Summary
Background
Iodine deficiency is associated with goiter and impaired brain function leading to cretinism. An increased frequency of thyroid-stimulating hormone (TSH) measurements above 5 mlU/L on newborn screening points toward an impaired iodine status of the population.
Methods
A 13-year retrospective analysis was performed in 228,266 newborns participating in the national thyroid newborn screening program. The TSH concentration was measured in dry blood spots collected by heel stick on filter paper, 48 hours after birth, using fluoroimmunometric DELFIA method.
Results
Out of 236,378 live-born infants, 228,266 (96.6%) have been screened for TSH, of which 198,213 (86.8%) were retrospectively evaluated for TSH levels above 5 mlU/L. Neonates with congenital hypothyroidism, prematurity, and low birth weight were excluded from the evaluation, as well as the inadequately sampled neonates (13.2%). A national prevalence of 3.08% newborns (n=6,105) with TSH > 5 mIU/L was found. Higher percentages were noted in two regions of the country, indicating possible mild iodine deficiency in these regions and shifting the overall average to above 3%.
Conclusions
Our results indicate overall iodine sufficiency in the Macedonian population. Additional assessment of the iodine intake in the regions with suspected mild iodine deficiency is needed to prevent suboptimal cognitive and psychomotor outcomes.
Keywords: iodine status, newborn screening, thyroid-
Kratak sadržaj
Uvod
Nedostatak joda je povezan sa strumom i kompromitovanom funkcijom mozga sa konsekutivnim kretenizmom. Povecćana učestalost nivoa tireostimulišućeg hormona (TSH) iznad 5 mU/L dobijena na skriningu novorolenih govori o poremecenom statusu joda u stanovništva.
Metode
Uralena je retrospektivna analiza kod 228,266 no- vorolenčadi koja su tokom proteklih 13 godina bila obuhvaćena nacionalnim neonatalnim tiroidnim skrining programom. Koncentracija TSH je merena u suvim kapima krvi pokupljene iz pete novorolencadi na filter hartiji, 48časova nakon rolenja, koristeći fluoroimunometrijsku DELFIA metodu.
Rezultati
Od 236,378 živorodenih, 228,266 (96,6%) testi- rani su za TSH, od kojih su 198,213 (86,8%) retrospek-tivno analizirani za nivo TSH iznad 5 mU/L. Novorolencčad sa urolenom hipotireozom, preranim rolenjem i niskom porolajnom tezinom, kao i novorolencad sa neadekvatno sakupljenim uzorcima krvi (13,2%) isključena su iz pro - cene. Dobijena je nacionalna prevalencija od 3,08% novorolenčadi (n=6,106) sa TSH >5 mU/L. Veći procenat od prosečnog je zabeležen u dva regiona u zemlji, ukazujući namoguć blagi nedostatak joda u ovim regionima.
Zaključak
Naži rezultati ukazuju na sveukupnu dovoljnost joda kod makedonskog stanovništva. Dodatna procena unosa joda u regionima sa sumnjom na blagi nedostatak joda je potrebna da bi se omogucio normalan kognitivni i psihomotorni razvoj kod dece.
Introduction
More than 1.9 billion people worldwide, including 285 million children, have an inadequate iodine intake (1).Iodine is necessary for the production of thyroid hormones which are essential for normal brain development, especially for the fetus and newborn who are particularly vulnerable to iodine deficiency (2). According to the World Health Organization (WHO), iodine deficiency is the main cause of preventable brain damage, producing a spectrum of neurological defects and intellectual impairment (3). Consequently, endemic goiter, hypothyroidism, cretinism and congenital anomalies are termed the iodine deficiency disorders – IDD (4). Neonatal thyroid-stimulating hormone (TSH) whole blood levels, median urinary iodine excretion, thyroid size and blood thyroglobulin concentration have been recommended as indicators for monitoring iodine status in the population (5–7). Frequency below 3% of TSH concentrations exceeding 5 mlU/L on neonatal thyroid screening indicates iodine sufficiency, frequency between 3 and 19.9% indicates mild iodine deficiency, frequency of 20–39.9% indicates moderate deficiency and frequency above 40% indicates severe iodine deficiency, according to the recommendations of the WHO (4, 6, 7).
