Abstract
Objectives
YWHAE-rearranged high-grade endometrial stromal sarcoma (HG-ESS) is a rare, recently defined uterine sarcoma harboring t(10;17)(q22;p13) resulting in YWHAE-NUTM2A/B fusion. Chemotherapy sensitivity of metastatic YWHAE-rearranged HG-ESS is unknown. We reviewed the response to chemotherapy in women with YWHAE-rearranged HG-ESS to provide guidance for clinical management.
Methods
We retrospectively identified patients diagnosed with YWHAE-rearranged HG-ESS who received treatment for metastatic disease at our institutions. Cytogenetics or fluorescence in situ hybridization were performed in all cases to confirm rearrangement and, in conjunction with histopathology, a diagnosis of YWHAE-rearranged HG-ESS. Patient demographics, tumor histology, surgical procedures, radiation therapy, chemotherapy and treatment responses were collected.
Results
Seven patients were identified with YWHAE-rearranged HG-ESS and met criteria for inclusion in this study. The median age at diagnosis was 45 (range 42–47). All patients had undergone hysterectomy with bilateral salpingo-oophorectomy. FIGO stage at diagnosis was IVB in four patients and a single patient each at stage IIIB, II or I. Median follow-up for the cohort was 27 months (range 6–123). Six patients received anthracycline-based chemotherapy, with two of six achieving a complete radiologic response. One patient received gemcitabine and docetaxel, resulting in a partial response. For three patients who died from metastatic disease, survival from initial diagnosis was 33, 100 and 123 months.
Conclusions
For metastatic YWHAE-rearranged HG-ESS, prolonged disease control following diagnosis was seen, with notable responses to anthracycline-based therapy. This emphasizes the need for appropriate molecular testing of uterine mesenchymal malignancies and suggests that chemotherapy is an effective treatment option for metastatic YWHAE-rearranged HG-ESS.
Introduction
Endometrial stromal sarcomas (ESSs) are the second most common mesenchymal neoplasm of the uterus and constitute less than 2% of all uterine tumors[1,2]. ESSs are subdivided into low-grade and high-grade forms, which are distinguished by clinical behavior, histologic appearance, immunohistochemical features and chromosomal translocations. Low-grade ESS (LG-ESS) is characterized by an indolent course, with relapse common and typically treated with multimodal therapy. These tumors commonly express estrogen (ER) and progesterone (PR) receptors, and hormonal therapy is effective in their treatment[3]. Several chromosomal translocations resulting in gene fusion have been identified in LG-ESS, with the most common being JAZF1-SUZ12; others include JAZF1-PHF1, EPC1-PHF1 and MEAF6-PHF1[1]. These fusion genes all involve members of the Polycomb Group Family, either together or in combination with transcription factors.
High-grade ESS (HG-ESS), harboring t(10;17)(q22;p13) resulting in YWHAE-NUTM2A/B fusion[4], is a recently described and more aggressive neoplasm with a poorer prognosis compared to its low-grade counterpart. Patients with this tumor type typically present with vaginal bleeding, a mean age near 50 and advanced disease[5]. YWHAE-rearranged HG-ESS characteristically contains a morphologically high-grade but uniform round to epithelioid cell component and may show an associated low-grade fibroblastic/myxoid cell component. The morphologically high grade round to epithelioid cell component often shows brisk mitotic activity and necrosis, and typically expresses strong and diffuse cyclin D1 and KIT[6,7]. The YWHAE gene encodes the 14-3-3ε protein, which functions as a molecular scaffold to coordinate cellular signaling by binding to phosphoserine- or phosphothreonine-containing proteins. The NUTM2A and NUTM2B genes are located on chromosome 10q and bear 99% amino acid identity. The NUT (nuclear protein in testis) family consists of nuclear proteins with poorly described function. The fusion between BRD4 or BRD3 genes to NUTM1 on chromosome 15 has been well described as the causative lesion in NUT midline carcinoma[8]. The fusion protein in YWHAE-rearranged HG-ESS has been shown to retain the functional domains of 14-3-3ε and, with fusion to the NUT proteins, prompt aberrant nuclear localization of 14-3-3ε[4]. How the fusion protein leads to a neoplastic cellular program has not yet been determined.
