Summary
Background
Subclinical hypothyroidism (SCH) is defined as high TSH and normal thyroxine. Data on the effects of early substitution by levothyroxine on psychophysical health in SCH are still not consistent enough to support its introduction.
Methods
Clinical parameters, biochemical data and quality of life (Short Form 36 questionnaire) were measured before the intervention and 3 months after the euthyroid state had been achieved in SCH patients.
Results
Significant reduction in body weight (p=0.030), systolic and diastolic blood pressure (p=0.024, p=0.019), homocysteine (p<0.001), leukocytes and neutrophils (p=0.011, p=0.001), INR (p=0.049), K levels (p=0.040, p=0.013), HbA1c (p=0.001), fasting insulin (p<0.001) and insulin resistance measured by HOMA index (p<0.001), lipid parameters (total cholesterol (p<0.001), LDL-cholesterol (p<0.001), triglycerides (p=0.007), apoB (p=0.022), Lp(a) (p<0.001), LDL/HDL (p=0.008), LAP (p=0.04) and apoB/apoA1 ratios (p<0.023)), TSH (p<0.001) and tAbs (p<0.001) was recorded. Frequency of fatty liver (20% to 2.9%, p=0.016), hyperlipidemia (85% to 65.7%, p=0.001) and metabolic syndrome (34.3% to 2.9%, p=0.070) significantly decreased. A statistically significant positive association was found between the average dose of levothyroxine and changes in physical functioning (r=0.391, p=0.020), vitality (r=0.393, p=0.020), mental health (r=0.374, p=0.027) and overall dimensions of mental health (r=0.376, p=0.026). With increasing doses of levothyroxine, the previously listed scores of SF 36 grew (r=0.296, p=0.084).
Conclusions
Early substitution of SCH improved the many clinical and biochemical parameters related to cardiovascular risk. Quality of life was also improved, and correlated only with thyroxine doses suggesting an indirect relationship between the degree of hypothyroidism and quality of life.
Keywords: subclinical hypothyroidism, biochemical parameters, quality of life, early T4 substitution
Kratak sadržaj
Uvod
Supklinička hipotireoza (SCH) definiše se kao povišen TSH uz normalne vrednosti tiroksina. Podaci o efektima rane supstitucije na psihofizički status individua sa SCH nisu dovoljno konzistentni da bi podržali njeno uvođenje.
Metode
Klinički i biohemijski parametri, kao i kvalitet života mereni su pre intervencije i 3 meseca nakon postiza nja eutiroidnog stanja.
Rezultati
Zabeležena je značajna redukcija telesne mase (p=0,030), sistolnog i dijastolnog krvnog pritiska (p=0,024, p=0,019), homocisteina (p<0,001), leukocita i neutrofila u krvi (p=0,011, p=0,001), INR-a (p=0,049), nivoa K (p=0,040, p=0,013), HbA1c (p=0,001), insulinemije bazno (p<0,001) i insulinske rezistencije merene HOMA indeksom (p<0,001), lipidnih parametara (ukupnog holesterola (p<0,001), LDL-holesterola (p<0,001), triglicerida (p=0,007), apoB (p=0,022), Lp(a) (p<0,001), LDL/HDL (p=0,008), LAP (p=0,04) i apoB/apoA1 (p<0.023)), TSH (p<0,001) i tAbs (p<0.001). Učestalost masne jetre (sa 20% na 2,9%, p=0,016), hiperlipidemije (sa 85% na 65,7%, p=0,001) i metaboličkog sindroma (sa 34,3% na 2,9%, p=0,070) značajno je snižena. Statistički značajna pozitivna povezanost nađena je između prosečne doze levotiroksina i promena u fizičkom funkcionisanju (r=0,391, p=0,020), vi - talnosti (r=0,393, p=0,020), mentalnom zdravlju (r=0,374, p=0,027) i ukupnoj dimenziji mentalnog zdravlja (r=0,376, p=0,026). Sa porastom doze levotiroksina, prethodno navedeni skorovi SF 36 su rasli (r=0,296, p=0,084).
Zaključak
Rana supstitucija supkliničke hipotireoze poboljšava brojne kliničke i biohemijske parametre, koji su u vezi sa kardiovaskularnim rizikom. Kvalitet života je takođe poboljšan i korelira jedino sa dozama levotiroksina, sugerišući indirektnu vezu između dubine hipotiroidnog stanja i kvaliteta života.
Introduction
Subclinical hypothyroidism (SCH) is defined as elevated serum thyroid-stimulating hormone (TSH) and normal serum free thyroxine (FT4). SCH is a biochemical diagnosis based on the TSH concentration higher than 4.12 mlU/L or above the age-adjusted upper normal level as it was proposed by The American and European Thyroid Associations (ATA/ETA) and the American Association of Clinical Endocrinologists (AACE) (1, 2).
The incidence of SCH is 4–10%, showing higher frequency in women. In people over the age of 60, the prevalence of SCH significantly increases and reaches 15% of females and 8% of males (3). The most common cause of elevated TSH is chronic autoimmune thyroiditis (4). Among the individuals with the TSH values of 5.0–9.9 mlU/L, SCH progresses to overt hypothyroidism in 5.6% of cases. A much higher percentage of progression (85.7%) is seen in patients with TSH values between 15.0 and 19.9 mlU/L (1, 5).
In patients aged less than 70 and with TSH <10 mlU/L, a diagnosis of SCH is not always followed by substitution with levothyroxine (LT4). According to current guidelines, a decision for treatment is based on the presence of mild symptoms suggestive of hypothyroidism, goitre and comorbidity. Authors advise LT4 treatment only if TSH elevation persists for more than 3 months and the patient has other risk factors for developing overt hypothyroidism (6). Patients who do not fit into these recommendations are followed in 6-month intervals, and in those who start the 3-month trial of LT4, the effect of treatment should be evaluated.
