Abstract
Objective
Disturbances in functional connectivity have been suggested to contribute to cognitive and emotion processing deficits observed in bipolar disorder (BD). Functional connectivity between medial prefrontal cortex (mPFC) and other brain regions may be particularly abnormal. The goal of the present study was to characterize the temporal dynamics of the default mode network (DMN) connectivity in BD and examine its association with cognition.
Method
In a preliminary study, euthymic BD (n=15) and healthy comparison (HC, n=19) participants underwent resting-state functional magnetic resonance imaging, using high-resolution sequences adapted from the Human Connectome Project, and completed neuropsychological measures of processing speed and executive function. A seed-based approach was used to measure DMN correlations in each participant, with regions of interest in the mPFC, posterior cingulate cortex (PCC), and lateral parietal cortex. Subsequently, to characterize temporal dynamics, correlational analyses between the mPFC and other DMN nodes were repeated using a sliding-window correlational analysis with subsets of the time series.
Results
When averaged across the entire scan, there were no group differences in overall connectivity strength between the mPFC and other regions of the DMN. However, dynamic connectivity between the mPFC and PCC was altered in BD, such that connectivity was less variable (i.e., more rigid) over time. Decreased connectivity variability was associated with slower processing speed and reduced cognitive set-shifting in BD patients.
Conclusions
Variability in resting-state functional connectivity may be an index of internetwork flexibility that is reduced in BD and a correlate of ongoing cognitive impairment during periods of euthymia.
Keywords: bipolar disorder, functional connectivity, resting-state fMRI, default mode network, cognition
INTRODUCTION
Bipolar disorder (BD) is a devastating psychiatric illness characterized by mood instability, with alternating periods of manic, depressive, and euthymic mood states. Neurocognitive dysfunction is a core feature of BD, particularly during acute episodes of mania and depression, but these deficits persist even between mood episodes during periods of euthymia when patients are stable on medications (Bearden, Woogen, & Glahn, 2010; Kurtz & Gerraty, 2009). Executive function, especially cognitive control and flexibility, as well as processing speed are worse in those with BD compared to those without, even during periods of euthymia. However, the neural underpinnings of these deficits are not completely understood.
Disturbances in functional connectivity have been suggested to contribute to cognitive and emotion processing deficits observed in bipolar disorder. Studies of functional connectivity have reported the existence of several functionally distinct organized networks that show highly correlated activity during rest (Greicius, Krasnow, Reiss, & Menon, 2003; van den Heuvel & Hulshoff Pol, 2010). Among the identified resting-state networks, the default mode network (DMN) consists of medial prefrontal cortex, posterior cingulate cortex, and bilateral angular gyrus regions that are thought to support self-reflective and emotional processing as well as other internally-directed cognitive functions (e.g., intrinsic attention, information processing, remembering autobiographical information, planning aspects of personal future, reasoning)(Andrews-Hanna, 2012; Buckner, Andrews-Hanna, & Schacter, 2008; Gusnard, Akbudak, Shulman, & Raichle, 2001; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008; Spreng, Mar, & Kim, 2009), which makes it particularly interesting in the context of BD. While the DMN has been observed to be active at rest and suspended during cognitive task states (Raichle et al., 2001), studies have also found functional connectivity in the DMN to be related to cognitive performance, including executive functions such as working memory and cognitive set-shifting (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Hampson, Driesen, Skudlarski, Gore, & Constable, 2006), suggesting that a common set of cognitive processes are spontaneously active even during passive states. Mazoyer and colleagues (2001) explored the content of spontaneous thought at rest using introspective questionnaires regarding subjects’ mental activity and observed that resting-state activity was associated with “generation and manipulation of mental images, reminiscence of past experiences based on episodic memory, and making plans.” Furthermore, inhibitory processes are also inherently recruited in order to refrain from moving and avoid structured mental activities (Mazoyer, et al., 2001). All these cognitive processes are components of working memory and executive systems; thus, these authors conclude that conscious resting state is sustained by large-scale fronto-parietal cortical networks working together under the supervision of an executive prefrontal network (Mazoyer, et al., 2001).
Notably, the medial prefrontal cortex (mPFC) was of particular interest in this study as it is central to emotional regulation, via connections to limbic regions, and exhibits mood-state dependent alterations in BD (Strakowski, Delbello, & Adler, 2005). Additionally, its connectivity with the posterior cingulate cortex (PCC) has been linked with executive function (Andrews-Hanna, et al., 2007; Hampson, et al., 2006). Both structural and functional abnormalities of the mPFC are seen in BD (Strakowski, et al., 2005), including how its activity is coordinated with that of other brain regions within the DMN and associated networks (Calhoun, Maciejewski, Pearlson, & Kiehl, 2008; Vargas, Lopez-Jaramillo, & Vieta, 2013). Although abnormalities in resting-state functional connectivity are well documented, the specific direction of these findings is varied across studies. For example, some studies observe hyperconnectivity of the mPFC with other cortical (e.g., dorsolateral prefrontal cortex, other DMN regions) and meso-limbic regions (e.g., amygdala, insula)(Chai et al., 2011; Favre, Baciu, Pichat, Bougerol, & Polosan, 2014), while others find it to be less functionally connected to these regions (Favre, et al., 2014; Liu et al., 2012; Ongur et al., 2010). Anticevic and colleagues (2013) observed both reduced mPFC global brain connectivity as well as increased mPFC-amygdala connectivity. Discrepancies across studies may be attributed to differences across studies in methods of analyses (e.g., region of interest analysis, independent component analysis, regional homogeneity approach) and sample characteristics (e.g., euthymic, vs. manic or depressed patients); nevertheless, the consistent finding is that patients with BD show differences in mPFC connectivity patterns compared to healthy controls.
