Summary
Background
In this study, the effects of olmesartan therapy on asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), L-arginine and inducible nitric oxide synthase (iNOS) levels were investigated in patients undergoing cardiopulmonary bypass.
Methods
Patients were randomly allocated to two groups, control and olmesartan. Olmesartan was administered 30 mg once a day beginning from preoperative day 5 to postoperative day 28 and on operation day. Blood was drawn from all patients and ADMA, SDMA, L-arginine and iNOS levels were analyzed at six time points (T1: before anesthesia induction, T2: during cardiopulmonary bypass, T3: five min after the cross-clamp was removed, T4: after protamine infusion, T5: on postoperative day 3 and T6: on postoperative day 28).
Results
In the olmesartan treated group, iNOS levels exhibited significant decreases at T2, T3, T4, T5 and T6 time points compared with control group (p<0.001, p<0.05, p<0.001, p<0.01, p<0.05 respectively). ADMA levels were significantly lower in olmesartan treated group than in control group at T3, T4, T5 and T6 time points (p<0.05, p<0.05, p<0.05, p<0.01 respectively). SDMA levels at T2, T3 and T6 time points were higher in control group than olmesartan group. L-Arginine levels were significantly higher at T2 and T3 time points in olmesartan treated group than control group (p<0.001, p<0.01).
Conclusions
It was concluded that administration of olmesartan reduced plasma ADMA, SDMA, iNOS levels and enhanced L-arginine level in CPB time and it could reduce potential postoperative complications through reducing oxidative stress and inflammatory response in the postoperative period after coronary bypass surgery.
Keywords: asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), L-arginine, cardiopulmonary bypass (CPB), renin–angiotensin system
Kratak sadržaj
Uvod
U ovoj studiji ispitivan je uticaj terapije olmesartanom na nivoe asimetričnog dimetilarginina (ADMA), simetričnog dimetilarginina (SDMA), L-arginina i inducibilne azot-oksid sintaze (iNOS) kod pacijenata podvrgnutih kardiopulmonarnom bajpasu.
Metode
Pacijenti su nasumično podeljeni u dve grupe, kontrolnu i lečenu olmesartanom. Grupa olmesartan dobijala je 30 mg jednom dnevno počev od petog dana pre operacije do 28. dana posle operacije i na dan operacije. Uzorci krvi sakupljeni su od svih pacijenata i analizirani su nivoi ADMA, SDMA, L-arginina i iNOS u šest vremenskih tačaka (T1: pre davanja anestezije, T2: tokom kardiopulmonarnog bajpasa, T3: pet minuta pošto je uklonjena aortna klema, T4: posle infuzije protamina, T5: trećeg dana posle operacije i T6: 28. dana posle operacije.
Rezultati
U grupi lečenoj olmesartanom nivoi iNOS pokazali su značajan pad u tačkama T2, T3, T4, T5 i T6 u poređenju s kontrolnom grupom (p<0,001, p<0,05, p<0,001, p<0,01, p<0,05). Nivoi ADMA bili su značajno niži u grupi tretiranoj olmesartanom nego u kontrolnoj grupi u vremenskim tačkama T3, T4, T5 i T6 (p<0,05, p<0,05, p<0,05, p<0,01). Nivoi SDMA u vremenskim tačkama T2, T3 i T6 bili su viši u kontrolnoj nego u grupi tretiranoj olmesartanom. Nivoi L-arginina bili su u vremenskim tačkama T2 i T3 značajno viši u grupi koja je dobijala olmesartan u odnosu na kontrolnu grupu (p<0,001, p<0,01).
Zaključak
Zaključeno je da primena olmesartana snižva nivoe ADMA, SDMA i iNOS u plazmi i podiže nivo L-arginina u vreme kardiopulmonarnog bajpasa te da bi mogla redukovati potencijalne postoperativne komplikacije kroz smanjenje oksidativnog stresa i inflamatornog odgovora u postoperativnom toku posle operacije koronarnog bajpasa.
Introduction
Cardiopulmonary bypass (CPB) is an important part of many cardiothoracic procedures but it is known to be related to an excessive undesirable systemic inflammatory response and the cardiac biomarkers released in reaction to CPB and surgical trauma. During extracorporeal circulation, blood and its elements are continuously in contact with artificial surfaces (1). As a result, the activation of leukocytes, production of free oxygen radicals, arachidonic acid metabolites, platelet-activating factor (PAF), nitric oxide cause a systemic inflammatory response. There is a crucial link between the systemic inflammatory response and several postoperative complications such as respiratory failure, pulmonary damage and brain damage.
Upregulation of inducible nitric oxide synthase (iNOS) is induced by proinflammatory mediators such as interferon (IFN) regulatory factor-1, nuclear factor- κB (NF-κB), IL6 and tumor necrosis factor α (TNF-α) (2). Myocardial iNOS2-derived NO, as a source of myocardial ROS may contribute to the formation of lethal brady-arrhythmia, LV hypertrophy and dilatation (3). Induction of iNOS was identified during CPB (4).