Numerous studies emphasize the link of SCH with a great number of conditions that increase morbidity and mortality rates, such as weight gain, diastolic hypertension, low-grade inflammation, elevated total homocysteine, hemostatic abnormalities, changes in renal function, non-alcoholic fatty liver disease (NAFLD), hypercholesterolemia, dyslipidemia, decreased insulin resistance, metabolic syndrome (MS), coronary heart disease and ischemic heart disease (1, 3–8). Still, there are insufficient data for a favourable effect of LT4 on many of them. Quality of life is also frequently disturbed. For this reason, health-related quality of life (HRQoL) questionnaires are used in SCH as an additional tool for the assessment of the need for treatment (9). They are also useful for the evaluation of the effects of early substitution since some studies have indicated that thyroxine improves scores related to complaints (including mental lethargy) and psychometric performance compared with a placebo (10–13).
The aim of our study is to quantify the effect of a 3-month trial of LT4 treatment on biochemical blood parameters and the quality of life in persistent SCH with TSH less than 10 mlU/L. Better validation of clinical benefit could help in making a decision whether to stop or continue the LT4 treatment and does the benefit support its early introduction. Another aim is to define the starting substitutive dose of LT4 and target TSH values.
Materials and Methods
This was a prospective open-label study. The including criteria were the presence of untreated SCH defined as TSH level between upper normal level to 10 mlU/L, normal FT4, positive thyroid antibodies (tAbs) and/or an ultrasound scan characteristic for chronic autoimmune thyroiditis. The exclusion criteria were: missing vital data (TSH), previous history of thyroid disease and treatment, conditions that affect thyroid status and lipid metabolism (14), taking any medicine that affects the thyroid or lipid metabolism in the past 6 months (7), past or current serious medical diseases including diabetes mellitus and coronary heart disease, using any medication, including aspirin or diuretics, that might affect the study parameters, having symptoms and signs of clinical bleeding, smoking.
Weight, height, BMI, waist circumference (WC) and blood pressure were measured before intervention with thyroxine and 3 months after the euthyroid state had been achieved. The following biochemical parameters of blood were chosen for analysis: CRP was measured by nephelometry, a latex particle enhanced immunoassay (TBA-200FR, Tokyo, Japan); fibrinogen, INR, APTT were done by analyzers using kits from Dade Behring Marburg GmbH; total homocysteine in plasma was measured by the Abbott Homocysteine (HCY) assay; whole blood analyses were made using the Advia 120 hematologic system (automated analyzer). By using the Auto Analyzer HITACHI 7020 (902), Japan, the following biochemical analyses were done: fasting plasma glucose, albumin, high-density lipoprotein cholesterol (HDL), triglycerides (TG), total cholesterol (TC), calcium (Ca), phosphorus (P), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine phosphokinase (CPK) and lactate dehydrogenase (LDH). Lp(a) was measured with a commercially available enzyme-linked immunosorbent assay (Strategic Diagnostics, Newark, DE); insulinemia was measured by DRG: HYBRiD-XL® Immunoassay and Clinical Chemistry Analyzer from DRG International, Inc.; potassium (K) was measured by Refurbished AVL 9180/Medica Easylyte Analyser. Immunoturbidimetric assays were used for measuring the apolipoproteins A1 and B using the Cobas Bio centrifugal analyser; serum FT3, FT4 and TSH levels were measured by a commercially available automated chemiluminescence system and associated kits (ACS: 180, Chiron Diagnostics, East Walpole, MA, USA); tAbs were measured by the RIA method (Inep, Belgrade, Serbia).
NAFLD was defined as AST/ALT < 1. LDL-cholesterol (LDL) was calculated by the Friedewald formula (LDL = TC – HDL – TG/2.2) (15). Total-body lipid accumulation products (LAP) were calculated as: (waist circumference (cm) – 65) × (triglyceride concentration (mmol/L)) for men and (waist circumference (cm) – 58) × (triglyceride concentration (mmol/L)) for women (16). MS was defined by the presence of three or more of the following five components: (1) central obesity defined as WC ≥ 90th percentile for age and gender; (2) elevated systolic and/or diastolic blood pressure ≥ 95th percentile for age, sex and height; (3) hypertriglyceridemia defined as TG ≥ 1.24 mmol/L, equal to the 90th percentile of the reference population; (4) low serum HDL (Low-HDL) defined as ≤ 1.03 mmol/L i.e., ≤ 5th percentile of the reference population and (5) impaired fasting glucose (IFG) defined as ≥ 5.6 mmol/L (17). The insulin resistance index was calculated by the homeostasis model assessment of insulin resistance (HOMA-IR) as (fasting insulin mU/L) × (fasting glucose mmol/L)/22.5 (18). The TG/HDL cholesterol ratio was calculated as a good predictor of cardiovascular events, and the LDL/HDL cholesterol ratio was estimated as an index of atherosclerosis. The Short Form 36 (SF-36) questionnaires, as a generic quality of life instrument, were self-administered by the patients and completed before the clinical examination. SF-36 is intended to measure the full range of health status and well-being, both physical and mental (19, 20). After the initial investigation, levothyroxine (LT4) treatment was started in all patients. Doses sufficient to normalize TSH were ranged from 25 μg to 75 μg daily with a mean dose of 50 μg. Three months after TSH normalization all blood tests were repeated. All patients gave informed consent before participating in the study.
Data were analyzed using methods of descriptive and analytical statistics. The methods of descriptive statistics were: measures of central tendency (mean and median), measures of variability (standard deviation and interquartile range) and the relative numbers. The methods of analytical statistics were: identification methods of empirical distributions, methods to assess the significance of differences: McNemar test for categorical variables and paired T test or Wilcoxon signed rank test for numerical variables. The Pearson or Spearman correlation coefficient was used to analyze the relationship between study variables. The p<0.05 was considered statistically significant. All statistical analyses were performed in SPSS 20.0 (SPSS Inc., Chicago, Illinois).
