Summary
Background
25 (OH) vitamin D3 (25(OH)D) and parathyroid hormone (PTH) are important regulators of calcium homeostasis. The aim of this study was to retrospectively determine the cut–off for sufficient 25(OH)D in a four-season region and the influence of age, seasons, and gender on serum 25(OH)D and PTH levels.
Methods
Laboratory results of 9890 female and 2723 male individuals aged 38.8±22.1 years who had simultaneous measurements of 25(OH)D and PTH were retrospectively analyzed by statistical softwares. Serum 25(OH)D and PTH levels were measured by a mass spectrometry method and by an electrochemiluminescence immunoassay, respectively.
Results
Mean serum 25(OH)D levels showed a sinusoidal fluctuation throughout the year and were significantly (p<0.01) higher in summer and autumn. On the other hand, PTH levels were significantly higher (p<0.01) in women and showed an opposite response to seasonal effects relative to 25(OH)D. Lowest levels of 25(OH)D were detected in people aged between 20 and 40 years whereas PTH hormone levels were gradually increasing in response to aging. The significant exponential inverse relationship that was found between PTH and 25(OH)D (PTH=exp(4.12–0.064*sqrt(25(OH)D)) (r=–0.325, R– squared=0.105, p<0.001)) suggested that the cut–off for sufficient 25(OH)D should be 75 nmol/L.
Conclusions
Our retrospective study based on large data set supports the suitability of the currently accepted clinical cut–off of 75 nmol/L for sufficient 25(OH)D. However, the issue of assessing Vitamin D deficiency remains difficult due to seasonal variations in serum 25(OH)D. Therefore, PTH measurements should complement 25(OH)D results for diagnosing Vitamin D deficiency. It is imperative that seasonally different criteria should be considered in future.
Keywords: vitamin D, 25(OH)D, vitamin D deficiency, parathyroid hormone
Kratak sadržaj
Uvod
25 (OH) vitamin D3 (25(OH)D) i paratireoidni hormon (PTH) imaju važnu ulogu u regulisanju homeostaze kalcijuma. Cilj ove studije bio je da se retrospektivno odrede cut-off vrednosti za dovoljan nivo 25(OH)D u regionu sa četiri godišnja doba, kao i uticaj starosti, godišnjeg doba i pola na nivoe 25(OH)D i PTH u serumu.
Metode
Laboratorijski rezultati 9890 žena i 2723 muška rca starosti 38,8±22,1 godina kod kojih su istovremeno mereni 25(OH)D i PTH retrospektivno su analizirani statističkim softverom. Nivoi 25(OH)D i PTH u serumu mereni su metodom masene spektrometrije, odnosno elektrohemiluminiscencije.
Rezultati
Srednji nivoi 25(OH)D pokazali su sinusoidnu fluktuaciju tokom cele godine i bili su značajno viši (p<0,01) u leto i jesen. S druge strane, nivoi PTH bili su značajno viši (p<0,01) kod žena i pokazali su suprotan odgovor na sezonske efekte u odnosu na 25(OH)D. Najniži nivoi 25(OH)D otkriveni su kod ljudi starosti između 20 i 40 godina, dok su hormonski nivoi PTH sa starenjem bili u postepenom porastu. Značajan eksponencijalni obrnut odnos koji je utvrđen između PTH i 25(OH)D (PTH=exp(4,12–0,064* koren2 (25(OH)D)) (r=–0,325, R-na kvadrat=0,105, p<0,001)) ukazao je na to da cut-off vrednost za dovoljan nivo 25(OH)D treba da bude 75 nmol/L.
Zaključak
Naša retrospektivna studija zasnovana na velikim skupinama podataka potvrđuje da je trenutno prihvaćena klinička cut-off vrednost od 75 nmol/L prigodna za dovoljan nivo 25(OH)D. Međutim, utvrđivanje nedostatka vitamina D ostaje teško izvodljivo usled varijacija u serumskom nivou 25(OH)D zbog godišnjih doba. Stoga, merenje PTH trebalo bi da posluži kao komplement rezultatima 25(OH)D prilikom dijagnostikovanja nedostatka vitamina D. Najvažnije je da se u budućnosti razmotre različiti kriterijumi prilagođeni različitim godišnjim dobima.
Introduction
Vitamin D and parathyroid hormone (PTH) are regulators of serum calcium levels in the body. Vitamin D deficiency causes the softening of bones leading to rickets in children or osteomalacia in adults (1). More recently, Vitamin D deficiency has also been associated with non-musculoskeletal disorders (2, 3). In this respect, a number of observational studies have linked Vitamin D deficiency to cancer, cardiovascular diseases, diabetes, depression, or multiple sclerosis (1, 4–8). Since Vitamin D deficiency can be prevented by supplements or injections, it is most important to determine the clinical decision thresholds to define the deficiency.
Vitamin D2 (ergocalciferol) and Vitamin D3 (cholecalciferol) are the most common forms of Vitamin D. Vitamin D3 is synthesized from 7– dehydrocholesterol in the skin upon sun exposure. This inactive form of Vitamin D3 is then converted to the prohormone form – 25(OH)D in the liver. The biologically active form, which is 1,25(OH)D3, is then produced from 25(OH)D in the kidney and serves a regulatory hormone role in the body. Therefore, 25(OH)D is the primary metabolite of Vitamin D in blood circulation which is commonly used to reflect vitamin D status (9).