YWHAE-rearranged HG-ESS was added to the World Health Organization classification system in 2014[1]. Because of this recent definition and the rare nature of this tumor, the clinical management of YWHAE-rearranged HG-ESS has not yet been described. Prior pathologic case series of translocation-confirmed HG-ESS[5,9,10] have not included modalities of treatment or responses to therapy. Many other reports describing case series of ESS have no or few translocation-confirmed cases [11–15]. Unlike LG-ESS which consistently express ER and PR and have demonstrated responses to hormonal therapy[3], YWHAE-rearranged HG-ESS stains variably for hormone receptors[5], with staining characteristically present in the morphologically low grade component. One case report of a HG-ESS with response to imatinib has been published[16], though the tumor was not tested for the YWHAE-NUTM2A/B rearrangement and may represent an alternative malignancy. Furthermore, no KIT or PDGFRA mutations have been found in YWHAE-NUTM2A/B rearranged tumors[7]. Recently, rare cases considered HG-ESS lacking YWHAE-rearrangement have been reported. These tumors were noted to have morphologic features resembling myxoid leiomyosarcoma and recurrent ZC3H7B-BCOR gene fusions[17].
We therefore now describe a case series of YWHAE-rearranged HG-ESS and their multimodal clinical management from two large cancer centers to begin to define the clinical management and outcomes of women with this recently defined disease. For each case treated with systemic therapy, an anthracycline-based regimen at any line of therapy resulted in a high response rate and, in two cases, complete radiologic response. Combination gemcitabine and docetaxel was also found to be effective as first-line therapy in one patient.
Methods
Institutional Review Board approval was obtained at the Dana-Farber Cancer Institute and Memorial Sloan Kettering Cancer Center to conduct this study. A retrospective search of all patients diagnosed with YWHAE-rearranged HG-ESS from 2012–2015 was undertaken. YWHAE-rearrangement testing was performed in six of the seven identified cases when initial pathology review raised suspicion for this diagnosis based on morphology and immunohistochemical features[5]. From review of our clinical database, one additional case was identified through retesting of archival tissue for YWHAE-rearrangement. Pathology slides and reports were retrospectively reviewed by two gynecologic pathologists and t(10;17)(q22;p13) YWHAE rearrangement confirmed by cytogenetics or fluorescence in-situ hybridization (FISH) with break-apart probes flanking the YWHAE gene. Patients who presented only for consultation or were lost to follow-up were excluded. Electronic medical records were reviewed for the date of diagnosis, stage at diagnosis, immunohistochemical and molecular pathology testing, surgical interventions, radiation treatments, chemotherapy regimens and response to treatment. Cancer stage was assigned based on the International Federation of Gynecology and Obstetrics (FIGO) 2009 criteria[18]. Response to treatment on imaging for patients with measurable disease was determined by radiographic images and radiology reports. Tumor response was determined as per RECIST[19]. Overall survival (OS) was defined as the interval from the date of diagnosis to date of death. Time to progression (TTP) for patients being treated with measurable disease was defined as the interval from the beginning of systemic therapy to evidence of disease progression on imaging studies.
Results
Patient Characteristics and Initial Treatment
We identified 7 patients with YWHAE-rearranged HG-ESS who received treatment and follow-up at one of the two institutions. The patients ranged in age from 42–47 years. One (Case-7) underwent supracervical hysterectomy with morcellation at an outside institution prior to presenting at one of the study institutions, with subsequent diagnosis of FIGO Stage I YWHAE-rearranged HG-ESS. All other patients underwent hysterectomy without morcellation, and salpingo-oophorectomy was performed at the time of initial surgery or at a later date. Most patients (5/7) presented with FIGO Stage IIIB or higher. Histologically, tumors contained areas exhibiting characteristic round cell morphology with brisk mitotic activity that diffusely expressed cyclin D1; all were found to harbor the YWHAE-rearrangement by FISH or cytogenetics (Table 1). Two patients with ER/PR-positive disease were treated with aromatase inhibitors (Case-1, Case-2) following initial surgery. One of these was treated with a second-line aromatase inhibitor following progression of disease and further surgical debulking. Two patients received 50 Gy pelvic radiation, one as post-operative therapy (Case-1) and the other as pre-operative therapy for recurrent disease (Case-6).
