Summary
Patient and sample misidentification may cause significant harm or discomfort to the patients, especially when incorrect data is used for performing specific healthcare activities. It is hence obvious that efficient and quality care can only start from accurate patient identification. There are many opportunities for misidentification in healthcare and laboratory medicine, including homonymy, incorrect patient registration, reliance on wrong patient data, mistakes in order entry, collection of biological specimens from wrong patients, inappropriate sample labeling and inaccurate entry or erroneous transmission of test results through the laboratory information system. Many ongoing efforts are made to prevent this important healthcare problem, entailing streamlined strategies for identifying patients throughout the healthcare industry by means of traditional and innovative identifiers, as well as using technologic tools that may enhance both the quality and efficiency of blood tubes labeling. The aim of this article is to provide an overview about the liability of identification errors in healthcare, thus providing a pragmatic approach for diverging the so-called patient identification crisis.
Keywords: errors, patient safety, identification, misidentification, laboratory medicine
Kratak sadržaj
Pogrešno identifikovanje pacijenata i uzoraka može naneti značajnu štetu ili neprijatnost pacijentima, naročito ako se netačni podaci koriste za izvođenje specifičnih zdravstvenih aktivnosti. Otud je jasno da efikasna i kvalitetna nega može početi samo od tačne identifikacije pacijenata. Postoji mnogo prilika za pogrešnu identifikaciju u zdravstvenoj nezi i laboratorijskoj medicini, uključujući homonimiju, netačnu registraciju pacijenta, oslanjanje na pogrešne podatke o pacijentu, greške u unošenju naloga, uzimanje bioloških uzoraka od pogrešnih pacijenata, neodgovarajuće obeleza- vanje uzoraka i netačan unos ili pogrešno prenošenje rezultata testova kroz laboratorijski informacioni sistem. Trenutno se mnogo radi na otklanjanju ovih važnih problema uz zdravstvu, što podrazumeva organizovane strategije za identifikovanje pacijenata u čitavoj zdravstvenoj industriji putem tradicionalnih i inovativnih identifikatora, kao i pomoću tehnoloških alatki koje mogu poboljšati kako kvalitet tako i efikasnost označavanja krvnih epruveta. Cilj ovog rada je da se pruži pregled o odgovornosti za greške u identifikaciji u okviru zdravstvene nege, kako bi se omogučio pragmatičan pristup za rešavanje takozvane krize identifikacije pacijenata.
Introduction
Due to several reasons, including largely sto-chastic demand, limitation of resources, time pressure and lack ofor imperfect staff education, many healthcare processes are inherently complicated, multifaceted and thereby intrinsically vulnerable to errors, which may ultimately jeopardize patient health (1). According to the US Institute of Medicine (IOM), the burden of preventable medical harm is dramatically high, accounting for approximately 98.000 deaths each year in the country (2), thus exceeding the number of deaths currently reported for firearms homicides by the Federal Bureau of Investigation (FBI) or the mortality burden caused by commercial airline accidents worldwide (3).
Among the various medical errors, unquestionable evidence has been gathered that failure to correctly identify patients throughout the healthcare industry may be associated with a number of unfavorable consequences, including direct patient harm, medication errors, diagnostic errors (i.e., diagnoses/ test results attributed to the wrong patient), procedures on wrong person or repetition, discharge of infants to wrong families, as well as discomfort, stress and anxiety for patients combined with stress, anxiety and time pressure for healthcare staff (4). Notably, treatment delays are also unavoidable, since the current guidelines mandate that diagnostic test results plagued by a reasonable suspicion of mismatch should not be delivered (5).
Unlike many other preventable causes of patient harm, the epidemiologic liability of identification errors in healthcare cannot be easily ascertained, and this is mostly attributable to challenges or failures in detecting identification errors across various healthcare settings, the impressive burden of underreporting or underestimation, fear of sanctioning, but also the fact that not all identification errors translate into real patient harm (i.e., near misses), thus being frequently overlooked by the healthcare personnel. Of particular concern is the issue of underreporting, especially because patient harm due to identification errors should be mandatorily reported (as a ≫sentinel event≪) and the Working Group on ≫Laboratory Errors and Patient Safety≪of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has included misidentification within the Priority 1 Quality Indicators of the preanalytical phase (6). Between November 2003 and July 2005, the National Reporting and Learning Service (NRLS) of the UK National Patient Safety Agency has received as many as 236 reports of patient safety incidents and near misses relating to missing wristbands or wristbands with incorrect information (7). As regards the US, the Joint Commission (JC) sentinel event statistics includes 130 cases of patient safety incidents attributable to identification errors in 2015 (including transfusion errors with wrong patient, wrong-site, wrong-procedure), all of which had variable degrees of impact on patient health (8). Henneman et al. carried out a prospective simulated study in which health care workers were asked to perform their daily activities (i.e., 28 nurses administering intravenous medications, 16 laboratory technicians labeling blood specimens and 17 emergency service associates applying identity bands) using an eye-tracking device for patient recognition (9). In 39% of all cases (39% nurses, 6% technicians, 71% emergency service associates) the staff performed the assigned activity on the wrong patient, in 74% of all cases (87% nurses, 49% technicians) the patient was not matched with the identity band and, even more importantly, in 15% of all cases the error was not recognized at the end of the procedure.