The extant literature suggests that resting-state connectivity may be more complex than simply describing an increase versus decrease in connectivity between regions/networks (Vargas, et al., 2013). All of the aforementioned studies were focused on overall connectivity strength—that is, they are based on the implicit assumption of stability or consistency throughout the measurement period. While this assumption is convenient and has allowed simplification of incredibly complex network interactions that underlie conscious mental activity during rest, it is also oversimplified. It is natural to expect that functional connectivity metrics will exhibit variation over time. Rather than just representing noise in the measurement processes, these fluctuations may reflect a fundamental feature of connectivity, which is non-stationary in nature (Allen et al., 2014; Chang & Glover, 2010). Particularly in the resting-state, spontaneous or intrinsic fluctuations of activity and connectivity are even more prominent because mental activity is unconstrained, and relatively subtle modulations in cognitive load can alter the spontaneous activity and patterns of functional connectivity throughout the brain, including DMN regions (Yan et al., 2009). Quantifying the changes in and dynamics of functional connectivity over time can provide greater insight into fundamental properties of brain networks (Hutchison et al., 2013).
Characterizing dynamic functional network connectivity is perhaps even more important in clinical populations given that many neuropsychiatric diseases exhibit altered structural and functional properties (for a review, see Greicius, 2008). Quantification of disrupted dynamics might capture differences in variability that may not exist in healthy populations and may serve as a sensitive biomarker and/or prognostic indicator of disease progression or cognitive capacity. As such, there is tremendous potential for clinical application of this methodology to make valuable contributions to our understanding of clinical populations. Exploration of the dynamic properties of functional connectivity has provided new insights into neuropsychiatric disease, including Alzheimer’s disease (Jones et al., 2012), autism (Starck et al., 2012), and schizophrenia (Damaraju et al., 2014; Rashid, Damaraju, Pearlson, & Calhoun, 2014; Sakoglu et al., 2010), and we apply this method to further our understanding of BD. Specifically, little is known about temporal dynamics of functional connectivity from mPFC to other regions within the DMN and how it relates to cognition in euthymic BD patients.
Therefore, in order to gain a better understanding of how the DMN is affected in BD, we investigated the temporal features of functional connectivity within this network. The goals of the present study were to 1) characterize the temporal dynamics of mPFC functional connectivity to other DMN regions at rest in euthymic BD using high-resolution sequences adapted from the Human Connectome Project, and 2) examine associations between variability of mPFC functional connectivity in the DMN and measures of executive function and processing speed. We hypothesized that temporal dynamic analysis of the DMN would reveal differences in functional connectivity between patients with BD and healthy individuals not captured by a traditional static (i.e., average) analysis. We were especially interested in the connectivity between the mPFC and posterior cingulate cortex (PCC) and bilateral angular gyrus (AG) because they represent major components of the DMN (Greicius, et al., 2003; Raichle, et al., 2001) and because functional disruption between these regions has been found to be associated with cognition (Andrews-Hanna, et al., 2007). Secondly, we hypothesized that differences in dynamic connectivity would underlie deficits in cognitive control/flexibility and processing speed that are subserved by these regions and typically persist in BD patients during periods of euthymia.
METHODS
Participants
Participants in this study were 21 euthymic bipolar I disorder patients (BD; 7M/14F, 47.2 ± 11.8 years of age) and 20 demographically-matched healthy controls (HC; 6M/14F, 47.3 ± 13.1 years of age). All participants were between the ages of 30–79 years, right-handed, native English speakers, appropriate for magnetic resonance imaging (MRI; e.g., no metal in body), and free of any serious neurological diagnoses, history of head injury with loss of consciousness greater than fifteen minutes, or current or recent diagnosis of substance abuse or dependence in the past 6 or 12 months, respectively.
All BD participants met criteria for a diagnosis of Bipolar I Disorder based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; American Psychiatric Association, 2000), with onset of first mood episode occurring between ages 13–30 years, and had no history of any other Axis I disorder, as determined by the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, & Williams, 1996). Patients were stable on medications for at least six weeks and were not currently experiencing a mood episode. Subjects were considered euthymic if they met cutoff scores on the Hamilton Rating Scale for Depression (HAM-D ≤ 7; Hamilton, 1960), Young Mania Rating Scale (YMRS ≤ 6; Young, Biggs, Ziegler, & Meyer, 1978), and Positive and Negative Syndrome Scale (PANSS positive ≤ 21 and PANSS negative ≤ 21; Kay, Fiszbein, & Opler, 1987). An index of psychotropic medication load was calculated for each BD participant using a method previously developed (Hassel et al., 2008). Current doses of antipsychotics, mood stabilizers, antidepressants, and anxiolytics were assessed, assigned a value based on dosage and duration of use (coded absent = 0, low = 1, or high = 2), and then summed across medication type for each patient. All BD patients were medicated, with 48% taking antidepressants, 52% taking antipsychotics, 67% taking mood stabilizers, and 43% taking anxiolytics or benzodiazepines. Also, 76% were on polytherapy involving two or more classes of these psychotropic medications. HC participants were free of any Axis I disorder, as determined by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998), and any first-degree relatives with bipolar disorder, unipolar depression, or schizophrenia. All procedures were approved by the Institutional Review Board of the Veteran’s Affairs San Diego Healthcare System. Written informed consent was obtained from all participants in the study. Demographic data and clinical rating scores are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the sample
| Variable | BD (n = 15) | HC (n = 19) | p-value |
|---|---|---|---|
| Age (years) | 44.3 (11.38) | 46.9 (13.28) | .547 |
| Gender [n (% female)] | 14 (67) | 14 (70) | .819 |
| Education (years) | 15.7 (2.19) | 15.6 (2.22) | .909 |
| AMNART estimated IQ | 115.3 (8.75) | 118.8 (6.92) | .201 |
| Age of illness onset (years) | 18.8 (4.39) | -- | |
| Illness duration (years) | 28.4 (11.00) | -- | |
| Medication Load | 3.8 (2.02) | -- | |
| HAM-D | 3.4 (2.50) | -- | |
| YMRS | 1.3 (1.70) | -- | |
| PANSS Positive | 8.8 (1.48) | -- | |
| PANSS Negative | 8.8 (2.13) | -- | |
| PANSS Total | 36.7 (4.1) | -- |
Data are presented as M (SD) unless otherwise specified. Comparisons between treatment groups were conducted using independent-samples t-tests for continuous variables and chi-square test for categorical variables.