Currently, measurement of serum 25(OH)D (either 25(OH)D3 or 25(OH)D2) concentration is a routine laboratory test to assess Vitamin D levels (10). However, determination of 25(OH)D levels still involves methodological and clinical challenges. From a clinical point of view, there are seasonal variations in 25(OH)D levels which may be accompanied by additional variations due to gender, age, and BMI (11–15). On the other hand, there are significant problems in methodology, such as the reference interval calculations cannot be made through classical methods (16, 17). Therefore, there is still no consensus in regards to determining the clinical decision levels for Vitamin D deficiency and, instead, the guidelines report recommended levels for health (18–24).
Vitamin D and PTH have a well–known inverse relationship, such that Vitamin D insufficiency causes an increase in serum PTH (19–21, 25–29). For example, Hollick et al. (21) reported a significant inverse correlation between serum PTH and 25(OH)D levels in post-menopausal North American women. In another study, it was shown that subjects who were placed on Vitamin D therapy had an overall decrease (∼20%) in their serum PTH concentrations (19).
Knowledge innovation from databases using data mining techniques is an invaluable methodology for extracting patterns from large data sets and comprehending the knowledge retained within these patterns (30, 31). This knowledge discovery process has several distinct steps or sub-processes that begin with data collection which is then followed by data refining, aggregation, and combination. After these steps, the data is ready to be utilized for data imagining followed by data mining. Sub-processes in the data mining procedure are iterative rather than being consecutive (i.e. movement from data imagining back to data refining if abnormalities are discovered in the data set) (32). Overall, data mining techniques provide a very effective way of retrospective laboratory data analysis to discover patterns otherwise unknown (15, 30, 31, 33).
In the present study, we employed data mining techniques to analyze the changes in 25(OH)D levels and PTH further by gender and season based on retrospective data obtained using the tandem mass spectrometry technique, which is currently accepted as the most stable method for measurements. Our analysis, based on one of the largest data sets obtained in the Eastern Europe region, deciphered the seasonal patterns of 25(OH)D and PTH levels as well as their dependence on gender and age. Additionally, use of the 75 nmol/L clinical decision threshold level for 25(OH)D was also supported by our retrospective study.
Material and Methods
The study included the results of 13026 individuals who had simultaneous measurements of serum 25(OH)D and PTH concentrations at Acıbadem LABMED Clinical Laboratories (Turkey) between the years 2009 and 2015. During the data refining process, approximately 3.3% of data points were excluded and a total of 12613 people were included in this study. The extreme values were excluded by the Generalized Extreme Studentized procedure, leaving data from 9890 female and 2723 male individuals aged between 1–97 years (38.8±22.1 years) to be used in analysis. Data mining techniques were applied in order to understand the correlation between 25(OH)D and PTH. For this purpose, different regression models were built and the effects of age, gender, and seasons on the relationship between 25(OH)D vs. PTH were investigated.
Serum 25(OH)D concentrations were measured by the Agilent Rapid Res 1200 LC system and Agilent 6420 and 6460 triple quadruple mass spectrometers (Agilent Technologies, Santa Clara, CA). Acıbadem LABMED Clinical Laboratories was a participant in the National Institute of Standards and Technology (NIST)/National Institute of Health (NIH) vitamin D metabolites Quality Assurance Program (VitDQAP) and 25(OH)D measurements are traceable as NIST SRM 972a, and the exact precision and bias values are 1.3–8.2% and 0.07–3.2%, respectively. We used a number of different cut–off values to define the degree of insufficiency. Based on several research articles and society recommendations (34–36), we used the following groups: >75 nmol/L sufficient, 50–75 nmol/L moderate deficiency, 25–50 nmol/L deficiency, <25 nmol/L severe deficiency.
Intact PTH concentrations were determined by an electrochemiluminescence immunoassay with an Elecsys analyzer (Roche Diagnostics, Mannheim, Germany). For intact PTH, values greater than 200 pg/mL were excluded because these values correspond to three-fold higher than the upper reference limit indicating the presence of primary hyper-parathyroidism (15).
Analyse-it for Microsoft Excel 4.0 (Analyse-it Software, Ltd. Leeds, UK), Statgraphics Centurion XVI (Statpoint Technologies, Inc. Warrenton, Virgi nia, USA), Minitab 16 (Minitab Inc, PA, USA), and IBM SPSS Statistics 23 (IBM Ltd, USA) were used for statistical analyses in the study. The data were analyzed by independent sample t test, One-Way ANOVA and Tukey’s post hoc test and regression analysis. The significance level of p was set to <0.01 throughout the analysis.