Table 1.
Patient Characteristics, Diagnosis and Outcomes in YWHAE-rearranged HG-ESS.
| Case | Age at Diagnosis | t(10;17) Diagnostic Method | FIGO Stage at Diagnosis | Time From Initial Resection to Metastasis (months) | Sites of Metastasis at Time of Chemotherapy | Overall Survival (months) |
|---|---|---|---|---|---|---|
| Case-1 | 44 | FISH | IVB | - | Peritoneum | - |
| Case-2 | 45 | FISH | IVB | - | Pelvis, Liver, Lung, Pleura | - |
| Case-3 | 46 | FISH | IVB | - | Abdomen, Lymph Node | 33 |
| Case-4 | 47 | FISH | II | - | - | - |
| Case-5 | 46 | FISH | IVB | - | Adnexa, Lymph Node, Bone | - |
| Case-6 | 45 | cytogenetics | IIIB | 65 | Abdomen, Pelvis | 100 |
| Case-7 | 42 | FISH | I | 64 | Lung, Mediastinum, Abdomen, Pelvis, Lymph Nodes, Bone | 123 |
IHC: Immunohistochemistry; FIGO: International Federation of Gynecology and Obstetrics. The dash in the overall survival column indicates the patient is alive and under observation or treatment for disease.
Treatment of Recurrent and Metastatic Disease
Six of seven patient developed metastatic disease. Locations and time to metastases are summarized in Table 1. All patients received at least one line of systemic therapy and up to five total lines for metastatic disease, as summarized in Table 2. Six patients received cytotoxic chemotherapy, and in the first-line, five received an anthracycline-based regimen and one received combination gemcitabine and docetaxel. Of the five who received an anthracycline-based regimen in the first-line setting, one achieved a CR, three a PR and one SD over seven cycles. One patient (Case-5) presenting with FIGO Stage IVB disease received chemotherapy with doxorubicin, ifosfamide and mesna (AIM) followed by liposomal doxorubicin resulting in a radiographic CR, with subsequent hysterectomy and salpingo-oophorectomy that revealed no viable tumor. The patient who received combination gemcitabine and docetaxel (Case-3) as first-line therapy achieved a near complete radiologic response and went on to resection of residual disease; pathology from this case showed no viable tumor in all resection specimens. Second-line therapy was anthracycline-based in four patients, all of whom achieved either a PR or CR. Other agents with known efficacy in sarcoma (ifosfamide, pazopanib, single agent gemcitabine, trabectedin) were given beyond the second-line, with generally poor disease control, with the exception of anthracyclines which showed activity as late as the third-line of therapy (Table 2, Figure 1). For patients with recurrent disease following the primary surgery, subsequent surgery was performed a median of 2 times (range 1–6) to remove residual disease, debulk tumor or manage sequela of metastatic disease.
Table 2.
Chemotherapy Response in YWHAE-rearranged HG-ESS.