AMNART, American National Adult Reading Test; BD, bipolar disorder; HAM-D, Hamilton Rating Scale for Depression; HC, healthy control; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale
MRI Acquisition
Participants were scanned at the UCSD Keck Center for Functional MRI on a General Electric (GE) Discovery MR750 3.0 Tesla scanner with a 32-channel phased-array head coil (Nova Medical). A high resolution anatomical T1-weighted MRI image was collected using a Fast Spoiled Gradient echo pulse (FSPGR) sequence (TE = 4 ms, FA = 8, TI = 600 ms, FOV = 25.6 × 19.2 mm, 176 1-mm thick sagittal slices, voxel size 1 × 1 × 1 mm). The resulting images were utilized to localize the functional signal. T2*-weighted resting-state functional images were acquired for 10 minutes using a gradient-echo multiband echoplanar imaging method (multiband factor = 8, TR = 720 ms, TE = 33 ms, FA = 52, FOV = 180 × 208 mm, 90 × 104 matrix, 72 oblique axial 2 mm slices, 833 TRs, voxel size 2 × 2 × 2 mm). Participants were instructed to remain still, stay awake, keep their eyes open, and focus on a fixation point. Slices were collected in the oblique plane aligned to the anterior-posterior commissure plane. In addition, two short scans were collected with reversed phase-encode blips, resulting in pairs of images with distortions going in opposite directions. These images were used for distortion correction of multiband echoplanar images using the TOPUP software in FSL (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004).
Physiological Signal Acquisition and Processing
Earlier resting-state studies have shown that blood oxygenation level dependent (BOLD) frequency oscillations can be confounded by multiple sources of physical and physiological noise (Shmueli et al., 2007; van Buuren et al., 2009; Wise, Ide, Poulin, & Tracey, 2004). Dynamic analysis is particularly sensitive to physiological noise since estimates of time-varying connectivity are based on relatively short time windows, and signal fluctuations generated from various sources of noise can be misinterpreted as temporary changes in connectivity patterns (Hutchison, Womelsdorf, Allen, et al., 2013). For this reason, continuous heart rate and respiration signals were collected using the standard physiological monitoring equipment associated with the MR750 MRI system. Cardiac pulse was recorded using a photopulse sensor attached to a finger to detect blood flow into the vascular bed of the finger. Breathing was recorded with a respiratory bellows placed around the diaphragm or abdomen where there was the greatest breathing motion when the participant was lying down. Signal processing was performed with an in-house Matlab algorithm.
MRI Processing and Analysis
Functional images were processed using Analysis of Functional NeuroImages (AFNI)(Cox, 1996) and custom code written in Matlab. Pre-processing of MR images included image reconstruction, FSL TOPUP correction for field inhomogeneities, registration, and automated motion correction. The first ten images were discarded to allow for T1-equilibrium. Images were spatially blurred with a Gaussian kernel full-width-half-max (FWHM) of 3 mm. Linear regression was applied to remove sources of spurious variance in the data. In order to correct for confounds due to physical and physiological noise, the following nuisance regressors and their temporal derivatives were included: linear and quadratic trends, six motion parameters estimated during image co-registration, physiological noise terms RETROICOR (Glover, Li, & Ress, 2000) and RVHRCOR (Chang & Glover, 2009) associated with the heart and respiratory rate, the mean BOLD signal averaged over the deep cerebral white matter, and the mean BOLD signal averaged over the ventricles. The corrected BOLD time series were then low-pass filtered using a cut-off frequency of 0.08 Hz. Individual subject data were registered to Montreal Neurological Institute (MNI) template using FSL FLIRT program at 2 × 2 × 2 mm voxel size. A DMN map was determined for each subject as an average correlation map for six 5-mm seeds at locations described by Yeo and colleagues (2011). DMN regions of interest (ROIs) were defined by intersecting a group average DMN map at r > 0.15 with a highly reliable 17-network cortical parcellation derived from the fMRI data of 1000 healthy controls (Baker et al., 2014; Yeo, et al., 2011). This resulted in twelve DMN ROIs, including nodes in the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), left and right angular gyrus and inferior parietal lobule (AG), left and right anterior middle temporal gyrus (aMTG), left and right ventral prefrontal cortex (vPFC), superior medial frontal cortex (sMFC), left and right superior frontal gyrus (SFG), and dorsal prefrontal cortex (dPFC). Based on our a priori hypotheses, primary analyses were conducted only for the canonical nodes of the DMN, which included the PCC and right and left AG. Medial PFC connectivity with other DMN nodes was explored in secondary, post-hoc analyses. The mean time course in each node was extracted and correlated with the average mPFC time course to calculate Pearson’s r. Pairwise correlations were then converted to Fisher r-to-z transformed correlation coefficients, z(r), which are normally distributed over the sample, and an average connectivity score of each node with the mPFC was calculated. Subsequently, to examine time-varying dynamics of resting-state connectivity, a sliding-window approach was applied to the entire time series (Allen, et al., 2014; Chang & Glover, 2010; Hutchison, Womelsdorf, Gati, Everling, & Menon, 2013). Correlational analyses between the mPFC and other DMN nodes were repeated with a truncated window size of 60 seconds (s). The window was advanced in increments of 30s (resulting in 50% overlap between windows), and correlations were obtained for each window and, hence, different temporal portions of the data. The variability (standard deviation2) over the sequence of sliding-window correlation coefficients was computed for each node. These variability scores were also explored for all pairwise connections between DMN nodes and nodes within two other networks, the dorsal attention network (DAN) and fronto-parietal control network (FPCN).
Motion
BD patients had increased relative motion (BP: 0.11 ± 0.06 mm vs. HC: 0.06 ± 0.04 mm, p < 0.01) compared to HC. In order to minimize potential effects of increased relative motion on connectivity results, six BD participants and one HC participant with higher relative motion (defined as 2 SD above the mean for HC) were excluded. Therefore, all analyses were performed with the remaining set of 15 BD (5M/10F, 44.3 ± 11.4 years of age) and 19 HC participants (6M/13F, 46.9 ± 13.3 years of age), who did not significantly differ on relative motion (p > .05). Individuals who were excluded from analyses did not differ on any demographic or clinical characteristic from the remaining sample (ps > .05), except that excluded BD patients scored higher on the PANSS Positive Scale.