Many experimental and clinical studies have demonstrated that increases in the renin–angiotensin–aldosterone system (RAAS) activity stimulate development, instability and rupture of atherosclerotic plaques by the activation of vascular inflammatory response (5). In addition, angiotensin II (ang II) impairs NO bioavailability and induces oxidative stress, resulting in endothelial dysfunction. Moreover, RAAS might be activated during cardiac surgery (6).
L-arginine is a foremost player in the regulation of vascular health and homeostasis, because arginine is essential in the synthesis pathway of NO (7). In this regard, deficiency of arginine contributes to the development of high blood pressure and endothelial cell dysfunction. Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (NOS), is produced by protein N-arginine methyltransferase (PRMT) and dimethylarginine dimethylaminohydrolase (DDAH) catalyzes the hydrolysis of ADMA (8). Type II PRMT produce symmetric dimethylarginine (SDMA) (9). SDMA is not able to inhibit NOS. Oxidative stress increases the activity of PRMT and attenuates induction of DDAH. ADMA diminishes NO bioavailability, leading to disturbed vasodilatation, antithrombotic, antiinflammatory and antiapoptotic actions that are associated with cardiovascular pathology. ADMA is taken up by endothelial cells and this competitive action inhibits the cellular uptake of arginine and NO production. On the other hand, increased concentrations of arginine may prevent harmful effects of ADMA (10). High ADMA levels have been determined in various cardiac diseases such as atherosclerosis, hypertension, diabetes. It has been reported that ADMA has prognostic capacities for disease progression and mortality in heart failure patients (11).
In this study, we investigated the effect of an angiotensin type II receptor antagonist (olmesartan) on ADMA, SDMA, iNOS and arginine serum levels during coronary artery bypass grafting done with CPB technique at six different time points, and thus we aimed to attempt different prophylactic strategies to improve clinical outcomes following CPB.
Materials and Methods
This clinical study was approved by the Local Ethics Committee. Informed consent was obtained. The study was performed in conformance with the Declaration of Helsinki ethical guidelines.
Patients
This prospective randomized study included 50 patients who were aged between 30 and 80. We excluded patients who had thyroid disease, congestive heart failure, chronic renal failure, liver failure, coagulation disorder, hypertension, diabetes mellitus, chronic lung disease, active infections or malignancy; patients who had undergone heart surgery or reoperations due to myocardial infarction in the previous month; patients who had ejection fraction less than 30% or heart rate less than 60/min; patients who were being treated with corticosteroids, salicylates, dipyridamole, anticoagulants or thrombolytics. We included all patients who had coronary artery disease and all of them were taken into the program for elective coronary bypass surgery.
The 50 patients included in the study were divided into 2 study groups. 1) Control group (n=25): no drug was given to patients. 2) Olmesartan group (n=25): olmesartan was started with 10 mg/day orally 5 days before surgery, 30 mg was given on the day of surgery, and 10 mg/day was given after surgery during 35 days.
Surgical procedures
Anesthesia induction and maintenance were similar for all patients and consisted of weight-appropriate doses of midazolam (0.1 mg/kg induction; 0.8 μg/kg/min maintenance), vecuronium (0.1 mg/kg induction) and fentanyl (20–40 μg/kg induction; 0.3–1 μg/kg/min maintenance).
The surgical approach was always performed through a standard median sternotomy. Vena saphena magna and the left internal mammary artery were prepared for use as a graft. The patients were anticoagulated with 3 mg/kg of heparin (Nevparin, Mustafa Nevzat). After heparinization the ascending aorta was cannulated with an arterial cannula, a two-stage venous cannula and an antegrade cardioplegia catheter was placed in the right atrium. When the activated clotting time (ACT) was greater than 450 seconds, CPB was partially initiated. Pulsatile roller pump (Stockert Instrumente, Germany) and a membrane type oxygenator (Dideco D 708 simplex III, Italy) were used. The pump prime solution contained 2000 mL of lactated Ringer’s solution to maintain hematocrit at a level of 20%. The body temperature of the patient was cooled down to 28–30 °C. Following cardiac arrest, an aortic cross-clamp was placed (full CPB). Cold crystalloid cardioplegic solution (15 mL/kg, 4 °C) was administered via an antegrade cardioplegia catheter at 100–110 mm Hg pressure. Prepared in cold blood cardioplegia (200 ml + 800 mL of blood Plegisol, Hb: 80 g/L), it was administered at 400 mL every 20 minutes in patients weighing less than 80 kg; 500 mL was administered in patients over 80 kg. Pump flow was set at 2.2–2.4 L/m/m2 to maintain the mean pulsatile arterial pressure between 50–80 mmHg.
According to coronary artery involvement, before the right coronary artery, the circumflex artery and its branches and left internal mammary artery were anastomosized to the anterior descending artery. All proximal anastomoses were performed under cross-clamping except for the left internal mammary artery. After all anastomoses the hot (36 °C) blood cardioplegia (hot shot, 3–4 min then 500 mL) was administered before removing the cross-clamp. When the blood pressure and temperature returned to normal, CPB was terminated. Arterial and venous cannulas were neutralized with heparin protamine sulfate (protamine, Roche) and then removed.
