Summary
Background
Until now, a proper biomarker(s) to evaluate sarcoidosis activity has not been recognized. The aims of this study were to evaluate the sensitivity and specificity of the two biomarkers of sarcoidosis activity already in use (serum angiotensin converting enzyme – ACE and serum chitotriosidase) in a population of 430 sarcoidosis patients. The activities of these markers were also analyzed in a group of 264 healthy controls.
Methods
Four hundred and thirty biopsy positive sarcoidosis patients were divided into groups with active and inactive disease, and groups with acute or chronic disease. In a subgroup of 55 sarcoidosis patients, activity was also assessed by F-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scanning. Both serum chitotriosidase and ACE levels showed non-normal distribution, so nonparametric tests were used in statistical analysis.
Results
Serum chitotriosidase activities were almost 6 times higher in patients with active sarcoidosis than in healthy controls and inactive disease. A serum chitotriosidase value of 100 nmol/mL/h had the sensitivity of .5% and specificity of 70.0%. A serum ACE activity cutoff value of 32.0 U/L had the sensitivity of 66.0% and the specificity of 54%. A statistically significant correlation was obtained between the focal granulomatous activity detected on 18F-FDG PET/CT and serum chitotriosidase levels, but no such correlation was found with ACE. The levels of serum chitotriosidase activity significantly correlated with the disease duration (P < 0.0001). Also, serum chitotriosidase significantly correlated with clinical outcome status (COS) categories (ρ =0.272, P =0.001).
Conclusions
Serum chitotriosidase proved to be a reliable biomarker of sarcoidosis activity and disease chronicity.
Keywords: sarcoidosis, serum chitotriosidase, serum angiotensin converting enzyme, biomarker
Kratak sadržaj
Uvod
Do sada, nisu nađeni odgovarajući biomarkeri za procenu aktivnosti sarkoidoze. Ciljevi studije su bili: procena osetljivosti i specifičnosti dva već korišćena biomarkera aktivnosti sarkoidoze (serumskog angiotenzin-konvertujućeg enzima (ACE) i serumske hitotriozidaze) u populaciji od 430 bolesnika sa sarkoidozom. Ovi markeri su takođe analizirani u kontrolnoj grupi od 264 zdrava ispitanika.
Metode
Četiristo trideset bolesnika sa sarkoidozom koja je dokazana biopsijom podeljeno je u grupe sa aktivnom i neaktivnom bolešću i u grupe sa akutnom i hroničnom sarkoidozom. U podgrupi od 55 bolesnika, aktivnost sarkoidoze je procenjivana i F18 fluorodeoksiglukoznom pozitronskom emisionom tomografijom (18F-FDG-PET). Nivoi serumske hitotriozidaze i ACE nisu pratili normalnu distribuciju, tako da su u statističkoj analizi korišćeni neparametrijski testovi.
Rezultati
Nivoi serumske hitotriozidaze su skoro 6 puta bili viši kod bolesnika sa aktivnom sarkoidozom u odnosu na kontrolnu grupu i na bolesnike sa neaktivnom bolešću. Nivo serumske hitotriozidaze od 100 nmol/mL/h pokazao je osetljivost od 82,5% i specifičnost od 70,0%. Cut off vrednost od 32,0 U/L za aktivnost ACE u serumu pokazala je osetljivost od 66,0% i specifičnost od samo 54,0%. Statistički značajna povezanost je nađena između fokalne granulomatozne aktivnosti koja je detektovana pomoću 18F-FDG PET/CT i nivoa serumske hitotriozidaze, dok takva korelacija nije nađena sa ACE. Nivoi serumske hitotriozidaze su bili u značajnoj korelaciji sa trajanjem bolesti (P < 0,0001). Serumska hitotriozidaza je bila značajno povezana sa kategorijama kliničkog ishoda (COS) (ρ = 0,272, P = 0,001).
Zaključak
Aktivnost hitotriozidaze u serumu je dokazano pouzdan biomarker za procenu aktivnosti i hroniciteta sarkoidoze.
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown etiology and unpredictable course (1). The unpredictable clinical course of sarcoidosis has encouraged the research of biomarkers useful for predicting disease activity and the outcome. Severity of sarcoidosis is routinely evaluated by a staging system based on the appearance of the chest X ray, supported by pulmonary function tests and evaluation of extrapulmonary disease (2). Significant efforts have been made for decades in order to identify biochemical parameters that can support the evaluation of disease activity (3–4).
Until recently, the increase in angiotensin-converting enzyme (ACE) has been the most frequently used laboratory test in sarcoidosis. The enzyme is also supposed to give indication of total body granuloma burden (7–8). However, the value of elevated serum ACE in sarcoidosis is still a matter of debate. Determination of serum ACE has an estimated sensitivity of only 57% (9). Especially in the first months of acute disease, ACE levels may be within the normal range. Despite the fact that the ACE is the most frequently used marker of sarcoidosis activity, the diagnostic and prognostic usefulness of the serum ACE remains uncertain. In a study of 1941 patients with sarcoidosis, 1575 healthy control subjects, and 1355 patients with other diseases, the sensitivity of elevated serum ACE for the diagnosis of sarcoidosis was 57%, the specificity 90%, positive predictive value 90%, but negative predictive value only 60% (10).
Polymorphisms of the ACE gene may lead to changes in the serum ACE level in both normal control subjects and patients with sarcoidosis. The polymorphism with insertion (I) or deletion (D) of a portion of the gene affects the enzyme activity, so that the subjects with DD have higher serum ACE levels than those with II polymorphism (11–12). Given the fact that I/D allele frequencies can differ greatly between ethnicities, genotype corrected reference values for ACE should be established locally (13).