In the past, Macedonia used to be an iodine deficient area, with a high incidence of goiter, which, in certain regions, was endemic (8). Mild to moderate iodine deficiency in Macedonia was detected in 1995/96, in a national survey conducted among school children, when median urinary iodine concentrations of 117 µg/L and goiter incidence of 18.7% were detected (9). New salt iodination regulations, salt iodized at 20–30 mg iodine per kg salt, were introduced and became effective in October 1999. Subsequent surveys were performed in 1999, 2000 and 2001 (10). Median urinary iodine excretion (UIE) of 241 mg/L and goiter incidence of 0.99% in 2007 indicated elimination of IDD as a public health problem in school children. The International Council for Control of Iodine Deficiency Disorders (ICCIDD) issued a certificate of iodine sufficiency for Macedonia (10).
This was the first report on the assessment of iodine status in the whole country, as well as by regions, using whole-blood spot TSH concentrations obtained through newborn screening.
Materials and Methods
Patients
Out of 236,378 live births in the country during a 13-year period (April 2002 – December 2014), 228,266 newborns (96.6%) have been screened for TSH in dry blood spots collected by heel stick on filter paper (Whatman 903, LKB Vertriebs GmbH, Vienna, Austria), 48 hours after birth. TSH concentrations are expressed as mIU/L whole blood. The blood samples were collected in 31 public and private birth centers all over the country (with approximately 24 000 births per year), and mailed to the screening laboratory every second day. The screening center within the University Children’s Hospital in the capital of Macedonia is responsible for conducting the national newborn thyroid screening program, which became mandatory in 2007, after 5 years as a pilot study. Of all the screened neonates, 198,213 (86.8%) were retrospectively evaluated for TSH concentrations above 5 mIU/L. Neonates with congenital hypothyroidism (CH), low birth weight or prematurity, as well as early or inadequate sampling (13.2%)were excluded from the study. All newborns with a birth weight lower than 2,500 g and/or less than 37 weeks gestational age were classified as premature neonates. The TSH cutoff value on screening was 10 mIU/L of whole blood. Therefore, whenever a TSH concentration on the Guthrie card was lower than 10 mIU/L of whole blood, it was considered negative for congenital hypothyroidism and no further action was pursued. Second card with dry blood spots was requested whenever TSH concentrations between 10 and 20 mIU/L were detected. Newborns with initial screening TSH concentrations higher than 20 mIU/L were suspicious for CH and, after confirmation of diagnosis by serum TSH measurement and clinical evaluation, were excluded from the study. For better assessment of the iodine status in different parts of the country, the study focused on each of the 8 separate regions (Skopje, Polog, Vardar, Pelagonia, Northeastern, Eastern, Southeastern and Southwestern Region) in Macedonia. The study was approved by the local Ethical Committee.
Measurements
A sensitive time-resolved DELFIA fluoroimmunoassay was applied to measure neonatal TSH concentration in dried blood spots, using a DELFIA neonatal hTSH (human TSH) kit, manufactured by Wallac Oy, Turku, Finland. The assay principle is based on a reaction between two monoclonal antibodies (derived from mice) and two separate antigenic determinants on the hTSH molecule, as described previously (11, 12). The analytical sensitivity of the DELFIA Neonatal TSH assay is 2 mU/L blood. Intra-assay and inter-assay were used for assessment of the accuracy of the TSH test. Intra-assay coefficients of variation at TSH concentrations of 15, 23.7 and 66.1 mIU/L were 7%, 7.8%, and 7.5% respectively, while the inter-assay coefficients of variation at the same TSH concentrations were 8%, 9%, and 7.9% respectively. TSH concentrations were read on a 1420 VICTOR 2 D Fluorometer, Wallac Oy, Turku, Finland. Quality control of the national newborn TSH screening program was conducted quarterly and certified by ≫Referenzinstitut für Bioanalytik≪, Bonn, Germany (www.dgkl-rfb.de).
Statistical analysis
Data were analyzed using simple descriptive statistics such as frequency and percentages, by the statistical software SPSS version 20 (Chicago, IL, USA).