Results
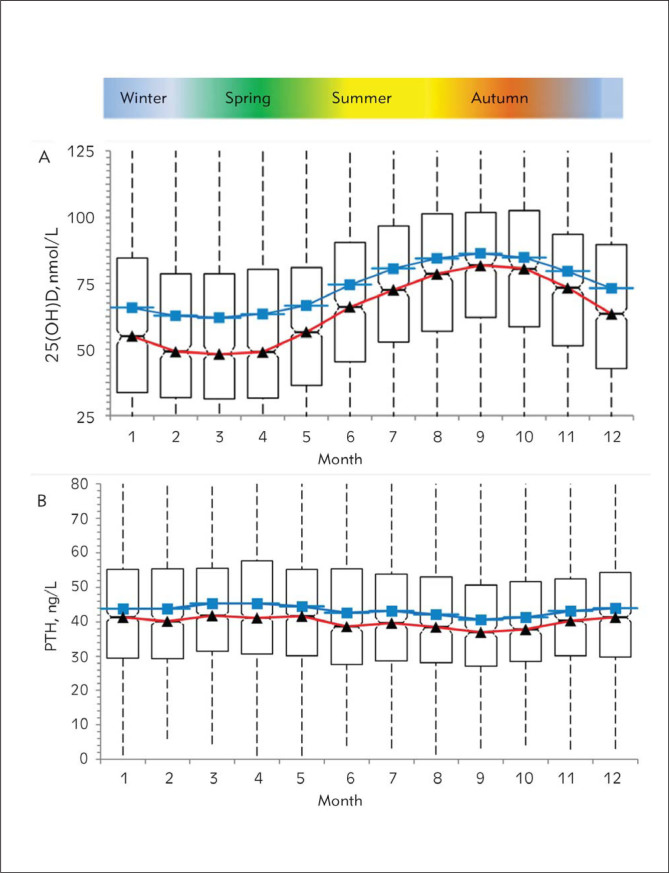
Analysis of seasonal changes in 25(OH)D and PTH hormone levels revealed a sinusoidal pattern where the 25(OH)D values increased starting in June, reaching a peak level in September, and then decreased to baseline levels by December (Figure 1A). It is important to note that there was a two–fold difference in the median 25(OH)D values between the months with the lowest (35.8 nmol/L, 95% CI 33.8–38.8, in March) and the highest (69.3 nmol/L, 95% CI 66.5–72.0, in September) levels as shown in Table I. On the other hand, seasonal changes in PTH levels showed a limited inverse sinusoidal pattern (Figure 1B) to what was observed for 25(OH)D levels. Response rate of PTH hormone levels to seasonal changes was near 12% based on the difference between the medians of lowest (36.9 ng/L, 95% CI 35.4–38.7, in September) and the highest (41.9 ng/L, 95% CI 40.7–42.7, in March) values.
Figure 1.
Monthly changes in 25(OH)D (A) and PTH (B) levels. Red line, median; blue line, mean. Seasons experienced in a year in Turkey are indicated in the ribbon above the graphs.
Table I.
Seasonal changes in 25(OH)D and PTH hormone levels (A), changes in 25(OH)D and PTH hormone levels by age (B).
| A | PTH, ng/mL | 25(OH)D, nmol/L | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | 95 CI% | SD | Median | 95 CI% | Mean | 95 CI% | SD | Median | 95 CI% | |||||
| January | 1057 | 43.7 | 42.5 | 45.0 | 20.2 | 41.2 | 40.2 | 42.8 | 53.3 | 50.8 | 55.8 | 43.0 | 42.5 | 39.3 | 44.8 |
| February | 1192 | 43.8 | 42.6 | 45.0 | 20.3 | 40.2 | 39.1 | 41.9 | 50.3 | 47.8 | 52.5 | 44.3 | 36.8 | 34.0 | 39.3 |
| March | 1580 | 45.3 | 44.3 | 46.3 | 20.8 | 41.9 | 40.7 | 42.7 | 49.5 | 47.5 | 51.5 | 42.0 | 35.8 | 33.8 | 38.8 |
| April | 1311 | 45.4 | 44.2 | 46.5 | 21.8 | 41.2 | 40.2 | 42.4 | 50.8 | 48.5 | 53.0 | 44.0 | 36.5 | 34.3 | 38.8 |
| May | 1392 | 44.5 | 43.4 | 45.6 | 21.7 | 41.6 | 40.3 | 42.4 | 54.0 | 51.8 | 56.3 | 41.0 | 44.0 | 42.3 | 46.0 |
| June | 971 | 42.6 | 41.3 | 43.9 | 21.6 | 38.6 | 37.1 | 39.8 | 62.0 | 59.3 | 64.5 | 41.3 | 53.5 | 51.0 | 56.5 |
| July | 791 | 43.1 | 41.7 | 44.6 | 20.5 | 39.7 | 38.0 | 41.3 | 68.0 | 65.0 | 71.0 | 42.3 | 60.0 | 57.3 | 63.0 |
| August | 631 | 42.1 | 40.4 | 43.7 | 20.4 | 38.5 | 36.5 | 39.8 | 71.8 | 68.5 | 75.0 | 41.8 | 66.0 | 63.3 | 69.3 |
| September | 689 | 40.6 | 39.1 | 42.2 | 19.1 | 36.9 | 35.4 | 38.7 | 73.8 | 70.8 | 77.0 | 40.0 | 69.3 | 66.5 | 72.0 |
| October | 843 | 41.3 | 39.9 | 42.7 | 18.7 | 37.7 | 36.2 | 39.3 | 72.3 | 69.5 | 75.0 | 38.5 | 68.0 | 65.3 | 71.5 |
| November | 944 | 43.2 | 41.9 | 44.5 | 19.4 | 40.3 | 39.1 | 41.6 | 67.0 | 64.3 | 69.8 | 41.5 | 60.8 | 58.3 | 63.0 |
