Abstract
Background
We tested the hypothesis that moxifloxacin monotherapy is equally effective and safe as a betalactam antibiotic based combination therapy in patients with acute respiratory distress syndrome (ARDS) evoked by severe community acquired pneumonia (CAP).
Methods
In a retrospective chart review study of 229 patients with adult respiratory distress syndrome (ARDS) admitted to our intensive care unit between 2001 and 2011, 169 well-characterized patients were identified to suffer from severe CAP. Patients were treated with moxifloxacin alone, moxifloxacin in combination with ß-lactam antibiotics, or with another antibiotic regimen based on ß-lactam antibiotics, at the discretion of the admitting attending physician. The primary endpoint was 30-day survival. To assess potential drug-induced liver injury, we also analyzed biomarkers of liver cell integrity.
Results
30-day survival (69% overall) did not differ (p = 0.89) between moxifloxacin monotherapy (n = 42), moxifloxacin combination therapy (n = 44), and other antibiotic treatments (n = 83). We found significantly greater maximum activity of aspartate transaminase (p = 0.048), alanine aminotransferase (p = 0.003), and direct bilirubin concentration (p = 0.01) in the moxifloxacin treated groups over the first 10–20 days. However, these in-between group differences faded over time, and no differences remained during the last 10 days of observation.
Conclusions
In CAP evoked ARDS, moxifloxacin monotherapy and moxifloxacin combination therapy was not different to a betalactam based antibiotic regimen with respect to 30-day mortality, and temporarily increased markers of liver cell integrity had no apparent clinical impact. Thus, in contrast to the current S3 guidelines, moxifloxacin may also be safe and effective even in patients with severe CAP evoked ARDS while providing coverage of an extended spectrum of severe CAP evoking bacteria. However, further prospective studies are needed for definite recommendations.
Keywords: Acute respiratory distress syndrome, CAP, 30-day mortality, Liver failure, S3 guideline, Consensus guideline
Background
Acute respiratory distress syndrome (ARDS) is a life-threatening disease evoked by a variety of precipitants, including community acquired pneumonia (CAP) with a current mortality of about 40% [1–4]. Severe CAP is the most frequent cause of ARDS [5] and acknowledged recommendations for antimicrobial therapy are provided in Germany by the S3-guidelines [6–8] and in the United States by the Consensus guidelines on the management of CAP in adults [9]. Along these recommendations, a combination of a broad-spectrum beta-lactam antibiotic (e.g. cefotaxim, ceftriaxone, piperacillin/tazobactam, ertapenem, or meropenem) in combination with a macrolide or fluoroquinolone is usually administered empirically to patients with severe CAP and septic disease progression or mechanically ventilated patients [7, 9, 10]. Fluoroquinolone monotherapy (levofloxacin or moxifloxacin) is reserved for patients without severe disease progression [7, 9, 11]. However, the recommendations to limit moxifloxacin monotherapy to patients with lesser CAP severity is supported only by evidence grade II A and is due to the lack of efficacy data on fluoroquinolones as monotherapy for the treatment of severe CAP [7, 9, 11]. Although, one study reported that an antibiotic combination therapy improved survival among critically ill patients with bacteriaemic pneumococcal illness compared to those treated with beta-lactam monotherapy [12]. Third generation fluoroquinolones were either not used for monotherapy in this study [12] or further studies dedicated to this issue did not exist at that time. However, several studies have suggested sufficient efficacy of fluoroquinolones monotherapy in CAP in the absence of septic disease progression or mechanical ventilation [13–22].
Specifically, moxifloxacin has a broad spectrum of activity against almost all microorganisms isolated from CAP patients and its activity is not opposed by relevant antimicrobial resistance, including multi-resistant pneumococci and pathogens such as Moraxella catarrhalis and Haemophilus influenzae. Those can carry resistance to penicillins, macrolides, and tetracyclines. Moxifloxacin also possesses the best activity against other pathogens such as Legionella pneumophila, Chlamydophila pneumoniae, and Mycoplasma pneumonia) and is therefore the first choice antibiotic for such infections [23, 24]. Meta-analysis data suggests that moxifloxacin alone has a higher pathogen eradication rate than a β-lactam-based combination therapy [25]. Accordingly, moxifloxacin may be sufficient even when used as a monotherapy in severe CAP. Nevertheless, studies addressing the effectiveness of moxifloxacin monotherapy in severe CAP evoked ARDS are lacking.
Accordingly, we assessed the hypothesis that moxifloxacin monotherapy is equally effective as a guideline recommended combination therapy in ARDS patients due to severe CAP.