Results
Our study included 35 patients, in the age range of 51.6±15.4 years, 29 females (82.9%) and 6 males patients (17.1%). Patients’ anthropometric data and clinical characteristics are listed in Table I.
Table I.
Anthropometric data, clinical characteristics before and after therapy (MV±SD).
| Before ±sd | After ±sd | p-value | |
|---|---|---|---|
| Body mass (kg) | 72.6±18.4 | 71.4±16.6 | 0.030 |
| BMI (kg/m2) | 25.5±4.0 | 24.9±4.0 | 0.320 |
| Waist circumference (cm) | 87.1±16.0 | 85.7±16.2 | 0.422 |
| BP systolic (mmHg) | 126.9±10.2 | 121.7±6.5 | 0.024 |
| BP diastolic (mmHg) | 75.3±6.8 | 71.6±5.8 | 0.019 |
| CRP (mg/L) | 1.3 (0.6–2.6) | 1.3 (0.8–2.2) | 0.788* |
| fibrinogen (g/L) | 3.9±1.4 | 3.7±1.2 | 0.114 |
| homocysteine (čmol/L) | 7.2 (3.5–10.9) | 4.7 (3.1–6.0) | <0.001* |
| Leu (x109/L) | 6.2±1.4 | 5.8±1 | 0.011 |
| Neut (%) | 63.6±5 | 59.9±4.4 | 0.001 |
| APTT (s) | 29.1±2.7 | 29.2±2.6 | 0.693 |
| INR | 1.2±0.4 | 1.1±0.2 | 0.049 |
| K (mmol/L) | 4.5±0.4 | 4.3±0.3 | 0.013 |
| Fasting glucose (mmol/L) | 5.2±0.6 | 5±0.5 | 0.192 |
| Fasting insulin (mU/L) | 6.8±2.4 | 5.7±1.9 | <0.001 |
| HOMA index | 2±0.5 | 1.8±0.4 | <0.001 |
| HbA1c (%) | 5.4±0.3 | 5.2±0.3 | 0.001 |
| total cholesterol (mmol/L) | 6.1±1.5 | 5.0±1.2 | <0.001 |
| LDL (mmol/L) | 4.1±1.2 | 2.8±1.0 | <0.001 |
| HDL (mmol/L) | 1.3±0.3 | 1.3±0.2 | 0.287 |
| TG (mmol/L) | 1.4 (1.1–2.0) | 1.2 (0.9–1.8) | 0.007* |
| LDL/HDL | 3.0±1.1 | 2.3±1.0 | 0.008 |
| TG/HDL | 1.0±0.5 | 1.2±1.0 | 0.209 |
| Apo A1 (g/L) | 1.7±0.3 | 1.7±0.2 | 0.335 |
| Apo B (g/L) | 1.0±0.3 | 0.9±0.3 | 0.022 |
| ApoB/ApoA1 | 0.65±0.25 | 0.53±0.18 | <0.023 |
| Lp(a) (mg/L) | 60±29 | 52±24 | <0.001 |
| LAP | 44.3 ± 32.6 | 42.0 ± 45.7 | 0.040 |
| fT4 (pmol/L) | 13.4 (12.5–14.7) | 16.6 (15.4–18.8) | <0.001* |
| TSH (mlU/L) | 7.0±2.1 | 3.0±1.0 | <0.001 |
| fT3 (pmol/L) | 1.7±0.2 | 1.6±0.2 | 0.019 |
| TPO Ab (U/mL) | 208.4 (16.2–903.1) | 25.4 (0.1–87.5) | <0.001* |
| AST (U/L) | 19.4±3.8 | 20.4±4.6 | 0.245 |
| ALT (U/L) | 22.3±5.6 | 25.4±7.2 | 0.016 |
p-value from t-test
p-value from Wilcoxon test, paired samples, SD – standard deviation; MV – mean value; p<0.05 and p<0.001.
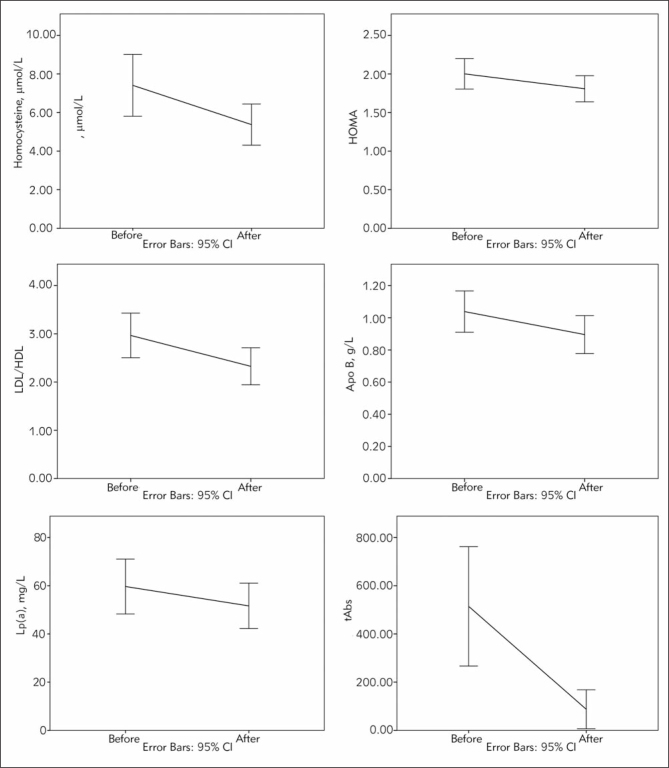
The body weight was significantly lower after treatment (p=0.030). There was no statistically significant change in BMI and waist circumference (p=0.320, p=0.422). A significant reduction in systolic and diastolic blood pressure is underlined (p=0.024, p=0.019). The analysis of cardiovascular markers, parameters of inflammation and hematological markers showed statistically significant reduction in homocysteine levels (p<0.001) (Figure 1), total leukocytes and neutrophils (p=0.011, p=0.001), while the level of CRP and fibrinogen remained unchanged (p=0.788, p=0.114). There was statistically significant reduction in INR (p=0.049), and no significant change (p=0.693) in APTT levels. The significant decline in serum potassium (p=0.013) was recorded after the therapy. There was a statistically significant drop in HbA1c (p=0.001), fasting insulin (p<0.001) and insulin resistance measured by HOMA index (p<0.001) (Figure 1), while the change in the value of fasting glycemia did not reach significance (p=0.192). Statistically significant decreases were registered in lipid parameters: total cholesterol (p<0.001), LDL-cholesterol (p<0.001), triglycerides (p=0.007), apoB (p=0.022), Lp(a) (p<0.001), LDL/HDL ratio (p=0.008) (Figure 1), apoB/apoA1 ratio (p<0.023) and LAP (p=0.040). Levels of HDL, TG/HDL ratio, Apo A1 levels remained unchanged after therapy (p=0.287, p=0.209, p=0.335 respectively). The significant decrease was recorded in the level of TSH (p<0.001) and tAbs (p<0.001) (Figure 1) and an increase in fT4 (p<0.001) and T3 (p=0.019) thyroid hormones. There was a statistically significant reduction in LDH after the therapy (p=0.019).