| December | 1216 | 44.0 | 42.8 | 45.1 | 20.9 | 41.2 | 40.0 | 42.5 | 60.5 | 58.3 | 63.0 | 43.0 | 51.0 | 48.5 | 53.0 |
| B | PTH, ng/mL | 25(OH)D, nmol/L | |||||||||||||
| Age | N | Mean | 95 CI% | SD | Median | 95 CI% | Mean | 95 CI% | SD | Median | 95 CI% | ||||
| 0–10 | 1476 | 30.6 | 29.6 | 31.6 | 18.8 | 26 | 25 | 26.7 | 71.0 | 68.8 | 73.0 | 43.3 | 64.0 | 61.3 | 66.3 |
| 10–20 | 681 | 38.6 | 37.1 | 40.1 | 18.4 | 34.8 | 33.4 | 36.8 | 47.3 | 44.0 | 50.3 | 33.8 | 40.0 | 37.8 | 42.3 |
| 20–30 | 1011 | 40.1 | 38.8 | 41.3 | 18.1 | 37 | 35.8 | 38.1 | 47.8 | 45.0 | 50.3 | 41.3 | 35.8 | 32.5 | 38.5 |
| 30–40 | 1929 | 43.5 | 42.6 | 44.4 | 18.9 | 40.6 | 39.9 | 41.7 | 53.0 | 51.3 | 55.0 | 42.5 | 43.3 | 40.5 | 45.0 |
| 40–50 | 2086 | 44.2 | 43.4 | 45.1 | 19.2 | 40.6 | 39.9 | 41.7 | 55.8 | 54.0 | 57.8 | 43.3 | 46.3 | 44.3 | 48.5 |
| 50–60 | 2361 | 45.8 | 45 | 46.6 | 19.4 | 42.8 | 41.9 | 43.6 | 61.5 | 59.8 | 63.3 | 40.3 | 55.0 | 53.3 | 57.3 |
| 60–70 | 1775 | 48.5 | 47.6 | 49.5 | 20.7 | 45.7 | 44.6 | 46.8 | 63.0 | 61.0 | 65.0 | 43.3 | 57.0 | 54.8 | 59.8 |
| 70–80 | 889 | 51.7 | 50.4 | 53 | 24 | 47.5 | 46 | 49.3 | 64.0 | 61.3 | 66.8 | 47.8 | 55.8 | 53.5 | 58.8 |
| 80–90 | 377 | 54.4 | 52.4 | 56.4 | 25 | 51.1 | 48.1 | 53.7 | 64.0 | 59.8 | 68.3 | 46.8 | 55.0 | 48.5 | 62.0 |
| <90 | 28 | 52.2 | 44.9 | 59.5 | 22.3 | 52.3 | 40.9 | 58.9 | 67.3 | 51.5 | 82.8 | 51.0 | 57.3 | 30.0 | 84.3 |
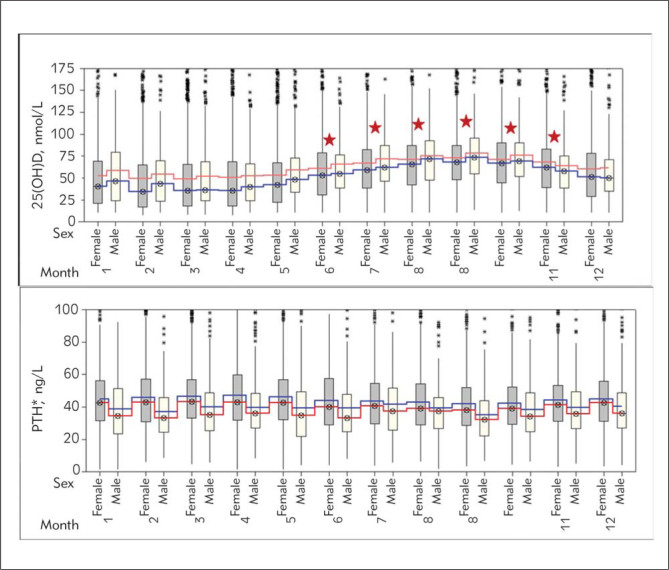
When gender and seasonal changes were considered (Figure 2), it was found that women had higher (p<0.001) PTH levels than men throughout the whole year. In addition, there was no statistical difference (p>0.01) in 25(OH)D levels in men and women while there were statistically significant (p<0.001 with ANOVA test) seasonal highs and lows in both genders. 25(OH)D levels were maximal (p<0.001) between July and October in comparison to the rest of the months in a year (Figure 2).
Figure 2.
Changes in 25(OH)D and PTH levels by gender and month. Red line, median; blue line, mean.
Stars: p<0.001 upon comparison with winter months (Tukey Test)
*PTH levels were statistically higher in women relative to men throughout all the seasons
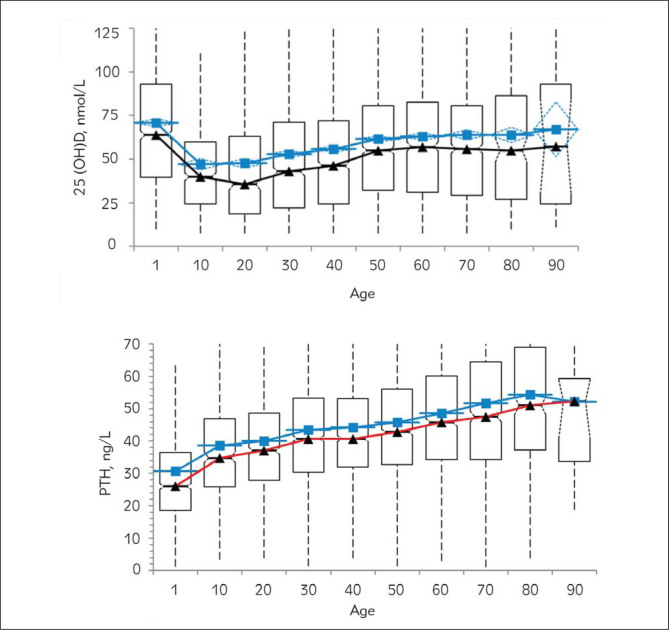
Next, the age dependence of 25(OH)D and PTH levels was examined (Figure 3). Perhaps not surprisingly, the highest levels of 25(OH)D were observed during the first decade (71 nmol/L, 95% CI 68.8–73), which is possibly due to vitamin D replacement during the childhood period. Moreover, there was a significant decrease in 25(OH)D between ages 10–40 (Figure 3). In later age groups, the mean values of 25(OH)D were increasingly higher (Figure 3).