Methods
This retrospective study was reviewed and approved by the Ethics Committee of the Medical Faculty of the University of Duisburg-Essen (no. 052776) and informed consent was obtained from patients or their guardians. We eventually included 169 patients with CAP evoked severe ARDS (98 males (58%), 71 females (42%), mean age: 43 years ±13.8 SD), with the need of antibiotic treatment due to an suspected or proven bacterial infection, selected from 229 patients with ARDS from our intensive care unit (ICU) ARDS database (Fig. 1). Patients with a proven or suspected combined viral infection with bacterial superinfection received antimicrobiotic treatment accordingly (n = 41). Forty-one Patients were excluded because ARDS was evoked by trauma or other diseases than CAP and in 19 cases essential data was not documented. Patients with no need of an antibiotic treatment were not included into this study, e.g. patient suffering from solitary viral infection.
Fig. 1.
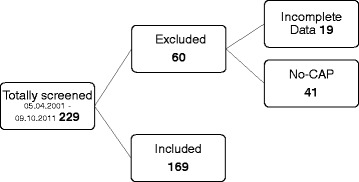
Consort flow chart of inclusion and exclusion of study patients
Community-acquired pneumonia was defined as a primary lung infection of bacterial or viral etiology, without history of prior hospitalization [6, 7]. Diagnosis of CAP and classification of severity was accomplished according to guidelines valid at the time of admission [6]. Patients had been admitted to our ICU between 2001 and 2011 and all fulfilled the joint American/European Consensus Committee criteria for ARDS [26]. Patients were treated with a multimodal concept, which included analgosedation, fluid administration, lung-protective mechanical ventilation, as well as hemodynamic, antibiotic, and diagnostic management. Continuous hemofiltration or hemodialysis was performed according to standardized protocols, if required. Microbiologic data were gathered from frequent samples in every patient (e.g., blood cultures, broncho-alveolar lavage, tracheal aspirates, catheter tip cultures). A summary of the pathogen-associated spectrum based on the first detected germ, including the pathogens triggering a secondary or superinfection is shown in Fig. 2.
Fig. 2.
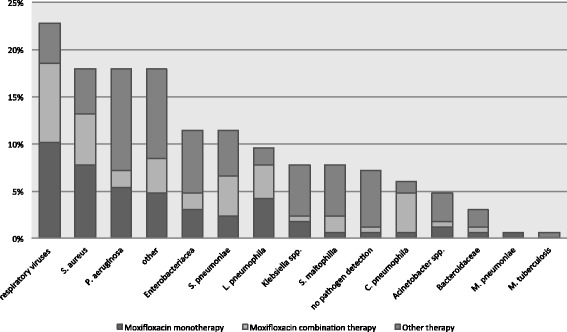
Cumulative frequencies of specific infectious agents detected in respiratory specimen, blood cultures, and/or by serologic and urine antigen tests in the whole cohort of 169 patients during the observation period of 30 days. Bacteria of the normal human flora were not considered
For study inclusion, a minimum of clinical and demographic data had to be documented, including preexisting morbidities and prior treatments (including antibiotics), Lung Injury Score, Simplified Acute Physiology Score II, Sepsis-related Organ Failure Assessment Score (SOFA), body mass index, necessity for continuous hemofiltration/dialysis, mode and settings of mechanical ventilation, PaO2/FiO2 ratio (Horowitz Index), establishment of extracorporal membrane oxygenation (ECMO) therapy, results of microbiologic cultures and related modifications of antibiotic therapy, and blood chemistry values. This included markers of liver cell integrity (such as aspartate transaminase: AST; alanine aminotransferase: ALT; glutamate dehydrogenase: GDH), of cholestasis (total bilirubin, direct bilirubin, gamma glutamyltransferase: GGT, alkaline phosphatase: ALP), and those of liver synthetic function (prothrombin time) in order to evaluate the influence of antibiotic therapy on drug-induced liver injury. The observation period was defined from admission to our ICU either to day 30 of hospital stay or death.
Antibiotic regimens
The patients were allocated to three groups according to the antibiotic regimens they received at the discretion of the ICU attending physician upon admission on day 1 of the observation period. 1) Monotherapy with moxifloxacin intravenously 1 × 400 mg per day (42 patients), 2) combination therapy with moxifloxacin intravenously 1 × 400 mg per day (44 patients) plus a broad-spectrum betalactamase resistant antibiotic (piperacillin, ceftazidime, cefotiam, meropenem, or imipenem), and 3) any other antibiotic regimen based on a broad-spectrum batalactamase resistent antibiotic (see above) and a macrolide (83 patients). Patient’s characteristics and clinical data are depicted in Table 1. Monotherapy was accepted as appropriate in all cases without high risk for multidrug-resistant bacteria. Evaluation of microbiological therapy was followed up on a daily basis for detected microorganisms and on antibiotic susceptibility reports. This was supported by weekly consultations of infectious disease physicians. The duration of antimicrobial therapy was based on subsequently available culture results, clinical recovery, and normalization of laboratory infection values.
Table 1.