Results
Between April 2002 and December 2014, a total of 228,266 newborns underwent newborn thyroid screening (coverage 96.6%). To assess iodine intake in the population, 198,213 (86.8%) of the screened newborns were retrospectively evaluated for TSH concentrations greater than 5 mlU/L. Among them, 104,458 (52.7%) were males and 93,755 (47.3%) were females, with a male to female ratio of 1.114. Average concentration of 6.24±1.23 mlU/L whole blood was noted in the analyzed samples with TSH>5 mlU/L (Table I). TSH concentrations exceeding 5 mlU/L were found in 6,105 neonates with a national prevalence of 3.08%. The frequency of TSH>5 mlU/L varied throughout the study period. It ranged from 1.34% in 2005 to 5.88% in 2003.Moreover, the frequency of TSH values above 5>mlU/L varied in different regions of the country. Whereas in most regions it ranged between 1% and 3%, it was higher than 3% in two regions, Polog (5.03%) and Vardar (5%), indicating possible mild iodine deficiency in these regions (Figure 1).
Table I.
Distribution of neonatal TSH levels > 5 mIU/L in Macedonia from 2002 to 2014.
| Year | n | TSH mean (SD) | % of TSH measurements |
|---|---|---|---|
| >5 mlU/L | |||
| *2002 | 6 004 | 6.73 (1.72) | 4.26 |
| *2003 | 8 213 | 6.32 (1.31) | 5.88 |
| *2004 | 10 239 | 5.81 (0.80) | 2.74 |
| *2005 | 8 509 | 5.77 (0.76) | 1.34 |
| *2006 | 16 407 | 5.92 (0.91) | 1.75 |
| 2007 | 18 162 | 5.86 (0.85) | 2.48 |
| 2008 | 18 206 | 6.49 (1.48) | 3.18 |
| 2009 | 18 684 | 6.31 (1.20) | 2.95 |
| 2010 | 18 686 | 6.38 (1.33) | 2.88 |
| 2011 | 18 259 | 6.30 (1.26) | 3.29 |
| 2012 | 19 013 | 6.29 (1.14) | 3.31 |
| 2013 | 18 764 | 6.16 (1.11) | 2.85 |
| 2014 | 19 067 | 6.72 (1.71) | 3.12 |
| Total | 198 213 | 6.24 (1.23) | 3.08 |
n – number of newborns evaluated for TSH > 5 mlU/L;
TSH – thyroid-stimulating hormone; TSH mean – average concentration of the analyzed samples with a TSH level of > 5 mlU/L whole blood;
pilot study
Figure 1.
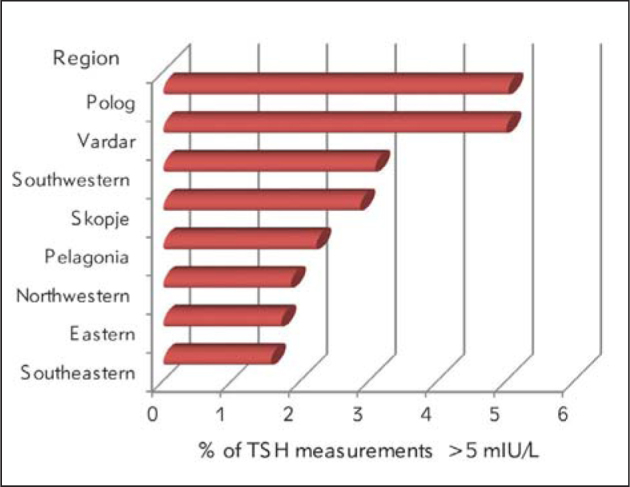
Frequency of neonatal TSH concentrations > 5 mIU/L by regions (2002–2014)
Discussion
The management of iodine deficiency allows for the prevention of possible intellectual impairment in regions with poor iodine intake (6, 7). A growing number of countries use the neonatal TSH screening results above 5 mlU/L as a universal indicator for tracking iodine deficiency in a population, as well as its severity, disappearance or reemergence data (13–16). Since the newborn thyroid gland is very sensitive to iodine deficiency, and an elevated TSH level at birth signifies that the thyroid hormone supply to the developing brain cells is insufficient (17), the neonatal TSH screening is a valuable test to predict possible cognitive and psychomotor impairments in a population (7). Furthermore, it is covered in the screening program costs, avoiding any additional expenses. In Macedonia, it is even more applicable since there is only one screening center using one assay method, enabling centralized evaluation.