The correlation between serum ACE levels and sarcoidosis activity or even response to treatment seems to be relatively poor (14). These limitations of serum ACE as the disease marker have stimulated an ongoing search for better ones. Regarding the crucial role of chronically activated macrophages in the pathogenesis of sarcoidosis, chitotriosidase – a new potential marker of the disease was introduced for the first time in 2004 by Grosso et al. (15).
The enzyme chitotriosidase is originally involved in the degradation of chitin; however, the enzyme is also functional in humans (15–16), although its exact physiological function in humans still remains uncertain (17–18). It has been already evaluated that in pathological conditions, chitotriosidase is considered a serum marker of macrophage activation. In these cases pathological tissue macrophages produce an additional amount of chitotriosidase as observed in a number of lysosomal storage diseases i.e. Gaucher syndrome (4–6).
The lack of reliable parameters for monitoring sarcoidosis activity led to a hypothesis that serum chitotriosidase may be a dependable parameter in identifying patients with active disease (4, 15–18). The idea of detecting serum chitotriosidase in patients with sarcoidosis originated from the evidence of direct involvement of activated macrophages in the pathogenesis of sarcoidosis and granulomatous inflammation (15–18).
A group of authors from Siena University has noticed a significantly higher activity of chitotriosidase in the serum of patients with sarcoidosis compared with controls; higher activity of serum chitotriosidase was also reported to be more elevated in active sarcoidosis than in patients with inactive disease. Significant correlations with the radiographic stages of lung sarcoidosis, radiological CT features, BAL cells characteristics, suggested that the enzyme could be a marker of disease severity (15–16).
The first objective of this study was to evaluate the utility of serum chitotriosidase in assessment of sarcoidosis activity and to correlate it with other markers of disease activity i.e. chest X ray, number of organs involved, disease duration and for the first time, F-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) scan findings. The second objective of this study was to correlate serum chitotriosidase levels in the largest until now cohort of sarcoidosis patients with the serum ACE levels, in order to assess which marker the clinicians can rely on better when determining sarcoidosis activity.
Material and Methods
Study design
In a large cohort of 430 biopsy positive sarcoidosis patients, the associations between serum chitotriosidase activity and ACE serum levels were tested in correlation with radiographic stage of sarcoidosis, number of organs involved and disease duration. It is conceivable that the extent of involvement and quantification of sarcoidosis activity can be accurately assessed by F-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scanning (19–21), so we also correlated serum chitotriosidase levels in a subgroup of 55 sarcoidosis patients, with the disease activity resulting from PET scanning examination.
All patients included in this study were diagnosed in the Clinic of Pulmonary Diseases, Clinical Center of Serbia, Belgrade, Serbia. The examined population included 299 females and 131 males, 23 to 77 years old. Healthy control group consisted of 264 subjects matched for age and gender with patients’ group. This study was approved by the institution’s ethics committee and all patients consented to participate.
Definition of activity
Activity of sarcoidosis was defined by the presence of clinical symptoms such as cough, dyspnea, chest pain or tightness of the chest, fever, bone or joint pains and fatigue, together with or without pathologic chest X ray findings. Activity was also suggested in clinically asymptomatic patients, but with absolute and marked roentgenographic worsening. Additionally, patients with major manifestations such as recently developed skin lesions, parotid or ocular involvement, peripheral lymphadenopathy, or deterioration of pulmonary function tests, were considered to have active disease. Inactive sarcoidosis was suggested in cases with absent clinical symptoms together with a negative or nonprogressive chest X-ray.
According to these criteria, at the time of the study, 230 patients were considered to have active sarcoidosis, while 200 patients had inactive sarcoidosis. Patients complained of more than one symptom, including cough 18% (41/230), chest pain or tightness of the chest 35% (80/230), fever 12% (27/230), bone pain 33% (76/230), joint pain or stuffiness 22% (51/230), and fatigue 33% (76/230).
Disease duration
Regarding disease duration, patients were also divided into a group of acute sarcoidosis (147 patients) and a group of chronic sarcoidosis (283 patients). The mean duration of sarcoidosis was 5.69±6.79 years, ranging from 6 months to 33 years. Acute sarcoidosis was defined as disease that persisted for less than two years. Frequently, acute sarcoidosis had an abrupt onset with symptoms including erythema nodosum, polyarthralgia, bilateral hilaradenopathy and occasionally diffuse parenchyma infiltration, but the disease usually remitted spontaneously (22). In contrast, chronic sarcoidosis was defined as disease with both symptoms and signs of sarcoidosis activity and parameters of the activity (measuring serum ACE and hypercalciuria) unremitting for more than two years. Unfortunately for these patients, therapy tends to only relieve symptoms and rarely leads to resolution of structural abnormalities (22).
The World Association of Sarcoidosis and Other Granulomatous disease (WASOG) established a task force to provide a better definition of clinical outcome in sarcoidosis (COS) which takes into account the variable patterns of outcome with/without therapy. The clinical outcome of patients was examined five years after diagnosis of sarcoidosis was established (23). The number of patients with an adequate follow-up (at least 5 years) was 159. They were classified in subgroups according to clinical outcome status – COS (23). The classification ranges from COS-1Ø(0/159) – patients in complete remission, not requiring therapy in the last year and never treated, and those not requiring therapy in the last year but previously treated – COS-2 (7/159); to those with minor involvement, defined as less than 25% of lung involvement at chest x-ray, not needing therapy in the last year, who were also classified into COS-3 (0/159)– never treated, and COS-4 (26/159) – not treated in the last year. Patients with persistent disease not on systemic therapy were divided into COS-5 (0/159) – never treated, and COS-6 (19/159) – not treated in the last year. Patients with persistent disease treated systemically were classified into COS-7 (58/159) – asymptomatic patients without clinical functional deterioration in the last year, COS-8 (14/159) – symptomatic patients without clinical functional deterioration in the last year, and COS-9 (35/159) – patients with clinical functional deterioration in the last year.