Baseline characteristics and 30-day survival of patients with ARDS for the whole cohort and stratified for in three antibiotic regimen
| Overall cohort | Moxifloxacin monotherapy | Moxifloxacin combination therapy | Other antibiotic therapy | P-value | |
|---|---|---|---|---|---|
| Total number (%) | 169 (100) | 42 (25) | 44 (26) | 83 (49) | |
| Survivors 30-days (%) | 117 (69) | 28 (67) | 31 (70) | 58 (70) | 0.915 |
| Non-Survivors 30-days (%) | 52 (31) | 14 (33) | 13 (30) | 25 (30) | |
| Characteristics | |||||
| Age yrs. (range/± SD) | 43 (18–75) | 45 (±13.1) | 41 (±12.5) | 43 (±14.7) | 0.473 |
| Male gender (%) | 98 (58) | 31 (74) | 25 (57) | 42 (51) | 0.045 |
| Female gender (%) | 71 (42) | 11 (26) | 19 (43) | 41 (49) | |
| BMI kg/m2 (±SD) | 27,65 (±7.3) | 30,0 (±8.6) | 27,7 (±6.3) | 26,4 (±6.7) | 0.016 |
| ECMO-Therapy (%) | 51 (30) | 19 (45) | 13 (30) | 19 (23) | 0.070 |
| Dialysis (%) | 83 (49) | 25 (59) | 22 (50) | 36 (43) | 0.337 |
| Antibiotics before ICU admission (%) | 142 (84) | 35 (83) | 38 (86) | 69 (83) | 0.885 |
| Cardiovascular disease (%) | 25 (14) | 4 (10) | 6 (14) | 15 (14) | 0.432 |
| Prior lung disease (%) | 20 (12) | 4 (10) | 7 (16) | 9(18) | 0.609 |
| SAPS II (±SD) | 46 (±19.5) | 47 (±19.2) | 40 (±17.7) | 49 (±19.5) | 0.057 |
| LIS (±SD) | 3,2 (±0.56) | 3,1 (±0.54) | 3,1 (±0.59) | 3,3 (±0.56) | 0.187 |
| SOFA (±SD) | 13 (±6.7) | 15 (±7.3) | 13 (± 5.6) | 12 (±4.4) | 0.131 |
| Horowitz-index upon ICU admission (±SD) | 152 (±115.7) | 135 (±99.5) | 221 (±153.0) | 124 (±80.4) | 0.001 |
Data are presented as n (%); mean (± SD) or median(range); BMI body-mass-index, ECMO extracorporeal membrane-oxygenation, SAPS II simplified acute physiology score, LIS Lung-Injury-Score, SOFA Sepsis-related organ failure assessment score; Horowitz-Index: paO2/FiO2; p-values were calculated using the Chi-squared test for categorical variables, ANOVA for continuous parametric variables, and the Kruskal-Wallis test for continuous nonparametric variables
Statistical analyses
Statistical analyses were performed using SPSS (version 21, IBM, Chicago, IL) and power analysis for endpoint survival using G*Power (version 3.1.9.2, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany, http://www.gpower.hhu.de/) revealed a power of 6.3% regarding the number of included patients. Continuous variables are presented as means and standard deviation or expressed as range. All values of variables were grouped according to the patient’s antibiotic treatment regimen. Comparison of values of variables between groups was performed using the Pearson’s chi-squared test for categorical variables, two-way analysis of variance (including Bonferroni’s post hoc test adjustment) for continuous parametric variables, and the Kruskal-Wallis-test (followed by post-hoc Mann-Whitney-U-tests) for continuous non-parametric variables, respectively. Measurements in survivors vs. non-survivors (Table 2) were investigated for differences using a two-sided Student t-test for continuous variables, a Mann-Whitney-U-test for continuous non-parametric variables, and Pearson’s chi-squared test for categorical variables. Differences with an alpha-error p of less than 0.05 were regarded as statistically significant.
Table 2.
Baseline characteristic of survivors and non-survivors (30 days)
| Survivors 30-days n = 117 (69) |
Non-survivors 30-days n = 52 (31) |
P-value | |
|---|---|---|---|
| Characteristics | |||
| Age yrs. (range/± SD) | 40,8 (±14.2) | 47,2 (±11.7) | 0.005 |
| Male gender (%) | 65 (56) | 33 (63) | 0.337 |
| Female gender (%) | 52 (44) | 19 (37) | |
| BMI kg/m2 (±SD) | 28,1 (±7.7) | 26,7 (± 6.2) | 0.348 |
| ECMO-Therapy (%) | 31 (27) | 20 (38) | 0.099 |
| Dialysis (%) | 49 (42) | 34 (65) | 0.002 |
| Antibiotics before ICU admission (%) | 97 (83) | 45 (87) | 0.552 |
| Cardiovascular disease (%) | 14 (12) | 11 (21) | 0.120 |
| Prior lung disease (%) | 11 (9) | 9 (17) | 0.142 |
| SAPS II (±SD) | 43 (±19.0) | 53 (±19.1) | 0.003 |
| LIS (±SD) | 3,2 (±0.56) | 3,2 (±0.56) | 0.702 |
| SOFA (±SD) | 12 (±5.5) | 15 (±5.8) | <0.001 |
| Horowitz-index upon ICU admission (±SD) | 158 (±122.5) | 140 (±98.5) | 0.480 |
Data are presented as n (%); mean (± SD) or median(range); BMI body-mass-index, ECMO extracorporeal membrane oxygenation, SAPS II simplified acute physiology score, LIS lung-injury-score, SOFA Sepsis-related organ failure assessment score; Horowitz-Index: paO2/FiO2; p-values were calculated using the Chi-squared test for categorical variables, t-test for continuous parametric variables, and the Mann&Whitney U-test test for continuous nonparametric variables
The antibiotic treatment regimens were also compared in relation to 30-day survival using the Kaplan-Meier method with a log-rank test. The impact on 30-day survival of the choice of antibiotic treatment, age, gender, height, weight, pulmonary or cardiac pre-illnesses, ECMO therapy, dialysis or hemofiltration, Simplified Acute Physiology II Score, Lung Injury Score, SOFA Score, and the Horowitz Index as prognostic factors for clinical outcome were analyzed by multivariate Cox regression analysis with stepwise removal of non-significant variables (p > 0.05) from the model. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated from the Cox regression analysis to describe the effect of covariates on the hazard.