Despite identification errors being conventionally related to quality issues in blood transfusion (10) and drug administration practices (11), diagnostics is not seen as a safer arena, inasmuch as misidentification is an important source of both medical imaging (12), pathology (13) and laboratory (14) errors. As specifically regards laboratory diagnostics, although the identification error rate typically ranges between 1–2% of all mistakes throughout the total testing process (15), the frequency has been reported to be as high as 9% in urgent testing (16). Notably, a large Q-Probes study endorsed by the College of American Pathologists (CAP) (17) revealed that despite the fact that a vast majority of identification errors (up to 86%) could be identified before test verification, the remaining were only detected after the test results were released, thus yielding to a final estimation of 55 post-verification errors per 1 million billable tests. The aggregate rate of adverse events out of the 6705 identification errors that occurred in the 120 different laboratory services participating in the Q-Probes survey was 5.1%, encompassing significant patient inconvenience with no change of treatment or patient outcome in 72.8% of such cases, change in patient treatment in 4.6% of cases and, finally, unfavorable clinical impact in 22.6% of cases. Along with inaccurate patient identification, specimen labeling errors have been identified as one of the most challenging tasks in laboratory diagnostics, occurring with a frequency of approximately 1.3% (18). The major problems reported are specimens mislabeled (30% of cases), partially labeled (23% of cases), unlabeled (22% of cases) or incompletely labeled (21% of all cases), whereas illegible labels could be identified in 6% of cases. Whether or not these figures really reflect the worldwide scenario, it seems reasonable to conclude that healthcare is suffering from a patient identity crisis, so that identification errors should now be regarded as a public healthcare issue and one of the leading concerns in laboratory diagnostics.
How can this happen?
There are several circumstances throughout the healthcare industry in which patient misidentification may occur, many of which may be causes of diagnostic errors and patient harm. These basically include homonymy, incorrect patient registration, reliance on wrong patient data, error in order entry (incorrect or incomplete data entry), mistranscription of orders, collection of biological specimens from the wrong patient, inappropriate sample labeling and inaccurate entry or transmission of test results in the laboratory information system (LIS) (Table I). Interesting statistics from the Harris County Hospital District (Texas, US) (19) revealed that out of 3,428,925 patients included in the Hospital District’s database, the number of 2 (or more) patients sharing the same last and first names was as high as 249,213 (7.3%), whereas the number of 2 (or more) patients sharing the same last and first names and date of birth was up to 69,807 (2.0%), thus imposing a considerably high risk of identity mismatch. Noteworthy, 2488 patients were named Maria Garcia in the Hospital District, and 231 of these (9.3%) also shared the same date of birth.
Table I.
Causes of identification errors in medical laboratories.
| • Homonymy |
| • Incorrect patient registration |
| • Reliance on wrong patient data |
| • Error in order entry (incorrect or incomplete data entry) |
| • Order mistranscription |
| • Collection of biological specimens from the wrong patient |
| • Inappropriate labeling of specimens |
| ○ Specimens mislabeled |
| ○ Specimens partially labeled |
| ○ Specimens unlabeled |
| ○ Illegible label |
| • Inaccurate entry or transmission of test results in the Laboratory Information System |
The barriers for accurate patient identification
Despite the fact that the history of (laboratory) medicine is plagued by several cases of identification errors and many efforts have been driven to overcome this important healthcare issue in the past decades, technical and practical barriers remain. The main hurdles include process variation among healthcare organizations and personnel, high expenditure associated with innovative and safer approaches, use of technical solutions that may be unsuitable for specific care settings, integration of new technology inside and across healthcare facilities, perception that the relationship with a patient may be jeopardized by repeated identity verification, increase of workload and time spent for patient and sample identification, the need for a cultural revolution as well as challenges in achieving behavioral changes complying with the available recommendations.