Figure 1.
Changes of the biochemical parameters after achieving adequate substitution by LT4.
Frequency of fatty liver (20% to 2.9%, p=0.016), hyperlipidemia (85% to 65.7%, p=0.001) and metabolic syndrome (34.3% to 2.9%, p=0.070) significantly decreased after the L-T4 therapy (Figure 2).
Figure 2.
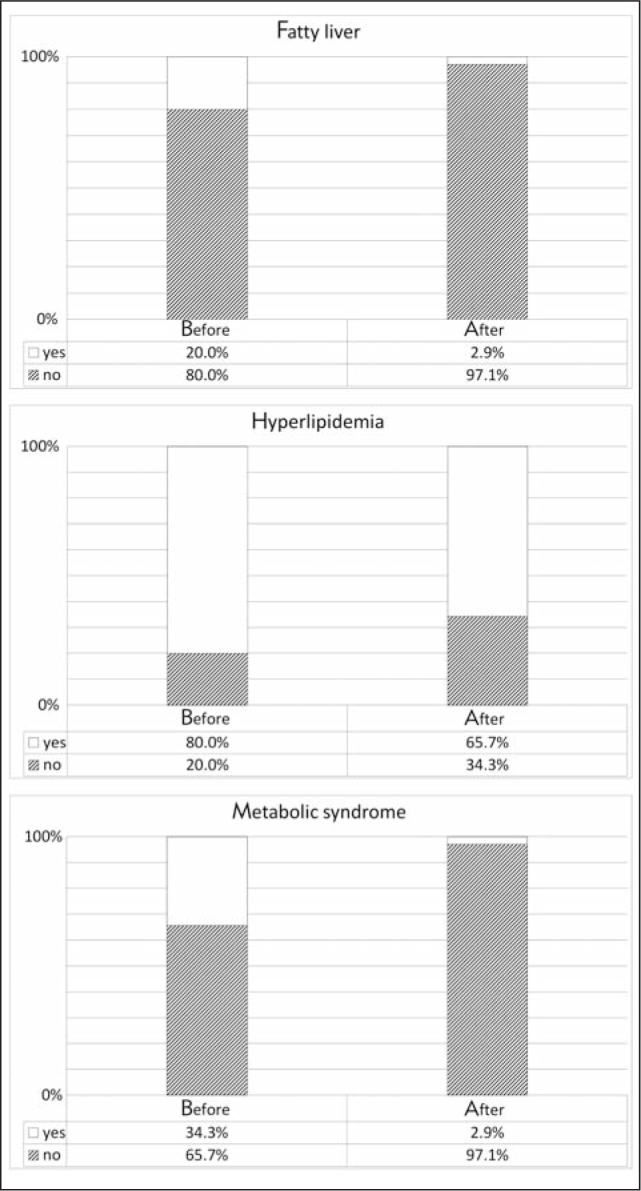
The frequency of fatty liver, metabolic syndrome, hyperlipidemia (HLP Ila, HLP Ilb, HLP IV) before and after therapy.
The correlation between quality of life parameters, thyroid hormone levels and dose of levothyroxine used for substitution was done and the results are shown in Table II. A positive, moderate but significant correlation among the average dose of levothyroxine and changes in physical functioning (r=0.391, p=0.020), vitality (r=0.393, p=0.020), mental health (r=0.374, p=0.027) and overall dimensions of mental health (r=0.376, p=0.026) was found. With increasing doses of levothyroxine, previously listed scores of SF 36 grew (r=0.296, p=0.084) (higher score means improving the health-related questionnaire results). There were no statistically significant correlations among the changes of TSH, FT4 and FT3, anti tAbs and the scores of the SF questionnaire (r=0.197, p=0.258; r=0.068, p=0.706; r=0.273, p=0.145 and r=-0.084, p=0.643 respectively).
Discussion
The highest incidence of subclinical hypothyroidism is in women aged between 50 and 60 years, as in our work (3, 4, 21). Since many studies showed that even mild thyroid hypofunction may represent an additional risk factor for metabolic and cardiovascular diseases, it is important to estimate the benefit of early levothyroxine replacement. Our decision to treat is based on these risks, patients’ complaints, quality of life and their TSH level. The insufficient amount of evidence to support the benefit of early LT4 substitution is the main limiting factor, since its restoring effect on body homeostasis is still under investigation.