In order to clarify the extent of organ involvement and quantification of sarcoidosis, a subgroup of 55 patients underwent 18F-FDG PET/CT examination on 64 slice hybrid PET/CT scanner (Siemens Biograph, Siemens Medical Solutions USA Inc., Hoffman Estates, Illinois, USA). Patients were chosen according to following criteria: chronicity, radiographic progression and multiorgan involvement. Findings of 18F-FDG PET/CT were scored as positive or negative for inflammation. The findings were considered positive in case of increased 18F-FDG uptake above blood vessels in the mediastinum and/or lung parenchyma, or in extrathoracic sites, including lymph nodes, visceral organs (like parotid, liver, spleen), nasopharynx, skin, muscle, or bone (20). Quantitative analysis of 18F-FDG uptake in the lesion was based on maximum standardized uptake value (SUVmax) per focus. The SUVmax was calculated as the activity concentration measured at the end of the scan and corrected for individual body weight and dose injected, as follows: SUVmax = Tissue Activity (counts/pixel/second) × Calibration Factor / Injected 18F-FDG Dose (MBq/ kilogram body weight) (21).
Serum ACE and serum chitotriosidase measurements
Blood samples for chitotriosidase and ACE serum levels were obtained from all patients. Patients included into this study were not taking medications that interfere with the renin–angiotensin–aldosterone system i.e. ACE inhibitors or angiotensin II receptor antagonists. Chitotriosidase activity in serum was determined using the developed and validated method with fluorogenic substrate 4-methyl-umbelliferyl-β-D-N-N′-N″-triacetylchitotrioside (Sigma Chemical Co, St. Louis, MO) in Mcllvaine’s citrate-phosphate buffer, pH 5.2, at 37 °C (24). Fluorescence was measured on an SPF-500TMC spectrofluorometer (SLM Instruments, Inc.). ACE activity was measured by the spectrophotometric method adapted in the laboratory utilizing synthetic tripeptide substrate N-[3-(2-furyl) acryloyl]-L phenylalanylglycylglycine, using a commercial test kit (Trinity Biotech, St. Louis, USA), on an Olympus AU 2700 automated analyzer (Beckman Coulter Biomedical Ltd.).
Patients
All patients had pulmonary sarcoidosis and 120 (28%) had extrapulmonary sarcoidosis, with different organs involvement. Chest X ray classification was done using Scadding’s modified staging system: stage 0 – no adenopathy or infiltrates; stage 1 – hilar and mediastinal adenopathy alone; stage 2 – adenopathy and pulmonary infiltrates; stage 3 – pulmonary infiltrates alone; and stage 4 – pulmonary fibrosis (25). Using the ACCESS organ index, we assessed the extent of the current sarcoidosis organ involvement (26–27). The diagnosis of sarcoidosis in any organ system was defined as definite, probable or possible. In this study, positive organ involvement included definite or probable criteria from the ACCESS study index (26–27). Regarding the treatment, at the time of the study, no treatment was required in 154/430 patients (36%), while others were treated regarding the stage of the lung disease, different organs involvement, symptoms and pulmonary function tests. Here, a group of patients with chronic sarcoidosis (46) was included. These were patients without significant symptoms suggesting sarcoidosis activity but treated with morbostatic doses of immunosuppressive therapy in order to prevent further deterioration of the chronic persistent disease.
Statistical methods
Since both serum chitotriosidase and ACE levels were not normally distributed (as revealed by Kolmogorov Smirnov and Shapiro-Wilk tests of normality), the nonparametric tests were used in statistical analysis (Kruskal-Wallis test and Spearman’s correlation), and results were presented as median values with interquartile ranges (IQR). Statistical analysis was performed using SPSS v 15.0 software (IBM, Chicago, Illinois, USA). ROC analysis and graphic representations of data were conducted using GraphPad Prism Version 4.0 software for Windows (GraphPad Software, San Diego, California, USA).
Results
Clinical characteristics of study participants are summarized in Table I. Table II represents serum ACE and chitotriosidase levels in healthy controls and patients with different forms of sarcoidosis. Both serum markers, ACE and chitotriosidase levels, showed no correlation with the age series of the analyzed population. Significantly higher were both serum levels of chitotriosidase and ACE in male patients (Table I).
Table I.