Patient identification
There is general consensus that the healthcare providers and health service organizations should identify pertinent items approved for patient identification to be used in their care settings. An accurate patient identification should actually occur at patient admission or registration; when matching patient identity with diagnostics, care, therapy or other services; whenever clinical handover, patient transfer or patient documentation is generated; and within specific care settings different from those used throughout the local facility. The approved patient identifiers are usually items of information that can be used when administering care services, and may basically include patient name (first, possibly middle, and last name), date and place of birth, gender, address, medical record number, individual healthcare identifier (IHI), identity card and/or passport and/or driving license and/or other valid documents as for the local legislation.
The process of accurate patient identification has originally been proposed by the JC many years ago, and was then reiterated until recently within the 2016 National Patient Safety Goals (NPSG) (20). More specifically, the NPSG.01.01.01 mandates that at least two patient identifiers should be used when collecting blood samples and other specimens for clinical testing, or when providing other treatments and procedures. Notably, the room number or physical location of the patient should not be used as identifiers, whereas containers used for blood and other specimens should be labeled in the presence of the patient. Rather understandably, no definitive advice is given about the solutions to be used, wherein the (human and economic) organization of the different facilities differs widely, so that one solution working for one healthcare organization may be unsuitable (or unsustainable) for others. Unlike this approach, the Clinical and Laboratory Standards Institute (CLSI) also recognizes that patient identification is always crucial (21), but suggests a specific sequence for patient identification, entailing asking the patient to provide the full name, address, identification number, and/or birth date. The data should then be compared with the information on the request form. Finally, a recent document of the Working Group for Preanalytical Phase (WG-PRE) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) recommends that a minimum of two (preferably three) unique patient identifiers (one of which is the full name of the patient) should be used for patient identification (22).
Conventional or two-dimensional (2D) barcoded wristbands are indeed the most used approach for patient identification around the globe. The many advantages and the potential drawbacks of this approach have been comprehensively discussed elsewhere (23). Alternative solutions, which became available thanks to information technology (IT) advances, are currently represented by radio-frequency identification (RFID) tags, infrared (IR)-based patient tracking, wireless networks, patient smart cards and biometric technologies. Several lines of evidence now attest that most of these systems are highly effective for reducing the burden of misidentification throughout different healthcare settings (24–26). However, each of these solutions, especially the most widely applied (i.e., RFID tags and biometric technologies), has its own advantages and limitations. The use of passive RFID devices implanted under the skin of the upper arm and storing medical data has been approved since 2004 by the US Food and Drug Administration (FDA). As compared to traditional barcodes, RFID tags are capable of detecting patient identity (and also localizing the patient within the healthcare facility) from a much greater distance, can be used without being positioned in line with a scanner, can be read quickly, can be used as read/write devices, are safer (e.g., data can be encrypted and secured), carry large data capabilities (e.g., up to 2,000 bytes), can be used with minimal human contribution and are substantially reusable. On the other hand, the potential disadvantages of RFID tags include necessary assembling into an expensive computerized chip, problems of information capturing through metals and/or liquids, collision of signals from different readers, possible block of radiofrequency signal, limited battery life, impossibility to provide an absolutely unique patient identification. The advantages of biometric tools are mainly represented by the simple technology, less invasive approach, rapidity, accuracy and minimal training required, whereas the potential limitations include incremental costs, challenging application on a large scale, concerns about informed consent, privacy and secondary uses, as well as changes in body shape or function that may jeopardize system efficiency.
Sample identification
Unlike patient recognition, many recommendations have been published about appropriate identification of biological specimens, sometimes providing different advices. The reference procedure published by the CLSI for collecting diagnostic blood specimens by venipuncture (21) contains the instruction that blood tubes should be labeled after filling and not before. This is in clear contradiction with the recommendations of the World Health Organization (WHO) (27) and those of the Italian Society of Clinical Biochemistry and Laboratory Medicine (SIBioC) (28, 29), both mandating that blood tubes should be labeled before and not after filling. Notably, the WG-PRE of the EFLM also recommends that patient and sample identity should always be checked in the presence of the patient, but also concludes that labeling the tubes before or after blood collection should be based on a local risk analysis of the phlebotomy process (22).