Biomarkers of liver cell integrity, cholestasis, and liver function were assessed by the Kruskal-Wallis and Monte Carlo tests, comparing the peak values of these markers. In case of a significant result, a time-dependent analysis of the respective data means over three periods (days 0 to 10, 11 to 20, and day 21 to 30, respectively) was performed by the Kruskal-Wallis test to describe a potential time-dependence. Variances in all calculations were regarded as statistically significant within an a priori alpha error p of less than 0.05.
Results
30-day survival (67% overall) did not differ (p = 0.89, Fig. 3) between the antibiotic regimen groups, with a statistical power of 6.3%. Fourteen patients (33%) died in the moxifloxacin monotherapy group, 13 patients (30%) in the moxifloxacin combination therapy group, and 25 patients (30%) in the group treated with other antibiotics.
Fig. 3.
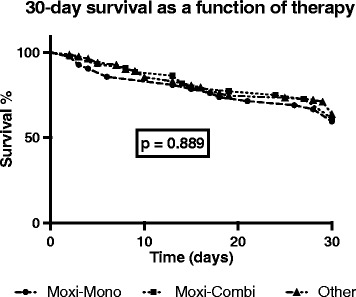
Thirty-day survival in patients with severe CAP evoked acute respiratory distress syndrome (ARDS) stratified for the antibiotic treatment regime after ICU admission. Kaplan-Meier estimates were used to calculate probabilities of 30-day survival. Thirty-day survival was not significantly different (p = 0.889) between moxifloxacin monotherapy (Moxi-Mono), moxifloxacin combination therapy (Moxi-Combi), and other regimens (Other)
The most common cause of CAP evoked severe ARDS were bacterial infections (n = 115, 68%), followed by viral infections (n = 41; 24%), 68% (n = 28) of the latter caused by Influenza-A (H1N1). No specific pathogen was detected in 8% (n = 13) of all patients. Most frequently detected bacteria were S. aureus (23%, n = 39), P. aeruginosa (11%, n = 19), S. pneumoniae (11%, n = 19), Enterobacteriacea (11%, n = 18), and L. pneumophila (10%, n = 17). In almost all patients moxifloxacin showed a suitable spectrum of antimicrobial activity against the initially detected bacterium. A summary of the pathogen-associated spectrum, based on the first detected germ and also including pathogens presumably triggering a secondary or superinfection, is shown in Fig. 2.
Looking at the antibiotic pretreatment the regimens were distributed equally between the three groups. In detail, 2 patients in the moxifloxacin-monotherapy group were pretreated with moxifloxacin alone, 6 patients were pretreated with moxifloxacin in combination with a ß-lactam antibiotic, 27 received other ß-lactam based regimen without using moxifloxacin and 7 patients were not treated with antibiotics till admission on our ICU. In the moxifloxacin combination-group 2 patients were pretreated with moxifloxacin alone, 3 in combination with beta-lactam antibiotics, 33 patients without using moxifloxacin and 6 patients did not receive any antibiotics till admission. In the other treatment group one patient received moxifloxacin alone and 2 patients received moxifloxacin in combination, 65 were pretreated with a regimen not using moxifloxacin and 14 patients were not given antibiotics until admission. Therefore, respective antibiotic treatments had been started in 84% of all patients prior to ICU admission, with no significant impact on 30-day survival (p = 0.42) compared to patients without antibiotic pretreatment.
Comparing mean duration of ICU stay, we found the shortest duration in the moxifloxacin-monotherapy group (24.6 days ±18.15) compared with the moxifloxacin combination therapy (25.1 days ±19.28), and the control group (27.6 days ±25.83), albeit without reaching significance (p = 0.797). Furthermore, there was no significant difference in the frequency of antibiotic changes between the three groups over the 30-day observation period (p = 0.805).