Clinical characteristics of patients.
| Serum ACE levels (U/L) | Serum chitctriosidase levels (nmol/mL/h) | ||||||
|---|---|---|---|---|---|---|---|
| Clinical characteristics of patients | N | Median | Interquartile range | P* | Median | Interquartile range | P* |
| Gender | |||||||
| Female | 297 | 35 | 20–51 | 0.0094 | 116.1 | 51.3–235.6 | 0.0811 |
| Male | 132 | 40 | 28–59 | 139.2 | 73.9–259.2 | ||
| Activity | |||||||
| Active | 230 | 43 | 26–62 | <0.0001 | 224.2 | 139.2–394.9 | <0.0001 |
| Inactive | 199 | 30 | 20–44 | 56.4 | 27.3–111.8 | ||
| Clinical course of sarcoidosis | |||||||
| Acute | 146 | 39 | 20–56 | 0.1534 | 141.3 | 54–259.9 | 0.2996 |
| Chronic | 283 | 36 | 20–51 | 115.4 | 51.3–236.9 | ||
| Stage of the lung disease | |||||||
| Stage 0 | 28 | 38 | 24–46 | 51.2 | 21.6–92.7 | ||
| Stage 1 | 204 | 36 | 22–54 | 0.7326 | 129.61 | 52.6–245.1 | 0.0001 |
| Stage 2 c | 187 | 39 | 21–56 | 137.71 | 54.8–256.8 | ||
| Stage 3 | 10 | 47 | 23–54 | 175.81 | 91.8–211 | ||
| Organ involvement | |||||||
| Pulmonary | 309 | 38 | 23–54 | 0.4925 | 129.6 | 53.7–249.9 | 0.1445 |
| Extrapulmonary | 120 | 37 | 19–51 | 105.3 | 51.3–228.4 | ||
| Number of organs involved | |||||||
| 1 | 308 | 38 | 23–54 | 129.6 | 52.6–247.9 | ||
| 2 | 87 | 39 | 20–52 | 0.8560 | 119.9 | 51–234.4 | 0.05667 |
| 3 | 30 | 38 | 19–50 | 83.2 | 51.3–218.7 | ||
| 4 | 4 | 28 | 16–46 | 56.7 | 56.7 (29.7–387.4) | ||
Kruskal-Wallis test, significant difference between subgroups P < 0.05
Significantly different from stage 0 by Conover post hoc test, P < 0.01
Table II.
Serum ACE and chitotriosidase levels in healthy controls and patients with different forms of sarcoidosis.
| Activity of sarcoidosis | Healthy controls | Inactive | Active | P* |
|---|---|---|---|---|
| ACE, U/L | 28 (13–36) | 30 (20–44) | 43 (26–62)1,2 | 0<0.0001 |
| Chitotriosidase, nmol/mL/h | 60 (35–83) | 56.4 (27.3–111.8) | 224.2 (139.2–394.9)1,2 | 0.0078 |
| Clinical course of sarcoidosis | Healthy controls | Chronic | Acute | P* |
| ACE, U/L | 28 (13–36) | 36 (20–51)1 | 39 (25–56)1 | <0.0001 |
| Chitotriosidase, nmol/mL/h | 60 (35–83) | 115.4 (51.3–236.9)1 | 141.3 (54.0–259.9)1 | <0.0001 |
Results are expressed as median (interquartile range).
Kruskal-Wallis test, significant difference between subgroups P < 0.05
Significantly different from healthy controls by Conover post hoc test, P < 0.01
Significantly different from inactive sarcoidosis by Conover post hoc test, P < 0.01
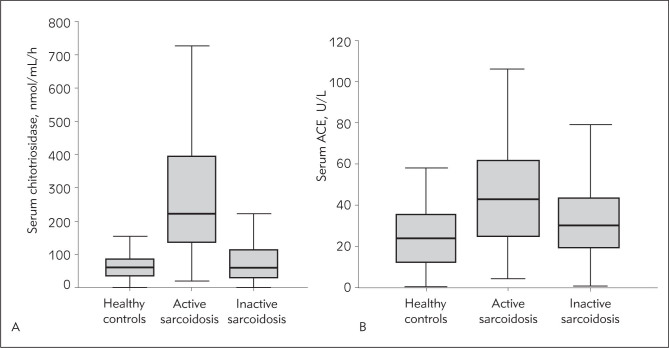
The median value of serum ACE in healthy controls was 28 U/L. Using Kruskal-Wallis test and Conover post hoc test, statistically significant difference was found between healthy controls and sarcoidosis patients with both acute and chronic disease. However, statistically significant difference was found only between healthy controls and active disease, while the difference was not significant between healthy controls and patients with inactive disease. Also, serum ACE levels were significantly different between patients with active and inactive sarcoidosis, but the difference was not significant between acute and chronic sarcoidosis patients (Figure 1).
Figure 1.
Box-plots graphs: A) Serum chitotriosidase in active sarcoidosis, inactive disease and healthy controls. B) Serum ACE values in healthy controls, patients with active and inactive disease.
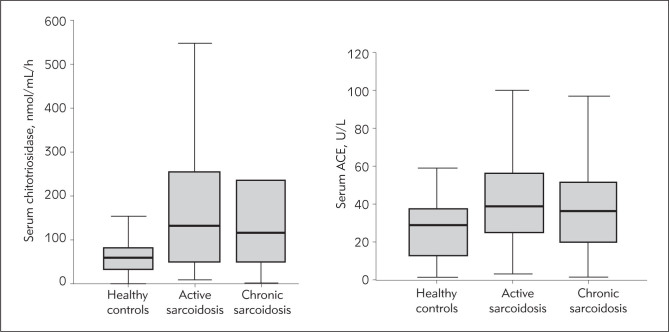
The median value of serum chitotriosidase in healthy controls was 60.0 nmol/mL/h. Kruskal-Wallis test and Conover post hoc test showed statistically significant difference between chitotriosidase levels in healthy controls and patients with both acute and chronic disease, but again the difference was not significant between acute and chronic course of sarcoidosis. On the other hand, besides the statistically significant difference between healthy controls and patients with active and inactive disease, the difference in serum chitotriosidase level was statistically significant between active and inactive sarcoidosis patients (Figure 2).
Figure 2.
Box-plots graphs: A) Serum chitotriosidase in acute versus chronic sarcoidosis and healthy controls. B) Serum ACE in healthy controls and sarcoidosis patients with acute and chronic disease.