After epicardial pacing wires and chest tubes were placed, sternal incision was then closed and the patients were transferred to an intensive care unit.
Biochemical analysis
In all patients, to evaluate the levels of ADMA, SDMA, arginine, iNOS and hsCRP blood samples were collected at six different time points. T1: before anesthesia induction, T2: during cardiopulmonary bypass, T3: five min after the cross-clamp was removed, T4: after protamine infusion, T5: postoperative day 3 and T6: postoperative day 28.
Five mL of blood was taken for each tube and collected in tubes with ethylenediaminetetraacetic acid (EDTA). To measure plasma levels of ADMA, SDMA, L-arginine and iNOS, the blood samples were centrifuged for 15 min at 1000 rpm at 4 °C and frozen at −80 °C until assayed.
ADMA, SDMA and L-arginine levels were measured using the High Performance Liquid Chromatography (HPLC) method via EUREKA brand test kits (Catalog No.: Z58010). The MS was operated in positive multiple reaction mode (MRM). The assay was linear over the range 50–1000 ng/mL. Within-batch precision at 100 ng/mL was < 10% (n = 138) and the between-batch precision was < 8%.
Levels of iNOS were determined with an Enzyme- Linked Immunosorbent Assay (ELISA) method using CUSAB O (Catalog No.: CSB-E08148h) brand test kits.
In addition, in all patients chest X-ray, complete blood tests (hemoglobin, hematocrit, leukocytes, platelets), blood sedimentation rate, and routine biochemical (glucose, total, AST, ALT, urea, creatinine, Na+, K+, Cl- and Ca2+) examinations were performed in the preoperative and postoperative period. Also, in the preoperative and intraoperative period and during the first 24 hours after operation blood gas parameters were measured.
Statistical analysis
Data were analyzed by using a commercially available statistical software package (SPSS 15). The Mann–Whitney U test was used to compare groups at different time points. Intragroup comparisons were performed using Wilcoxon test. Data are shown as mean values ± standard deviation (SD); p<0.05 was regarded as statistically significant.
Results
Table I shows the demographic characteristics of patients. There were no significant differences between the two groups including age, sex, weight, length, the number of coronary artery bypass grafting, cardiopulmonary bypass time and cross-clamp time.
Table I.
Patients’ characteristics.
| Study groups | Group K (n = 25) | Group O (n=25) |
|---|---|---|
| Male sex | 18 (% 79.2) | 18 (% 79.2) |
| Age | 60.64±10.53 | 63.36±10.73 |
| Weight (kg) | 71.16±8.52 | 71.56±8.45 |
| Height (cm) | 168.4±7.69 | 168.36±7.53 |
| Total CABG number | 3.2±0.86 | 3.08±0.9 |
| Total CPB time (min) | 80.2±12.58 | 80.72±12.34 |
| Cross-clamp time (min) | 50.12±4.24 | 50.40±4.60 |
CABG: Coronary artery bypass grafting, CPB: cardiopulmonary bypass.
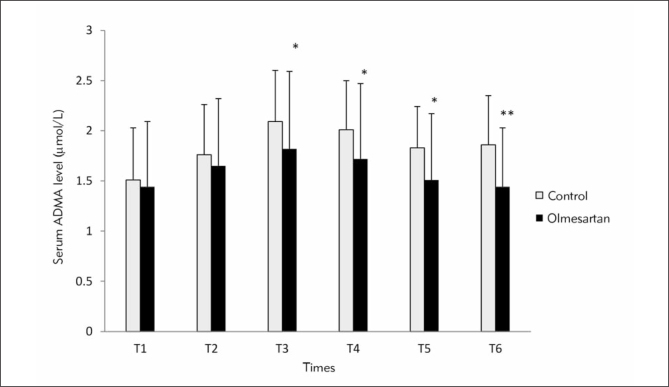
In Figure 1, comparison of ADMA levels is shown between control and olmesartan treatment groups at six different time points. In olmesartan treatment group, ADMA levels were significantly lower than in control group at T3, T4, T5 and T6 (p<0.05, p<0.05, p<0.05, p<0.01 respectively). Intra-group comparison of ADMA levels revealed significant elevation at time points T2, T3, T4, T5 and T6 compared with T1 time point in control group (p<0.001; p<0.001; p<0.001; p<0.001 and p<0.0l respectively). In the olmesartan treatment group, serum levels of ADMA were significantly higher at time points T2, T3 and T4 compared with T1 time point (p<0.01; p<0.001; p<0.01 respectively).
Figure 1.