Extracorporeal gas exchange for severe lung failure was necessary in 51 of 169 patients (30%), and hemodialysis/hemofiltration was established in 83 patients (49%) within the 30-day observation period. Overall duration of stay in the ICU averaged 26 days ±22.4. Patient characteristics are illustrated in Tables 1 and 2.
Age (p = 0.473), ECMO therapy (p = 0.07), dialysis (p = 0.337), antibiotic treatment before ICU admission (p = 0.89), pulmonary (p = 0.61) or cardiac disease (p = 0.43), SOFA Score (p = 0.13), Lung Injury Score (p = 0.19), and the SAPS II Score (p = 0.057) were distributed equally across groups.
We found some differences regarding the demographic characteristics between groups (Table 1), i.e., gender (p = 0.045), body mass index (p = 0.016), and Horowitz Index (p = 0.001). Body mass index was 13.6% greater (p = 0.006) and there were 23% more males in the moxifloxacin monotherapy group compared to other groups (Table 1). Furthermore, the Horowitz Index was 38.9% less in the moxifloxacin monotherapy group compared to the moxifloxacin combination therapy group (p < 0.001, Table 1). The Horowitz Index did not differ between the moxifloxacin monotherapy group and the combination therapy group (p = 0.59).
Multivariate proportional hazard analysis by Cox regression revealed age (hazard ratio (HR): 1.03; 95% confidence interval (CI) 1.0–1.05), p = 0.029), body mass index (HR 0.95; 95% CI 0.89–1.00), p = 0.042), cardiac preconditions (HR 2.2 (95% CI 1.1–4.4), p = 0.026) and hemodialysis/hemofiltration (HR 2.5; 95% CI 1.3–4.8), p = 0.004) as independent risk factors for 30-day survival (Table 3). Thus, ARDS patients without cardiac preconditions showed a more than twofold greater 30-day survival compared to patients with preexisting cardiac disease and patients needing hemodialysis or hemofiltration showed a 2.5 greater mortality (Table 3).
Table 3.
Multivariate stepwise Cox regression analysis with Hazard ratio
| p-value | HR (95% CI) | |
|---|---|---|
| Antibiotic regime | ||
| Other antibiotic therapy | ||
| Moxifloxacin monotherapy | 0.86 | |
| Moxifloxacin combination-therapy | 0.99 | |
| Sex | 0.653 | |
| Age | 0.029 | 1.03 (1.00–1.05) |
| BMI | 0.042 | 0.95 (0.90–1.00) |
| History of prior pulmonary disease | 0.481 | |
| History of prior cardiovascular disease | 0.027 | 2.12 (1.06–4.35) |
| ECMO-Therapy | 0.222 | |
| Dialysis | 0.005 | 2.48 (1.31–4.71) |
| Horowitz-Index upon ICU admission | 0.987 | |
| SAPS-II | 0.313 | |
| LIS | 0.477 | |
| SOFA-Score | 0.358 | |
HR hazard ratio (specified in case of significant p-value of multivariate stepwise Cox regression analysis), CI confidence interval, BMI body-mass-index, ECMO extracorporeal membrane Oxygenation, SPAS: simplified acute physiology score, LIS Lung-Injury-Score, SOFA Sepsis-related organ failure assessment; Horowitz-Index: pO2/FiO2
Liver tests were abnormal in all groups and there were significant differences between groups when comparing maximum AST activity (p = 0.048), ALT activity (p = 0.03), and direct bilirubin concentration (p = 0.01). When moxifloxacin was used within the first 10 days (both in monotherapy and combination therapy groups), a 65–123% increased AST activity (p < 0.001, Fig. 4), a 81–16% greater ALT activity (p < 0.001, Fig. 4), and a 23–69% greater direct bilirubin concentration (p < 0.001, Fig. 4) was seen compared to the regimens not using moxifloxacin. However, initially abnormal liver function variables diminished over time and between group differences faded over time. Accordingly, during days 21–30 we found no significant differences for AST activity (p = 0.06), ALT activity (p = 0.68), or direct bilirubin concentration (p = 0.64) under moxifloxacin regimens when compared to the non-moxifloxacin group (Fig. 4). Other liver related tests (GDH, GLDH, GGT, ALP, prothrombin time) showed no significant differences between groups.
Fig. 4.