Figure 3.
Changes in 25(OH)D and PTH levels by age. Red line, median; blue line, mean.
Table II.
Frequency distribution among the 25(OH)D concentration categories in various months, decades of life and gender.
| Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D, nmol/L | January | February | March | April | May | June | July | August | September | October | November | December | Total |
| < 25 | 315 | 416 | 553 | 448 | 370 | 172 | 84 | 54 | 56 | 71 | 109 | 224 | 2872 |
| 25–50 | 296 | 337 | 436 | 362 | 426 | 267 | 206 | 142 | 117 | 170 | 242 | 367 | 3368 |
| 50–75 | 201 | 209 | 285 | 225 | 299 | 260 | 237 | 191 | 230 | 248 | 296 | 302 | 2983 |
| > 75 | 245 | 230 | 306 | 276 | 297 | 270 | 264 | 242 | 286 | 354 | 297 | 323 | 3390 |
| Total | 1057 | 1192 | 1580 | 1311 | 1392 | 969 | 791 | 629 | 689 | 843 | 944 | 1216 | 12613 |
| Age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D, nmol/L | 1 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | Total |
| < 25 | 170 | 175 | 358 | 562 | 537 | 426 | 351 | 196 | 90 | 7 | 2872 |
| 25–50 | 360 | 255 | 284 | 548 | 582 | 625 | 430 | 196 | 82 | 6 | 3368 |
| 50–75 | 359 | 162 | 194 | 390 | 493 | 615 | 449 | 237 | 80 | 4 | 2983 |
| > 75 | 587 | 89 | 175 | 429 | 474 | 695 | 545 | 260 | 125 | 11 | 3390 |
| Total | 1476 | 681 | 1011 | 1929 | 2086 | 2361 | 1775 | 889 | 377 | 28 | 12613 |
| Gender | ||||
|---|---|---|---|---|
| Female | Male | |||
| 25(OH)D, nmol/L | n | % | n | % |
| < 25 | < 25 | 24.5 | 445 | 16.3 |
| 25–50 | 25–50 | 25.8 | 821 | 30.2 |
| 50–75 | 50–75 | 23.0 | 711 | 26.1 |
| > 75 | > 75 | 26.7 | 746 | 27.4 |
| Total | 9890 | 2723 | ||
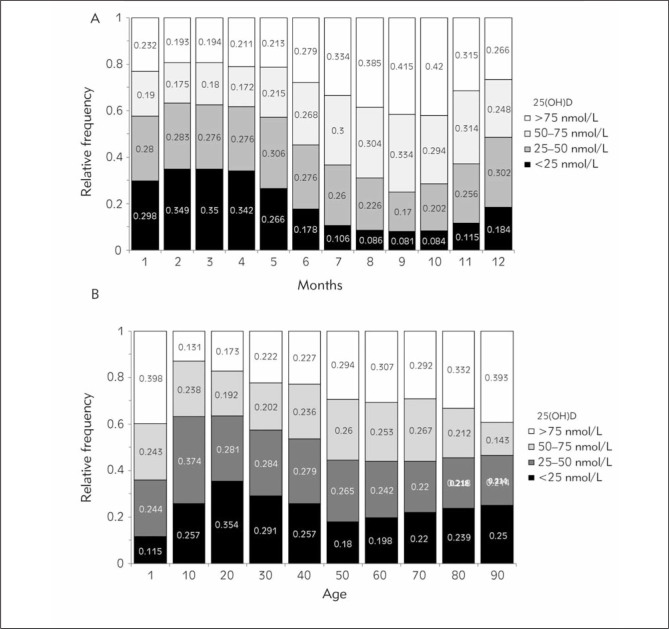
We also analyzed the seasonal, gender, and age effects on relative deficiencies or insufficiencies in 25(OH)D levels. In particular, about 80% of people (severe deficiency in 35%) had 25(OH)D lower than 75 nmol/L between February and May (Figure 4A and Table I). The percentage of people with low 25(OH)D (<75 nmol/L) levels dropped to 58% during the summer months (severe deficiency in 8%) (Figure 4A and Table I). When the effect of age on 25(OH)D deficiencies was analyzed, 25(OH)D deficiency was seen in 83% of the cases (<75 nmol/L) (severe deficiency in 35% (<25 nmol/L)) (Figure 4B, Table I). The highest prevalence of 25(OH)D deficiency was seen between ages 20 and 30 (Figure 4B, Table I). In addition, a review of the entire group by gender revealed that only 27% of the population had adequate 25(OH)D levels regardless of season and age (Table I).
Figure 4.
25(OH)D deficiency rates by months (A) and age (B).