Even the cause of weight gain in hypothyroidism is complex, including excessive retention of salt and water, an increase in lean body mass, but is rarely related to the excess fat accumulation (22). Erdogan et al. emphasized that BMI is significantly higher in SCH compared with euthyroid subjects (23). The same was shown by Pešić et al. (3), but they found that waist circumference was not increased in the SCH group compared with controls. Only a few studies were dealing with the effects of euthyroid state restoration on body composition. During the therapy, our patients reduced their weight, but there was no significant reduction in BMI and WC used as a surrogate marker of abdominal fat mass.
It is well known that the lack of thyroid hormones causes impaired vascular smooth muscle relaxation, increased venous resistance, and diastolic hypertension (4, 24). Regain of normal thyroid hormone level contributes to the normalization of blood pressure (24). After three months, our patients significantly reduced both systolic and diastolic blood pressure, with maximal measured values less than 130/80 mmHg.
Besides blood pressure, we monitored CRP, fibrinogen and homocysteine as widely used surrogate cardiovascular markers (25, 26). Our results showed a profound reduction only in homocysteine level on treatment (Table I, Figure 1). According to Monzani et al., homocysteine level is higher in patients with SCH compared to euthyroid individuals (27). Hyperhomocysteinemia contributes to atherosclerosis by accelerating oxidation of LDL and leading to endothelial dysfunction (28, 29).
Although in the reference range, total leukocytes and neutrophils were significantly reduced in our group of patients after treatment. Christ-Crain et al. (30) published similar changes in leukocyte level, but with the subtle alterations of the leuko-lymphomonocytic distribution. Our results suggest a leukocyte shift to a more favorable profile and may imply an improvement of sub-inflammation state.
Limited published data suggest the existence of reversible coagulation and fibrinolysis derangement in hypothyroidism. In mild hypothyroidism, including SCH, it is more likely that the risk of thrombosis is increased, whereas in patients with more severe hypothyroidism a bleeding tendency is more frequent. In this study APTT levels were normal with no change on treatment, and INR showed a significant reduction within the normal range. Hypothyroidism decreases the catabolism of the vitamin K clotting factors and therefore potentially may influence anticoagulant treatment. The relevance of these findings in SCH is not quite established (15, 31–33).
Among many effects of SCH on the whole body, renal function indices (i.e. serum creatinine, sodium, potassium and estimated glomerular filtration rate (eGFR)) may also be unfavorably changed and improved after achieving euthyroidism on therapy (34). Our results supported previous studies considering potassium which was significantly reduced within the normal range, probably due to better renal excretion, or also due to better intracellular transport affected by insulin. Other mentioned renal function indices (not shown in the Table) were normal and unchanged by therapy.
According to many data, SCH is associated with early insulin resistance and fasting hyperinsulinemia (35–37). On the other side, several reports emphasize no difference in fasting glucose level or hemoglobin A1C and the prevalence of diabetes mellitus between the SCH and euthyroid subjects (38–40). Our results demonstrated a significant reduction in HbA1c, fasting insulin and insulin resistance measured by HOMA index (Table I, Figure 1) after levothyroxine treatment. Having in mind the role of insulin resistance in the development of many cardiovascular events, this finding seems very important.
Thyroid hormones regulate lipid metabolism in several different ways. According to a recent study, an increase of 1 mlU/L in serum TSH was associated with a rise in serum cholesterol of 0.09 mmol/L in women and 0.16 mmol/L in men (24). The LDL/HDL and TG/HDL ratios are frequently used for a more accurate assessment of cardiovascular risk, related to the metabolic pathway of cholesterol (41). In a recent study, elevated triglyceride levels were also found to be an independent risk factor for cardiovascular disease in SCH (7). Lipid disorder in SCH is represented by an increase of TC and LDL cholesterol, as well as increased LDL/HDL and TG/HDL ratios which even better represent disturbance of lipid profile (24). Our study pointed out that early substitution and achievement of a euthyroid state significantly improves the LDL/HDL ratio (Table I, Figure 1) which might decrease cardiovascular risk. Also, a significant decrease of total cholesterol, LDL-cholesterol and triglycerides was registered, with no change in HDL cholesterol and otherwise normal TG/HDL ratio. The increase of apoB/apoA1 ratio reflects the increasing of cardiovascular risk in hypothyroidism (41). Some data report higher values of apoB in SCH compared to euthyroid individuals, which are corrected by LT4 treatment (41). Our results demonstrated significant decreases in apoB (Table I, Figure 1) and apoB/apoA1 ratio while apoA1 remained unchanged. The frequency of hyperlipidemia also decreased (Figure 2).
Lipoprotein (a) is considered as one of the blood markers for atherosclerosis and thrombogenesis risk. The association between increased levels of Lp(a) and SCH has been found only in patients with TSH value above 12 mlU/L and in postmenopausal women regardless of their serum TSH value (42–44). Our patients had a significant decrease of Lp(a) level after the treatment, but generally the mean values were lower than 100 mg/L even at the time of the diagnosis (Table I, Figure 1). Still, taking in mind the deterioration of lipid profile and insulin sensitivity, age and sex distribution in SCH, measurement of Lp(a) may have clinical importance. A positive effect of LT4 replacement on Lp(a) level and lipid metabolism also may be expected. In accordance with this, we found a decrease of LAP that is caused by improvement of lipid profile and weight loss.
The level of thyroid antibodies and TSH are found predictive for the progression of SCH in clinical hypothyroidism (1). Restoring of euthyroid state was followed by reduction of tAbs (Figure 1) in this study, suggesting an amelioration of autoimmune thyroiditis.
Liver enzymes AST and ALT are reported to be increased in subclinical hypothyroid patients compared to euthyroid individuals (29). Our results are in accordance with the literature data since the percent of positive criteria for fatty liver are decreased after the therapy delivery (Figure 2).