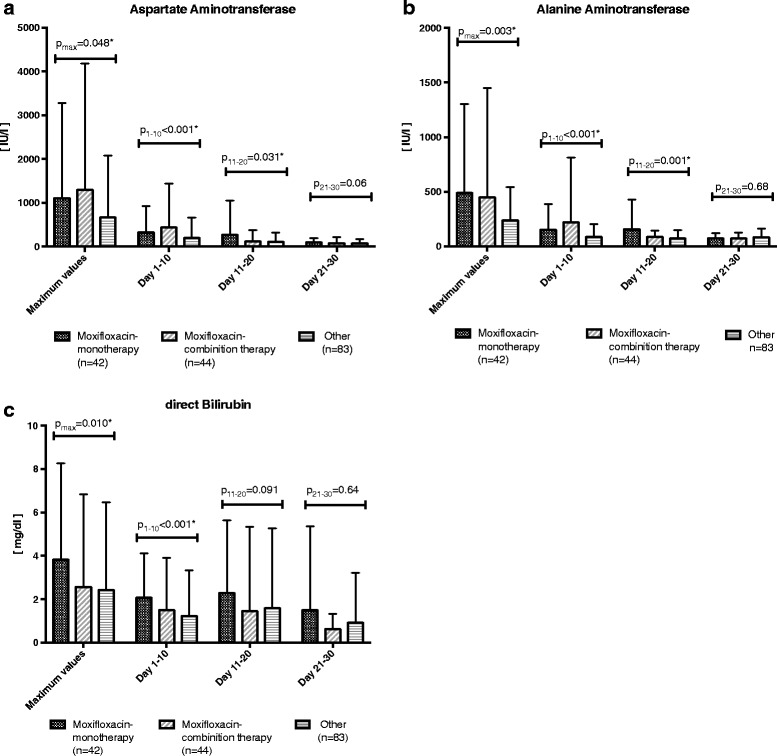
Time course of liver function markers in patients with ARDS as stratified by antibiotic regime after admission to ICU. Group means are depicted as a bar chart with corresponding standard deviation. a Aspartate aminotransferase activity. The maximum values of each patient (pmax = 0.048) and the average values of the first 10 days (p1–10 < 0.001) and from days 11–20 (p11–20 = 0.031) were significantly different between moxifloxacin monotherapy (Moxi-Mono), moxifloxacin combination therapy (Moxi-Combi), and other regimens (Other). In, there was no significant difference between during the last 10 days (p21–30 = 0.06). b Alanine aminotransferase activity. The maximum values of each patient (pmax = 0.003) and the average values in the first 10 days (p1–10 < 0.001) and from day 11–20 (p11–20 = 0.001) were significantly different between moxifloxacin monotherapy, moxifloxacin combination therapy (Moxi-Combi), and other regimens (Other). In contrast, there was no significant difference in the last 10 days (p21–30 = 0.68). c Direct bilirubin concentration. The maximum values of each patient (pmax = 0.010) and the average values in the first 10 days (p1–10 < 0.001) were significantly different between moxifloxacin monotherapy (Moxi-Mono), moxifloxacin combination therapy (Moxi-Combi), and other regimens (Other). In contrast, there was no significant difference from day 11–20 (p11–20 = 0.091) and in the last 10 days (p21–30 = 0.64)
Discussion
This retrospective study shows, that moxifloxacin monotherapy is effective and not associated with excess 30-day mortality in patients with severe CAP evoking ARDS (Fig. 3). Furthermore, initially and temporarily increased liver function markers had no apparent influence on outcome and elevated values normalized over time. Therefore, moxifloxacin monotherapy may be an effective and safe antibiotic treatment even in CAP evoked severe ARDS while extending the spectrum of susceptible bacteria.
Our findings supplement the recommendations of both the current German and American guidelines, which prefer beta-lactam antibiotic based combination therapies [6, 7, 9] over moxifloxacin monotherapy. However, other studies have also reported that moxifloxacin monotherapy can be as effective and safe as a combination regimen in patients with severe CAP and is not associated with relevant disadvantages for the patient’s recovery [18, 20–22]. Furthermore, Vardakas et al. [21] showed in a meta-analysis that the use of respiratory fluoroquinolones was associated with higher treatment success and a better clinical outcome when compared to the combination therapy of a ß-lactam antibiotic and a macrolide. In particular, treatment success as defined by resolution of two or more baseline symptoms or signs of infection, especially in cases with more severe CAP, was significantly higher among patients who received fluoroquinolones. While we could not demonstrate superiority of moxifloxacin monotherapy, even showing no difference is an important finding since using one drug instead of multiple antibiotics might be beneficial alone by reducing costs and, potentially, development of antibiotic resistance in other ICU patients. Comparing duration of ICU stay our data correspond well with the above mentioned studies. In addition, the shortest duration was found in the moxifloxacin-monotherapy group (24.6 days) compared to the control group (27.6 days), albeit without showing statistical difference. Additionally, our data showed no significant different frequency of antibiotic-changes between the three groups.
Moxifloxacin covers most bacteria evoking CAP and is more effective against of the whole spectrum of CAP related pathogens compared to beta-lactam antibiotic based combination therapies [9, 18, 19]. A better microbiological effect and lower risk of treatment failures of moxifloxacin [19, 27] can be attributed to its excellent tissue penetration, high bioavailability, a bactericidal mechanism, an effect even on otherwise resistant pathogens, and high effectiveness against pathogens of atypical pneumonia, such as Chlamydia, Legionella, or Mycoplasma. Therefore moxifloxacin is the first choice in pneumonia cases caused by Legionella. Our germ spectrum showed in almost 10% of the cases an infection with Legionella causing severe CAP and ARDS (Fig. 2). Thus, we would submit that a third generation fluoroquinolone should be part of an initially used antibiotic regimen in CAP evoked ARDS, at least unless proper culture results and resistograms have been obtained.