| Line of Chemotherapy | First | Second | Third | Fourth | Fifth | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Therapy | Response | TTP(months) | Therapy | Response | TTP(months) | Therapy | Response | TTP (months) | Therapy | Response | TTP (months) | Therapy | Response | TTP (months) |
| Case-1 | Doxorubicin | SD | S | ||||||||||||
| Case-2 | Doxorubicin | PR | S | ||||||||||||
| Case-3 | Gemcitabine, Docetaxel | PR | 7.5 | Doxorubicin, olaratumab | CR | 14 | Ifosfamide | PD | - | Trabectedin1 | PD | - | |||
| Case-5 | AIM | PR | * | liposomaldoxorubicin | PR | S | |||||||||
| Case-6 | AIM | CR | 65 | AIM/IM | PR | 11 | liposomaldoxorubicin | PR | 10 | ||||||
| Case-7 | Doxorubicin | PR | 10 | liposomaldoxorubicin | PR | 7 | Pazopanib | PD | - | Gemcitabine | PD | - | IM | PD | - |
AIM: doxorubicin, ifosfamide and mesna; IM: ifosfamide and mesna; CR: complete response; PR: partial response; SD: stable disease; PD: progression of disease. S = patient went on to resection of residual disease following chemotherapy with no disease recurrence through the follow-up date.
patient switched from AIM to liposomal doxorubicin due to intolerance.
Trabectedin was given at an outside institution.
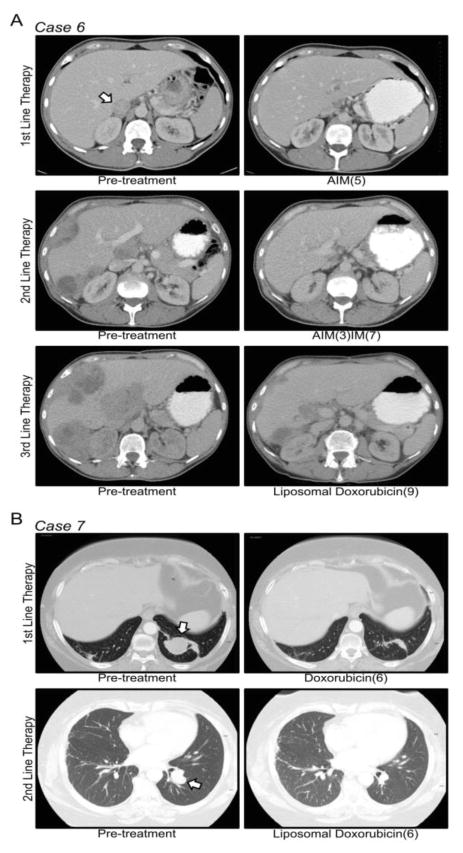
Figure 1. Response to Anthracyclines in YWHAE-rearranged HG-ESS.
(A) Pre- (left panel) and post-treatment (right panel) CT scans for the three lines of anthracycline-based chemotherapy in Case-6. (B) Pre- and post-treatment scans for the two lines of anthracycline-based chemotherapy in Case-7. The chemotherapy regimen and number of cycles are listed below the post-treatment CT scans. Arrowheads indicate locations of pre-treatment metastatic disease. AIM: doxorubicin, ifosfamide and mesna; IM: ifosfamide and mesna.
Discussion
This study represents the first case series describing the management and response to therapy of YWHAE-rearranged HG-ESS, an entity molecularly and prognostically distinct from its low-grade counterpart and only recently described by the World Health Organization[1]. The unique course and treatment responses of this malignancy merit pathologic evaluation to confirm the presence of the t(10;17)(q22;p13) translocation in cases of suspected HG-ESS. Remarkable among these cases are the substantial responses to cytotoxic chemotherapy. Complete radiographic responses were seen in two patients treated with anthracyclines as first- or second-line therapy, and at least stable disease as best response was seen in all cases and in all lines of therapy using anthracyclines. Activity was seen with AIM, doxorubicin monotherapy and liposomal doxorubicin monotherapy. Further, combination gemcitabine and docetaxel was found to have activity in the one patient who received it as first-line therapy, suggesting this combination as another active regimen in this disease. The high rate of response to chemotherapy in this case series compares favorably to the most common uterine sarcoma, leiomyosarcoma, which has a response rate of 36% to gemcitabine and docetaxel[20]. YWHAE-rearranged HG-ESS also compares favorably to high-grade undifferentiated uterine sarcomas, which in advanced disease exhibit a more modest response rate to systemic therapy and subsequent rapid progression and high mortality from disease[21].
