receptor (GPCR), neurokinin 1 receptor (NK1-R), activates several oncogenic pathways (for review see ref. (9)) in non-neuronal human cancers (10,11). The SP-NK1-R axis, for example, can stimulate the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) (12) signaling pathway, which is also an important driver of Kras–mediated oncogenesis in PDAC (13).
We hypothesized sensory nerves in the PanIN microenvironment communicated directly with the PanIN epithelium to drive tumorigenesis. We identified a small population of unique neoplastic neuroendocrine cells expressing the neuropeptide receptor NK1-R in both murine and human PanINs. Using organoid culture techniques we demonstrated that these neuron-responsive NK1-R+ cells exerted trophic influences and potentiated global PanIN organoid growth. Accordingly, sensory denervation in a PDAC mouse model decreased the NK1-R+ cell population and protected against PanIN progression, confirming a pro-tumorigenic role of sensory nerves.
Materials and Methods
Mouse strains
Pdx1:Cre, LSL-KrasG12D and LSL-Trp53R172H mice (Jackson Laboratories) were bred to generate KC Pdx1 and KPCPdx1 mice. Mist1:CreERT2; LSL-KrasG12D and LSL-tdTomato mice were bred to generate KCMist1tdT mice. KCMist1tdT mice were injected with Tamoxifen and treated with cerulein as described previously (14). Animal studies were approved by the Animal Care and Use Committees of Johns Hopkins University School of Medicine, Memorial Sloan Kettering Cancer Center and Cold Spring Harbor Laboratory.
Cell and organoid lines
A6L, MIA Paca-2 and Capan-2 cells (Iacobuzio-Donahue lab) and 3T3 and 293T (ATCC) cells were maintained in RPMI 1640 or DMEM (Gibco) supplemented with 10% FBS (Sigma-Aldrich), GlutaMAX (Gibco) and 1x penicillin-streptomycin (Gibco). Human pancreatic ductal epithelial (HPDE) cells were cultured according to established protocols (15). Murine PanIN organoids (derived from KCPdx1 and KCMist1tdT mice) were cultured according to published protocols (16). A6L cells (2008) were authenticated with whole exome sequencing and confirmed to have mutant Kras (17). HPDE (2013), MIA Paca-2 (2016), Capan-2 (2016), 3T3 (2015) and 293T (2016) cells were authenticated using short tandem repeat profiling. Organoids underwent lox-KrasG12D allele genotyping (2016).
Neuronal co-culture
Dorsal root ganglia (DRGs) from 4 to 8 week C57BL/6J mice were harvested and cultured in supplemented Neurobasal medium (NBM; Gibco) as previously described (18). Growth factor-free NBM was used for all co-culture experiments. DRG neurons were plated with PDAC cell lines (5×103 cells/well) in the microfluidic device for 48 hours (Figure S1A) or with organoids (1 × 103 cells/well) in transwells (Corning) for 96 hours (Figure S1B). Organoid proliferation was measured with Cell Titer Glo assay for ATP (Promega) or MTT (Promega). Inhibitors L-733,060, RP 67580 and Stattic were used in some experiments.
Sensory denervation of KPCPdx1 mice
7 day old KPCPdx1 mice underwent a single subcutaneous injection of resiniferatoxin (RTX) or vehicle solutions. Early prophylactic denervation prior to the development of PanINs obviated the concern of possible interactions of RTX with PanIN cells. Physiologic testing for somatic denervation was performed using the capsaicin-induced eye wipe response.
Immunohistochemistry and immunofluorescence
Fixed cells and deparrafinized murine and human sections were subject to immunofluorescence (IF) and H&E analyses as per standard protocols. Organoids were stained in whole mount in chamber slides (Lab-Tek). Pancreas tissues were optically cleared and stained as per established protocols for 3D analysis (19) and each view was scanned to a depth of 150 µm. Axon length densities (µm/µm3) were calculated using Avizo 7.1 software (Burlington, MA, USA).
Histopathologic analysis
Histopathological analyses were performed on de-identified slides from 8, 12 and16-week old KPCPdx1 pancreas. For each pancreas, 3–5 sections were sampled 150–200 µm apart and 5–15 random views were taken for each section. Lesions were classified as acinar to ductal metaplasia (ADM), PanIN1 (1A and 1B; “early”), PanIN2/3 (“late”) and PDAC (tumor) based on the classification consensus (20). PanIN and stroma burden were expressed as percentage of total analyzed surface area occupied per lesion as previously described (Figure S2A; ref. (21)). Proliferative activity, which correlates with degree of PanIN dysplasia (22), was assessed with Ki67 labeling. Cytokeratin 19 (CK19) staining of tumors further confirmed the presence of invasive cells in the stroma (Figure S2B). The fraction of mice with tumors at all ages was calculated.
Flow cytometry and clonogenic assays
Flow cytometry on live PanIN organoid and PDAC cells were performed using a FITC-conjugated anti-NK1-R antibody (Alomone) in a LSRFortessa 3 cell analyzer (BD) based on established protocols (14). tdTomato+ or NK1-R+ and NK1-R− PanIN cells (using the conjugated anti-NK1-R antibody) from KCMist1tdT mice were FACS sorted on a FACSAria III cell sorter (BD) based on established protocols (14). In clonogenic assays, sorted cells were cultured in 50% Matrigel (MG; Corning) with neurons (5000 sorted cells/well) or in organoid media (500 sorted cells/well) for 7 days (Figure S3A–D).
Reassociation assays
FACS sorted NK1-R− and NK1-R+ cells were used to derive NK1-Rlow and NK1-Rhi, organoids, respectively (Figure S3A–D). NK1-Rlow and NK1-Rhi organoids were dissociated into single cells and transduced with lentiviral constructs containing pLKO.1-puro-CMV empty vector and pLKO.1-puro-CMV-TurboGFP (Sigma), respectively, as per manufacturer’s protocol. NK1-Rlow and NK1-Rhi/GFP organoids were plated as single cells at a 20:1 ratio (5×103 total cells/well) and imaged every 4 hours for 4 days in a microscope incubation chamber. NK1-Rlow and NK1-Rhi/GFP were plated at a 1:1 ratio (2×104 total cells/well) and subject to flow cytometry analysis at several time points.
Quantitative real-time PCR (qPCR)
RNA extraction and cDNA synthesis was done as described previously (21). qPCR was performed on a QuantStudio 6 Flex System (Applied Biosystems). TaqMan Universal PCR Master Mix (Applied Biosystems) was used with CgA and GAPDH primers, Mm99999915_g1 and Mm00514341_m1 (Thermo Fisher), respectively. All other cDNAs were amplified with PowerUp SYBR Green Master Mix (Applied Biosystems) with the primers listed in Supplementary Table S1. Relative amounts of mRNA were calculated by the comparative ΔCt method with Gapdh and/or Hprt as house-keeping genes.
Western blotting
Organoids were starved for 24 hours, dissociated and incubated with media with or without SP. Cell extracts were prepared according to standard protocols using cell lysis buffer (RIPA; Pierce) with protease and phosphatase inhibitors (Roche). Membranes were incubated with antibodies and developed with Trident ECL (Genetex).
Densitometric analysis was done in ImageJ.
Substance P (SP) Elisa
SP concentration was quantified using a Fluorescent Immunoassay Kit as per the manufacturer’s protocol (Phoenix Pharmaceuticals, Inc.).
Statistical analysis
Data were analyzed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA) and were expressed as mean ± SEM. Comparisons between groups where data were normally distributed were made with Student t test, and comparisons with categorical independent variables were performed using Fisher’s exact test. Detailed protocols and standard procedures are described in Supplementary Materials and Methods.