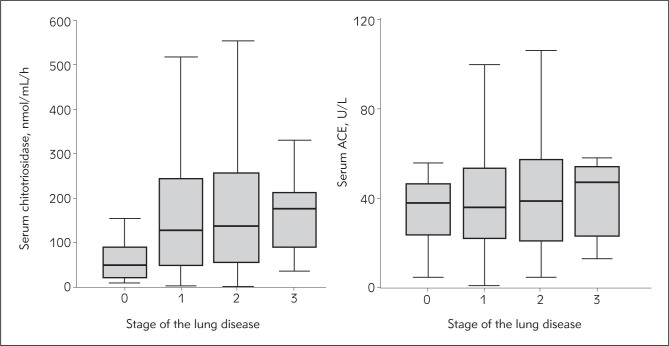
Important difference was noticed between the two potential serum markers (ACE and chitotriosidase) considering sarcoidosis acivity. Investigating the area under the receiver operating characteristic (ROC) curve (AUC) as a performance measure of the potential strength of the analyzed parameters to predict sarcoidosis activity, for chitotriosidase the AUC was 0.870, and for serum ACE it was only 0.643 (Figure 3). A serum chitotriosidase level of 100 nmol/mL/h had 82.5% sensitivity (to confirm active sarcoidosis) and 70% specificity (to exclude active sarcoidosis). Serum ACE cut off value of 32 U/L had the sensitivity of 66% and specificity of 54%. The difference between serum chitotriosidase levels in the different radiographic stages of lung disease was statistically significant, unlike the ACE levels (Figure 4). However, Conover post hoc test revealed that the difference was significant only between stage 0 and later stages of lung sarcoidosis, but not between stages 1, 2 and 3 (Table 1). In total, 159 patients were pheno-typed in COS categories. Serum chitotriosidase levels significantly correlated with COS categories (ρ = 0.272, P = 0.001). However, the correlation between COS categories and serum ACE levels was not significant (ρ = 0.132, P = 0.097).
Figure 3.
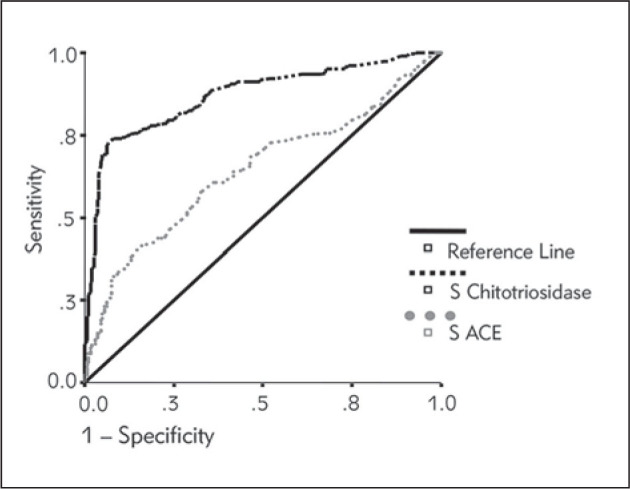
ROC curve: serum chitotriosidase and serum ACE levels.
Figure 4.
Box-plots graphs. The correlation of serum chitotriosidase level with the radiographic stage of lung sarcoidosis.
Analyzing patients with pulmonary and extrapulmonary sarcoidosis, neither serum chitotriosidase nor serum ACE levels were statistically significantly different between these two groups of patients. Also, neither serum chitotriosidase nor serum ACE levels revealed statistically significant difference between the groups of patients with different number of organs involved in sarcoidosis (Table I).
Statistically significant correlation was obtained with the analysis, between the SUV representing sarcoidosis focal granulomatous activity detected on 18F- FDG PET/CT examination and serum chitotriosidase levels (Figure 5). Serum ACE levels showed no correlation with 18F-FDG PET/CT detected sarcoidosis activity (ρ = −0.102, P = 0.459).
Figure 5.
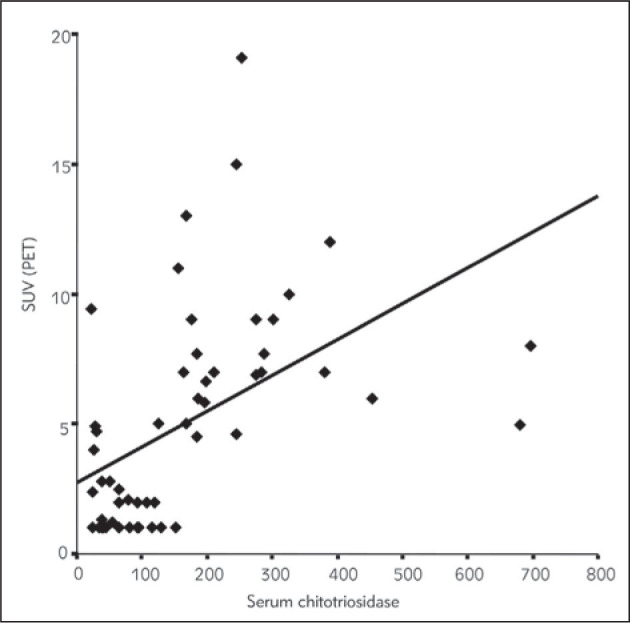
Serum chitotriosidase levels and sarcoidosis activity assumed on 18F-FDG PET/CT.
