Abstract
We report the unusual case of a 63-year-old man with spinocerebellar ataxia (SCA) type 31 who developed neuromyelitis optica spectrum disorder (NMOSD) 14 years after the onset of cerebellar symptoms. In addition to cerebellar atrophy, magnetic resonance imaging showed multiple high-intensity areas in the brain and a long thoracic cord lesion from Th1/2 to Th11. The combination of NMOSD and SCA31 is accidental. However, our case suggests that inflammatory processes could be involved in the pathogenesis of NMOSD and SCA31.
Keywords: Neuromyelitis optica spectrum disorder, Anti-aquaporin-4 antibody, Spinocerebellar ataxia type 31, Neurodegeneration, Autoimmune disease, Central nervous system disease
Introduction
Spinocerebellar ataxia type 31 (SCA31) is an autosomal dominant cerebellar ataxia, characterized by a slowly progressive pure cerebellar ataxia [1, 2]. The genetic defect of SCA31 is an expansion of complex penta-nucleotide repeats containing (TGGAA)n in the 16q22.1 region [1, 2]. This is the most common familial SCA in Japan [3]. Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system (CNS) that mainly affects the optic nerve and spinal cord [4, 5]. The anti-aquaporin-4 (AQP4) antibody has been identified as a disease-specific autoantibody in NMO patients [4, 5]. Here, we report the unusual case of a male SCA31 patient who developed NMO spectrum disorder (NMOSD) 14 years after the onset of cerebellar symptoms.
Case Presentation
A 49-year-old man developed speech disturbance and an unsteady gait. His mother had similar symptoms. He went to the hospital at the age of 52 years. The neurological examination disclosed saccadic eye movement, slurred speech, poor coordination, and wide-based and poor tandem walking. Deep tendon reflexes were normal and no pathological reflexes were noted. His Scale for the Assessment and Rating of Ataxia (SARA) score was 13. Brain magnetic resonance imaging (MRI) revealed a mild cerebellar atrophy with widening of cerebellar sulci (Fig. 1a, arrow). Genetic analysis revealed a mutation of −16 G>T q.22 and insertion of a penta-nucleotide repeat expansion (3.3 kb). The patient's mother showed the same mutation and repeat expansion of SCA31. He was diagnosed with SCA31. Differential diagnoses revealed no signs of metabolic or inflammatory etiologies. The cerebellar symptoms gradually worsened. His SARA score was 21 at the age of 62 years.
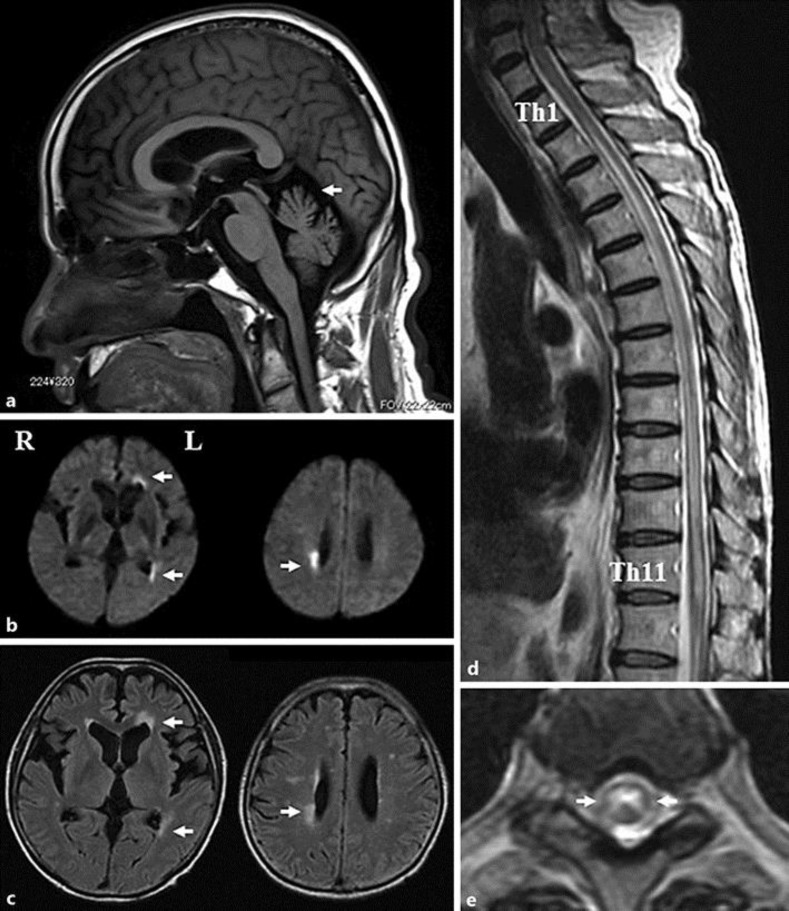
Fig. 1.
Sagittal T1-weighted MRI showing cerebellar atrophy (a, arrow). Axial diffusion-weighted (b) and fluid-attenuated inversion recovery (c) MRI showing high signals in the right posterior horn as well as the left anterior and posterior horns (arrows). Sagittal thoracic spine T2-weighted MRI showing high signal extending from Th1/2 to Th11 (d). Axial thoracic spine T2-weighted MRI showing high signal in the central part of the cord at the Th6/7 level (e, arrows).