Results
PanINs and PDAC cells demonstrate neurotropism
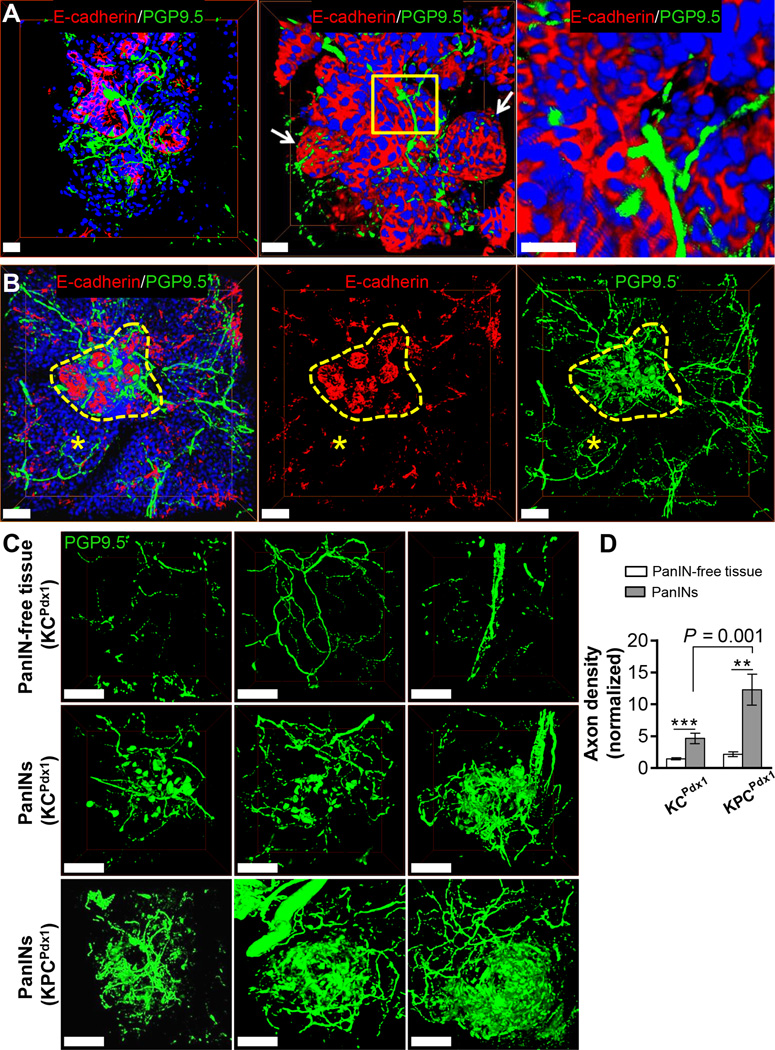
Increased global nerve density has been suggested in pancreas from human PDAC (3) and murine PanINs (23) compared to densities in pancreas from disease-free subjects. We aimed to characterize the nerve density in the immediate PanIN microenvironment compared to respective PanIN-free pancreas in isogenic mice to control for neural remodelling changes that may occur with genotype and disease state variables. We used 3D whole mount staining to reconstruct the fine intra-visceral axons (<5µm) that are not easily visualized on 2D histology (Figure S4A–B; ref (19)). In 12 week-old KPCPdx1 mice, axons were observed in close proximity to PanIN epithelial cells in the immediate microenvironment (Figure 1A–B and Video V1). We quantified the nerve density surrounding PanINs versus their respective PanIN-free pancreas tissue in 12 week KC Pdx1 mice, which have only early PanINs, and age-matched KPC Pdx1 mice which have mostly late PanINs (Figure 1C–D and Figure S4C). KC Pdx1 and KPC Pdx1 mice had a 3.2± 0.84 and 10.1±2.4 fold increase in nerves densities compared to adjacent tissues, respectively. PanIN-associated fibers had a grossly aberrant branching pattern compared to acinar axons. Similar to our findings, neoplasia associated nerves in prostate cancer show evidence of new sprouting and axonogenesis (1).
Figure 1. Innervation of PanINs.
3D IF projections (scan depths = 150 µm) of PanIN epithelium stained with E-cadherin (red), axons with the pan-neuronal marker PGP9.5 (green) and nuclei with DAPI (blue) in mouse pancreas. (A) Late PanIN in a 12 week KPCPdx1mouse is a multilobar structure (arrows) with infiltrating axons (insert). Scale bars = 25 µm. (B) Increased innervation of an early PanIN lesion (yellow dashes) in a 12 week KCPdx1 mouse pancreas compared to adjacent pancreas tissue (asterisks). Scale bars = 100 µm. (C) Sample projections of axons in PanIN-free tissue and PanIN lesions used for quantification of nerve densities (PanIN epithelial staining not shown to highlight axonal network). Scale bars = 100 µm. (D) Axon density quantifications (n=6–13 scans for each group; n = 3–4 mice per group). The data represent mean ± SEM (two-tailed unpaired t test; **P ≤ 0.01, ***P ≤ 0.001).
Having observed markedly increased axon density around PanIN lesions in vivo, we investigated whether neoplastic pancreatic cells actively recruited more axons. We co-cultured DRG neurons with human PDAC or non-neoplastic human pancreatic ductal epithelial (HPDE) cells in a microfluidic system that allows axonal interactions and models intrapancreatic sensory innervation (Figure S1A). We used PDAC cell lines because of technical limitations of 3D culture of organoids in the device. The co-cultured PDAC cell lines, A6L and MIAPaCa-2, recruited 3.30 ± 0.31 and 3.43 ± 0.46 times more sensory axons, respectively, than the HPDE cells (Figure S4D–E). Co-cultured axons also expressed synapsin proteins, indicating active vesicular transport and neurotransmitter release (Figure S4F–G). Reflective of these in vitro findings, PanIN cells likely actively recruit axons to their microenvironments. Interestingly, mutations in axon guidance genes, such as semaphorins, in human PDAC are significant, associated with a worse prognosis and may explain increased axon recruitment (24).