Cognitive Assessment
Participants completed the Delis-Kaplan Executive Function System (D-KEFS) Trail Making (TM) and Color-Word Interference (CWI) Tests. The TM Number Sequencing and Letter Sequencing conditions as well as the CWI Color Naming and Word Reading conditions were used as measures of psychomotor processing speed. The TM Number-Letter Switching and CWI Inhibition conditions were considered executive function measures of cognitive control and flexibility. For each domain, raw completion times were converted into Z-scores and averaged to create composite scores of processing speed and executive function that were subsequently used in analyses. Higher, more positive values on composite scores are indicative of worse performance.
Statistical Analyses
Statistical analyses were conducted using SPSS Statistics version 14.0 (SPSS Incorporation, 2005). An alpha level of p < .05 was used to determine statistical significance. Demographic data were analyzed using Chi-square statistics for categorical variables (gender) and independent samples t-tests for continuous variables (age, years of education). Groups were compared on cognitive performance using univariate analyses of variance (ANOVA). Demographic variables were included as covariates if they were significantly correlated with the dependent variable and did not interact with either the dependent or independent variables. Group differences in the static (i.e., average) and dynamic (i.e., temporal variability) functional connectivity between the mPFC and other DMN ROIs were evaluated using univariate analysis of variance, controlling for age and relative motion. A Bonferroni-adjusted family-wise alpha level of p < .017 (p < .05/3 a priori connections) was used to correct for multiple comparisons within static and dynamic analyses. To examine whether variability in functional connectivity was related to cognition, regions that demonstrated significant group differences in temporal variance of mPFC connectivity were included in subsequent multiple linear regression analyses. In the regression model, mPFC connectivity variability, Group (BD, HC), and the interaction term served as predictors of cognitive performance on tasks of executive function and processing speed. Finally, correlations were performed within the BD group to evaluate the relationship of medication load, disease chronicity (e.g., age of onset, disease of illness duration, number of psychiatric hospitalizations, and number of mood episodes), and symptoms severity (e.g., HAM-D, YMRS, and PANSS) with cognitive performance and functional connectivity.
RESULTS
Demographics and Cognitive Performance
Groups did not differ on age, gender, years of education, or estimated verbal IQ (Table 1). Mean scores for each group on each D-KEFS measure and cognitive composites are presented in Table 2. Demographic variables were not significantly related to cognitive performance scores and were not included as covariates in group comparisons. No significant group differences were observed for either composite scores of processing speed or executive function (ps > .15); BD and HC groups did not statistically differ on cognition.
Table 2.
Mean scores by group for each cognitive variable
| Cognitive Domain/Task | BD | HC | p-value | Effect size (Cohen’s d) |
|---|---|---|---|---|
| Processing Speed Composite | .806 (3.10) | −.637 (2.88) | .170 | .482 |
| TM Number Sequencing | .341 (1.20 | −.270 (.733) | ||
| TM Letter Sequencing | .243 (1.18) | −.192 (.812) | ||
| CWI Color Naming | .150 (.984) | −.118 (1.02) | ||
| CWI Word Reading | .072 (.970) | −.057 (1.05) | ||
| Executive Function Composite | .414 (1.61) | −.415 (1.61) | .151 | .515 |
| TM Number-Letter Switching | .345 (1.24) | −.288 (.654) | ||
| CWI Inhibition | .069 (.781) | −.054 (1.16) |
Data are presented as M (SD). All variables are presented as Z-scores.
p ≤ 0.05
BD, bipolar disorder; HC, healthy control
Static Functional Connectivity
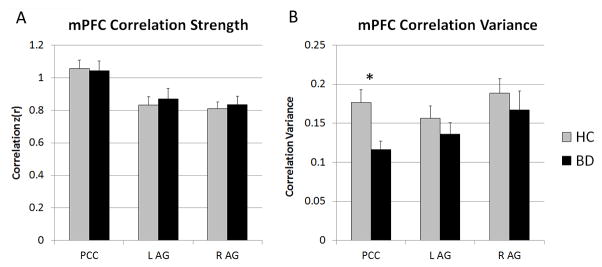
Average connectivity scores between the mPFC and each DMN seed region are presented in Figure 1A and Table 3. Analyses revealed no significant group differences in average mPFC connectivity strength with the PCC or AG regions (ps > .306). Exploratory analyses with other DMN regions did not reveal any differences in average mPFC connectivity strength (ps > .053).
Figure 1.
Static (A) and dynamic (B) resting-state functional connectivity between the mPFC and canonical DMN regions. (A) Scores represent the average connectivity strength of the mPFC with other DMN regions for the entire resting-state scan. BD and HC did not differ on average connectivity of the mPFC to the PCC or AG regions. (B) Scores are presented as the variance in correlation strength, across 19 sliding windows, between the mPFC and other DMN regions. mPFC connectivity strength with PCC was less variable over time in BD than in HC.
*Bonferroni-corrected p ≤ .017
BD, bipolar disorder; HC, healthy control; AG, angular gyrus and inferior parietal lobule; mPFC, medial prefrontal cortex; L, left; PCC, posterior cingulate cortex; R, right
Table 3.
Average correlation strength between the mPFC and each DMN seed region
| mPFC Correlation Strength z(r) | BD (n = 15) | HC (n = 19) | p-value | Effect size (Cohen’s d) |
|---|---|---|---|---|
| PCC | 1.04 (.233) | 1.06 (.229) | .796 | −.087 |
| L AG | .871 (.252) | .832 (.229) | .306 | .162 |
| R AG | .835 (.199) | .809 (.182) | .351 | .136 |
| L aMTG | .829 (.226) | .845 (.180) | .787 | −.078 |
| R aMTG | .869 (.224) | .868 (.161) | .778 | .005 |
| L vPFC | .743 (.135) | .605 (.258) | .060 | .675 |
| R vPFC | .813 (.185) | .625 (.271) | .053 | .810 |
| sMFC | 1.12 (.252) | 1.09 (.209) | .549 | .130 |
| L SFG | .898 (.273) | .856 (.306) | .584 | .145 |
| R SFG | .931 (.280) | 1.01 (.269) | .647 | −.288 |
| dPFC | .704 (.226) | .618 (.250) | .273 | .361 |
Data are presented as M (SD).