In the present study, 3.08% neonates with TSH concentrations above 5 mlU/L were detected during a 13-year period, indicating iodine sufficiency in Macedonia. It was around or below 3% for all study years with the exception of the first two years, when the newborn screening was being introduced. The findings were not surprising because of the new salt iodination regulations which were instituted and became effective in 1999 (9). Median urinary iodine excretion (UIE) of 241 µg/L and the goiter incidence of 0.99% in 2007 indicated elimination of IDD as a public health problem in school children (10). Our results have confirmed general iodine sufficiency in Macedonia, according to WHO/UNICEF/ICCIDD guidelines, certified by the ICCIDD (10). An unexpected finding was a prevalence of TSH concentrations >5 mIU/L higher than 3% in two regions of the country, Polog and Vardar. Additionally, the incidences of congenital hypothyroidism of 1/1520 in the Polog region and 1/1045 in the Vardar region were significantly higher than the overall incidence in the country (1/2486) for the same period, due to a higher number of cases (40%) with transient CH unpublished data). These data indicate possible mild iodine deficiency in these regions. Furthermore, some environmental factors may play a role for the higher percentage of slightly elevated TSH levels in the samples from Polog and Vardar because both provinces are placed in the valley of the river Vardar.
Since Macedonia was an iodine deficient area in the past and mild to moderate iodine deficiency was detected in 1995/96 (9), a detailed assessment program of iodine deficiency by evaluation of urinary iodine concentration among school children in these regions is warranted. Mild iodine deficiency impairs intellectual development and can reduce the average population cognitive scores by 10–15% (18). Though neonatal TSH screening is a universal indicator of the severity of iodine deficiency, the proposed TSH cutoff level of 5 mIU/L has been regarded as not sensitive enough to detect mild iodine deficiency (17, 19–23). Since frequency of TSH results > 5 mIU/L below 3% has been found in populations with mild iodine deficiency (17), the frequency below 3% which indicates iodine sufficiency should be reevaluated (24).
In conclusion, our study has confirmed iodine sufficiency in Macedonia. The whole-blood spot national neonatal TSH screening database is a valuable indicator for monitoring the population iodine status over time. Additional analysis for confirmation of suspected regional mild iodine deficiency, as well as national policies and good practices in food supplementation for achieving adequate iodine nutrition are necessary.
Acknowledgements
Thanks to the Departments of Neonatology in all the birth centers in the country for their cooperation with the blood spots sampling.
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.de Benoist B, Andersson M, Takkouche B, Egli I.. Prevalence of iodine deficiency worldwide. Lancet. 2003;(362):1859–60. doi: 10.1016/S0140-6736(03)14920-3. [DOI] [PubMed] [Google Scholar]
- 2.Assessment of iodine deficiency disorders and monitoring their elimination. World Health Organization; Geneva: 2001. World Health Organization, United Nations Children’s Fund, International Council for Control of Iodine Deficiency Disorders. [Google Scholar]
- 3.Iodine status worldwide. World Health Organization; Geneva: 2004. World Health Organization. [Google Scholar]
- 4.International council for the control of iodine deficiency disorders: Indicators for assessing iodine deficiency disorders and their control through salt iodization. World Health Organization, United Children’s Fund. World Health Organization; Geneva: 1994. [Google Scholar]
- 5.Delange F.. Neonatal screening for congenital hypothyroidism: results and perspectives. Horm Res. 1997;(48):51–61. doi: 10.1159/000185485. [DOI] [PubMed] [Google Scholar]
- 6.Delange F.. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl. 1999;(88):21–4. doi: 10.1111/j.1651-2227.1999.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 7.Delange F.. Screening for congenital hypothyroidism used as an indicator of the degree of iodine deficiency and of its control. Thyroid. 1998;(8):1185–92. doi: 10.1089/thy.1998.8.1185. [DOI] [PubMed] [Google Scholar]