Although one of the authors of this article is an active member of the WG-PRE of the EFLM, there are several reasons prompting us to suggest that the practice of labeling blood collection tubes after venipuncture should be discouraged, and these mostly converge on the evidence that post-collection labeling of tubes carries a higher risk of identification errors (30). By taking into account the available evidence and the current expert recommendations, a tentative approach to dealing with patient and sample identification can hence be proposed, as shown in Figure 1.
Figure 1.
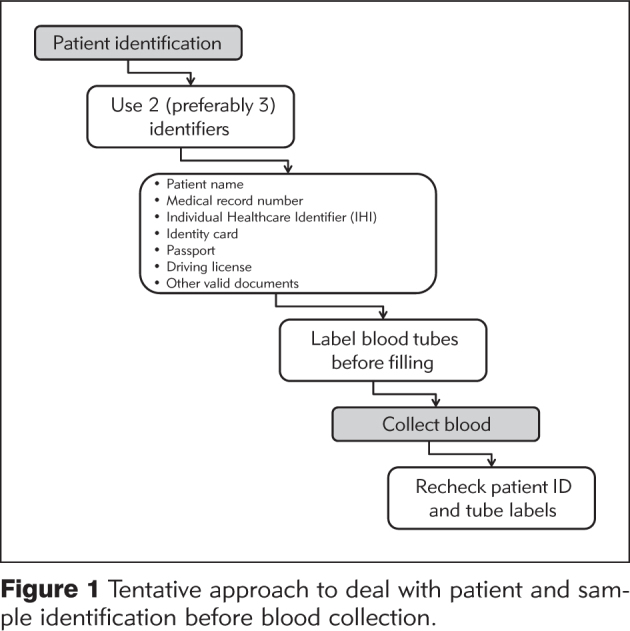
Tentative approach to deal with patient and sample identification before blood collection.
What the future holds
Patient and/or sample identification errors are significantly associated with harm or discomfort for the patients, especially when incorrect data is used for performing specific healthcare tasks or activities. It is hence obvious that efficient and quality care can only start with accurate patient identification, so that whatever technologic aid can be reliably proposed to limit the risk of misidentification in healthcare should be welcomed. Recent advances in mobile, digital identification, printing and labeling techniques have made it easier to print patient-related documents close to the patient and confirm the identity, thus improving the efficiency and the safety of both patient identification and tube labeling before venipuncture, while contextually lowering the overall cost attributable to blood collection (31). Innovative approaches are also emerging for univocal patient identification, such as human hand back skin texture detection (32), probabilistic matching (33) or near field communication (NFC). Interestingly, Hawker et al. recently developed and validated an automated device based on four cameras which photographs the outside of a sample tube and then recognizes discrepancies between patient identity in the LIS versus that on the blood tube label by means of optical character recognition (OCR) (34). The system was found to have a high sensitivity for labeling errors (i.e., out of 742,977 total images that were passed, zero were mislabeled by patient name or exhibited patient name spelling discrepancies, yielding to 1.00 sensitivity; 95% CI 0.97–1.00), whereas the specificity was still modest (only 121 true patient mislabels were identified in the 266,853 images classified as fails by the system, yielding to 0.74 specificity; 95% CI 0.73–0.74). The recently developed chip-size passive RFID Tag also offers a locating capability and read range that are comparable to an active tag, but in a form factor and price that would allow to attach the tags to disposable labels. A final consideration can be made regarding more active patient involvement in healthcare. In accord with the joint recommendations of the WHO and the JC (35), healthcare providers carry the primary responsibility of checking and verifying patient identity, but patients should also actively participate in the process and should receive education about the importance of correct identification throughout the healthcare industry.
Conclusions
The issue of diagnostic errors, which has only received minor attention for decades, has now become the focus of many important initiatives such as the recent report on Improving Diagnosis in Health Care (36). Considerable improvements in analytical performances have indeed contributed to enhancing both the quality and safety of laboratory diagnostics, so leading the way to a redirection of further efforts toward more vulnerable tasks of the total testing process, especially in the pre-analytical phase, such as patient and biological sample identification (37–39). We hope that this article provides a useful overview that will encourage good laboratory practices in this important aspect of testing that has not received the attention it deserves in the past.
Acknowledgement
None.