According to some data, the increase of TSH is followed by the increase of MS frequency (45). Even a high normal TSH (2.5–4.5 mmol/L) is associated with the metabolic syndrome (46). Normalization of TSH led to the decrease of frequency of MS in our patients after the therapy (Figure 2).
Thyroid diseases have a large influence upon both the physical and the mental dimensions of health status. According to an Italian study, even a subclinical thyroid disturbance alters the perceived health status (47). Another study demonstrated that thyroid autoimmunity with normal thyroid function is associated with mental health in postpartum depression (48). Inconsistent with the above findings are results from the study that included elderly subjects and reports that SCH is not clearly associated with cognitive impairment, depression or poor quality of life (QoL) (49).
There are many controversies about replacement therapy in SCH. According to some authors, the patients have improved well–being if the LT4 dosage is adjusted until TSH reaches lower part of the reference range (under 2 mmol/L) (10, 50–53). In a double-blind, randomized, controlled trial, Walsh et al. compared the individuals with TSH values in lower (0.3–1.99 mmol/L) and upper (2.0–4.8 mmol/L) reference range after T4 dosage adjustment. They did not confirm the influence of those TSH level variations on the measurable hypothyroid symptoms, wellbeing, or quality of life (9). Our results showed that even average replacement doses of levothyroxine are significantly associated with the improvement of physical performance, vitality and mental health found in untreated SCH. An increase of SF-36 questionnaire score(s) showed a positive correlation with the increment of LT4 doses. There were no significant correlations among the changes of TSH, FT4, FT3, tAbs and the scores of SF questionnaire. Although the changes of hormone levels have not correlated with the changes of quality of life, the treatment replacement dosage of levothyroxine has, suggesting an indirect relationship between the degree of hypothyroidism and the quality of life.
Conclusion
The current guidelines on the diagnosis and treatment of SCH define who the screening candidates are (1, 2). Still, there is no clear evidence to support the benefits of early treatment of SCH and recommendations for the introduction of levothyroxine are inconsistent for TSH less than 10 mlU/L. According to available data, this is the most complete study on the effect of early substitution of subclinical hypothyroidism on the biochemical parameters and the quality of life in Serbia. This data can be used to produce new approaches for the creation of SCH treatment. The new approach should be based on treatment with a higher starting dose of levothyroxine (50–100 μg) or target values TSH less than 2.5 mmol/L except for the very old individuals. The improvement of clinical parameters (blood pressure, body mass), biochemical parameters (homocysteine, lipid profile, LAP, glucose metabolism) and quality of life seems to be a relevant index or indicator in monitoring the therapeutic effect.
Limitation of the study
This is a pilot study, with 35 patients that are followed for 3 months after achieving a euthyroid state.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Commission of Belgrade University of Medical Sciences and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Acknowledgements
This work was not financed nor funded.
Glossary
List of abbreviations
- SCH
subclinical hypothyroidism;
- TSH
thyroid-stimulating hormone;
- LT4
levothyroxine;
- FT4
free thyroxine;
- FT3
free triiodothyronine;
- tAbs
thyroid antibodies;
- BMI
body mass index;
- TC
total cholesterol;
- HDL
high-density lipoprotein cholesterol;
- LDL
low density lipoprotein cholesterol;
- TG
triglycerides;
- ALT
alanine aminotransferase;
- AST
aspartate aminotransferase;
- Lp(a)
lipoprotein a;
- LAP
lipid accumulation product;
- HOMA-IR
homeostasis model assessment of insulin resistance.
Footnotes
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Hennessey JV, Espaillat R.. Subclinical hypothyroidism: a historical view and shifting prevalence. Int J Clin Pract. 2015;69(7):771–82. doi: 10.1111/ijcp.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce SHS, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S. et al. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur Thyroid J. 2013;2(4):215–28. doi: 10.1159/000356507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesic MM, Radojkovic D, Antic S, Kocic R, Stankovic-Djordjevic D.. Subclinical hypothyroidism: association with cardiovascular risk factors and components of metabolic syndrome. Biotechnol Biotechnol Equip. 2015;29(1):157–63. doi: 10.1080/13102818.2014.991136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyati A, Dhuttargi S, Das D.. Assessment of the Cardiovascular Risk in Subclinical Hypothyroidism. Int J Pharm Biol Sci. 2012:2230–7605. [Google Scholar]
- 5.Karmisholt J, Andersen S, Laurberg P.. Variation in thyroid function in subclinical hypothyroidism: importance of clinical follow-up and therapy. Eur J Endocrinol. 2011;164(3):317–23. doi: 10.1530/EJE-10-1021. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Tang Z.. Subclinical hypothyroidism: to treat or not to treat? Med Princ Pract. Karger Publishers. 2015;24(1):99–100. doi: 10.1159/000365743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Tang X, Yang T, Zhang B, Guan Q, Shao S. et al. Lipotoxicity, a Potential Risk Factor for the Increasing Prevalence of Subclinical Hypothyroidism? J Clin Endocrinol Metab. 2015;100(5):1887–94. doi: 10.1210/jc.2014-3987. [DOI] [PubMed] [Google Scholar]