PanIN neuroendocrine cells express the substance P neuropeptide receptor
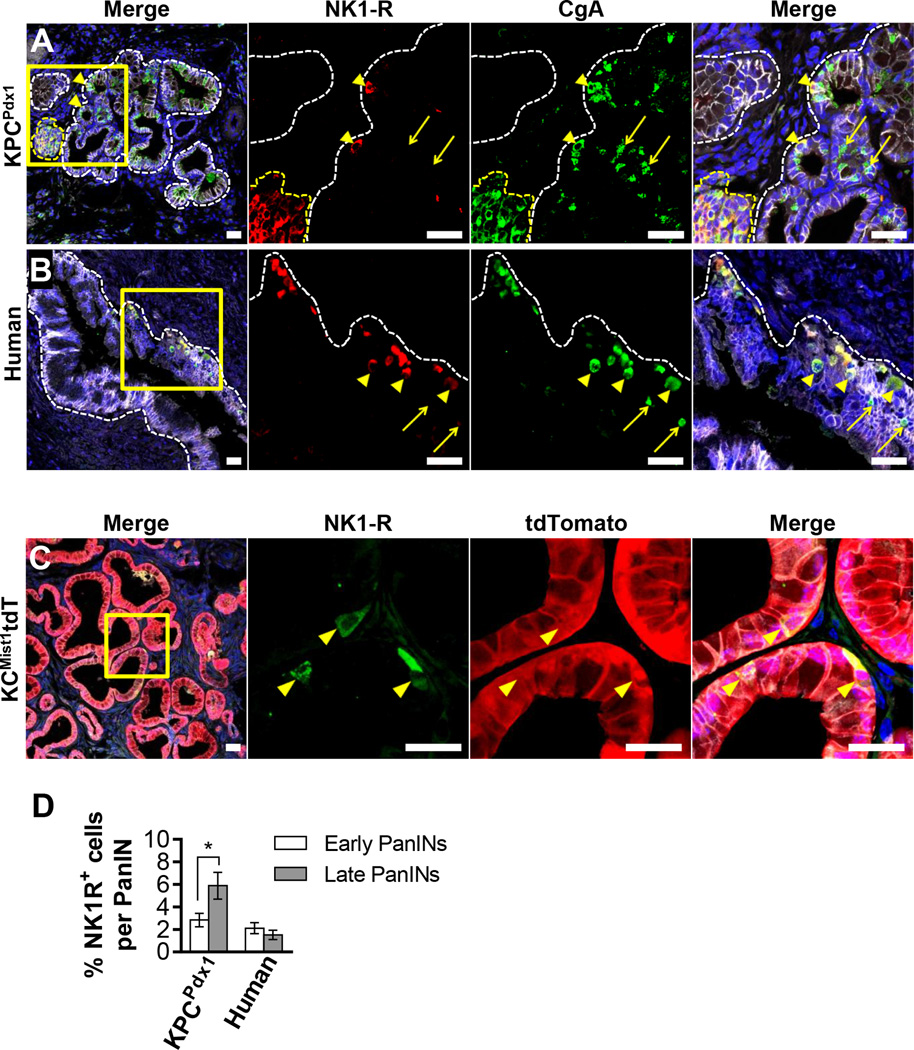
Since we observed axons in close proximity to PanIN epithelial cells and showed that PDAC cells robustly recruited sensory axons, we hypothesized that nerves, like other stromal components (21,25), may communicate with and regulate the PanIN epithelium. The KPCPdx1 PanIN epithelia were negative for expression of several nerve-responsive elements such as the neurotrophic receptors p75, TrkA, TrkB and TrkC that can be expressed in human PDAC (4) (data not shown). The well-characterized sensory neuropeptide SP and its receptor, NK1-R, have been shown to mediate neurogenic inflammation in the pancreas (26) and promote mitogenesis in several cancers (9–11). We discovered that a subpopulation of PanIN epithelial cells in the KPCPdx1 mouse expressed NK1-R. These cells were present as a low-abundance population in the PanIN epithelium and expressed NK1-R in a cytoplasmic and membranous pattern (Figure 2A and Figure S5A–C). In areas of PanIN-free pancreas, NK1-R was expressed only in islets (Figure 2A, Figure S5A). NK1-R expression in islets raised the question of whether PanIN NK1-R+ cells also displayed features of endocrine differentiation. PanIN NK1-R+ cells expressed the pan-endocrine marker chromogranin A (CgA) but not insulin, confirming their identity as neuroendocrine cells.
Figure 2. PanIN neuroendocrine cells express the neuropeptide receptor NK1-R.
Confocal IF analyses of murine and human PanINs stained with E-cadherin (gray) and nuclei with DAPI (blue). (A) PanIN in a KPCPdx1 pancreas (white dashes) next to an islet (yellow dashes). Note PanIN NK1-R+/CgA+ cells (arrowheads) and NK1-R−/CgA+ cells (arrows). (B) Human PanIN lesion with NK1-R+ /CgA+ cells (arrowheads) and NK1-R−/CgA+ cells (arrows). (C) tdTomato+ PanINs in a KCMisttdT pancreas. All PanIN NK1-R+ cells are tdTomato+ (arrowheads). All scale bars = 25 µm. (D) Quantification of NK1-R+ cells/PanIN. 70 sections from mice of different ages (n = 10) and 33 sections from patients (n=16) were quantified. The data represent mean ± SEM (two-tailed unpaired t test; *P ≤ 0.05).
We surveyed PDAC tumor sections from16 patients containing synchronous PanIN lesions. NK1-R+ and CgA+ neuroendocrine cells were identified in one or more PanINs of all patients (Figure 2B and Figure S5D–E). As in the mouse, NK1-R expression was limited to islets in disease-free areas. The frequency of NK1-R+ cells per lesion increased with PanIN grade in KPCPdx1 mouse but not in human PanINs (Figure 2D). Even early KPCPdx1 PanINs composed of a few neoplastic cells also expressed the NK1-R, suggesting a potential early and persistent role in PanIN evolution (Figure S5A).
Since NK1-R and CgA are generally expressed by islet cells, we investigated whether the NK1-R+ cells were intrinsic neoplastic cells, or alternatively, of an endocrine lineage that had been incorporated into the PanIN epithelium. We stained for NK1-R in the lineage traced KCMist1tdT mouse that expresses tdTomato only in cells that have undergone Cre-mediated recombination as directed by the acinar-specific Mist1 promoter (27). All NK1-R+ cells in the PanIN epithelium co-expressed tdTomato, confirming that these were acinar cell-derived neoplastic PanIN epithelial cells (Figure 2C and Figure S5F).
We incidentally saw expression of NK1-R on a subpopulation of CgA+ enteroendocrine cells (EECs) in human duodenal villi (Figure S6A), suggesting similarities between PanIN and intestinal neuroendocrine cells. Even though PanIN neuroendocrine cells were a subpopulation in the epithelium, we hypothesized they may have the potential to affect global PanIN biology through paracrine niche influences, similar to the way EECs can indirectly regulate crypt stem cells (28).
Sensory neurons promote PanIN organoid proliferation
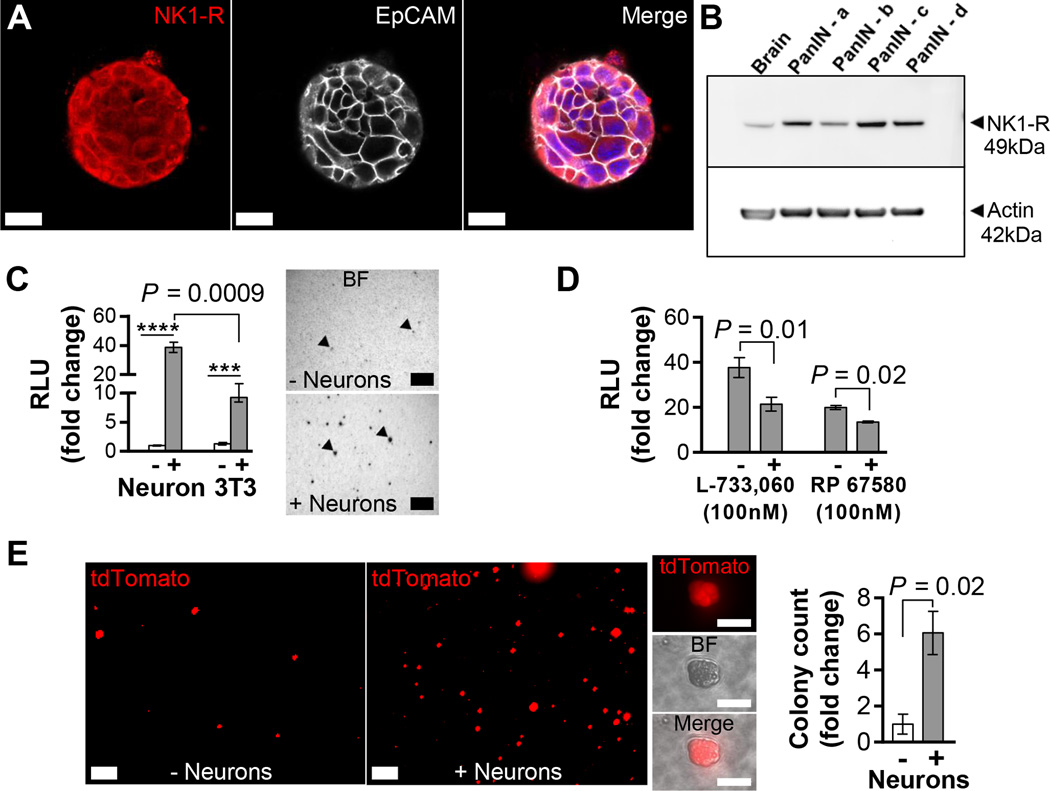
To investigate whether PanIN NK1-R expression could mediate neuroepithelial crosstalk, we utilized KCPdx1 PanIN organoids in co-culture experiments. The organoid culture is a powerful tool to study non-cancerous pancreatic epithelial cells, such as PanIN cells, as it allows for propagation of these cells without the need of a mesenchymal niche while preserving the characteristics of the source epithelium (16,29). All PanIN organoid lines studied expressed cytoplasmic and membranous NK1-R as detected on IF staining and immunoblotting (Figure 3A–B). In contrast to the low abundance NK1-R+ cells in vivo, PanIN organoid cells uniformly expressed NK1-R, possibly indicating enrichment of receptor expression in organoid culture. The universal expression of NK1-R in organoid cells and minimal expression in PDAC cell lines was confirmed by flow cytometry studies (Figure S7A). PanIN organoids also diffusely expressed CgA (Figure S7B).