Fourteen years later at the age of 63 years, he suddenly developed paraplegia, as well as bladder and rectal disturbance. On admission, the general physical examination was normal. His past medical history included diabetes mellitus. Neurological examinations revealed saccadic eye movement, slurred speech, poor coordination, and muscle weakness of grade 1/5 affecting the lower limbs. He had no disturbance in the visual field and acuity. Deep tendon reflexes were absent in the lower limbs without pathological reflexes. There was loss of position and vibration sense combined with hypesthesia below T4. He had bladder and rectal disturbances. His SARA score was 32. The Expanded Disability Status Scale (EDSS) score was 8.5. Findings from routine serum studies, including blood chemistry and enzymes, were normal except for a HbA1c of 6.3%. He had normal or negative studies for HTLV-1, serum angiotensin converting enzyme, syphilis serology, and PR3- and MPO-ANCA. Cerebrospinal fluid analysis revealed a cell count of 12/ml (100% monocytes) with an increased protein concentration of 64 mg/dl and a myelin basic protein level of 754 mg/dL. Brain MRI showed high-intensity areas in the right posterior horn as well as the left anterior and posterior horns on diffusion-weighted (Fig. 1b, arrows), fluid-attenuated inversion recovery (FLAIR) (Fig. 1c, arrows), and T2-weighted image (T2WI). Spinal MRI on T2WI revealed long lesions extending from Th1/2 to Th11 (Fig. 1d). Axial T2WI at the level of Th6/7 showed high-signal intensity in the central part of the cord (Fig. 1e, arrows). The patient was treated 3 times with high-dose methylprednisolone (1,000 mg/day for 3 days). His serum was positive for anti-AQP4 antibody (cell-based assay) 25 days after the onset. He was diagnosed with NMOSD and treated with immunoadsorption plasmapheresis and followed up with daily prednisolone (30 mg). Follow-up brain MRI on T2WI and FLAIR 50 days after the onset showed slowly diminishing high-intensity areas in the right posterior horn and left anterior and posterior horns. However, follow-up spinal MRI had no change. He was bedridden and the paraplegia persisted. He discharged to another hospital for rehabilitation 3 months after the onset with a low-dose daily prednisolone (10 mg). His EDSS score was still 8.5.
Discussion
We presented a case of NMOSD developing in a patient with SCA31. The cerebral lesions in our patient were not specific for NMOSD. However, 10% of NMO patients had multiple sclerosis-like lesions [6]. Our patient presented with paraplegia, as well as bladder and rectal disturbance. He had severe symptoms, even though immunosuppressive therapy was initiated. Late onset and male gender were atypical for NMO. They most likely affected the clinical severity and also the cause of the poor prognosis.
SCA31 is an autosomal dominant disorder with adult onset and a slowly progressive pure cerebellar ataxia. The disorder is the result of a specific insertion of complex penta-nucleotide repeats containing (TGGAA)n in the 16q22.1 region [1]. Neuropathological examination revealed severe Purkinje cell loss that was accentuated in the anterior lobe of the cerebellum [2]. Although the present combination of NMOSD and SCA31 is accidental, only one previous report showed multiple sclerosis coinciding with SCA3, in which the loss of neuronal integrity by SCA3-related CAG expansion was speculated to contribute the inflammatory CNS disease [7]. In fact, inflammatory genes were upregulated in cell lines and patient brains of SCA3 [8], and a secretion of eotaxin was elevated in astrocyte in brains of asymptomatic SCA3 carries [9]. Although there are no reports on cytokine upregulation in SCA31 so far, our case suggests that inflammatory processes could be involved in the pathogenesis of NMOSD and SCA31. It is necessary to continue efforts to increase the number of case series, such as our case report, in the future.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest and no financial disclosure to declare.
References
- 1.Niimi Y, Takahashi M, Sugawara E, Umeda S, Obayashi M, Sato N, Ishiguro T, Higashi M, Eishi Y, Mizusawa H, Ishikawa K. Abnormal RNA structures (RNA foci) containing a penta-nucleotide repeat (UGGAA)n in the Purkinje cell nucleus is associated with spinocerebellar ataxia type 31 pathogenesis. Neuropathology. 2013;33:600–611. doi: 10.1111/neup.12032. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Asakawa M, Suzuki-Kouyama E, Tabata K, Shintaku M, Ikeda S, Oyanagi K. Distinctive features of degenerating Purkinje cells in spinocerebellar ataxia type 31. Neuropathology. 2014;34:261–267. doi: 10.1111/neup.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi T, Kitayama M, Nakano T, Adachi Y, Kato S, Nakashima K. Autopsy case of spinocerebellar ataxia type 31 with severe dementia at the terminal stage. Neuropathology. 2015;35:273–279. doi: 10.1111/neup.12184. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63:390–396. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 7.Röhl JE, Lünemann JD, Zimmer C, Zschenderlein R, Valdueza JM. Multiple sclerosis coinciding with Machado-Joseph disease. Neurol Sci. 2005;26:135–136. doi: 10.1007/s10072-005-0447-0. [DOI] [PubMed] [Google Scholar]
- 8.Evert BO, Vogt IR, Kindermann C, Ozimek L, de Vos RA, Brunt ER, Schmitt I, Klockgether T, Wullner U. Inflammatory genes are upregulated in expanded ataxin-3-expressing cell lines and spinocerebellar ataxia type 3 brains. J Neurosci. 2001;21:5389–5396. doi: 10.1523/JNEUROSCI.21-15-05389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silvia Carvalho G, Saute JA, Haas CB, Torrez VR, Brochier AW, et al. Cytokines in Machado Joseph disease/spinocerebellar ataxia 3. Cerebellum. 2016;15:518–525. doi: 10.1007/s12311-015-0719-z. [DOI] [PubMed] [Google Scholar]