- 8.Andersen MN, Olsen A-MS, Madsen JC, Faber J, Torp-Pe dersen C, Gislason GH. et al. Levothyroxine Substitution in Patients with Subclinical Hypothyroidism and the Risk of Myocardial Infarction and Mortality. Eugenin EA, editor. PLoS One. Public Library of Science. 2015;10(6):e0129793. doi: 10.1371/journal.pone.0129793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D. et al. Small Changes in Thyroxine Dosage Do Not Pro duce Measurable Changes in Hypothyroid Symptoms, Well-Being, or Quality of Life: Results of a Double-Blind, Randomized Clinical Trial. J Clin Endocrinol Metab. 2006;91(7):2624–30. doi: 10.1210/jc.2006-0099. [DOI] [PubMed] [Google Scholar]
- 10.Carr D, McLeod DT, Parry G, Thornes HM.. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 1988;28(3):325–33. doi: 10.1111/j.1365-2265.1988.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 11.Nyström E, Caidahl K, Fager G, Wikkelsö C, Lundberg PA, Lindstedt G.. A double-blind cross-over 12-month study of l-thyroxine treatment of women with »subclinical« hypothyroidism. Clin Endocrinol (Oxf) Blackwell Publishing Ltd; 1988;29(1):63–76. doi: 10.1111/j.1365-2265.1988.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC.. L-Thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med. 1984;101(1):18–24. doi: 10.7326/0003-4819-101-1-18. [DOI] [PubMed] [Google Scholar]
- 13.Monzani F, Del Guerra P, Caraccio N, Pruneti CA, Pucci E, Luisi M. et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig. 1993;71(5):367–71. doi: 10.1007/BF00186625. [DOI] [PubMed] [Google Scholar]
- 14.Kaur V, Singh K, Verma M.. Changes in biochemical markers of renal function in subclinical and overt hypothyroidism. Int J Bioassays. 2015;4(4):3799–802. [Google Scholar]
- 15.Gullu S, Sav H, Kamel N.. Effects of levothyroxine treatment on biochemical and hemostasis parameters in patients with hypothyroidism. Eur J Endocrinol. 2005;152(3):355–61. doi: 10.1530/eje.1.01857. [DOI] [PubMed] [Google Scholar]
- 16.Kahn HS.. The Lipid Accumulation Product Is Better Than BMI for Identifying Diabetes. Diabetes Care. 2005;29(1) doi: 10.2337/diacare.29.1.151. [DOI] [PubMed] [Google Scholar]
- 17.Lakka H-M, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J. et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 18.Wallace TM, Matthews DR.. The assessment of insulin resistance in man. Diabet Med. 2002;19(7):527–34. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- 19.Pekmezovic T, Kisic Tepavcevic D, Kostic J, Drulovic J.. Validation and cross-cultural adaptation of the diseasespecific questionnaire MSQOL-54 in Serbian multiple sclerosis patients sample. Qual Life Res. 2007;16(8):1383–7. doi: 10.1007/s11136-007-9234-0. [DOI] [PubMed] [Google Scholar]
- 20.Basta I, Pekmezović T, Padua L, Stojanović V, Stević Z, Nikolić A. et al. Validation of Serbian version of the disease-specific myasthenia gravis questionnaire. Acta Neurol Scand. 2010;122(2):110–4. doi: 10.1111/j.1600-0404.2009.01269.x. [DOI] [PubMed] [Google Scholar]
- 21.Bose A, Sharma N, Hemvani N, Chitnis DS.. A Hospital Based Prevalence Study on Thyroid Disorders in Malwa region of Central India. Int J Curr Microbiol App Sci. 2015;4(6):604–11. [Google Scholar]
- 22.Cerit ET, Akturk M, Altinova AE, Tavil Y, Ozkan C, Yayla C. et al. Evaluation of body composition changes, epicardial adipose tissue, and serum omentin-1 levels in overt hypothyroidism. Endocrine. 2015;49(1):196–203. doi: 10.1007/s12020-014-0460-2. [DOI] [PubMed] [Google Scholar]
- 23.Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. J Endocrinol Invest. 7. Vol. 34. Springer International Publishing; Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters; pp. 488–92. [DOI] [PubMed] [Google Scholar]
- 24.Kottagi SS, Rathi DB, Dongre NN.. Evaluation of LDLCholesterol / HDL-Cholesterol Ratio as Predictor of Dyslipidemia in Subclinical Hypothyroidism. Journal of Krishna Institute of Medical Sciences University. 2014;3(1):34–40. [Google Scholar]
- 25.Marchiori RC, Pereira LAF, Naujorks AA, Rovaris DL, Meinerz DF, Duarte MMMF. et al. Improvement of blood inflammatory marker levels in patients with hypothyroidism under levothyroxine treatment. BMC Endocr Disord. 2015;15(1):32. doi: 10.1186/s12902-015-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksoy DY, Cinar N, Harmanci A, Karakaya J, Yildiz BO, Usman A. et al. Serum resistin and high sensitive CRP levels in patients with subclinical hypothyroidism before and after L-thyroxine therapy. Med Sci Monit. International Scientific Literature, Inc.; 2013;19:210–5. doi: 10.12659/MSM.883847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monzani F, Caraccio N, Kozàkowà M, Dardano A, Vittone F, Virdis A. et al. Effect of Levothyroxine Replacement on Lipid Profile and Intima-Media Thickness in Subclinical Hypothyroidism: A Double-Blind, Placebo-Controlled Study. J Clin Endocrinol Metab. 2004;89(5):2099–106. doi: 10.1210/jc.2003-031669. [DOI] [PubMed] [Google Scholar]
- 28.Gunduz M, Gunduz E, Kircelli F, Okur N, Ozkaya M.. Role of surrogate markers of atherosclerosis in clinical and subclinical thyroidism. Int J Endocrinol. Hindawi Publishing Corporation. 2012;2012:109797. doi: 10.1155/2012/109797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav A, Arora S, Saini V, Arora MK, Singh R, Bhattacharjee J.. Influence of thyroid hormones on biochemical parameters of liver function: a case-control study in North Indian population. Internet J Med Updat. 2013;8(1):4–8. [Google Scholar]