Lastly, our analysis showed an inverse signifi cant correlation between PTH and 25(OH)D levels (r=–0.277, p<0.001), which is supported by other experimental studies in literature (4, 20, 26–29). However, we performed other regression analyses and determined that the highest correlation was bet ween log PTH and the minus square root of 25(OH)D. In the linear regression model, the formula was as follows: PTH=51.5–0.133*25(OH)D (r=–0.277, R-squared =0.076, p<0.001) while this was PTH=exp (4.12–0.064*sqrt(25(OH)D)) (r=–0.325, R-squared =0.105 p<0.001) in the current regression analysis.
We observed that PTH levels showed a steeper increase when 25(OH)D levels were below 75 nmol/L. Moreover, PTH levels were at 35 ng/mL when 25(OH)D levels were at 75 nmol/L (Figure 5).
Figure 5.
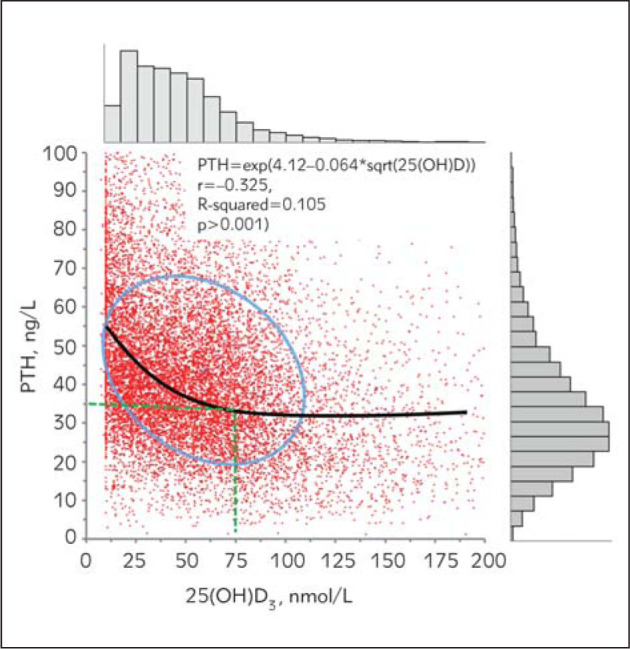
Relation between PTH and 25(OH)D levels.
Discussion
There is ongoing controversy over optimal serum levels of 25(OH)D recommended in current clinical guidelines. This is mostly due to the fact that the determination of reference intervals for 25(OH)D cannot be made according to IFCC recommendations because 25(OH)D levels show seasonal variations. Consequently, varying levels of 25(OH)D were recommended to be used as clinical thresholds of deficiency by different research groups. For example, Heaney et al. (17) suggested that 80–90 nmol/L was necessary for appropriate calcium absorption, while Malabanan et al. recommended a 25(OH)D level of 50 nmol/L considering also the PTH levels (19). Additionally, Chapuy et al. (20) and Holick et al. (21) reported the most optimal 25(OH)D levels to be between 75–77.5 nmol/L based on PTH hormone levels which tended to show a steep increase above optimal 25(OH)D levels chosen. A cross–sectional study by Bischoff–Ferrari et al. (24) on 4100 elder individuals suggested that a 100 nmol/L level was adequate for minimum musculoskeletal functions. In our analysis, PTH levels reach a stable plateau above 25(OH)D levels of 75 nmol/L suggesting this value to be the clinical decision threshold for 25(OH)D (Figure 5). This finding is a reconfirmation of the clinical decision threshold level that others found previously (20, 21) using a much larger retrospective data set.
One of the most interesting findings of this study is the examination of seasonal and age de pendence of PTH levels. The progressive increase in PTH due to aging was expected as previously seen (37) but these levels were statistically higher in women relative to men throughout all the seasons. It is possible that women spend less time outside compared to men in Turkey, which would indicate reduced sun exposure and, therefore, reduced Vitamin D synthesis. The latter may result in increased levels of PTH. However, the observed difference in PTH levels may also arise from reproductive hormones playing a role in PTH metabolism (38, 39). It would be important to study the effect of androgen and estrogen on PTH levels in the future.
Also, the higher levels of 25(OH)D above 40 years old relative to the 10–40-year old group are rather unexpected. However, it is possible that this older age group is receiving replacement therapy in Turkey. It is also possible that the hormonal changes above this age are playing a role in the observed results (38, 39). Further analysis is required to understand the mechanism underlying this observation.
25(OH)D deficiency has previously been detected at different rates depending upon societies, geographical location, and traditions. In addition, other variations in the levels of 25(OH)D have been observed due to methodological differences in measurements and the seasonal effects on the measurements (40). A previous review of Vitamin D deficiency in Turkey showed a rather wide severe deficiency rate which spans an interval of 8 to 84% (22). In our study, severe deficiency was found in 25% of the cases (<25 nmol/L) and deficiency in 75% of the cases (<75 nmol/L). The present study brought together the largest data set ever collected in the region which experiences four seasons.
Recently, Kroll et al. (15) performed one of the largest data mining studies to investigate seasonal and latitude effects on 25(OH)D and PTH hormones using 3.8 million laboratory results (Quest Diagnostics) of American adults. Based on samples taken from people residing in different regions of the country, both PTH and 25(OH)D levels showed a remarkable seasonal variation that showed a sinusoidal pattern (15). Similar to our results that represent Turkey, these sinusoidal patterns were inverted between PTH and 25(OH)D. Moreover, the response of PTH to seasonal variations was about 10% in both Kroll et al. (15) and our study. However, the response of 25(OH)D to seasonal variations was about 30% in the US population while this was found to be 50% in our population. This difference may be the result of higher mean levels of 25(OH)D in the US population because of higher consumption of Vitamin D fortified milk and its products relative to our region.