Comparison of ADMA levels (μmol/L) between the groups at six time points. (*) Compared with control group. * p<0.05; **p<0.01
(T1: before anesthesia induction, T2: during cardiopulmonary bypass, T3: five min after the cross-clamp was removed, T4: after protamine infusion, T5: postoperative day 3 and T6: postoperative day 28)
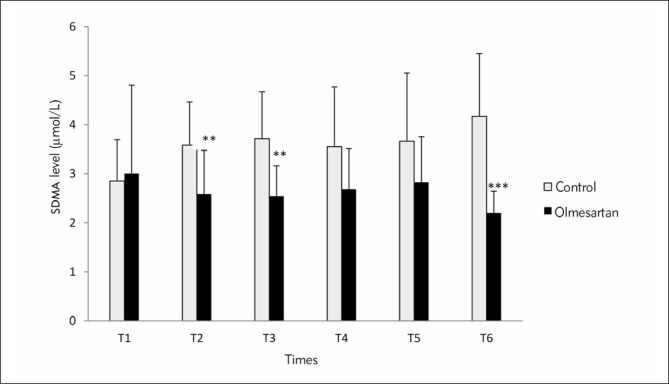
In Figure 2, comparison of SDMA levels is shown between the control and olmesartan treatment groups at six different time points. SDMA levels at T2, T3 and T6 time points were significantly higher in the control group than the olmesartan treatment group (p<0.01, p<0.01, p<0.001 respectively). Intragroup comparisons of SDMA levels exhibited significant elevations at T2, T3, T4, T5 and T6 (p<0.001, p<0.001, p<0.05, p<0.05, p<0.01 respectively) in the control group. SDMA revealed significant reduction at T6 compared with T1 time point in the olmesartan treatment group (p<0.05).
Figure 2.
Comparison of SDMA levels (μmol/L) between the groups at six time points (*) Compared with control. *p<0.05; **p<0.01; ***p<0.001
(T1: before anesthesia induction, T2: during cardiopulmonary bypass, T3: five min after the cross-clamp was removed, T4: after protamine infusion, T5: postoperative day 3 and T6: postoperative day 28)
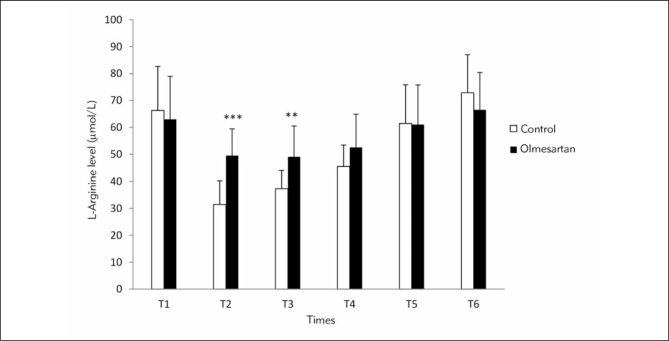
Figure 3 shows the comparison of L-arginine levels between the control and olmesartan treatment groups at the six different time points. L-arginine levels were significantly higher at T2 and T3 time points in the olmesartan treatment group than control group (p<0.001, p<0.01). Intra-group comparisons of L-arginine levels exhibited significant reduction at T2, T3 and T4 (p<0.001) and significant elevation at T6 compared with T1 time point in control group (p<0.01, Figure 4). In the olmesartan treatment group, L-arginine levels were decreased at T2, T3 and T4 time points (p<0.001) and increased at T6 compared with T1 (p<0.01).
Figure 3.
Comparison of L-arginine levels (μmol/L) between the groups at six time points (*) Compared with control. *p<0.05; **p<0.01; ***p<0.001
(T1: before anesthesia induction, T2: during cardiopulmonary bypass, T3: five min after the cross-clamp was removed, T4: after protamine infusion, T5: postoperative day 3 and T6: postoperative day 28)
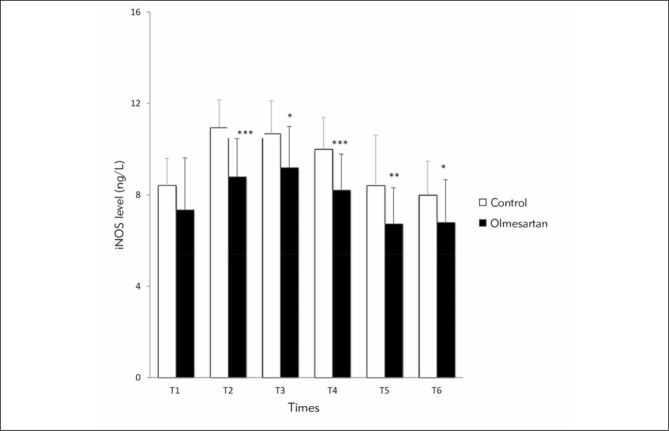
Figure 4.
Comparison of iNOS levels between the groups at six time points (*) Compared with control group. * p<0.05; **p<0.01; ***p<0.001
(T1: before anesthesia induction, T2: during cardiopulmonary bypass, T3: five min after the cross-clamp was removed, T4: after protamine infusion, T5: postoperative day 3 and T6: postoperative day 28)
In Figure 4, comparison of iNOS levels is shown between the control and the olmesartan treatment group at six different time points. In the olmesartan treatment group, iNOS levels exhibited significant decrease at time points T2, T3, T4, T5 and T6 compared with the control group (p<0.001, p<0.05, p<0.001, p<0.01, p<0.05 respectively). Intra-group comparisons of iNOS levels revealed significant elevation at T2, T3 and T4 compared with the T1 time point in control group (p<0.001). In the olmesartan treatment group, iNOS levels were increased at T2 and T3 time points (p<0.01); by contrast, iNOS level was significantly decreased at T5 compared with the T1 time point (p<0.05).