Bonferroni-corrected p ≤ 0.017
AG, angular gyrus and inferior parietal lobule; aMTG, anterior middle temporal gyrus; BD, bipolar disorder; dPFC, dorsal prefrontal cortex; HC, healthy control; L, left; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; R, right; SFG, superior frontal gyrus; sMFC, superior medial frontal cortex; vPFC, ventral prefrontal cortex
Dynamic Functional Connectivity
Variance over time in connectivity scores between the mPFC and each DMN seed region are presented in Figure 1B and Table 4. Analyses revealed a significant main effect of Group for the connectivity variability between the mPFC and the PCC, F(3,30) = 7.22, p = .012, η2 = .194. The BD group demonstrated reduced variability in connectivity between the mPFC and PCC (See Figure 2). No group differences in temporal variance between the mPFC and AG regions were observed (ps > .253). Additionally, post-hoc analyses did not reveal group differences in dynamic connectivity between the mPFC and other exploratory DMN regions (ps > .228).
Table 4.
Variance in connectivity strength between the mPFC and each DMN seed region in sliding-window analysis
| mPFC Correlation Variance | BD (n = 15) | HC (n = 19) | p-value | Effect size (Cohen’s d) |
|---|---|---|---|---|
| PCC | .117 (.040) | .177 (.071) | .012* | −1.04 |
| L AG | .136 (.057) | .156 (.068) | .333 | −.319 |
| R AG | .167 (.094) | .188 (.082) | .253 | −.238 |
| L aMTG | .210 (.122) | .191 (.073) | .645 | .189 |
| R aMTG | .201 (.105) | .182 (.068) | .623 | .215 |
| L vPFC | .176 (.089) | .194 (.056) | .419 | −.242 |
| R vPFC | .179 (.097) | .176 (.079) | .969 | .034 |
| sMFC | .162 (.084) | .164 (.059) | .592 | −.028 |
| L SFG | .147 (.052) | .156 (.074) | .485 | −.141 |
| R SFG | .145 (.077) | .167 (.084) | .228 | −.273 |
| dPFC | .169 (.068) | .174 (.071) | .591 | −.072 |
Data are presented as M (SD).
Bonferroni-corrected p ≤ 0.017
AG, angular gyrus and inferior parietal lobule; aMTG, anterior middle temporal gyrus; BD, bipolar disorder; dPFC, dorsal prefrontal cortex; HC, healthy control; L, left; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; R, right; SFG, superior frontal gyrus; sMFC, superior medial frontal cortex; vPFC, ventral prefrontal cortex
Figure 2.
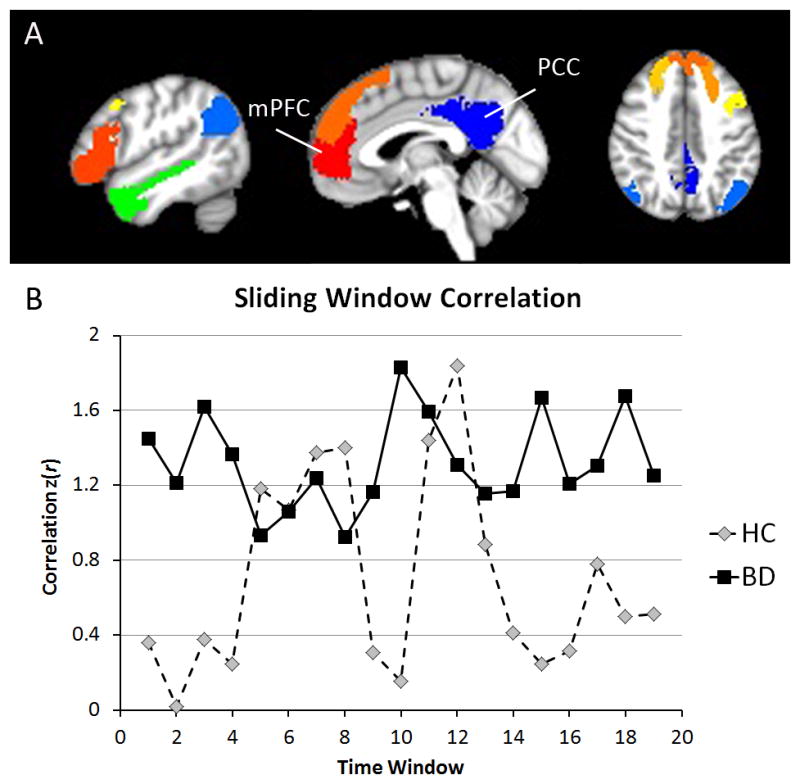
mPFC-PCC connectivity has reduced variability in BD compared to HC but not reduced average correlation strength. (A) Mask locations of the mPFC, PCC, and DMN ROIs. (B) Representative sliding-window correlation time courses between the mPFC and PCC for a HC and BD participant.
BD, bipolar disorder; HC, healthy control; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex
Additionally, Supplemental Figure 1 shows exploratory analyses of the group differences in variance of pairwise connectivity between all nodes of the DMN and all nodes of the FPCN and DAN. These exploratory analyses show that, on the whole there are more pairwise connections in which HC have greater connectivity variance than BD (i.e., overall more warm than cool colors). Also, connections of all DMN nodes to right inferior parietal lobule and right precuneus are consistently less variable in the BD compared to HC group.
Finally, to rule out the potential effect of caffeine usage as a confounding factor on our results, exploratory correlational analyses were conducted to determine whether caffeine intake was related to mPFC connectivity. For measures of static and dynamic connectivity that were significantly correlated with caffeine intake (p < .05), analyses were re-run with caffeine as a covariate, and results did not change.