Figure 3. Sensory neurons increase PanIN organoid proliferation.
(A) IF confocal projection of a PanIN organoid stained with NK1-R (red), EpCAM (gray) and nuclei (blue). Scale bars = 25 µm. (B) Western blot analysis for NK1-R expression in mouse brain (positive control) and PanIN organoids (β actin = loading control). (C) Proliferation of co-cultured PanIN organoids (RLU = random luminescence units; n=3–4). Note break in y-axis. Representative bright field (BF) images of viable MTT stained organoids (arrowheads) in this experiment. Scale bars = 200 µm. (D) Effects of NK-1R inhibitors on organoids co-cultured with neurons (n=4–6). Monoculture controls not shown. RLU values are normalized to monoculture controls in each experiment. (E) Live confocal images of organoid colonies from FACS sorted and co-cultured tdTomato+ single PanIN cells from a KCMisttdT mouse pancreas. Scale bars = 500 µm. All cells are tdTomato+ (inserts). Scale bars = 50 µm. Organoid colony (>20 µm diameter) counts (n=2). The data represent mean ± SEM (two-tailed unpaired t test; ***P ≤ 0.001, ****P ≤ 0.0001).
To recapitulate in vivo conditions with the potential for reciprocal signaling between neurons and cancer cells, we co-cultured DRG neurons with PanIN organoids in a transwell system (Figure S1B). Neurons increased organoid proliferation to a greater degree than 3T3 murine fibroblast cells (38.7 ± 3.5 fold versus 9.3 ± 0.80 fold), an established tumorigenic stromal cell type in the microenvironment (Figure 3C) (30). Neuron-mediated PanIN organoid proliferation could be partially but significantly blocked by two NK1-R antagonists L-733,060 and RP 67580, by 43.2 and 32.3%, respectively (Figure 3D and Figure S7C). To confirm that the neuronal effect on organoid proliferation was mediated by a soluble factor, we incubated organoids with neuronal conditioned media and found 3.1±1.4 fold increased proliferation that was also blocked by L-733,060 by 31.5% (Figure S7D). Neuronal conditioned media contained SP (20–40 pg/mL) as measured by ELISA. The decreased degree of proliferation in conditioned media compared to co-culture suggested that reciprocal crosstalk between organoids and neurons may help mediate neoplastic proliferation. Unlike neuronal co-culture, SP incubation alone did not cause organoid proliferation likely due to known peptide instability in solution (31) (data not shown). Very limited studies have shown that SP alone mediates proliferation of PDAC cell lines and the data are inconsistent (32,33). Since NK1-R antagonism only partly blocked PanIN organoid proliferation, additional neuronal factors such as nerve growth factor (NGF) (4) and glial derived neurotrophic factor (GDNF) (5) could drive PanIN oncogenesis but were not explored in this study.
Having observed that DRG sensory neurons exerted a robust proliferative influence on PanIN organoids, we hypothesized that neurons could support the survival of primary PanIN cells. FACS-sorted PanIN cells from the KCMist1tdT pancreas formed 6.1 ± 1.2 fold more organoid colonies in the presence of neurons (Figure 3E) as additionally confirmed by the proliferation assay (Figure S7E). Thus, sensory neurons supported the growth of organoid colonies from single primary cells in a 3D matrix, a process that additionally requires multiple growth factors (16).
PanIN neuroendocrine cells exert trophic influences
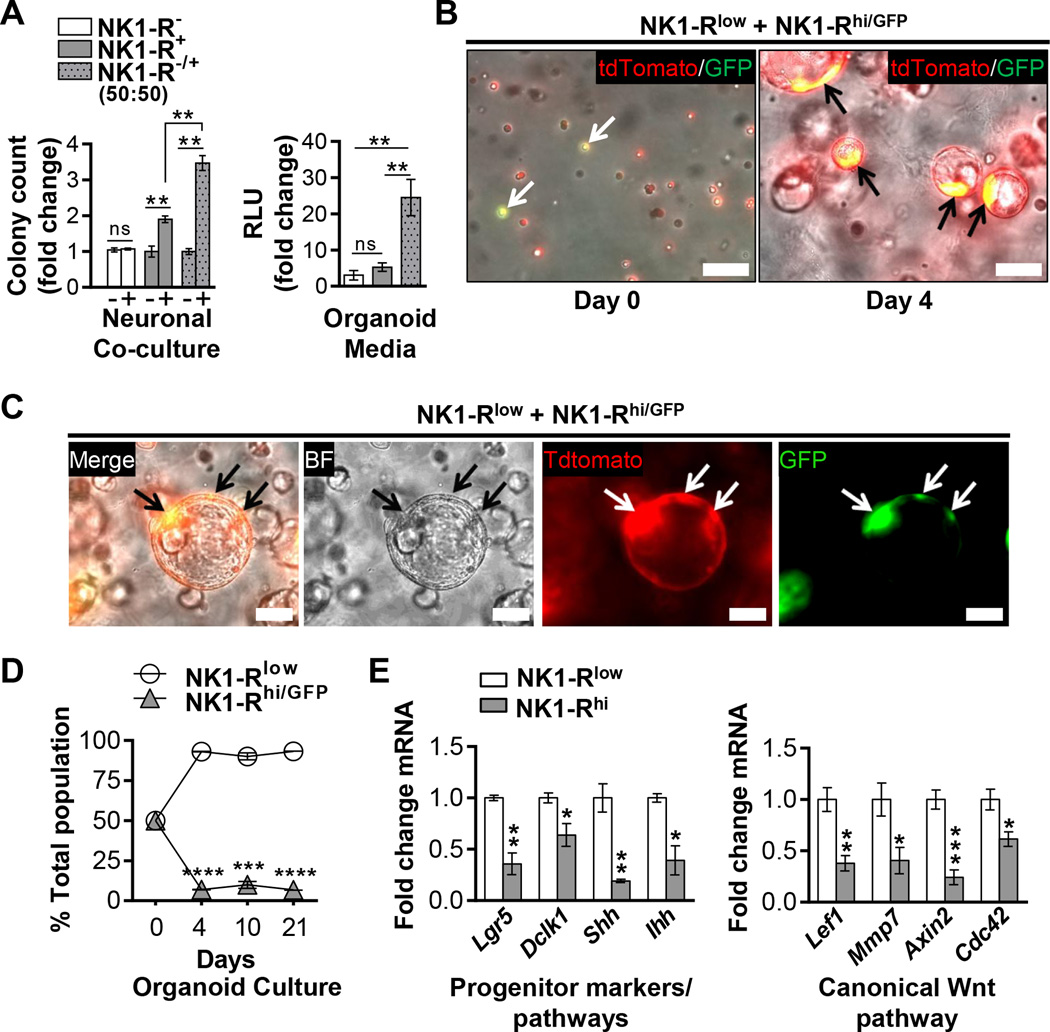
Given their similarity to intestinal enteroendocrine cells that are known to function as nerve-responsive niche cells (28), we investigated whether the subpopulation of NK1-R+ PanIN cells could influence global PanIN growth. In clonogenic assays, FACS sorted NK1-R− and NK1-R+ cells from KCMist1Tdt PanINs were plated at low density with neurons or in organoid media (Figure 4A and Figure S3A). Strengthening our studies of SP-NK1-R mediated neuroepithelial crosstalk, only NK1-R+ cells responded to DRG co-culture and formed 1.9 ± 0.09 fold more organoids than monoculture controls. Even though NK1-R inhibitors partially inhibited organoid proliferation in our prior studies, NK1-R+ cells exclusively responded to neuronal co-culture. Thus, NK1-R+ cells may be the sole sensory nerve-responsive PanIN cells in vivo. Surprisingly, the combination of NK1-R− and NK1-R+ cells formed the most colonies (3.5±0.21 fold over control) compared to the individual cultures, suggesting that the neuron-responsive NK1-R+ cells potentiated global organoid growth. This effect was accentuated in organoid media where combined NK1-R− and NK1-R+ cells formed ∼20 fold more colonies than the individual cultures. These data resonated studies of Paneth cells’ striking potentiation of intestinal organoid growth (34).