- 8.Karanfilski B. Results of iodine prophylaxis in Macedonia. 4 Vol. 8. IDD Newsletter November; 1992. International Council for Control of Iodine Deficiency Disorders. [Google Scholar]
- 9.Karanfilski B, Bogdanova V, Vaskova O, Loparska S, Miceva-Ristevska S, Sestakov G. et al. Correction of iodine deficiency in Macedonia. J Pediatr Endocrinol Metab. 2003;(16):1041–5. doi: 10.1515/jpem.2003.16.7.1041. [DOI] [PubMed] [Google Scholar]
- 10.Karanfilski B, Bogdanova V, Vaskova O, Miceva-Ristevska S, Loparska S, Kuzmanovska S. Macedonia begins to monitor IDD in pregnant and lactating women along with school-age children. 3. Vol. 33. IDD Newsletter August; 2009. International Council for Control of Iodine Deficiency Disorders; pp. 17–9. [Google Scholar]
- 11.Soini E, Kojola H.. Time-resolved fluorometer for lanthanide chelates – a new generation of nonisotopic immunoassays. Clin Chem. 1983;29(1):65–8. [PubMed] [Google Scholar]
- 12.Kocova M, Anastasovska V, Sukarova-Angelovska E, Tanaskoska M, Taseva E.. Clinical Practice: Experience with newborn screening for congenital hypothyroidism in the Republic of Macedonia–a multiethnic country. Europ J Pediatr. 2015;174(4):443–8. doi: 10.1007/s00431-014-2413-4. [DOI] [PubMed] [Google Scholar]
- 13.Costante G, Grasso L, Ludovico O, Marasco MF, Nocera M, Schifino E. et al. The statistical analysis of neonatal TSH results from congenital hypothyroidism screening programs provides a useful tool for the characterization of moderate iodine deficiency regions. J Endocrinol Invest. 1997;(20):251–6. doi: 10.1007/BF03350296. [DOI] [PubMed] [Google Scholar]
- 14.Mikelsaar RV, Viikmaa M.. Neonatal thyroid-stimulating hormone screening as an indirect method for the assessment of iodine deficiency in Estonia. Horm Res. 1999;(52):284–6. doi: 10.1159/000023496. [DOI] [PubMed] [Google Scholar]
- 15.Gyurjyan RH, Lugovska R, Vevere P, van der Haar F.. Newborn thyrotropin screening confirms iodine deficiency in Latvia. Eur J Clin Nutr. 2006;(60):688–90. doi: 10.1038/sj.ejcn.1602364. [DOI] [PubMed] [Google Scholar]
- 16.Kung AW, Lao TT, Low LC, Pang RW, Robinson JD.. Iodine insufficiency and neonatal hyperthyrotropinaemia in Hong Kong. Clin Endocrinol (Oxf) 1997;(46):315–9. doi: 10.1046/j.1365-2265.1997.1310960.x. [DOI] [PubMed] [Google Scholar]
- 17.Vandevijvere S, Coucke W, Vanderpas J, Trumpff C, Fauvart M, Meulemans A. et al. Neonatal Thyroid-Stimulating Hormone Concentrations in Belgium: A Useful indicator for Detecting Mild Iodine Deficiency? PLoS ONE. 2012;7(10):e47770. doi: 10.1371/journal.pone.0047770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maberly G.. Iodine deficiency. WHO Bull. 1998;(76):118–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Rajatanavin R.. Iodine deficiency in pregnant women and neonates in Thailand. Public Health Nutrition. 2007;(10):1602–5. doi: 10.1017/S1368980007360990. [DOI] [PubMed] [Google Scholar]
- 20.Gruneiro-Papendieck L, Chiesa A, Mendez V, Bengolea S, Prieto L.. Neonatal TSH level as an index of iodine sufficiency: differences related to time of screening sampling and methodology. Horm Res. 2004;62(6):272–6. doi: 10.1159/000081786. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Eastman JC.. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in population? Best Pract Res Clin Endocrinol Metab. 2010;(24):63–75. doi: 10.1016/j.beem.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Lampret RB, Murko S, Tanšek ŽM, Podkrajšek TK, Debeljak M, Šmon A, Battelino T.. Selective screening for metabolic disorders in THE Slovenian pediatric population. J Med Biochem. 2015;(34):58–63. doi: 10.2478/jomb-2014-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridefelt P.. Population-based pediatric reference intervals in general clinical chemistry: a swedish survey. J Med Biochem. 2015;(34):64–65. doi: 10.2478/jomb-2014-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trumpff C, Vanderfaeillie J, Vercruysse N, De Schepper J, Tafforeau J, Van Oyen H. et al. Protocol of the PSYCHOTSH study: association between neonatal thyroid stimulating hormone concentration and intellectual, psychomotor and psychosocial development at 4-5 year of age: a retrospective cohort study. Arch Public Health. 2014;(72):27–34. doi: 10.1186/2049-3258-72-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