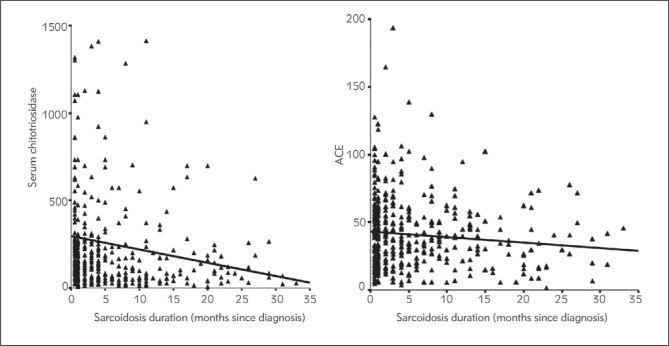
Analyzing sarcoidosis duration, significant negative correlation with the levels of chitotriosidase was found suggesting that longer disease duration correlated with lower serum levels of chitotriosidase (ρ = −0.205, P < 0.0001). No significant correlation was estimated between sarcoidosis duration and serum ACE activity (ρ = −0.076, P = 0.1155) (Figure 6).
Figure 6.
Disease duration and S-ACE levels and S-Chitotriosidase levels.
Discussion
The aims of this study were to evaluate the sensitivity and specificity of chitotriosidase in assessment of sarcoidosis activity in a population of 430 sarcoidosis patients, and to correlate it with other markers of disease activity, i.e. radiographic features, number of organs involved, disease duration and for the first time PET/CT findings, as well as to compare it with serum ACE. To our knowledge, this is the largest study done to evaluate the activity of serum chitotriosidase in a sarcoidosis population and healthy controls, comparing it with serum ACE levels.
The study has confirmed the findings of Grosso et al. (15) that individuals with sarcoidosis have higher serum chitotriosidase levels than healthy controls. Similar findings were noted in the study of Bargagli et al. (28) on 232 sarcoidosis patients, where serum chitotriosidase concentrations were significantly higher in patients than in healthy controls and were directly correlated with ACE levels (28).
However, we found serum ACE levels, the most frequently used marker of sarcoidosis activity, also significantly increased in patients with sarcoidosis. Both markers showed significantly higher values in the male patients’ population. The higher serum ACE levels in male adults do not correlate with the previous findings from the literature, but might confirm the fact that the reference values for ACE should be established locally (29, 30).
Comparing the two biomarkers (serum chitotriosidase and ACE levels) of sarcoidosis activity with the chest X ray findings (radiographic staging of sarcoidosis), significant correlation was found between the serum chitotriosidase levels and stage of the lung disease, while no correlation between serum ACE levels and radiographic staging was observed (Figure 4). As it is believable that the radiographic stage of sarcoidosis in general predicts prognosis (31), a positive association between chitotriosidase levels and radiographic stage suggests that chitotriosidase may be an important biochemical marker of valuable prognostic significance. Similar findings were confirmed by several other authors (15, 17, 28, 32). Golab et al. (32) even suggested that because of the correlation with the radiological stages of disease, the determination of chitotriosidase activity might decrease the number of X-ray examinations.
Considering the peak value of serum chitotriosidase and the stage of the lung disease, results from this study differ from the study of Bargagli et al. (17), who found the highest serum chitotriosidase levels in stage 3 of the lung disease. Our data showed the highest serum chitotriosidase values in the stage 2 of the lung disease. This only adds to the still open question: is chitotriosidase the enzyme that induces the over-expression of profibrotic type-2 (Th2) cytokines, the observation which is more in tune with stage 3 of the lung disease, or does it express the activity of the »fresh« granuloma, which is still identifiable in the stage 2 of the lung disease. Analyzing the disease duration, we found significant correlation between serum chitotriosidase levels and sarcoidosis duration. The correlation was negative (Figure 6); the longer the duration, the lower the serum chitotriosidase levels in sarcoidosis. This finding also reflects the fact that in patients with long lasting sarcoidosis, thus evidently the chronic form, the activity of the granulomatous inflammation is very low or even suppressed. These are patients on long lasting immunosuppressive therapy regimes, or at least on morbostatic doses. Unfortunately, chitotriosidase deficiency and carrier frequency occur in 6% and 30–40% of the general population, respectively, due to mutations (33).
Interpretation of plasma chitotriosidase levels is complicated due to the occurrence of the 24 bp duplication in the chitotriosidase gene, which results in an inactive protein in 5–6% of the general population. The carrier prevalence is as high as 30–40% and chitotriosidase activity is half of that in individuals with the wildtype gene (33). The most important difference was noticed between the two potential serum markers (ACE and chitotriosidase) considering sarcoidosis acivity. Our study revealed a high sensitivity of 82.5% for serum levels of chitotriosidase of only 100 nmol/mL/h to confirm active sarcoidosis, and specificity of 70.0% to exclude sarcoidosis activity (Figure 3). (Cut off of 32.0 U/L for serum levels of ACE had the sensitivity of 66.0% and the specificity of 54%.) Similarly, in the study of Bargagli et al. (28) on 232 sarcoidosis patients, ROC curve analysis for chitotriosidase revealed 88.6% sensitivity and 92.8% specificity.
The comparison of 18F-FDG PET/CT findings with the two markers of disease activity, done in a subgroup of 55 patients from the analyzed cohort, revealed most strongly the positive correlation between sarcoidosis activity and serum chitotriosidase levels. This is a unique finding of this study. Inflammatory lesions are metabolically active, and they can change quickly, growing in a short interval of time, and disagreement with the chest X ray may occur; besides the whole body 18F-FDG-PET illuminates the total granulomatous inflammation in individuals with already proven sarcoidosis (Figure 5).