Our data suggest that moxifloxacin monotherapy may be as effective as a betalactam antibiotic based combination therapy with or without fluoroquinolones. In fact, non-difference of antibiotic monotherapy might be evidence that besides the selection of the initial antibiotic and general supportive treatment, outcome likely depends more on the appropriateness of the hosts’ immune response than the antibiotic regime chosen.
Limitations
Although all ARDS patients were treated with a standardized multimodal concept, we cannot exclude that unknown and potentially confounding factors exist. In fact, we found a somewhat unequal distribution in some variables such as the Body-Mass-Index, gender, and the initial Horowitz Index but well-known independent risk factors for survival, such as cardiac preconditions, hemodialysis/−filtration, or age were not differently distributed between the therapy groups. In fact, a worse gas exchange in the moxifloxacin group, as suggested by a lesser Horowitz index, is a conservative error with respect to the non-difference hypothesis and did not turn out as independent risk factor both in our Cox regression analysis and in previous studies [28]. The spectrum of cultured infectious agents demonstrates for the large part the pathogens typical of community acquired pneumonia in ARDS patients, but their incidence did not show the classic CAP profile. One explanation might be that we examined a peculiar cohort with only the severest CAP evoking ARDS and that such a cohort has a slightly different pathogen spectrum. Another factor might be that 84% of patients had already received an antibiotic treatment before ICU admission, i.e., before we bronchoscopically sampled specimen or collected blood for cultures and therefore the targeted microbiological detection is much more imprecise and difficult, which may have influenced our detected microbiologic spectrum significantly. Additional the exact time and indication as well as the position of the gained samples are not reconstructible based on our recorded facts in our ARDS sheet. Another limitation is the inclusion of viral patients with the need of antibiotic therapy due to suspected or proven bacterial superinfection or co-infection. We did not exclude these patients since the empirical antimicrobial treatment was applicated, this special cohort my have additionally confounding impact on our result. Finally, although our data were derived from a cohort of patients with severe ARDS, our analysis is retrospective and associated with the substantial ß-error due to an underpowered sample size. Accordingly, we cannot exclude that a significant differencemay still be present between groups with a much larger sample size. Furthermore, our exploratory analysis revealed some differential effects on liver enzyme activities in the early observation period when using moxifloxacin which merits further analysis.
Conclusions
In summary, our retrospective data in a large cohort of ARDS patients allows the conclusion, that moxifloxacin monotherapy may also be an effective and safe empirical treatment option in patients with ARDS caused by severe CAP, with the advantage of covering the largest spectrum of severe CAP evoking infectious agents. Clearly, prospective, randomized controlled trials are needed to further investigate the effects of moxifloxacin monotherapy in patients with ARDS evoked by community-acquired pneumonia to provide definite recommendations.
Acknowledgments
None.
Funding
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum (Ref. No. IN-1214264), just for financial support for publication costs. This had no impact on our design or study, collection, analysis and interpretation of data.
Availability of data and materials
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
Dr. med. TR: Main author of this manuscript. Dr. med. SA: Supporting statistical calculation and participated in the design of this study. Dr. JK: Supporting data collection and participated in the design of this study. PD Dr. JS: Supporting microbiologic interpretation of the results and improving design of the study. Prof. Dr. MA: Supporting data collection, participated in the design of this study and revising the manuscript. Prof. Dr. JP: Senior author of this manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This retrospective study was reviewed and approved by the Ethics Committee of the Medical Faculty of the University of Duisburg-Essen (no. 052776) and informed consent was obtained from patients or their guardians.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ARDS
Acute respiratory distress syndrome
- AST
Aspartate aminotransferase
- CAP
Community acquired pneumonia
- CI
Confidence interval
- ECMO
Extracorporal membrane oxygenation
- FiO2
Inspired fraction of oxygen
- GDH
Glutamate dehydrogenase
- GGT
Gamma-glutamyl transferase
- HR
Hazard ratio
- ICU
Intensive care unit
- PaO2
Arterial partial pressure of oxygen
- SAPS II
Simplified Acute Physiology Score II
- SOFA
Sepsis-related organ failure assessment score
Contributor Information
Tim Rahmel, Phone: (+49)(234) 299-80025, Email: tim.rahmel@ruhr-uni-bochum.de.
Sven Asmussen, Email: sven.asmussen@kk-bochum.de.
Jan Karlik, Email: jan.karlik@uk-essen.de.
Jörg Steinmann, Email: joerg.steinmann@uk-essen.de.