Relationship of mPFC Functional Connectivity Variability to Cognition
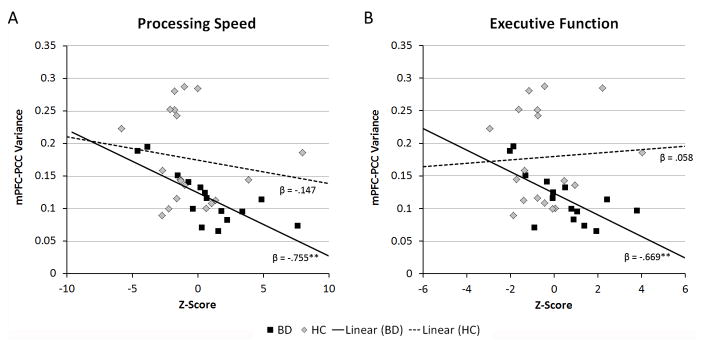
Given that the functional connectivity between the mPFC and PCC demonstrated reduced temporal variability in the BD group compared to the HC group, mPFC-PCC connectivity variance was considered in multiple linear regression analyses to determine its association with measures of processing speed and executive function. For processing speed, regression analysis revealed a main effect of Group, β = 1.20, p = .012, and Group × Variance interaction, β = −1.12, p = .011 (overall model fit: R2 = .32, p = .008). Follow-up of this interaction revealed that reduced variance in mPFC-PCC connectivity was associated with decreased processing speed in the BD group (β = −.755, p = .001), but there was no significant relationship in the HC group (β = −.147, p = .548). Additionally, for executive function, the regression model revealed a main effect of Group, β = 1.30, p = .011, and Group × Variance interaction, β = −1.12, p = .017 (overall model fit: R2 = .26, p = .033). Further examination of the interaction effect revealed that decreased variability in mPFC-PCC connectivity was associated with reduced cognitive control and flexibility in the BD group (β = −.669, p = .006) but not HC group (β = .058, p = .821). Figure 3 depicts the relationship between mPFC-PCC connectivity variability and measures of executive function and processing speed for BD and HC groups.
Figure 3.
Scatterplots depicting the significant interaction of Group (BD vs. HC) and mPFC-PCC connectivity variability on composite measures of (A) processing speed and (B) executive function.
**p ≤ .01
BD, bipolar disorder; HC, healthy control; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex
Given that there appeared to be a bimodal distribution of variance scores in the HC group, we divided the HC group into two subgroups (i.e., those with variance > 0.20 versus those with variance < 0.20) and repeated the above analyses with 3 groups (BD vs. HC-High vs. HC-Low). These follow-up analyses for both composites showed Group × Variance interactions (ps < .027), such that neither HC subgroup showed a relationship between cognition and variability of mPFC-PCC connectivity (ps > .252), in contrast to the significant associations in the BD group (ps < .006).
Medication Burden and Disease Severity
Medication load was examined as a potential contributor to cognitive performance and functional connectivity and was not found to be correlated with either cognitive composite scores or connectivity strength or variability in our BD sample (ps > .551). Moreover, accounting for medication load did not significantly diminish the relationship between connectivity variance and processing speed (Sobel’s statistic = −.224; p = .822) or executive function (Sobel’s statistic= .219; p = .827). Furthermore, we evaluated the relationship between current antipsychotic medication use and DMN connectivity and found no significant associations (ps > .295).
Finally, the relationship of disease chronicity and symptoms severity with altered mPFC connectivity variability was evaluated within the BD group. None of the chronicity variables were related (ps > .285), but measures of positive and negative psychotic symptoms were positively correlated with mPFC-PCC connectivity variability (PANSS positive: r = .573, p = .025; PANSS negative: r = .574, p = .025), such that increased symptomatology was associated with increased connectivity variability.
DISCUSSION
The present study examined the temporal features of resting-state functional connectivity within the DMN, with particular focus on the mPFC, in euthymic patients with BD. This study is one of few to apply dynamic functional connectivity metrics in BD, and no other study to date has investigated how temporal variations may be related to cognition. We characterized the static functional connectivity, using traditional methods, between the mPFC and defined regions of the DMN. Subsequently, to explore the effects of time-varying dynamics, correlational analyses of seed regions were repeated using a sliding-window approach with subsets of the time series. Results demonstrated that there were no group differences in static mPFC connectivity to any region of the DMN. When averaged across a 10-minute period, mPFC connectivity was not different in euthymic BD patients compared to healthy volunteers. However, the temporal dynamics of mPFC functional connectivity with the PCC was altered in BD participants, such that mPFC connectivity strength with the PCC was less variable over time compared to healthy controls. Secondly, we sought to identify whether variability in functional connectivity may underlie deficits in executive function and processing speed that are prominent in BD patients, even during periods of euthymia. Consistent with our hypotheses, BD patients with the most abnormal (i.e., least variable and most rigid) connectivity dynamics between the mPFC and PCC showed the slowest processing speed and most difficulty with cognitive set-shifting. These findings do not appear to be related to effects of psychotropic medications. Although these findings support our hypotheses that dynamic analysis of the DMN highlights underlying differences in functional connectivity that may not be captured by traditional static analysis, alone, the direction of the relationship was initially unexpected. Given that BD is characterized by dysfunctional emotion regulation (Green, Cahill, & Malhi, 2007), it might be expected that BD individuals would show increased fluctuation or greater variability in functional connectivity during rest. However, just as a high degree of heart rate variability is observed in well-functioning hearts and suggests optimal cardiac and autonomic adaptability to changing environmental demands (e.g., stress, negative affective states)(Thayer, Yamamoto, & Brosschot, 2010), it might also be argued that an increased degree of neural variability would allow for greater dynamic range and the flexibility to switch between networks in response to task demands. Thus, a certain degree of variability is important to a general readiness to change, and reduced connectivity variability suggests an inability to flexibly switch between networks. This explanation is supported by the observation that reduced mPFC-PCC connectivity variability was associated with slower processing speed and reduced cognitive set-shifting, or the ability to redirect one’s attention from one topic to another, in BD patients but not in healthy controls.