Patients with uncontrolled sarcoidosis (active persistent disease) despite the therapy had very high serum chitotriosidase levels, a finding which is in line with Bargagli et al. (28). Speaking in the words of a recently published new phenotype classification for patients with sarcoidosis (COS) by R Baughman et al. (23), these are patients with persistent disease on steroids and with functional deterioration within the last year. In our study, serum chitotriosidase levels significantly correlated with COS categories; however, the correlation between COS categories and serum ACE levels was not significant. In the study of Bargagli et al. (28) on 232 sarcoidosis patients, the lowest activities of chitotriosidase were found in untreated patients in remission (COS-1), while the highest enzyme activities were found in symptomatic patients with persistent disease on steroids and with functional deterioration in the last year (COS-9). Boot et al. (4) have noted that the increase in plasma chitotriosidase correlated with the stage of disease, being highest in active sarcoidosis with extrapulmonary involvement. Therapy with steroids resulted in clear reduction of plasma chitotriosidase and relapse of disease activity was preceded by increase in this parameter. The correlations of chitotriosidase activity and duration of sarcoidosis may be even stronger if we took chitotriosidase genotype status into account; however, we did not perform chitotriosidase genotyping, which is a limitation of this study (34, 35). A limitation of the study at first glance might also be that we did not analyze our patient group considering the therapy. As described, the majority of our patients were on therapy; due to active sarcoidosis (230 patients) or in order to prevent further deterioration of the chronic persistent disease (46 patients).
Although on therapy, a certain number of patients experienced symptoms of sarcoidosis activity, or activity was suggested in clinically asymptomatic patients, but with unquestionable and marked roentgenographic deterioration, or with major manifestations such as recently developed skin lesions, parotid or ocular involvement, peripheral lymphadenopathy, or deterioration of pulmonary function tests, suggesting disease activity. So, considering only the parameters of disease activity distinguishing them from the treatment, this study succeeded in its major goal to evaluate the utility of these different markers for the assessment of activity in sarcoidosis.
Conclusion
In conclusion, this is the largest study done with the major task to evaluate the significance of serum chitotriosidase in verifying sarcoidosis activity. The study added to the research of other authors in the field of possible biomarkers of sarcoidosis, validating the significance of serum chitotriosidase in assessing not only the activity of sarcoidosis at first glance, but also in investigating the possible disease chronicity.
Acknowledgement
This study was supported by the Serbian Ministry of Education and Science (grants no. 175018, 175046 and 175081).
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Am J Respir Crit Care Med. February 1999;1999;(160):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. Statement on Sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Culver DA, Judson MA.. A Concise Review of Pulmonary Sarcoidosis. Am J Respir Crit Care Med. 2011;(183):573–81. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller-Quernheim J.. Serum markers for the staging of disease activity of sarcoidosis and other interstitial lung disease of unknown etiology. Sarcoidosis Vasc Diffuse Lung Dis. 1998;(15):22–37. [PubMed] [Google Scholar]
- 4.Boot GR, Hollak C, Verhoek M, Alberts C, Jonkers ER, Aerts JM.. Plasma chitotriosidase and CCL18 as surrogate markers for granulomatous macrophages in sarcoidosis. Clinica Chimica Acta. 2010;(411):31–6. doi: 10.1016/j.cca.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Šumarac Z, Suvajdžić N, Ignjatović S, Majkić-Singh N, Janić D, Petakov M, Đorđević M, Mitrović M, Dajak M, Golubović M, Rodić P.. Biomarkers in Serbian patients with Gaucher disease. Clinical Biochemistry. 2011;(44):950–4. doi: 10.1016/j.clinbiochem.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Basok BI, Kucur M, Kizilgul M, Yilmaz M, Balci O, Ekmekci M, Uzunlulu F, Isman K.. Increased Chitotriosidase Activities in Patients with Rheumatoid Arthritis: A Possible Novel Marker? J Med Biochem. 2014;(33):245–51. [Google Scholar]
- 7.Muthuswamy PP, Lopez-Majano V, Panginwala M, Trainor WD.. Serum angiotensin-converting enzyme as an indicator of total body granuloma load and prognosis in sarcoidosis. Sarcoidosis. 1987;(4):142–8. [PubMed] [Google Scholar]
- 8.Lynch JP III, Ling Ma Y, Koss MN, White ES.. Pulmonary Sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):53–74. doi: 10.1055/s-2007-970333. [DOI] [PubMed] [Google Scholar]
- 9.Sharma OP.. Markers of sarcoidosis activity. Chest. 1986;(90):471–3. doi: 10.1378/chest.90.4.471. [DOI] [PubMed] [Google Scholar]
- 10.Studdy PR, James DG. Chretien J, Marsac J, Saltiel JC. Sarcoidosis. Paris: Pergamon Press; 1983. The specificity and sensitivity of serum angiotensin-converting enzyme in sarcoidosis and other diseases; pp. 332–44. [Google Scholar]