Figure 4. PanIN neuroendocrine cells exert trophic influences.
(A) Clonogenic assays of FACS sorted NK1-R− and NK1-R+ KCMisttdT single PanIN cells (n=3–4 for each experiment; RLU = random luminescence units). Data are normalized to the monoculture controls and the NK1-R− group in the co-culture and organoid media experiments, respectively. (B–C) Z-stack live IF and bright field (BF) images of the reassociation of single NK1-Rhi/GFP (yellow and green; arrows) and NK1-Rlow (red) organoid cells into composite organoids. Scale bars = 100 µm. (D) Population fractions of NK1-Rlow (GFP−) and NK1-Rhi/GFP (GFP+) organoid cells cultured together as single cells at a 1:1 ratio (on day 0) as determined by FACS analysis over time. (E) qPCR analysis. The data represent mean ± SEM (two-tailed unpaired t test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001).
Interestingly, conditioned media from NK1-R− and NK1-R+ cells did not influence the cells’ clonogenic potential in reciprocal experiments (data not shown). This indicated likely contact-dependent signaling. To better study NK1-R+ contact-mediated influences, we derived and validated new NK1-Rlow and NK1-Rhi organoids (Figure S3A). Freshly sorted NK1-R− cells, although initially depleted for Tacr1 relative to NK1-R+ cells, expressed the receptor after one week in organoid culture (Figure S3B). Since the NK1-R may support Wnt signaling (35), receptor upregulation and universal expression in our organoid progenitor culture (Figure 3A) was not surprising. NK1-Rhi organoids were enriched for NK1-R protein on immunoblotting (Figure S3C) and did not contain any non-neoplastic cells as determined by lox-KrasG12D genotyping (Figure S3D). In the reassociation assay, NK1-Rlow and NK1-Rhi/GFP organoids formed composite organoids when plated as single cells at a roughly in vivo ratio of 20:1 (Figure 4B–C and Figure S8A). This plating ratio was important so as to not drive the reaction in a biased direction. Surprisingly, the NK1-Rhi/GFP cells maintained restrictive expansion within isolated areas of the composite organoids and no homogenous NK1-Rhi/GFP organoids were seen. This finding was reminiscent of Paneth cell reassociation with intestinal cells into distinct regions within heterogeneous organoids (34). Furthermore, NK1-Rlow and NK1-Rhi/GFP organoid reassociation was a dynamic process with highly mobile organoids that appeared to preferably merge heterogeneously (Video V2). NK1-Rhi/GFP cells also maintained a constant low cell population in composite organoids over time when plated at equal starting ratios (Figure 4D and Figure S8B). Strikingly, the NK1-Rhi/GFP cell frequencies (ranging from 6.7% ± 0.11 to 9.9% ± 2.9) closely reflected their observed ratios in PanIN epithelium in vivo (Figure 2D). Even though NK1-Rhi/GFP organoids grew comparatively to NK1-Rlow organoids in individual cultures, they showed restricted expansion in the composite cultures.
Further characterization of NK1-Rhi organoids with qPCR showed significantly decreased expression of proposed pancreatic, and potentially neoplastic, progenitor markers relative to NK1-Rlow cells, including Lgr5 (29) and Doublecortin-like kinase-1 (Dclk1) (14,36) (Figure 4E). Hedgehog ligands, Shh and Ihh, which cooperate with mutant Kras in early PanIN development and are potentially activated in neoplastic “progenitor” cells (37), were also decreased. The Wnt pathway is a critical mediator of progenitor biology (for review see ref.(38)) and is notably activated in pancreatic injury and in organoid cultures (29). Like Hedgehog signaling, the canonical Wnt pathway is also critical for early PanIN development (39). Despite expressing many Wnt ligands similar to NK1-Rlow organoids, NK1-Rhi organoids had decreased activation of the downstream canonical versus non-canonical pathway (Figure 4E and Figure S8C) as well as decreased expression of several Wnt receptor Fzd family members (Figure S8D). Thus, the expression profile of NK1-Rhi cells was less “progenitor”-like than of NK1-Rlow cells.
Considering together their ability to greatly potentiate composite organoid growth despite being a restricted subpopulation and their lower expression of known pancreatic progenitor markers and pathways, NK1-R+ cells appeared to function as trophic “niche” cells with the potential to provide growth signals to NK-1R− cells. The NK1-R+ PanIN cells were also Ki67− on IF analysis, further suggesting that they were not proliferating progenitor cells in vivo (Figure S8E).
Substance P stimulates Stat3 phosphorylation in PanIN organoids
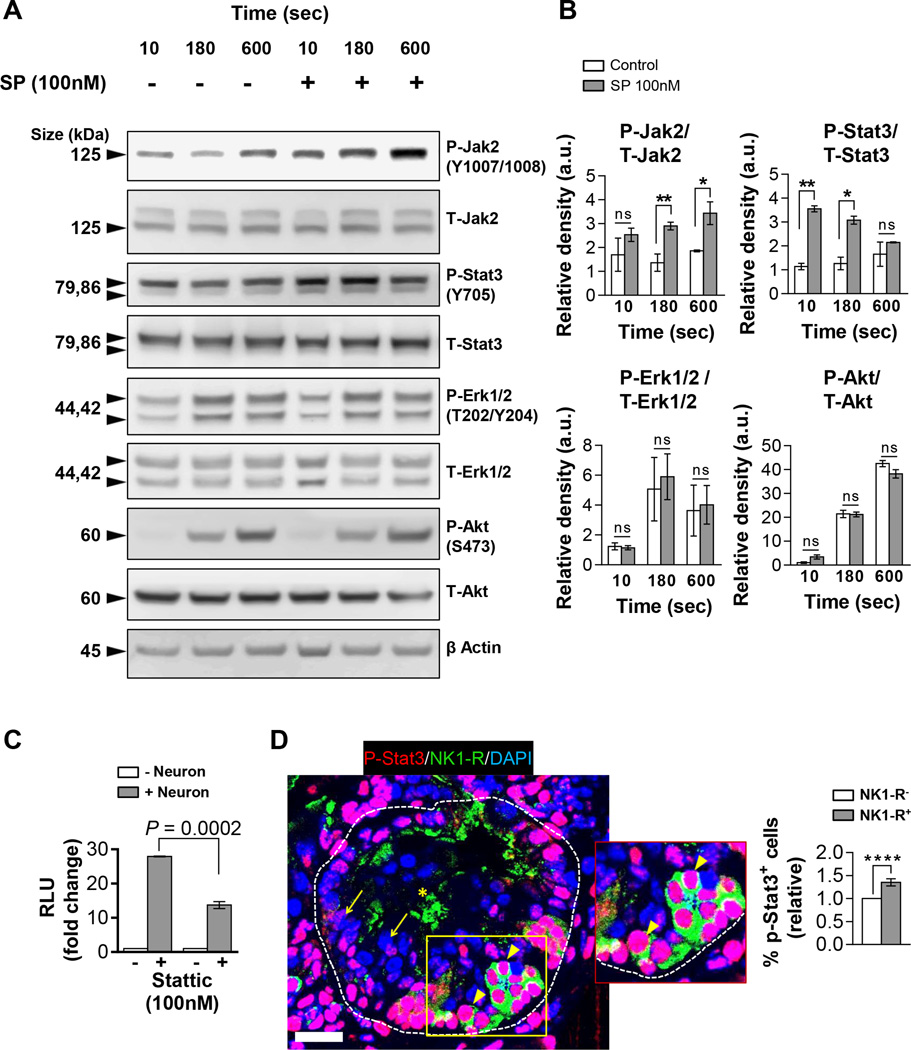
Since sensory neurons robustly increased PanIN organoid proliferation and selectively signaled to NK1-R+ PanIN cells, we hypothesized that SP should activate oncogenic pathways. Organoids incubated with SP were screened for known SP-mediated signaling pathways (9,12,40). SP rapidly phosphorylated Jak2 and Stat3 in the PanIN organoids but did not affect Mapk or Akt phosphorylation (Figure 5A–B). Stattic, a small molecule inhibitor of Stat3, decreased the proliferation of co-cultured organoids by 50.8%, suggesting that the neuron-mediated organoid proliferation is significantly mediated through Stat3 activation (Figure 5C). Indeed, the NK1-R+ PanIN cells in vivo had a significantly increased p-Stat3 expression (by 35.4 ± 7.9%) compared to NK1-R− cells within the same PanIN lesion, suggesting that NK1-R+ PanIN cells may be more responsive to neuropeptide signaling (Figure 5D).