Michael Adamzik, Email: michael.adamzik@kk-bochum.de.
Jürgen Peters, Email: juergen.peters@uk-essen.de.
References
- 1.Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol. 2012;4:159–169. doi: 10.2147/CLEP.S28800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Braune S, Kluge S. ARDS--an update. Dtsch Med Wochenschr. 2013;138:1019–1022. doi: 10.1055/s-0032-1333051. [DOI] [PubMed] [Google Scholar]
- 5.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 6.Hoffken G, Lorenz J, Kern W, et al. Epidemiology, diagnosis, antimicrobial therapy and management of community-acquired pneumonia and lower respiratory tract infections in adults. Guidelines of the Paul-Ehrlich-Society for Chemotherapy, the German Respiratory Society, the German Society for Infectiology and the Competence Network CAPNETZ Germany. Pneumologie. 2009;63:e1–68. doi: 10.1055/s-0029-1215037. [DOI] [PubMed] [Google Scholar]
- 7.Ewig S, Hoffken G, Kern WV, et al. Management of Adult Community-acquired Pneumonia and Prevention - Update 2016. Pneumologie. 2016;70:151–200. doi: 10.1055/s-0042-101873. [DOI] [PubMed] [Google Scholar]
- 8.Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016;42:699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frei CR, Attridge RT, Mortensen EM, et al. Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clin Ther. 2010;32:293–299. doi: 10.1016/j.clinthera.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez A, Mendia A, Sirvent JM, et al. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 12.Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 13.Zhanel GG, Ennis K, Vercaigne L, et al. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs. 2002;62:13–59. doi: 10.2165/00003495-200262010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Zhanel GG, Fontaine S, Adam H, et al. A Review of New Fluoroquinolones : Focus on their Use in Respiratory Tract Infections. Treat Respir Med. 2006;5:437–465. doi: 10.2165/00151829-200605060-00009. [DOI] [PubMed] [Google Scholar]
- 15.Finch R, Schurmann D, Collins O, et al. Randomized controlled trial of sequential intravenous (i.v.) and oral moxifloxacin compared with sequential i.v. and oral co-amoxiclav with or without clarithromycin in patients with community-acquired pneumonia requiring initial parenteral treatment. Antimicrob Agents Chemother. 2002;46:1746–1754. doi: 10.1128/AAC.46.6.1746-1754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzman I, Bezlepko A, Kondova Topuzovska I, et al. Efficacy and safety of moxifloxacin in community acquired pneumonia: a prospective, multicenter, observational study (CAPRIVI) BMC Pulm Med. 2014;14:105. doi: 10.1186/1471-2466-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forstner C, Rohde G, Rupp J, et al. Community-acquired Haemophilus influenzae pneumonia - New insights from the CAPNETZ study. J Inf Secur. 2016;72:554–563. doi: 10.1016/j.jinf.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Torres A, Garau J, Arvis P, et al. Moxifloxacin monotherapy is effective in hospitalized patients with community-acquired pneumonia: the MOTIV study--a randomized clinical trial. Clin Infect Dis. 2008;46:1499–1509. doi: 10.1086/587519. [DOI] [PubMed] [Google Scholar]
- 19.Wenisch C, Krause R, Szell M, Laferl H. Moxifloxacin versus standard therapy in patients with pneumonia hospitalized after failure of preclinical anti-infective treatment. Infection. 2006;34:190–195. doi: 10.1007/s15010-006-5120-x. [DOI] [PubMed] [Google Scholar]
- 20.Albertson TE, Dean NC, El Solh AA, Gotfried MH, Kaplan C, Niederman MS. Fluoroquinolones in the management of community-acquired pneumonia. Int J Clin Pract. 2010;64:378–388. doi: 10.1111/j.1742-1241.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- 21.Vardakas KZ, Siempos II, Grammatikos A, Athanassa Z, Korbila IP, Falagas ME. Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:1269–1277. doi: 10.1503/cmaj.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroy O, Saux P, Bedos JP, Caulin E. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005;128:172–183. doi: 10.1378/chest.128.1.172. [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt O, Welte T. 10 years' experience with the pneumococcal quinolone moxifloxacin. Expert Rev Anti-Infect Ther. 2009;7:645–668. doi: 10.1586/eri.09.46. [DOI] [PubMed] [Google Scholar]
- 24.Miravitlles M, Anzueto A. Moxifloxacin: a respiratory fluoroquinolone. Expert Opin Pharmacother. 2008;9:1755–1772. doi: 10.1517/14656566.9.10.1755. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X, Liang BB, Wang R, et al. Treatment of community-acquired pneumonia with moxifloxacin: a meta-analysis of randomized controlled trials. J Chemother. 2012;24:257–267. doi: 10.1179/1973947812Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 26.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med. 1994;20:225-232. [DOI] [PubMed]
- 27.Menendez R, Torres A, Zalacain R, et al. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59:960–965. doi: 10.1136/thx.2003.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995;152:1818–1824. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.