Bolland et al. (41) found that the seasonal changes in 25(OH)D levels in New Zealand were at such rates that might impair clinical diagnosis. The authors urged the clinicians to treat the results with caution during the deficiency assessment process. Their recommended Vitamin D levels to assess deficiency were 75 nmol/L and 50 nmol/L for the summer and winter months, respectively (23, 41). In our study, we also found high rates of change, at approximate levels of 50–80%, in Vitamin D levels. In addition, the results obtained in New Zealand were a mirror image of our study, where the highest levels of 25(OH)D were measured between February and April and the lowest levels of 25(OH)D were measured between August and September. Therefore, the results from both studies highlight the influence of summer and winter months on adjustment of Vitamin D levels. Another population based study (14) analyzed a total of 7,449 adult samples and recommended a realistic 25(H)D level determination through a formulation that took into consideration vitamin use, gender, BMI, and seasonal changes. Our results also emphasize the need for such studies to be conducted at the regional level and the findings implemented in new formulations to define the deficiency according to seasonal changes.
The present study has some limitations and one of them is the lack of demographic information on the individuals (BMI, level of income, vitamin drug use, and other diseases, etc.) whose test results were used. The second one is the number of male and female individuals is different (9890 female and 2723 male). Despite this fact, the number of male subjects is high enough for acceptable statistical evaluations. Nevertheless, the present study is valuable on the grounds that it was based on retrospective data employed from the largest study group ever in the Eastern Europe region in a country which experiences four seasons.
In conclusion, based on our analysis of the effects of age, gender, and seasons on 25(OH)D and PTH levels, we suggest that the determination of age dependent and winter values alone is not adequate for assessment of 25(OH)D levels since all these factors can possibly impair the clinical diagnosis. Therefore, as for the monthly reference intervals, each center may need to optimize their values examining the retrospectively determined data and PTH levels and the changes brought by factors such as age and seasons.
Acknowledgements
M.A.S., M.K., F.B.A., M.S., A.C. and I.U. collected data; M.A.S and B.B.C. processed and statistically analyzed data, M.A.S., Z.A.D., and A.O interpreted data and wrote the manuscript; all authors contributed to editing and reviewed the manuscript. The authors declare no competing financial interests.
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Holick MF.. The D-Lightful Vitamin D for Health. J Med Biochem. 2013;32:1–10. [Google Scholar]
- 2.Guessous I.. Role of Vitamin D deficiency in extraskeletal complications: predictor of health outcome or marker of health status? Biomed Res Int. 2015;2015:563403. doi: 10.1155/2015/563403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q.. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013;11:187. doi: 10.1186/1741-7015-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF.. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98:1024–7. doi: 10.1097/01.SMJ.0000140865.32054.DB. [DOI] [PubMed] [Google Scholar]
- 5.Guessous I, Bochud M, Bonny O, Burnier M.. Calcium, vitamin D and cardiovascular disease. Kidney Blood Press Res. 2011;34:404–17. doi: 10.1159/000328332. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez M, Martinez-Moreno JM, Rodríguez-Ortiz ME, Muñoz-Castañeda JR, Almaden Y.. Vitamin D and vascular calcification in chronic kidney disease. Kidney Blood Press Res. 2011;34:261–8. doi: 10.1159/000326903. [DOI] [PubMed] [Google Scholar]
- 7.Penckofer S, Kouba J, Wallis DE, Emanuele MA.. Vitamin D and diabetes: let the sunshine in. Diabetes Educ. 2008;34:939–40. doi: 10.1177/0145721708326764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertone–Johnson ER.. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67:481–92. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick MF.. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennel KA, Drake MT, Hurley DL.. Vitamin D Deficiency in Adults: When to Test and How to Treat. Mayo Clin Proc. 2010;85:752–8. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingberg E, Oleröd G, Konar J, Petzold M, Hammarsten O.. Seasonal variations in serum 25–hydroxy vitamin D levels in a Swedish cohort. Endocrine. 2015;49:800–8. doi: 10.1007/s12020-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozkurt S.. Age, Sex, and Seasonal Variations in the Serum Vitamin D3 Levels in a Local Turkish Population. Arch Rheumatol. Turkish League Against Rheumatism; 2014;29:14–9. [Google Scholar]
- 13.Darling AL, Hart KH, Gibbs MA, Gossiel F, Kantermann T, Horton K, Johnsen S, Berry JL, Skene DJ, Eastell R. et al. Greater seasonal cycling of 25-hydroxy vitamin D is associated with increased parathyroid hormone and bone resorption. Osteoporos Int. 2014;25:933–41. doi: 10.1007/s00198-013-2493-4. [DOI] [PubMed] [Google Scholar]