The rarity of YWHAE-rearranged HG-ESS and lack of clinical data lead to several limitations in interpreting these results. The role of pelvic radiation therapy is unclear, and in this series two patients received treatment and both ultimately progressed. Hormonal therapy also has an unclear role, and of the two patients treated in this series in the adjuvant setting, one had progression of disease. Though regimens other than those containing anthracyclines or combination gemcitabine and docetaxel were not found to be efficacious, they were trialed only as third-line or later therapies and effectiveness earlier in the disease course is unknown.
The pathogenic t(10;17) fusion of the YWHAE and NUTM2A/B genes leads to an oncoprotein of unclear function. Limited investigation to date has determined that the fusion protein leads to abnormal localization of 14-3-3ε to the nucleus and enhanced cell growth and migration in vitro[4]. The prior recognition that 14-3-3 proteins can function as chromatin regulators[22] suggests that, like LG-ESS bearing fusions of Polycomb family members, YWHAE-rearranged HG-ESS is a disease driven by aberrant epigenetic regulation. Additional translational research is needed to understand this neoplastic driver in YWHAE-rearranged HG-ESS and develop more rational therapeutic strategies.
In summary, this study is the first to detail the diagnosis and treatment course of a cohort of patients with YWHAE-rearranged HG-ESS. Though interpretations are limited by the rarity of this disease and the small number of patients, this combined series from two large cancer centers demonstrates the activity of anthracycline-based chemotherapy through many lines of treatment, and combination gemcitabine and docetaxel as first-line therapy. Prolonged disease control was seen with anthracycline-based therapy, and its efficacy suggests a standard for future treatment and trial design. Testing for this translocation is warranted when suspected to establish the diagnosis, guide therapeutic decisions for initial and subsequent lines of systemic therapy, provide prognostic information and allow for additional clinical investigation of this disease.
Highlights.
YWHAE-rearranged high-grade endometrial stromal sarcoma is an uncommon uterine malignancy.
Identification of t(10;17)(q22;p13) together with histopathology can support this diagnosis.
Metastatic YWHAE-rearranged HG-ESS appears quite sensitive to anthracycline-based chemotherapy
Acknowledgments
Funding. The authors declare no funding support related to this research.
The authors are grateful to study participants and their families.
Footnotes
Disclosure. Sarah Chiang, MD reports honoraria from Harvard Medical School as an invited lecturer. All other authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 4. World Health Organization; 2014. [Google Scholar]
- 2.Ali RH, Rouzbahman M. Endometrial stromal tumours revisited: an update based on the 2014 WHO classification. Journal of Clinical Pathology. 2015;68:325–32. doi: 10.1136/jclinpath-2014-202829. [DOI] [PubMed] [Google Scholar]
- 3.Reich O, Regauer S. Hormonal therapy of endometrial stromal sarcoma. Curr Opin Oncol. 2007;19:347–52. doi: 10.1097/CCO.0b013e3281a7ef3a. [DOI] [PubMed] [Google Scholar]
- 4.Lee C-H, Ou W-B, Marino-Enriquez A, Zhu M, Mayeda M, Wang Y, et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc Natl Acad Sci USa. 2012;109:929–34. doi: 10.1073/pnas.1115528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C-H, Marino-Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641–53. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 6.Lee C-H, Ali RH, Rouzbahman M, Marino-Enriquez A, Zhu M, Guo X, et al. Cyclin D1 as a diagnostic immunomarker for endometrial stromal sarcoma with YWHAE-FAM22 rearrangement. Am J Surg Pathol. 2012;36:1562–70. doi: 10.1097/PAS.0b013e31825fa931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C-H, Hoang LN, Yip S, Reyes C, Marino-Enriquez A, Eilers G, et al. Frequent expression of KIT in endometrial stromal sarcoma with YWHAE genetic rearrangement. Mod Pathol. 2013;27:751–7. doi: 10.1038/modpathol.2013.199. [DOI] [PubMed] [Google Scholar]