- 30.Christ-Crain M, Meier C, Huber P, Zulewski H, Staub J-J, Müller B.. Effect of restoration of euthyroidism on peripheral blood cells and erythropoietin in women with subclinical hypothyroidism. Hormones (Athens) 2(4):237–42. doi: 10.14310/horm.2002.11105. [DOI] [PubMed] [Google Scholar]
- 31.Chadarevian R, Bruckert E, Giral P, Turpin G.. Relationship between thyroid hormones and fibrinogen levels. Blood Coagul Fibrinolysis. 1999;10(8):481–6. doi: 10.1097/00001721-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Chadarevian R, Bruckert E, Leenhardt L, Giral P, Ankri A, Turpin G.. Components of the Fibrinolytic System Are Differently Altered in Moderate and Severe Hypo thyroidism. J Clin Endocrinol Metab. 2001;86(2):732–7. doi: 10.1210/jcem.86.2.7221. [DOI] [PubMed] [Google Scholar]
- 33.Cantürk Z, Çetinarslan B, Tarkun I, Cantürk NZ, Özden M, Duman C.. Hemostatic System as a Risk Factor for Cardiovascular Disease in Women with Subclinical Hypothyroidism. Thyroid. 2003;13(10):971–7. doi: 10.1089/105072503322511382. [DOI] [PubMed] [Google Scholar]
- 34.Lioudaki E, Mavroeidi NG, Mikhailidis DP, Ganotakis ES.. Subclinical hypothyroidism and vascular risk: an update. Hormones (Athens) 12(4):495–506. doi: 10.14310/horm.2002.1437. [DOI] [PubMed] [Google Scholar]
- 35.Al Sayed A, Al Ali N, Bo Abbas Y, Alfadhli E.. Subclinical hypothyroidism is associated with early insulin resistance in Kuwaiti women. Endocr J. 2006;53(5):653–7. doi: 10.1507/endocrj.k06-018. [DOI] [PubMed] [Google Scholar]
- 36.Dessein PH, Joffe BI, Stanwix AE.. Subclinical Hypothyroidism is Associated with Insulin Resistance in Rheuma toid Arthritis. Thyroid. Mary Ann Liebert, Inc.; 2004;14(6):443–6. doi: 10.1089/105072504323150750. [DOI] [PubMed] [Google Scholar]
- 37.Tuzcu A, Bahceci M, Gokalp D, Tuzun Y, Gunes K.. Subclinical hypothyroidism may be associated with elevated high-sensitive c-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr J. 2005;52(1):89–94. doi: 10.1507/endocrj.52.89. [DOI] [PubMed] [Google Scholar]
- 38.Wang C-Y, Chang T-C, Chen M-F.. Associations between subclinical thyroid disease and metabolic syndrome. Endocr J. 2012;59(10):911–7. doi: 10.1507/endocrj.ej12-0076. [DOI] [PubMed] [Google Scholar]
- 39.Anagnostis P, Efstathiadou ZA, Slavakis A, Selalmatzidou D, Poulasouchidou M, Katergari S. et al. The effect of Lthyroxine substitution on lipid profile, glucose homeostasis, inflammation and coagulation in patients with subclinical hypothyroidism. Int J Clin Pract. 2014;68(7):857–63. doi: 10.1111/ijcp.12394. [DOI] [PubMed] [Google Scholar]
- 40.Garduno-Garcia J d. J, Alvirde-Garcia U, Lopez-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O. et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163(2):273–8. doi: 10.1530/EJE-10-0312. [DOI] [PubMed] [Google Scholar]
- 41.Priyanka G., Rakshasmare PNJ.. Apolipoprotein b / apolipoprotein a1 ratio: an improved marker of cardiovascular risk in hypothyroidism. Int J Curr Res Rev. 2014;6(10):86–9. [Google Scholar]
- 42.Cheserak MJ, Wu GR, Ntazinda A, Shi YH, Shen LY, Le GW.. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;35:323–31. doi: 10.2478/jomb-2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsimihodimos V, Bairaktari E, Tzallas C, Miltiadus G, Liberopoulos E.. The Incidence of Thyroid Function Abnormalities in Patients Attending an Outpatient Lipid Clinic. Thyroid. 1999;9(4):365–8. doi: 10.1089/thy.1999.9.365. [DOI] [PubMed] [Google Scholar]
- 44.Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M.. Changes in Lipoprotein(a) Levels in Overt and Subclinical Hypothyroidism Before and During Treatment. Thyroid. 2000;10(9):803–8. doi: 10.1089/thy.2000.10.803. [DOI] [PubMed] [Google Scholar]
- 45.Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I. et al. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf). NIH Public Access. 2012;76(6):911–8. doi: 10.1111/j.1365-2265.2011.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J. et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf) 2010;72(5):696–701. doi: 10.1111/j.1365-2265.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 47.Bianchi GP, Zaccheroni V, Solaroli E, Vescini F, Cerutti R, Zoli M. et al. Health-related quality of life in patients with thyroid disorders. Qual Life Res. 2004;13(1):45–54. doi: 10.1023/B:QURE.0000015315.35184.66. [DOI] [PubMed] [Google Scholar]
- 48.Harris B, Othman S, Davies JA, Weppner GJ, Richards CJ, Newcombe RG. et al. Association between postpartum thyroid dysfunction and thyroid antibodies and depression. BMJ. BMJ Group. 1992;305(6846):152–6. doi: 10.1136/bmj.305.6846.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park YJ, Lee EJ, Lee YJ, Choi SH, Park JH, Lee SB. et al. Subclinical hypothyroidism (SCH) is not associated with metabolic derangement, cognitive impairment, depression or poor quality of life (QoL) in elderly subjects. Arch Gerontol Geriatr. 50:e68–73. doi: 10.1016/j.archger.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry J-F. et al. Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Thyroid. 2003;13(1):3–3. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 51.Wiersinga WM DL. DeGroot LJ HG. Thyroid disease manager. Dartmouth, MA: Endocrine Education; 2002. Adult hypothyroidism. [Google Scholar]
- 52.Toft AD, Beckett GJ.. Thyroid function tests and hypothyroidism. BMJ. BMJ Group. 2003;326(7384):295–6. doi: 10.1136/bmj.326.7384.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts CG, Ladenson PW.. Hypothyroidism. Lancet. 2004;363(9411):793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]