Discussion
Cardiopulmonary bypass-assisted surgery induces a systemic inflammatory response via extrinsic and intrinsic factors such as anesthesia, endothelial cell activation, tissue damage, contact activation within the extracorporeal circuit, endotoxemia and ischemiareperfusion injury of the myocardium (12, 13). The systemic inflammatory response and the super-imposed period of ischemia-reperfusion are situations that enhance the production of oxygen-derived free radical species, which promote lipid peroxidation and a series of events that cause cell membrane damage, tissue injury, functional impairment and postoperative complications such as respiratory failure, pulmonary damage, cognitive dysfunction and brain damage (14). Moreover, these complications contribute to the mortality and failure of cardiopulmonary bypass. Hence, to prevent these complications, the importance of pharmacological treatment strategies has been increasingly emphasized.
Increased renin–angiotensin–aldosterone system activity is associated with various cardiac diseases such as hypertension, atherosclerosis, congestive heart failure, type 2 diabetes mellitus, and renal disease (15). Angiotensin II, the major effector peptide of the renin–angiotensin–aldosterone system, promotes generation of oxidative radicals via AT1 receptors and initiates inflammatory processes including atherosclerosis and vascular ageing. Inflammation is a key mechanism in the advent and perpetuation of cardiovascular diseases especially atherogenesis. Likewise, there is a significant positive correlation between oxidative stress and many cardiovascular pathological conditions. Moreover, it has been found that proinflammatory cytokines such as tumor necrosis factor (TNF), IL-1β and growth factors such as platelet derived growth factor (PDGF) and basic fibroblast growth factor might provoke production of free radical species. It has been indicated that the renin–angiotensin system might be activated during cardiac surgery under CPB as evaluated by an increased angiotensin II plasma concentration (6).
Asymmetrical dimethylarginine (ADMA) is a risk factor for cardiovascular diseases including hypertension, coronary artery disease, peripheral arterial occlusive disease, pulmonary hypertension, and preeclampsia (16). In addition, it has been found that ADMA predicts clinical outcome in patients with coronary heart disease (17). Oxidative stress may also induce production of ADMA as evidenced by the increased expression of PRMT1 in endothelial cells exposed to excess of ROS. DDAH may also be inactivated by ROS and TNF-α. In addition, there is growing evidence that ADMA is not only a risk factor for endothelial dysfunction but also a novel proinflammatory mediator (18). It has been established that ADMA may induce a vascular inflammation reaction to promote the development of cardiovascular diseases by the activation of leukocyte adhesion and cytokines production (19, 20). Therefore, ADMA may be a therapeutic target for preventing inflammation and its complications due to cardiac surgery.
This is the first study to show the serum levels of ADMA, SDMA, L-arginine and iNOS at six time points associated with CPB and the effects of olmesartan therapy on these parameters. In the present study, when ADMA levels were compared between control and olmesartan treatment groups, they were found to be significantly lower in olmesartan than control at the time points after cross-clamping, after protamine infusion, and on postoperative days 3 and 28. Valkonen et al. (21) determined that the risk for acute coronary events is 3.9-fold increased with the highest plasma levels of ADMA in a prospective case-control study including middle-aged, nonsmoking men. In mice, marked accumulation of ADMA was observed in myocardial tissue after ischemia reperfusion injury and it was argued that ADMA could be a potential new target for the treatment of myocardial ischemia reperfusion injury (22). In elderly people with high ADMA, simvastatin treatment did not ameliorate endothelium-dependent vasodilation but administration of L-arginine alone or in combination with simvastatin enhanced endothelial function (23). In the current study, ADMA level was significantly increased at CPB time, after cross-clamping and protamine infusion, and on postoperative days 3 and 28 in the control group. It has been proposed that this result may be associated with enhancement of oxidative stress and inflammatory mediators via ischemia/reperfusion injury during cardiac surgery. In patients with essential hypertension, treatment with perindopril (ACE) (angiotensin-converting enzyme), inhibitor losartan (AT1 receptor antagonist) and bisoprolol (β-blocker) decreased blood pressure similarly, but only losartan or perindopril decreased serum ADMA and hence it has been put forward that RAAS may contribute to the elevation of serum ADMA (24).
In the olmesartan treatment group, ADMA level rose at CPB time, after cross-clamping and protamine infusion compared with before induction of anesthesia but these increases were not as high as in the control group. In a previous investigation, infusion of ADMA in wild type rats caused significant coronary microvascular lesions, and supplementation of L-arginine did not prohibit the development of lesions, in contrast to olmesartan treatment that suppressed the vascular effects of ADMA (25). In the present study, we have demonstrated that olmesartan treatment reduced the SDMA level at CPB time, after cross-clamping and on postoperative day 28 compared with control group. In patients with chronic kidney disease, elevation of serum SDMA and ADMA was found and there was a positive correlation between these results and cardiovascular events (26). Moreover, accumulation of serum SDMA concentrations may play an additive role for the renal outcome besides serum creatinine and hemoglobin levels.