Footnotes
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Lippi G, Simundic AM, Mattiuzzi C.. Overview on patient safety in healthcare and laboratory diagnostics. Biochem Med (Zegreb) 2010;20:131–43. [Google Scholar]
- 2.Kohn KT, Corrigan JM, Donaldson MS. Building a Safer Health System. Washington, DC: National Academy Press; 1999. To Err Is Human. [Google Scholar]
- 3.Lippi G, Plebani M, Graber ML.. Building a bridge to safe diagnosis in health care. The role of the clinical laboratory. Clin Chem Lab Med. 2016;54:1–3. doi: 10.1515/cclm-2015-1135. [DOI] [PubMed] [Google Scholar]
- 4.Thomas EJ, Petersen LA.. Measuring errors and adverse events in health care. J Gen Intern Med. 2003;18:61–7. doi: 10.1046/j.1525-1497.2003.20147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Banfi G, Buttarello M, Ceriotti F, Daves M, Dolci A. et al. Recommendations for detection and management of unsuitable samples in clinical laboratories. Clin Chem Lab Med. 2007;45:728–36. doi: 10.1515/CCLM.2007.174. [DOI] [PubMed] [Google Scholar]
- 6.Plebani M, Astion ML, Barth JH, Chen W, de Oliveira Galoro CA, Escuer MI. et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med. 2014;52:951–8. doi: 10.1515/cclm-2014-0142. [DOI] [PubMed] [Google Scholar]
- 7.UK National Patient Safety Agency. Safer patient identification. http://www.nrls.npsa.nhs.uk/resources/patient-safety-topics/patient-admission-transfer-discharge/?entryid45=59799 Last Access: 30 September 2016. [Google Scholar]
- 8.Joint Commission. Sentinel Event Data Summary. https://www.jointcommission.org/sentinel_event_statistics_quarterly/ Last Access: 30 September 2016. [Google Scholar]
- 9.Henneman PL, Fisher DL, Henneman EA, Pham TA, Campbell MM, Nathanson BH.. Patient identification errors are common in a simulated setting. Ann Emerg Med. 2010;55:503–9. doi: 10.1016/j.annemergmed.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Lippi G, Plebani M.. Identification errors in the blood transfusion laboratory: a still relevant issue for patient safety. Transfus Apher Sci. 2011;44:231–3. doi: 10.1016/j.transci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Acheampong F, Anto BP, Koffuor GA.. Medication safety strategies in hospitals–a systematic review. Int J Risk Saf Med. 2014;26:117–31. doi: 10.3233/JRS-140623. [DOI] [PubMed] [Google Scholar]
- 12.Danaher LA, Howells J, Holmes P, Scally P.. Is it possible to eliminate patient identification errors in medical imaging? J Am Coll Radiol. 2011;8:568–74. doi: 10.1016/j.jacr.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Nakhleh RE, Zarbo RJ.. Surgical pathology specimen identification and accessioning: A College of American Pathologists Q-Probes Study of 1 004 115 cases from 417 institutions. Arch Pathol Lab Med. 1996;120:227–33. [PubMed] [Google Scholar]
- 14.Valenstein PN, Sirota RL.. Identification errors in pathology and laboratory medicine. Clin Lab Med. 2004;24:979–96. doi: 10.1016/j.cll.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Guidi GC.. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med. 2007;45:720–7. doi: 10.1515/CCLM.2007.167. [DOI] [PubMed] [Google Scholar]
- 16.Carraro P, Plebani M.. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 17.College of American Pathologists. Valenstein PN, Raab SS, Walsh MK.. Identification errors involving clinical laboratories: a College of American Pathologists Q-Probes study of patient and specimen identification errors at 120 institutions. Arch Pathol Lab Med. 2006;130:1106–13. doi: 10.5858/2006-130-1106-IEICL. [DOI] [PubMed] [Google Scholar]
- 18.Wagar EA, Stankovic AK, Raab S, Nakhleh RE, Walsh MK.. Specimen labeling errors: a Q-probes analysis of 147 clinical laboratories. Arch Pathol Lab Med. 2008;132:1617–22. doi: 10.5858/2008-132-1617-SLEAQA. [DOI] [PubMed] [Google Scholar]
- 19.Harris County Hospital District. Harris County Hospital District Puts Patient Safety in the Palm of Your Hand. https://www.harrishealth.org/en/news/pages/patient-safety-biometric-palm-scanner.aspx Last Access: 30 September 2016. [Google Scholar]
- 20.Joint Commission. 2016 National Patient Safety Goals 2016. https://www.jointcommission.org/hap_2016_npsgs/ Last Access: 30 September 2016. [Google Scholar]