- 8.French CA. Pathogenesis of NUT Midline Carcinoma. 2012;7:247–65. doi: 10.1146/annurev-pathol-011811-132438. Http://DxDoiorgEzp-Prod1HulHarvardEdu/101146/Annurev-Pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CJR, Leung YC, Murch A, Peverall J. Evaluation of fluorescence in-situhybridization in monomorphic endometrial stromal neoplasms and their histological mimics: a review of 49 cases. Histopathology. 2014;65:473–82. doi: 10.1111/his.12406. [DOI] [PubMed] [Google Scholar]
- 10.Sciallis AP, Bedroske PP, Schoolmeester JK, Sukov WR, Keeney GL, Hodge JC, et al. High-grade endometrial stromal sarcomas: a clinicopathologic study of a group of tumors with heterogenous morphologic and genetic features. Am J Surg Pathol. 2014;38:1161–72. doi: 10.1097/PAS.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 11.Geller MA, Argenta P, Bradley W, Dusenbery KE, Brooker D, Downs LS, Jr, et al. Treatment and recurrence patterns in endometrial stromal sarcomas and the relation to c-kit expression. Gynecologic Oncology. 2004;95:632–6. doi: 10.1016/j.ygyno.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Donertas A, Nayki U, Nayki C, Ulug P, Gultekin E, Yildirim Y. Prognostic Factors, Treatment and Outcome in a Turkish Population with Endometrial Stromal Sarcoma. Asian Pacific Journal of Cancer Prevention. 2015;16:881–7. doi: 10.7314/APJCP.2015.16.3.881. [DOI] [PubMed] [Google Scholar]
- 13.Croce S, Hostein I, Ribeiro A, Garbay D, Velasco VER, Stoeckle E, et al. YWHAE rearrangement identified by FISH and RT-PCR in endometrial stromal sarcomas: genetic and pathological correlations. Mod Pathol. 2013;26:1390–400. doi: 10.1038/modpathol.2013.69. [DOI] [PubMed] [Google Scholar]
- 14.Brohl AS, Li L, Andikyan V, Obi an SG, Cioffi A, Hao K, et al. Age-Stratified Risk of Unexpected Uterine Sarcoma Following Surgery for Presumed Benign Leiomyoma. The Oncologist. 2015;20:433–9. doi: 10.1634/theoncologist.2014-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gremel G, Liew M, Hamzei F, Hardell E, Selling J, Ghaderi M, et al. A prognosis based classification of undifferentiated uterine sarcomas: Identification of mitotic index, hormone receptors and YWHAE-FAM22 translocation status as predictors of survival. Int J Cancer. 2014;136:1608–18. doi: 10.1002/ijc.29141. [DOI] [PubMed] [Google Scholar]
- 16.Salvatierra A, Tarrats A, Gomez C, Sastre JM, Balaña C. A case of c-kit positive high-grade stromal endometrial sarcoma responding to Imatinib Mesylate. Gynecologic Oncology. 2006;101:545–7. doi: 10.1016/j.ygyno.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Hoang LN, Aneja A, Conlon N, Delair DF, Middha S, Benayed R, et al. Novel High-grade Endometrial Stromal Sarcoma: A Morphologic Mimicker of Myxoid Leiomyosarcoma. Am J Surg Pathol. 2016:1. doi: 10.1097/PAS.0000000000000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutch DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecologic Oncology. 2009;115:325–8. doi: 10.1016/j.ygyno.2009.10.050. [DOI] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecologic Oncology. 2008;109:329–34. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner EJ, Garg K, Leitao MM, Jr, Soslow RA, Hensley ML. High grade undifferentiated uterine sarcoma: Surgery, treatment, and survival outcomes. Gynecologic Oncology. 2012;127:27–31. doi: 10.1016/j.ygyno.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Winter S, Simboeck E, Fischle W, Zupkovitz G, Dohnal I, Mechtler K, et al. 14-3-3 Proteins recognize a histone code at histone H3 and are required for transcriptional activation. Embo J. 2007;27:88–99. doi: 10.1038/sj.emboj.7601954. [DOI] [PMC free article] [PubMed] [Google Scholar]