Figure 5. SP induces Jak2 and Stat3 phosphorylation in PanIN organoids.
(A) Western blot analysis for the proteins shown from PanIN organoids incubated with media -/+ SP. (B) Quantification of relative band densities (a.u. = arbitrary units) of phospho-protein/total protein (β actin = loading control) (n=3). (C) Proliferation of co-cultured PanIN organoids with Stat3 inhibitor, Stattic (n=3). RLU (random luminescence units) values are normalized to monoculture controls. (D) Representative IF confocal projection of a PanIN lesion (dashes) in a 12 week KPCPdx1 pancreas. p-Stat3 (red) expression in NK1-R+ cells (green; short arrows) versus NK1-R− cells (long arrows) within the same lesion. Non-specific staining in PanIN lumen (asterisks). Scale bar = 25 µm. Quantification of p-Stat3+ nuclei within the same PanIN lesions of 12-week control KPCPdx1 mice (n=5; n=17 scans). The data represent mean ± SEM (two-tailed unpaired t test; *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001).
Sensory denervation of KPCPdx1 mice decreases PanIN progression
Since nerves were abundant in the PanIN microenvironment and promoted PanIN organoid proliferation, we hypothesized that sensory axons may play an important role in PanIN growth in vivo. A possible role of sensory nerves on PanIN tumorigenesis has been suggested by studies that show capsaicin, a TRPV1 antagonist at high doses, decreases tumor growth in PDAC mouse models (41,42). However, the mechanism for capsaicin’s chemoprotective effects and the specific role of decreased intrapancreatic innervation is unknown. We systemically denervated KPCPdx1 mice with resiniferatoxin (RTX), a super agonist of the TRPV1 receptor which causes highly selective and rapid degeneration of the TRPV+ sensory afferent nerve fibers (43).
In these studies, effective sensory denervation was confirmed by both the capsaicin-induced eye wipe response as well as sensory axon density counts in pancreas tissue. Denervated KPCPdx1 mice had significantly decreased corneal sensitivity to capsaicin for up to 12 weeks compared to control mice, indicating significant loss of TRPV1+ somatic innervation (Figure S9A). The 16 week control mice were more withdrawn and had a significantly decreased eye wipe response compared to earlier time points, likely reflecting tumor-associated morbidity. Although we had evidence for robust somatic sensory denervation, it was critical to confirm intrapancreatic loss of TRPV1+ fibers as we hypothesized local effects of nerves on PanINs. Pancreatic sensory nerve densities were quantified with SP staining, which overlaps exclusively with TRPV1 (Figure 6E and Figure S9B; ref. (44)). RTX treated mice had a significant decrease in pancreas sensory nerve density compared to control mice at 8 and 12 weeks but not at 16 weeks, suggesting likely axon regeneration over time. Interestingly, at this later time point the regrowth of pancreatic visceral sensory fibers was not associated with an increase in the eye wipe response (Figure S9A), suggesting maintenance of peripheral somatic denervation.
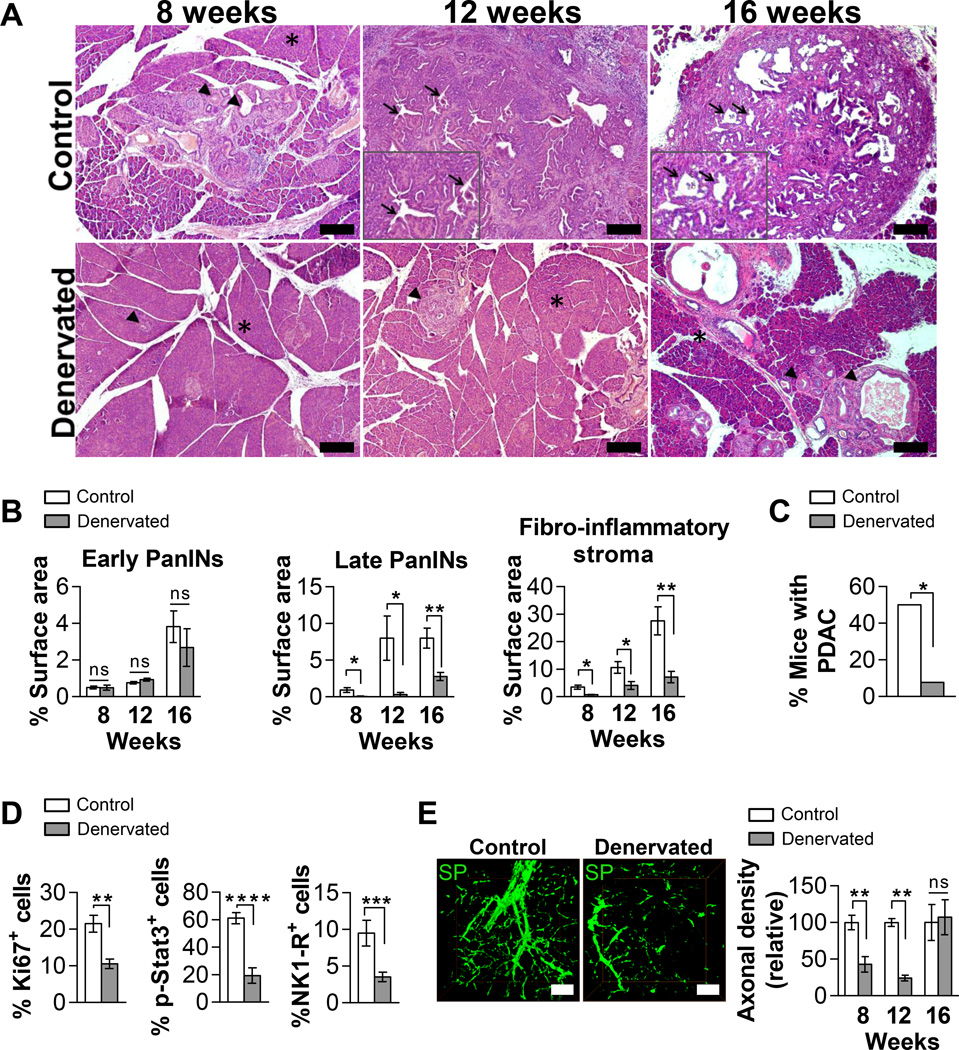
Figure 6. Sensory denervation decreases PanIN progression to PDAC.