These findings are consistent with those of other studies of dynamic functional connectivity in BD (Rashid, et al., 2014) and schizophrenia (Damaraju, et al., 2014; Sakoglu, et al., 2010). Rashid and colleagues (2014) also observed regional differences in functional connectivity variability in several DMN components, including the dorsal mPFC, bilateral angular gyrus, and bilateral precuneus in patients with BD and schizophrenia. Notably, their results suggested that psychiatric patients tend to be confined to a single connectivity state and make fewer transitions to other states compared to healthy controls. Similarly, in a sample of patients with schizophrenia, Damaraju et al. (2014) found that patients with schizophrenia spend significantly more time in a state of relatively weak and rigid connectivity, whereas healthy controls were able to switch more often between different connectivity states. We, on the other hand, observed that BD patients with the greatest psychotic symptoms had the most mPFC-PCC connectivity variability. Nevertheless, our findings, together with the extant literature, suggest that psychiatric patients demonstrate reduced connectivity variability (or increased connectivity rigidity) across various brain networks, which may impact the speed with which they are able to recruit necessary resources, particularly in the face of changing task demands. As such, variability in resting-state functional connectivity may be an index of flexibility of inter-network switching that is reduced in BD.
In particular, variability in the functional connectivity between the mPFC and PCC may be a correlate of ongoing cognitive impairment in BD during periods of euthymia. The mPFC is a region known to be associated with the processing of self-generated information, primarily emotional material involving representing one’s own thoughts, actions, and feelings as well as those of others (D’Argembeau, 2013; Denny, Kober, Wager, & Ochsner, 2012; Gilbert et al., 2006; Gusnard, et al., 2001). Similarly, the PCC is most widely known for its involvement in supporting internally-directed cognition (Andrews-Hanna, Smallwood, & Spreng, 2014; Brewer, Garrison, & Whitfield-Gabrieli, 2013; Buckner, et al., 2008), primarily due to its central role in the DMN. However, the PCC is a heterogenous brain structure with functional connections to the DAN and FPCN in addition to the DMN (Leech, Kamourieh, Beckmann, & Sharp, 2011). Viewed in this context, the PCC is an important transition site and plays an active role in the coordination between various intrinsic connectivity networks, controlling the balance between internal and external focus of attention and optimizing cognitive function (Leech & Sharp, 2014). This model implies that the connectivity of the PCC is inherently dynamic—it must be sufficiently stable to maintain coherent activity with one network (e.g., DMN) yet capable of responding flexibly to another (e.g., DAN or FPCN). This dynamic system appears to be impaired in BD. Euthymic BD patients exhibit neuropsychological deficits that suggests an inability to regulate or “tune” the breadth of attention (i.e., either too wide and unfocused or too narrow and rigid)(Bearden, et al., 2010). Thus, our findings suggest that these cognitive difficulties may be related to poor connectivity dynamics between the mPFC and PCC.
Abnormalities in the mPFC-PCC connectivity are observed in other populations—most notably, healthy aging. PCC connectivity with the mPFC is markedly reduced in older adults, and individuals with lowest functional correlation exhibit the greatest impairment in memory, executive function, and processing speed (Andrews-Hanna, et al., 2007). Thus, aging is associated with a disruption and weakening of the coordinated activity between the anterior and posterior components of the DMN. A similar observation in our sample of BD patients hints at the possibility that BD may be associated with an underlying accelerated aging process (Cardoso, Bauer, Meyer, Kapczinski, & Soares, 2015; Rizzo et al., 2014). Although overall connectivity strength was preserved, reduced variability may be an early sign of age-related changes that precedes further disruption of these networks, contributing to cognitive decline. Although BD patients in the current sample did not have substantial cognitive differences from healthy controls or demonstrate cognitive impairment by clinical standards, these patients may exhibit preclinical, neural signs of aging that may not yet manifest as frank cognitive deficits. Further examination of connectivity dynamics in a longitudinal, aging BD population is needed to evaluate the neuroprogression hypothesis of BD (Cardoso, et al., 2015). Additionally, future studies should examine how mood state and affective variability may impact connectivity variability.
Strengths and Limitations
A few limitations to the present study should be acknowledged. This study was focused on the DMN and did not primarily investigate the dynamic functional connectivity of other networks, such as the DAN or FPCN. The decision to concentrate on the DMN was based on its relevance to emotional processing and cognitive function (Buckner, et al., 2008; Gusnard, et al., 2001). By limiting our investigation to a single network, we also attempted to maximize power given our modest sample size. We have included exploratory analyses of group differences in dynamic connectivity with other networks in the Supplemental Material, but larger future studies should test more powerfully whether differences in dynamic functional connectivity extend to inter-network connections. Additionally, the resting-state scan in this study was only 10 minutes in duration, which recent research suggests may not be sufficiently long to capture a precise representation of stationary functional connectivity features of individual subjects (Anderson, Ferguson, Lopez-Larson, & Yurgelun-Todd, 2011; Birn et al., 2013; Hutchison, Womelsdorf, Allen, et al., 2013; Laumann et al., 2015). The precision of correlation matrix estimates greatly improves as the quantity of data increases up to 25 minutes and beyond (Anderson, et al., 2011; Laumann, et al., 2015). Nevertheless, the within-subject variability of the DMN is relatively lower and more stable than that of other networks (Laumann, et al., 2015). Moreover, the multiband echo planar imaging approach adapted from the Human Connectome Project allows for the acquisition of multiple slices simultaneously, thereby increasing the number of observations acquired in the same time period (Moeller et al., 2010). Furthermore, we did not examine the contribution of individual medications on cognition or brain function, but our analyses do not suggest a large role of medication in the findings. Although history of psychotropic medication use has been associated with changes in brain function in BD (Phillips, Travis, Fagiolini, & Kupfer, 2008), this study was not designed to disentangle the effects of individual psychotropic medications on functional connectivity. Finally, as previously mentioned, our relatively modest sample size may have limited our power to detect other clinically relevant differences between groups, particularly in overall correlation strength and neuropsychological performance, given considerable inter-individual variability.