In the olmesartan treatment group the SDMA level was significantly decreased on postoperative day 28. By contrast, SDMA level was significantly elevated in the control group at CPB time, after cross-clamping and protamine infusion, and on postoperative days 3 and 28. This finding may suggest that there is a positive correlation between oxidative stress, inflammation and SDMA. In a previous study it was demonstrated that concentrations of ADMA and SDMA were significantly correlated with each other and it was proposed that ADMA was not better than SDMA in estimating cardiovascular diseases risk in the general population (27).
L-arginine is a substrate for the generation of NO so it is a key chemical in cardiovascular health (28). Dysfunction of the endothelial L-arginine–nitric oxide pathway is a common mechanism for cardiovascular diseases. We have observed that in the olmesartan treatment group L-arginine levels were significantly higher than in the control group at CPB time and after cross-clamping. Diminished L-arginine levels were identified in cardiovascular diseases in previous examinations (29). Furthermore, it was demonstrated that oxidative stress enhanced the activity of arginase enzyme which converts arginine to ornithine and confines NO bioavailability in endothelial cells through increased arginine consumption (28–30). In another publication, it has been argued that plasma levels of ADMA, SDMA, L-arginine and the L-arginine/ADMA ratio are reliable and feasible indicators of early ischemia-reperfusion injury (31). We found that in the control group, L-arginine levels decreased at CPB time, after cross-clamping and protamine infusion and increased on postoperative day 28. In the olmesartan group, L-arginine levels decreased at CPB time, after cross-clamping and protamine infusion and increased on postoperative day 28 but the decline of L-arginine level was not as pronounced as in the control group. Our findings suggest that there may be a negative correlation between oxidative stress and L-arginine at CPB time points.
iNOS levels were significantly lower in the olmesartan group than in control at CPB time, after crossclamping and protamine infusion, and on postoperative days 3 and 28. Antiinflammatory effects of olmesartan may reduce iNOS levels. Our results are in agreement with previous examinations. In a previous study, myocardial infarction induced left ventricular damage was reduced in iNOS deficient mice compared with wild type (32). In the left ventricular (LV) tissue of 24 patients with end-stage heart failure, iNOS expression levels were increased and high iNOS activity was correlated with early relaxation and impaired responsiveness to β-adrenergic stimulation; moreover, the inotropic response to isoproterenol in failing hearts was inversely related to iNOS activity (33).
We have observed that in the control group, iNOS level was significantly increased at CPB time, after cross-clamping and protamine infusion, and on postoperative days 3 and 28. Moreover, in the olmesartan group iNOS was increased at CPB time and after cross-clamping but this enhancement was lower than in the control group and the iNOS level was decreased by the 3rd day postoperatively. These results were similar to previous studies.
Zhang et al. (34) determined that iNOS-deficient mice presented much less hypertrophy, dilation, fibrosis, and dysfunction. In an experimental animal model, L-NAME administration induced the expression of iNOS in the aorta; furthermore, ICAM-1 and VCAM-1 expression was also increased in the aortas of L-NAME–treated rats. These changes of the vascular wall were prohibited by the angiotensin II antagonist irbesartan (35). Kataoka et al. (36) suggested that AT1 receptor antagonists are able to improve TNF-α-dependent eNOS reduction or cell injury by inhibiting superoxide production or nuclear factor-κB activation. Yaylak et al. (37) demonstrated that lipopolysaccharide (LPS) induced hepatic injury was ameliorated by the selective iNOS inhibitor aminoguanidine. In left ventricular biopsies from two-vessel disease unstable angina and stable angina patients undergoing coronary bypass surgery, the expression levels of TNF-α, IL-6 IFN-gamma and iNOS were upregulated, and ramipril and valsartan treatment significantly decreased the expression levels of TNF-α, IL-6, IFN-gamma and iNOS (38). These data support our findings. In patients with coronary artery disease AT1 blockade reduced hsCRP IL-6, and platelet aggregation and this effect of AT1-blockade was stronger than ACE inhibition (39).
Conclusions
The current study is the first that has demonstrated the levels of novel and classical parameters associated with inflammation and oxidative stress such as ADMA, SDMA, L-arginine, and iNOS at six time points of CPB and the effects of olmesartan on these parameters.
We have observed elevation of ADMA, SDMA, iNOS and reduction of L-arginine during cardiac surgery. These results have been attributed to enhancement of oxidative stress and inflammatory response. In this regard, these parameters may participate in the formation of postoperative complications. The application of olmesartan reduced serum ADMA, SDMA, iNOS and enhanced L-arginine levels at CPB time points. It was concluded that olmesartan could reduce potential postoperative complications through diminishing oxidative stress and inflammatory response in the postoperative period following coronary bypass surgery. Preoperative and postoperative therapy with RAAS blockers could be a new strategy in reducing postoperative morbidity and mortality following CPB but further studies are required to confirm this hypothesis.
Acknowledgement
We would like to thank Firat University Scientific Research Projects Coordination Unit (FUBAP) for their financial support.
