Abstract
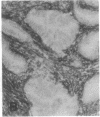
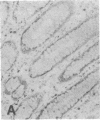
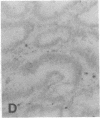
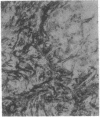
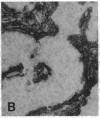
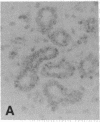
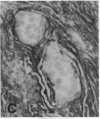
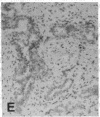
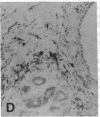
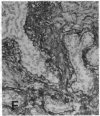
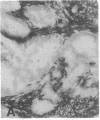
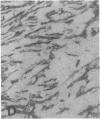
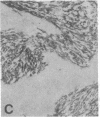
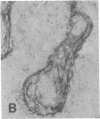
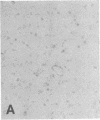
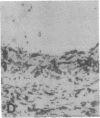
The F19 antigen is a cell surface glycoprotein (Mr, 95,000) of human sarcomas and proliferating, cultured fibroblasts that is absent from resting fibroblasts in normal adult tissues. Normal and malignant epithelial cells are also F19-. The present immunohistochemical study describes induction of F19 in the reactive mesenchyme of epithelial tumors. F19+ fibroblasts were found in primary and metastatic carcinomas, including colorectal (18 of 18 cases studied), breast (14/14), ovarian (21/21), bladder (9/10), and lung carcinomas (13/13). In contrast, the stroma of benign colorectal adenomas, fibrocystic disease and fibroadenomas of breast, benign prostate hyperplasia, in situ bladder carcinomas, and benign ovarian tumors showed no or only moderate numbers of F19+ fibroblasts. Analysis of dermal incision wounds revealed that F19 is strongly induced during scar formation. Comparison of F19 with the extracellular matrix protein tenascin, a putative marker of tumor mesenchyme, showed a cellular staining pattern for F19 vs. the extracellular matrix pattern for tenascin and widespread expression of tenascin in F19- normal tissues and benign tumors. Our results suggest that the F19+ phenotype correlates with specialized fibroblast functions in wound healing and malignant tumor growth. Because of its abundance in tumor mesenchyme, F19 may serve as a target for antibodies labeled with radioisotopes or toxic agents, or inflammatogenic antibodies, in carcinoma patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdon M. A., Wikstrand C. J., Furthmayr H., Matthews T. J., Bigner D. D. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res. 1983 Jun;43(6):2796–2805. [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. M., Taylor C. R., Epstein A. L. Tumor necrosis treatment of ME-180 human cervical carcinoma model with 131I-labeled TNT-1 monoclonal antibody. Cancer Res. 1989 Aug 15;49(16):4578–4585. [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Garin-Chesa P., Melamed M. R., Rettig W. J. Immunohistochemical analysis of human neuronectin expression in normal, reactive, and neoplastic tissues. J Histochem Cytochem. 1989 Dec;37(12):1767–1776. doi: 10.1177/37.12.2685107. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Loridon-Rosa B., Vielh P., Matsuura H., Clausen H., Cuadrado C., Burtin P. Distribution of oncofetal fibronectin in human mammary tumors: immunofluorescence study on histological sections. Cancer Res. 1990 Mar 1;50(5):1608–1612. [PubMed] [Google Scholar]
- Mackie E. J., Chiquet-Ehrismann R., Pearson C. A., Inaguma Y., Taya K., Kawarada Y., Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Takio K., Titani K., Greene T., Levery S. B., Salyan M. E., Hakomori S. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J Biol Chem. 1988 Mar 5;263(7):3314–3322. [PubMed] [Google Scholar]
- Rettig W. J., Chesa P. G., Beresford H. R., Feickert H. J., Jennings M. T., Cohen J., Oettgen H. F., Old L. J. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res. 1986 Dec;46(12 Pt 1):6406–6412. [PubMed] [Google Scholar]
- Rettig W. J., Chesa P. G., Beresford H. R., Melamed M. R., Old L. J. Definition of an extracellular matrix protein in rostral portions of the human central nervous system. Brain Res. 1988 Jan 12;438(1-2):315–322. doi: 10.1016/0006-8993(88)91355-8. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Garin-Chesa P., Beresford H. R., Oettgen H. F., Melamed M. R., Old L. J. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A. 1988 May;85(9):3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Garin-Chesa P. Cell type-specific control of human neuronectin secretion by polypeptide mediators and phorbol ester. J Histochem Cytochem. 1989 Dec;37(12):1777–1786. doi: 10.1177/37.12.2685108. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Triche T. J., Garin-Chesa P. Stimulation of human neuronectin secretion by brain-derived growth factors. Brain Res. 1989 May 15;487(1):171–177. doi: 10.1016/0006-8993(89)90954-2. [DOI] [PubMed] [Google Scholar]
- Vogetseder W., Feichtinger H., Schulz T. F., Schwaeble W., Tabaczewski P., Mitterer M., Böck G., Marth C., Dapunt O., Mikuz G. Expression of 7F7-antigen, a human adhesion molecule identical to intercellular adhesion molecule-1 (ICAM-1) in human carcinomas and their stromal fibroblasts. Int J Cancer. 1989 May 15;43(5):768–773. doi: 10.1002/ijc.2910430504. [DOI] [PubMed] [Google Scholar]
- Welt S., Mattes M. J., Grando R., Thomson T. M., Leonard R. W., Zanzonico P. B., Bigler R. E., Yeh S., Oettgen H. F., Old L. J. Monoclonal antibody to an intracellular antigen images human melanoma transplants in nu/nu mice. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4200–4204. doi: 10.1073/pnas.84.12.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky M. R., Moseley R. P., Coakham H. B., Coleman R. E., Bigner D. D. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989 May 15;49(10):2807–2813. [PubMed] [Google Scholar]