Despite these limitations, the current study offers a novel perspective on resting-state functional connectivity in bipolar disorder and presents preliminary findings that warrant further investigation and validation in a larger sample. This study is one of the few that have investigated the temporal dynamics of functional connectivity in BD, and our results extends the existing literature by demonstrating that reduced DMN connectivity dynamics may be a correlate of cognitive dysfunction during euthymia. Specifically, variability in mPFC-PCC connectivity may be an index of inter-network flexibility that modulates the focus of attention, and reduced variability may contribute to the difficulty of BD patients to efficiently process information or flexibly switch from one cognitive task to another. Future studies are needed to determine whether similar variability is observed between and within other intrinsic connectivity networks and how these dynamics may change with age and mood state.
Supplementary Material
38 × 38 grids depicting (A) HC-BD group differences in connectivity variance between BD and HC for pairs of ROIs across the default mode network (DMN), frontoparietal control network (FPCN), and dorsal attention network (DAN) and (B) –log10 p-values for each pairwise difference. Positive values indicate greater connectivity variance in HC (warm colors), while negative values indicate greater connectivity variance in BD (cool colors). AG, angular gyrus; aMTG, anterior middle temporal gyrus; aPFC, anterior prefrontal cortex; dPFC, dorsal prefrontal cortex; dPFC, dorsal prefrontal cortex; FEF, frontal eye fields; IPL, inferior parietal lobule; L, left; MFG, medial frontal gyrus; mPFC, medial prefrontal cortex; MTG, middle temporal gyrus; ParOcc, parieto-occipital; PCC, posterior cingulate cortex; pCun, precuneus; PFC, prefrontal cortex; pMTG, posterior medial temporal gyrus; postC, postcentral gyrus; preC, precentral gyrus; R, right; SFG, superior frontal gyrus; sMFC, superior medial frontal cortex; TempOcc, temporal occipital; vPFC, ventral prefrontal cortex
Acknowledgments
The authors acknowledge the efforts of Ashley Sutherland Owens and Rebecca Daly for database management, and Yadira Maldonado for data entry.
Research in this manuscript was supported by the Desert-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC) and NIMH grant numbers R01 MH083968 and MH083968-01-S1 to Lisa T. Eyler, Ph.D.
References
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24(3):663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Text Revision (DSM-IV-TR) Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Reproducibility of single-subject functional connectivity measurements. AJNR Am J Neuroradiol. 2011;32(3):548–555. doi: 10.3174/ajnr.A2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73(6):565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Ongur D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woogen M, Glahn DC. Neurocognitive and neuroimaging predictors of clinical outcome in bipolar disorder. Curr Psychiatry Rep. 2010;12(6):499–504. doi: 10.1007/s11920-010-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Front Hum Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29(11):1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso T, Bauer IE, Meyer TD, Kapczinski F, Soares JC. Neuroprogression and Cognitive Functioning in Bipolar Disorder: A Systematic Review. Curr Psychiatry Rep. 2015;17(9):75. doi: 10.1007/s11920-015-0605-x. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage. 2009;47(4):1381–1393. doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA, Vaidya JG, van Erp TG, Calhoun VD. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre P, Baciu M, Pichat C, Bougerol T, Polosan M. fMRI evidence for abnormal resting-state functional connectivity in euthymic bipolar patients. J Affect Disord. 2014;165:182–189. doi: 10.1016/j.jad.2014.04.054. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103(1–3):29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. 2013;34(9):2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, Przybelski SA, Gregg BE, Kantarci K, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr Non-stationarity in the “resting brain’s” modular architecture. PLoS One. 2012;7(6):e39731. doi: 10.1371/journal.pone.0039731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;(13):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23(5):551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, Schlaggar BL, Mumford JA, Poldrack RA, Petersen SE. Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron. 2015;87(3):657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Ma X, Li F, Wang YJ, Tie CL, Li SF, Chen TL, Fan TT, Zhang Y, Dong J, Yao L, Wu X, Wang CY. Regional homogeneity within the default mode network in bipolar depression: a resting-state functional magnetic resonance imaging study. PLoS One. 2012;7(11):e48181. doi: 10.1371/journal.pone.0048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo LB, Costa LG, Mansur RB, Swardfager W, Belangero SI, Grassi-Oliveira R, McIntyre RS, Bauer ME, Brietzke E. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. Neurosci Biobehav Rev. 2014;42:157–169. doi: 10.1016/j.neubiorev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA. 2010;23(5–6):351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38(2):306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- SPSS Incorporation. SPSS for Windows, Version 14.0. Chicago: SPSS Inc; 2005. [Google Scholar]
- Starck T, Nikkinen J, Remes J, Rahko J, Moilanen I, Tervonen O, Kiviniemi V. Organization for Human Brain Mapping. Beijing, China: 2012. Temporally varying connectivity between ICA default-mode sub-networks—ASD vs. controls. [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, van den Heuvel M, Ramsey NF, Kahn RS, Vink M. Cardiorespiratory effects on default-mode network activity as measured with fMRI. Hum Brain Mapp. 2009;30(9):3031–3042. doi: 10.1002/hbm.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vargas C, Lopez-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013;150(3):727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21(4):1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Long X, Zang Y. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One. 2009;4(5):e5743. doi: 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
38 × 38 grids depicting (A) HC-BD group differences in connectivity variance between BD and HC for pairs of ROIs across the default mode network (DMN), frontoparietal control network (FPCN), and dorsal attention network (DAN) and (B) –log10 p-values for each pairwise difference. Positive values indicate greater connectivity variance in HC (warm colors), while negative values indicate greater connectivity variance in BD (cool colors). AG, angular gyrus; aMTG, anterior middle temporal gyrus; aPFC, anterior prefrontal cortex; dPFC, dorsal prefrontal cortex; dPFC, dorsal prefrontal cortex; FEF, frontal eye fields; IPL, inferior parietal lobule; L, left; MFG, medial frontal gyrus; mPFC, medial prefrontal cortex; MTG, middle temporal gyrus; ParOcc, parieto-occipital; PCC, posterior cingulate cortex; pCun, precuneus; PFC, prefrontal cortex; pMTG, posterior medial temporal gyrus; postC, postcentral gyrus; preC, precentral gyrus; R, right; SFG, superior frontal gyrus; sMFC, superior medial frontal cortex; TempOcc, temporal occipital; vPFC, ventral prefrontal cortex