Glossary
List of abbreviations
- ACE
angiotensin converting enzyme
- ADMA
asymmetric dimethylarginine
- Ang II
angiotensin II
- ARB
angiotensin receptor blocker
- AT1
angiotensin II type 1 receptor
- AT2
angio tensin II type 2 receptor
- CPB
cardiopulmonary bypass
- DDAH
dimethylarginine dimethylaminohydrolase
- IFN
interferon
- iNOS
inducible nitric oxide synthase
- L-NAME
NGmonomethyl-L-arginine methyl ester
- NO
nitric oxide
- PDGF
platelet derived growth factor
- PRMT
protein N-arginine methyl transferase
- RAAS
renin–angiotensin–aldosterone system
- SDMA
symmetric dimethylarginine
- TNF a
TNF α, tumor necrosis factor alpha
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Bayram H, Erer D, Iriz E, Hakan Zor M, Gulbahar O, Ozdogan ME. Comparison of the effects of pulsatile cardiopulmonary bypass, non-pulsatile cardiopulmonary bypass and off-pump coronary artery bypass grafting on the respiratory system and serum carbonyl. Perfusion. 2012;27:378–85. doi: 10.1177/0267659112449036. [DOI] [PubMed] [Google Scholar]
- 2.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–82. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 3.Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010;333:191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Inducible nitric oxide production is an adaptation to cardiopulmonary bypass-induced inflammatory response. Ann Thorac Surg. 2001;72:149–55. doi: 10.1016/s0003-4975(01)02637-6. [DOI] [PubMed] [Google Scholar]
- 5.Mason RP. Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: focus on olmesartan medoxomil. Vasc Health Risk Manag. 2011;7:405–16. doi: 10.2147/VHRM.S20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasocki S, Iglarz M, Seince PF, Vuillaumier-Barrot S, Vicaut E, Henrion D. et al. Involvement of reninangiotensin system in pressure-flow relationship: role of angiotensin-converting enzyme gene polymorphism. Anesthesiology. 2002;96:271–5. doi: 10.1097/00000542-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Vasdev S, Gill V. The antihypertensive effect of arginine. Int J Angiol. 2008;17:7–22. doi: 10.1055/s-0031-1278274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki H. Activities of asymmetric dimethylargininerelated enzymes in white adipose tissue are associated with circulating lipid biomarkers. Diabetol Metab Syndr. 2012;4:17. doi: 10.1186/1758-5996-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakrzewicz D, Eickelberg O. From arginine methylation to ADMA: a novel mechanism with therapeutic potential in chronic lung diseases. BMC Pulm Med. 2009;9:5. doi: 10.1186/1471-2466-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser M, Paulus WJ, Vermeulen MA, Richir MC, Davids M, Wisselink W. et al. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur J Heart Fail. 2010;12:1274–81. doi: 10.1093/eurjhf/hfq158. [DOI] [PubMed] [Google Scholar]
- 11.Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ. et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew MS, Brandslund I, Brix-Christensen V, Ravn HB, Hjortdal VE, Pedersen J. et al. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: a descriptive study. Anesthesiology. 2001;94:745–53. doi: 10.1097/00000542-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 13.de Jong PR, Schadenberg AW, van den Broek T, Beekman JM, van Wijk F, Coffer PJ. et al. STAT3 regulates monocyte TNF-alpha production in systemic inflammation caused by cardiac surgery with cardiopulmonary bypass. PLoS One. 2012;7:35070. doi: 10.1371/journal.pone.0035070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melek FE, Baroncini LA, Repka JC, Nascimento CS, Precoma DB. Oxidative stress and inflammatory response increase during coronary artery bypass grafting with extracorporeal circulation. Rev Bras Cir Cardiovasc. 2012;27:61–5. doi: 10.5935/1678-9741.20120010. [DOI] [PubMed] [Google Scholar]
- 15.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12:205–13. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 16.Schulze F, Lenzen H, Hanefeld C, Bartling A, Osterziel KJ, Goudeva L. et al. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152:493–501. doi: 10.1016/j.ahj.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Hui Y, Wong M, Kim JO, Love J, Ansley DM, Chen DD. A new derivatization method coupled with LC-MS/MS to enable baseline separation and quantification of dimethylarginines in human plasma from patients to receive on-pump CABG surgery. Electrophoresis. 2012;33:1911–20. doi: 10.1002/elps.201100536. [DOI] [PubMed] [Google Scholar]
- 18.Chen XM, Hu CP, Li YJ, Jiang JL. Cardiovascular risk in autoimmune disorders: role of asymmetric dimethylarginine. Eur J Pharmacol. 2012;696:5–11. doi: 10.1016/j.ejphar.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Siervo M, Corander M, Stranges S, Bluck L. Post-challenge hyperglycaemia, nitric oxide production and endothelial dysfunction: the putative role of asymmetric dimethylarginine (ADMA) Nutr Metab Cardiovasc Dis. 2011;21:1–10. doi: 10.1016/j.numecd.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Antoniades C, Demosthenous M, Tousoulis D, Anto nopoulos AS, Vlachopoulos C, Toutouza M. et al. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension. 2011;58:93–8. doi: 10.1161/HYPERTENSIONAHA.110.168245. [DOI] [PubMed] [Google Scholar]