- 21.Procedures for collection of diagnostic blood specimens by venipuncture; approved guideline – 6th ed. CLSI document GP41-A6. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. 2007. Clinical and Laboratory Standards Institute. [Google Scholar]
- 22.van Dongen-Lases EC, Cornes MP, Grankvist K, Ibarz M, Kristensen GB, Lippi G. et al. Patient identification and tube labelling – a call for harmonisation. Clin Chem Lab Med. 2016;54:1141–5. doi: 10.1515/cclm-2015-1089. [DOI] [PubMed] [Google Scholar]
- 23.Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V. et al. Causes, consequences, detection, and prevention of identification errors in laboratory diagnostics. Clin Chem Lab Med. 2009;47:143–53. doi: 10.1515/CCLM.2009.045. [DOI] [PubMed] [Google Scholar]
- 24.Ajami S, Rajabzadeh A.. Radio Frequency Identification (RFID) technology and patient safety. J Res Med Sci. 2013;18:809–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Nalla PR, Chalavadi KM.. Iris classification based on sparse representations using on-line dictionary learning for large-scale de-duplication applications. Springerplus. 2015;4:238. doi: 10.1186/s40064-015-0971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ning HC, Lin CN, Chiu DT, Chang YT, Wen CN, Peng SY. et al. Reduction in Hospital-Wide Clinical Laboratory Specimen Identification Errors following Process Interventions: A 10-Year Retrospective Observational Study. PLoS One. 2016;11:e0160821. doi: 10.1371/journal.pone.0160821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO guidelines on drawing blood: best practices in phlebotomy. WHO Press; Geneva, Switzerland: 2010. World Health Organization. [PubMed] [Google Scholar]
- 28.Lippi G, Caputo M, Banfi G, Buttarello M, Ceriotti F, Daves M. et al. Recommendations for collection of venous blood. Biochim Clin. 2008;32:569–77. [Google Scholar]
- 29.Lippi G, Mattiuzzi C, Banfi G, Buttarello M, Caputo M, Daves M. et al. Proposal of a checklist for venous blood collection. Biochim Clin. 2013;37:312–7. [Google Scholar]
- 30.Lippi G, Sonntag O, Plebani M.. Appropriate labelling of blood collection tubes: a step ahead towards patient’s safety. Clin Chem Lab Med. 2011;49:1921–3. doi: 10.1515/CCLM.2011.736. [DOI] [PubMed] [Google Scholar]
- 31.Piva E, Tosato F, Plebani M.. Pre-analytical phase: The auto mated ProTube device supports quality assurance in the phlebotomy process. Clin Chim Acta. 2015;451:287–91. doi: 10.1016/j.cca.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Xie J, Zhang L, You J, Zhang D, Qu X.. A study of hand back skin texture patterns for personal identification and gender classification. Sensors (Basel) 2012;12:8691–709. doi: 10.3390/s120708691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomatam S, Carter R, Ariet M, Mitchell G.. An empirical comparison of record linkage procedures. Stat Med. 2002;21:1485–96. doi: 10.1002/sim.1147. [DOI] [PubMed] [Google Scholar]
- 34.Hawker CD, McCarthy W, Cleveland D, Messinger BL.. Invention and validation of an automated camera system that uses optical character recognition to identify patient name mislabeled samples. Clin Chem. 2014;60:463–70. doi: 10.1373/clinchem.2013.215434. [DOI] [PubMed] [Google Scholar]
- 35.Patient Identification. Patient Safety Solutions. 2. Vol. 1. WHO Press, World Health Organization; Geneva, Switzerland: 2007. World Health Organization, Joint Commission and Joint Commission International. [Google Scholar]
- 36.Graber ML.. The IOM report on improving diagnosis: new concepts. Diagnosis. 2015;2:201–3. doi: 10.1515/dx-2015-0029. [DOI] [PubMed] [Google Scholar]
- 37.Plebani M.. Laboratory-associated and diagnostic errors: a neglected link. Diagnosis. 2014;1:89–94. doi: 10.1515/dx-2013-0030. [DOI] [PubMed] [Google Scholar]
- 38.Lima-Oliveira G, Lippi G, Salvagno GL, Picheth G, Guidi GC.. Laboratory Diagnostics and Quality of Blood Collection. J Med Biochem. 2015;34:288–94. doi: 10.2478/jomb-2014-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aykal G, Keşapli M, Aydin O, Esen H, Yeğin A, Güngör F. et al. Pre-Test and Post-Test Applications to Shape the Education of Phlebotomists in A Quality Management Pro gram: An Experience in A Training Hospital. J Med Biochem. 2016;35:347–53. doi: 10.1515/jomb-2016-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]