(A) Representative H&E pancreas sections. Normal pancreas (asterisks), PanINs (arrowheads) and highly dysplastic ducts of tumors (inserts; arrows) are shown. Scale bars = 200 µm. (B) Histopathologic quantifications of PanIN and stroma burden (n=4–5 mice per group per time point). (C) Percentage of 8, 12 and 16 week-old mice with tumors (n=12 control and n=13 denervated). (D) Quantifications of Ki-67+, p-Stat3+ and NK1-R+ cells per PanIN (n=13–44 PanINs per analysis and 3–5 mice per group). (E) Representative 3D IF projections of substance P (SP) axons (green). Pancreatic axon density quantifications (n=3 views per mouse; n=3 mice per group per time point). Scan depth = 150 µm. Scale bars = 50 µm. The data represent mean ± SEM (two-tailed unpaired t test or Fisher’s exact test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001).
The denervated mice had reduced late-stage, but not early-stage, PanIN burden at all time points compared to the control group, suggesting an influence on PanIN progression but not PanIN initiation (Figure 6A–B). Supporting this interpretation, there was no difference in ADM lesions between the two groups (Figure S9C). In another study, Stat3 inactivation in the neoplastic epithelium of a different PDAC murine model also prevented PanIN progression but not initiation (13). Therefore, Stat3 may have minimal or no effects on PanIN initiation which most likely depends on cell-autonomous mutation-driven mechanisms. Denervated mice also had a significant decrease in the PanIN-associated fibro-inflammatory stroma. Consistent with their impaired PanIN progression, overall fewer denervated mice developed PDAC (7.7% of denervated versus 50% of control mice) (Figure 6C). The decreased dysplasia of denervated PanINs was further confirmed by a significantly lower Ki67 expression (Figure 6D and Figure S9D). Furthermore, consistent with the observation that SP activated Stat3 in PanIN organoids, PanINs in axotomized pancreata had profoundly decreased Stat3 phosphorylation in PanIN epithelial cells (Figure 6D and Figure S10A–B) as well as in NK1-R+ cells (Figure S11A). Interestingly, denervation also led to a decrease in NK1-R+ PanIN cell abundance (Figure 6D). Taken together, these findings demonstrated that decreased sensory intrapancreatic innervation reduced PanIN progression to PDAC in vivo, potentially through both impaired epithelial Stat3 activation and neuroendocrine cell maintenance.
Discussion
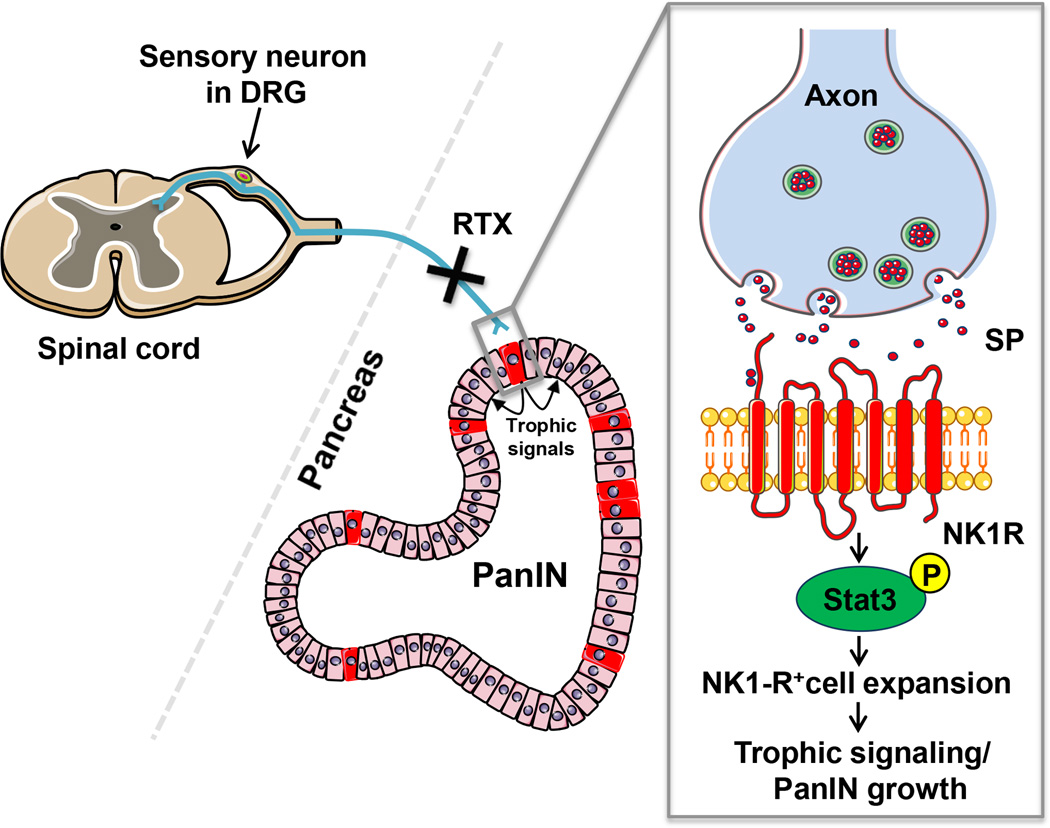
PanIN progression is not only accompanied by accumulating genetic mutations and cellular atypia but also the development of a complex tumor-associated stroma that promotes tumor progression through direct and indirect influences on the neoplastic epithelium (for review see ref. (25,45)). Here, we describe nerves as bona fide members of the PanIN microenvironment and demonstrate that they support PanIN tumorigenesis likely through crosstalk with a novel population of neoplastic neuroendocrine cells (Figure 7).
Figure 7. Neuroepithelial crosstalk.
Sensory innervation of a pancreatic PanIN lesion. The sensory neuron residing in the dorsal root ganglion (DRG) projects a visceral sensory afferent nerve into the pancreas. Vagal nodose ganglia (NG) projections are omitted. The terminal axon in the microenvironment is in close proximity to the PanIN epithelium. SP binding its membrane receptor, NK-1R expressed on NK1-R+ neuroendocrine cells (red), leads to Stat3 phosphorylation and maintenance of the NK1-R+ cells which signal to potentiate PanIN growth. Treatment with resiniferatoxin (RTX) results in loss of the intrapancreatic sensory fibers regardless of the origin of the projections.
Other examples of neuronal influences on non-neuronal tumors via crosstalk with the neoplastic stem cell niche are emerging. For example, autonomic nerves signal directly to gastric stem cells to upregulate the oncogenic Wnt pathway and drive gastric tumorigenesis (2). Here, we propose neuromodulation of PanIN neuroendocrine cells that influences global PanIN progression. Even though human PDACs commonly express endocrine markers (46,47), a functional role of endocrine differentiation in PanIN and PDAC has not been established. In our study, NK1-R+ neuroendocrine cells in PanIN epithelium appeared in the earliest murine neoplastic lesions, exclusively responded to neuronal signaling and functioned as trophic “niche” cells in organoid culture. NK1-R+ cells’ dramatic potentiation of composite organoid proliferation depended on contact mediated signaling, the exact mechanism of which needs to be elucidated. NK1-R+ cells may serve as a source of Wnt ligands that are critical for PanIN tumorigenesis (39) and which can depend on close cell contact for signal transduction (48). Interestingly, the NK1-R+ organoids highly expressed Wnt7b (Figure S8C), which mediates pancreatic progenitor cell growth during development (49). To formally establish whether NK1-R+ cells are required for PanIN progression in vivo, future studies should employ such strategies as toxin-dependent elimination of NK1-R+ cells in PanIN epithelium, as has been done for Dclk1+ PanIN progenitor cells (36).