- 21.Valkonen VP, Paiva H, Salonen JT, Lakka TA, Lehtimaki T, Laakso J. et al. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–8. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 22.Stuhlinger MC, Conci E, Haubner BJ, Stocker EM, Schwaighofer J, Cooke JP. et al. Asymmetric dimethyl Larginine (ADMA) is a critical regulator of myocardial reperfusion injury. Cardiovasc Res. 2007;75:417–25. doi: 10.1016/j.cardiores.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Boger GI, Rudolph TK, Maas R, Schwedhelm E, Dumbadze E, Bierend A. et al. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin: Effect of combination with oral L-arginine. J Am Coll Cardiol. 2007;49:2274–82. doi: 10.1016/j.jacc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Ito A, Egashira K, Narishige T, Muramatsu K, Takeshita A. Renin-angiotensin system is involved in the mechanism of increased serum asymmetric dimethylarginine in essential hypertension. Jpn Circ J. 2001;65:775–8. doi: 10.1253/jcj.65.775. [DOI] [PubMed] [Google Scholar]
- 25.Suda O, Tsutsui M, Morishita T, Tasaki H, Ueno S, Nakata S. et al. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1682–8. doi: 10.1161/01.ATV.0000136656.26019.6e. [DOI] [PubMed] [Google Scholar]
- 26.Busch M, Fleck C, Wolf G, Stein G. Asymmetrical (ADMA) and symmetrical dimethylarginine (SDMA) as potential risk factors for cardiovascular and renal outcome in chronic kidney disease – possible candidates for paradoxical epidemiology? Amino Acids. 2006;30:225–32. doi: 10.1007/s00726-005-0268-8. [DOI] [PubMed] [Google Scholar]
- 27.Kiechl S, Lee T, Santer P, Thompson G, Tsimikas S, Egger G. et al. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis. 2009;205:261–5. doi: 10.1016/j.atherosclerosis.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Hoang HH, Padgham SV, Meininger CJ. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care. 2013;16:76–82. doi: 10.1097/MCO.0b013e32835ad1ef. [DOI] [PubMed] [Google Scholar]
- 29.Boger RH, Ron ES. L-Arginine improves vascular function by overcoming deleterious effects of ADMA a novel cardiovascular risk factor. Altern Med Rev. 2005;10:14–23. [PubMed] [Google Scholar]
- 30.Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–19. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cziraki A, Ajtay Z, Nemeth A, Lenkey Z, Sulyok E, Szabados S. et al. Effects of coronary revascularization with or without cardiopulmonary bypass on plasma levels of asymmetric dimethylarginine. Coron Artery Dis. 2011;22:245–52. doi: 10.1097/MCA.0b013e3283441d5c. [DOI] [PubMed] [Google Scholar]
- 32.Liu YH, Carretero OA, Cingolani OH, Liao TD, Sun Y, Xu J. et al. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:2616–23. doi: 10.1152/ajpheart.00546.2005. [DOI] [PubMed] [Google Scholar]
- 33.Besedina A. NO-synthase activity in patients with coronary heart disease associated with hypertension of different age groups. J Med Biochem. 2016;35:3–9. doi: 10.1515/jomb-2015-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–98. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luvara G, Pueyo ME, Philippe M, Mandet C, Savoie F, Henrion D. et al. Chronic blockade of NO synthase activity induces a proinflammatory phenotype in the arterial wall: prevention by angiotensin II antagonism. Arterioscler Thromb Vasc Biol. 1998;18:1408–16. doi: 10.1161/01.atv.18.9.1408. [DOI] [PubMed] [Google Scholar]
- 36.Kataoka H, Murakami R, Numaguchi Y, Okumura K, Murohara T. Angiotensin II type 1 receptor blockers prevent tumor necrosis factor-alpha-mediated endothelial nitric oxide synthase reduction and superoxide production in human umbilical vein endothelial cells. Eur J Pharmacol. 2010;636:36–41. doi: 10.1016/j.ejphar.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Yaylak F, Canbaz H, Caglikulekci M, Dirlik M, Tamer L, Ogetman Z. et al. Liver tissue inducible nitric oxide synthase (iNOS) expression and lipid peroxidation in experimental hepatic ischemia reperfusion injury stimulated with lipopolysaccharide: the role of aminoguanidine. J Surg Res. 2008;148:214–23. doi: 10.1016/j.jss.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Neri Serneri GG, Boddi M, Modesti PA, Coppo M, Cecioni I, Toscano T. et al. Cardiac angiotensin II participates in coronary microvessel inflammation of unstable angina and strengthens the immunomediated component. Circ Res. 2004;94:1630–7. doi: 10.1161/01.RES.0000130944.49657.b8. [DOI] [PubMed] [Google Scholar]
- 39.Schieffer B, Bunte C, Witte J, Hoeper K, Boger RH, Schwedhelm E. et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:362–8. doi: 10.1016/j.jacc.2004.03.065. [DOI] [PubMed] [Google Scholar]