- 11.Tomita H, Ina Y, Sugiura Y, Sato S, Kawaguchi H, Morishita M. et al. Polymorphism in the angiotensin-converting enzyme (ACE) gene and sarcoidosis. Am J Respir Crit Care Med. 1997;(156):255–9. doi: 10.1164/ajrccm.156.1.9612011. [DOI] [PubMed] [Google Scholar]
- 12.Pietinalho A, Furuya K, Yamaguchi E, Kawakami Y, Selroos O.. The angiotensin-converting enzyme DD gene is associated with poor prognosis in Finnish sarcoidosis patients. Eur Respir J. 1999;(13):723–6. doi: 10.1034/j.1399-3003.1999.13d04.x. [DOI] [PubMed] [Google Scholar]
- 13.Kruit A, Grutters JC, Gerritsen WB, Kos S, Wodzig WK, van den Bosch JM. et al. ACE I/D-corrected Z-scores to identify normal and elevated ACE activity in sarcoidosis. Respir Med. 2007;101(3):510–5. doi: 10.1016/j.rmed.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Turner-Warwick M, McAllister W, Lawrence R, Britten A, Haslam PL.. Corticosteroid treatment in pulmonary sarcoidosis: do serial lavage lymphocyte counts, serum angiotensin-converting enzyme measurements and gallium-67 scans help management? Thorax. 1986;(41):903–13. doi: 10.1136/thx.41.12.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosso S, Margollicci MA, Bargagli E, Buccoliero QR, Perrone A, Galimberti D. et al. Serum levels of chitotriosidase as a marker of disease activity and clinical stage in sarcoidosis. Scand J Clin Lab Invest. 2004;(64):57–62. doi: 10.1080/00365510410004092. [DOI] [PubMed] [Google Scholar]
- 16.Bargaglia E, Margollicci M, Luddi A, Nikiforakis N, Perari MG, Grosso S. et al. Chitotriosidase activity in patients with interstitial lung diseases. Respiratory Medicine. 2007;(101):2176–81. doi: 10.1016/j.rmed.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Bargagli E, Maggiorelli C, Rottoli P.. Human Chitotriosidase: A Potential New Marker of Sarcoidosis Severity. Respiration. 2008;(76):234–8. doi: 10.1159/000134009. [DOI] [PubMed] [Google Scholar]
- 18.Brunner J, Scholl-Bürgi S, Zimmerhackl LB.. Chitotriosidase as a marker of disease activity in sarcoidosis. Rheumatol Int. 2007;(27):1171–2. doi: 10.1007/s00296-007-0363-0. [DOI] [PubMed] [Google Scholar]
- 19.Braun JJ, Kessler R, Constantinesco A, Imperiale A.. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008;(35):1537–43. doi: 10.1007/s00259-008-0770-9. [DOI] [PubMed] [Google Scholar]
- 20.Mostard RLM, Voo S, van Kroonenburgh MJ, Verschakelen JA, Wijnen PA, Nelemans PJ. et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011;(105):1917–24. doi: 10.1016/j.rmed.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, Vucinic-Mihailovic V, Artiko V, Saranovic D, Popevic S. et al. The Utility of 18F-FDG PET/CT for Diagnosis and Adjustment of Therapy in Patients with Active Chronic Sarcoidosis. J Nucl Med. 2012;53:1543–9. doi: 10.2967/jnumed.112.104380. [DOI] [PubMed] [Google Scholar]
- 22.Sharma O. Sarcoidosis: clinical management. London: Butterworth; 1984. [Google Scholar]
- 23.Baughman RP, Nagai S, Balter M, Costabel U, Drent M, du Bois R. et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:56–64. [PubMed] [Google Scholar]
- 24.Hollak CEM, Van Weely S, Van Oers MHJ, Aerts JMFG.. Marked elevation of plasma chitotriosidase activity. J Clin Invest. 1994;(93):1288–92. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scadding JG.. Prognosis of intrathoracic sarcoidosis in England. BMJ. 1961;(4):1165–72. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD. et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;(20):204–11. [PubMed] [Google Scholar]
- 27.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA. et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;(164):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 28.Bargagli E, Bennett D, Maggiorelli C, Di Sipio P, Margollicci M, Bianchi N. et al. Human Chitotriosidase: a Sensitive Biomarker of Sarcoidosis. J Clin Immunol. 2013;33(1):264–70. doi: 10.1007/s10875-012-9754-4. [DOI] [PubMed] [Google Scholar]
- 29.Beneteau-Burnat B, Baudin B, Morgant G, Baumann F. Ch, Giboudeau J.. Serum Angiotensin-Converting Enzyme in Healthy and Sarcoidotic Children: Comparison with the Reference Interval for Adults. Clin Chem. 1990;36(2):344–6. [PubMed] [Google Scholar]
- 30.Csaszar A, Halmos B, Palitz T, Szalai C, Romics L.. Interopulation effect of ACE I/D polymorphism on serum concentrations of ACE in diagnosis of sarcoidosis. Lancet. 1997;350(9076):518. doi: 10.1016/S0140-6736(05)63108-X. [DOI] [PubMed] [Google Scholar]
- 31.Sharma OP, Ratto D.. Pulmonary sarcoidosis: radiographic features. Clin Dermatol. 1986;(4):96–107. doi: 10.1016/0738-081x(86)90038-6. [DOI] [PubMed] [Google Scholar]
- 32.Gołab K, Passowicz-Muszyńska E, Jankowska R, Warwas M.. Serum chitotriosidase activity as a biomarker of pulmonary sarcoidosis. Pol Merkur Lekarski. 2011;31(183):179–82. [PubMed] [Google Scholar]
- 33.Aerts JMFG, Boot RG, Blammaart EFC, Renkema GH, Van Weely S, Hollak CEM.. Chitotriosidase: applications and features of the enzyme. Gaucher Clin Perspect. 1999;(7):4–8. [Google Scholar]
- 34.Ulusu NN.. Curious cases of the enzymes. J Med Biochem. 2015;(34):271–81. doi: 10.2478/jomb-2014-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlovic S, Zukić B, Stojiljković Petrović M.. Molecular genetic markers as a basis for personalized medicine. J Med Biochem. 2014;(33):8–21. [Google Scholar]