The NK1-R+ cells were Ki67− in vivo but expectedly proliferated under permissive organoid culture conditions (29). Intriguingly, the NK1-R+ cells recapitulated their in vivo role as a restricted subpopulation when associated with NK1-R− cells. Thus, neuronal activation of Stat3 in NK1-R+ cells in vivo may promote neuroendocrine cell maintenance rather than proliferation, reflecting the complex role of Stat3 in cancer epithelium (for review see ref. (50)). Furthermore, NK1-R+ cells may exclusively influence PanIN versus tumor biology as they were not expressed in tumors or PDAC cells lines. The long-term effects of intrapancreatic sensory denervation on tumor progression and survival require further investigation. Also, the SP-NK1-R axis is likely not involved in axon recruitment by PDAC cell lines that do not notably express the NK1-R. Such time- and context-specific roles of oncogenic factors are well described in PanIN and PDAC tumorigenesis (for review see ref. ((25)).
SP-induced Jak2 and Stat3 phosphorylation in the PanIN organoids provided evidence for neuropeptide activation of a key transcription factor in PanIN cells. Epithelial Stat3 activation by stromal cell-derived IL-6 is critical to Kras-mediated PanIN tumorigenesis (13). Here, we show neuronal SP in the microenvironment can serve as an additional activator of Stat3, as axotomized mice had markedly decreased PanIN p-Stat3 expression. Notably, p-Stat3 was also reduced in NK1-R− cells, suggesting the potential for a more complex role of nerves in mediating stromal-epithelial signaling. While we did not explore the effects of sensory denervation in altering the inflammatory compartment, our studies established an avenue of direct crosstalk between sensory nerves and the PanIN epithelium.
In summary, sensory nerves promote PanIN tumorigenesis potentially through direct crosstalk to unique neoplastic neuroendocrine cells. Our studies define a unique subpopulation of neural-responsive PanIN neuroendocrine cells capable of exerting trophic influences to stimulate global PanIN growth. These results highlight the heterogeneous nature of the neoplastic epithelium and its associated microenvironment and illustrate the potential for their dynamic interactions. Disruption of neuronal influences, perhaps even at the tumor stage, may have therapeutic implications in the chemoprevention and/or treatment of human pancreatic cancer.
Supplementary Material
Acknowledgments
We thank Dr. Mark Donowitz and Mr. John Gibas at JHH for microscopy support. We thank Dr. Christine Iacabuzio-Donahue for histology support and providing the A6L cells and Dr. Ming S. Tao for providing the HPDE cells. We acknowledge the utilized MSKCC core facilities supported by the Cancer Center Support Grant (CCSG) P30 CA008748.
Financial Support: National Institutes of Health R01DK073558 to P.J. Pasricha and R01DK097087 to S.D. Leach and grants P30DK089502 and T32-DK007632 to M. Donowitz. P30 CA008748 Cancer Center Support Grant (CCSG) to MSKCC.
Footnotes
The authors declare no conflict of interest.
References
- 1.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science (New York, NY) 2013;341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 2.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Science translational medicine. 2014;6(250) doi: 10.1126/scitranslmed.3009569. 250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136(1):177–186. doi: 10.1053/j.gastro.2008.09.029. e1. [DOI] [PubMed] [Google Scholar]
- 4.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. International journal of cancer Journal international du cancer. 1999;81(3):417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Annals of surgery. 2006;244(2):274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasanella KE, Christianson JA, Chanthaphavong RS, Davis BM. Distribution and neurochemical identification of pancreatic afferents in the mouse. The Journal of comparative neurology. 2008;509(1):42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Peng J. Sensory nerves and pancreatitis. Gland surgery. 2014;3(4):284–292. doi: 10.3978/j.issn.2227-684X.2013.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiological reviews. 2014;94(1):265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Recio S, Fuster G, Fernandez-Nogueira P, Pastor-Arroyo EM, Park SY, Mayordomo C, et al. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer research. 2013;73(21):6424–6434. doi: 10.1158/0008-5472.CAN-12-4573. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie E, Leeman SE, Watts LA, Coukos JA, O’Brien MJ, Cerda SR, et al. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(42):17420–17425. doi: 10.1073/pnas.1114275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. Journal of immunology. 2006;176(8):5050–5059. doi: 10.4049/jimmunol.176.8.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer cell. 2011;19(4):456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146(1):245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, et al. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. The American journal of pathology. 2000;157(5):1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, NY) 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89(2–3):181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 19.Fu YY, Tang SC. At the movies: 3-dimensional technology and gastrointestinal histology. Gastroenterology. 2010;139(4):1100–1105. doi: 10.1053/j.gastro.2010.08.025. 05 e1. [DOI] [PubMed] [Google Scholar]
- 20.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer research. 2006;66(1):95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 21.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer cell. 2014;25(5):621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2002;15(4):441–447. doi: 10.1038/modpathol.3880544. [DOI] [PubMed] [Google Scholar]
- 23.Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer research. 2014;74(6):1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha S, Leach SD. New insights in the development of pancreatic cancer. Curr Opin Gastroenterol. 2016 doi: 10.1097/MOG.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95(8):4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136(4):1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amcheslavsky A, Song W, Li Q, Nie Y, Bragatto I, Ferrandon D, et al. Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila. Cell reports. 2014;9(1):32–39. doi: 10.1016/j.celrep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32(20):2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer research. 2008;68(3):918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson SP. Rapid degradation of [3H]-substance p in guinea-pig ileum and rat vas deferens in vitro. British journal of pharmacology. 1983;79(2):543–552. doi: 10.1111/j.1476-5381.1983.tb11029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friess H, Zhu Z, Liard V, Shi X, Shrikhande SV, Wang L, et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Laboratory investigation; a journal of technical methods and pathology. 2003;83(5):731–742. doi: 10.1097/01.lab.0000067499.57309.f6. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Ma G, Ma Q, Li W, Liu J, Han L, et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Molecular cancer research : MCR. 2013;11(3):294–302. doi: 10.1158/1541-7786.MCR-12-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilmer M, Garnier A, Vykoukal J, Alt E, von Schweinitz D, Kappler R, et al. Targeting the Neurokinin-1 Receptor Compromises Canonical Wnt Signaling in Hepatoblastoma. Mol Cancer Ther. 2015;14(12):2712–2721. doi: 10.1158/1535-7163.MCT-15-0206. [DOI] [PubMed] [Google Scholar]
- 36.Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, et al. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell stem cell. 2016;18(4):441–455. doi: 10.1016/j.stem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes & development. 2006;20(22):3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Morris JPt, Yan W, Schofield HK, Gurney A, Simeone DM, et al. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer research. 2013;73(15):4909–4922. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(6):2013–2018. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai H, Li H, Zhang W, Matkowskyj KA, Liao J, Srivastava SK, et al. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis. 2011;32(11):1689–1696. doi: 10.1093/carcin/bgr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. The Journal of clinical investigation. 2004;113(9):1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. The journal of pain : official journal of the American Pain Society. 2007;8(3):263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neesse A, Algul H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Baithun SI, Pollock DJ, Berry CL. Argyrophilic and hormone immunoreactive cells in normal and hyperplastic pancreatic ducts and exocrine pancreatic carcinoma. Virchows Archiv A, Pathological anatomy and histopathology. 1988;413(5):399–405. doi: 10.1007/BF00716988. [DOI] [PubMed] [Google Scholar]
- 47.Eusebi V, Capella C, Bondi A, Sessa F, Vezzadini P, Mancini AM. Endocrine-paracrine cells in pancreatic exocrine carcinomas. Histopathology. 1981;5(6):599–613. doi: 10.1111/j.1365-2559.1981.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 48.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530(7590):340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 49.Afelik S, Pool B, Schmerr M, Penton C, Jensen J. Wnt7b is required for epithelial progenitor growth and operates during epithelial-to-mesenchymal signaling in pancreatic development. Developmental biology. 2015;399(2):204–217. doi: 10.1016/j.ydbio.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 50.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.