- 14.Vuistiner P, Rousson V, Henry H, Lescuyer P, Boulat O, Gaspoz J– M, Mooser V, Vollenweider P, Waeber G, Cornuz J. et al. A Population–Based Model to Consider the Effect of Seasonal Variation on Serum 25(OH)D and Vitamin D Status. Biomed Res Int. 2015:168–89. doi: 10.1155/2015/168189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, Zhang K, Clarke N, Xie M, Reitz RE. et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One. Public Library of Science; 2015;10:e0118108. doi: 10.1371/journal.pone.0118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, Wendler I, Fabian D, Hauptlorenz S, Halwachs-Baumann G.. 25- hydroxy-Vitamin D status: limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab. 2014;60:1541–50. doi: 10.7754/clin.lab.2014.131114. [DOI] [PubMed] [Google Scholar]
- 17.Heaney RP, Dowell MS, Hale CA, Bendich A.. Calcium absorption varies within the reference range for serum 25– hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 18.Janssen MJW, Wielders JPM, Bekker CC, Boesten LSM, Buijs MM, Heijboer AC, van der Horst FAL, Loupatty FJ, van den Ouweland JMW.. Multicenter comparison study of current methods to measure 25-hydroxyvitamin D in serum. Steroids. 2012;77:1366–72. doi: 10.1016/j.steroids.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Malabanan a, Veronikis IE, Holick MF.. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 20.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ.. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE.. Prevalence of Vitamin D Inadequacy among Postmenopausal North American Women Receiving Osteoporosis Therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer IM, Middelkoop BJC, Boeke AJP, Lips P.. Prevalence of vitamin D deficiency among Turkish, Moroccan, Indian and sub-Sahara African populations in Europe and their countries of origin: an overview. Osteoporos Int. 2011;22:1009–21. doi: 10.1007/s00198-010-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR.. The effects of seasonal variation of 25–hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007;86:959–64. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff–Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B.. Higher 25-hydroxyvitamin D concentrations are associated with better lower extremity function in both active and inactive persons aged >=60 y. Am J Clin Nutr. 2004;80:752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 25.Freaney R, McBrinn Y, McKenna MJ.. Secondary hyperparathyroidism in elderly people: combined effect of renal insufficiency and vitamin D deficiency. Am J Clin Nutr. 1993;58:187–91. doi: 10.1093/ajcn/58.2.187. [DOI] [PubMed] [Google Scholar]
- 26.Atapattu N, Shaw N, Högler W.. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr Res. International Pediatric Research Foundation, Inc.; 2013;74:552–6. doi: 10.1038/pr.2013.139. [DOI] [PubMed] [Google Scholar]
- 27.Saliba W, Barnett O, Rennert HS, Lavi I, Rennert G.. The relationship between serum 25(OH)D and parathyroid hormone levels. Am J Med. 2011;124:1165–70. doi: 10.1016/j.amjmed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO.. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab. 2005;90:5576–81. doi: 10.1210/jc.2005-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adami S, Viapiana O, Gatti D, Idolazzi L, Rossini M.. Relationship between serum parathyroid hormone, vitamin D sufficiency, age, and calcium intake. Bone. 2008;42:267–70. doi: 10.1016/j.bone.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Hand DJ, Hand DJ, Mannila H, Mannila H, Smyth P, Smyth P.. Principles of data mining. Drug safety: an international journal of medical toxicology and drug experience. 2001:322. [Google Scholar]
- 31.Han J, Kamber M.. Data Mining: Concepts and Techniques. Ann Phys (N Y) 2006;54:770. [Google Scholar]
- 32.Ramaprasad A.. A methodology for data mining. J Database Mark. 1996;4:65–75. [Google Scholar]
- 33.Shah NH, LePendu P, Bauer–Mehren A, Ghebremariam YT, Iyer SV, Marcus J, Nead KT, Cooke JP, Leeper NJ.. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults Recommendations abstracted from the American Geriatrics Society Con sensus Statement on vitamin D for Prevention of Falls and Their Consequences. J Am Geriatr Soc. 2014;62:147. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 35.Ross AC, Manson JE, Abrams SA. et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deschasaux M, Montourcy M, Sutton A, Charnaux N, Kesse-Guyot E, Fezeu LK, Latino-Martel P, Druesne-Pecollo N, Malvy D, Galan P, Hercberg S, Ezzedine K, Souberbielle JC.. Interpretation of plasma PTH concentrations according to 25OHD status, gender, age, weight status, and calcium intake: importance of the reference values. J Clin Endocrinol Metab. 2014;99(4):1196–203. doi: 10.1210/jc.2013-3349. [DOI] [PubMed] [Google Scholar]
- 37.Valcour A, Blocki F, Hawkins DM, Rao SD.. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab. 2012;97:3989–95. doi: 10.1210/jc.2012-2276. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Lv F, Zhang Z, Deng W, Li Y, Deng Z, Jiang Y, Wang O, Xing X, Xu L, Xia W.. Establishment of a normal reference value of parathyroid hormone in a large healthy Chinese population and evaluation of its relation to bone turnover and bone mineral density. Osteoporos Int. 2016;27(5):1907–16. doi: 10.1007/s00198-015-3475-5. [DOI] [PubMed] [Google Scholar]
- 39.Ebeling PR.. Vitamin D and bone health: epidemiologic studies. Bonekey Rep. 2014;3:511. doi: 10.1038/bonekey.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonelli P, Buonocore R, Aloe R, Lippi G.. Blood sampling seasonality as an important preanalytical factor for assessment of vitamin D status. J Med Biochem. 2016;35:3–7. doi: 10.1515/jomb-2015-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolland MJ, Chiu WW, Davidson JS, Grey A, Bacon C, Gamble GD, Reid IR.. The effects of seasonal variation of 25–hydroxyvitamin D on diagnosis of vitamin D insufficiency. N Z Med J. 2008;121:63–74. [PubMed] [Google Scholar]