Abstract
Ischemia/reperfusion (I/R) injury is a considerable insult to skeletal muscle, often resulting in prolonged functional deficits. The purpose of the current study was to evaluate the controlled release of the pro-regenerative growth factor, insulin-like growth factor-I (IGF-I), from a biodegradable polyethylene glycol (PEG)ylated fibrin gel matrix and the subsequent recovery of skeletal muscle from I/R. To accomplish this, the hind limbs of male Sprague–Dawley rats were subjected to 2-h tourniquet-induced I/R then treated with saline, bolus IGF-I (bIGF), PEGylated fibrin gel (PEG-Fib), or IGF-I conjugated PEGy-lated fibrin gel (PEG-Fib-IGF). Functional and histological evaluations were performed following 14 days of reperfusion, and muscles from 4-day reperfusion animals were analyzed by Western blotting and histological assessments. There was no difference in functional recovery between saline, bIGF, or PEG-Fib groups. However, PEG-Fib-IGF treatment resulted in significant improvement of muscle function and structure, as observed histologically. Activation of the PI3K/Akt pathway was significantly elevated in PEG-Fib-IGF muscles, compared to PEG-Fib treatment, at 4 days of reperfusion, suggesting involvement of the pathway PI3K/Akt as a mediator of the improved function. Surprisingly, myoblast activity was not evident as a result of PEG-Fib-IGF treatment. Taken together, these data give evidence for a protective role for the delivered IGF. These results indicate that PEG-Fib-IGF is a viable therapeutic technique in the treatment of skeletal muscle I/R injury.
Keywords: regenerative medicine, muscle regeneration, tourniquet, protein kinase B, satellite cells
Introduction
Ischemia/reperfusion (I/R) injury is a considerable perturbation to the extremities, often occurring in instances of vascular surgeries, orthopedic surgeries, or tourniquet (TK) use (Blaisdell, 2002; Walsh et al., 2009). The ischemic phase of I/R includes the accumulation of metabolites and depletion of ATP, while the more detrimental reperfusion phase is characterized largely by a burst of free radicals, causing severe damage to affected cell membranes, that can ultimately lead to apoptosis and/or necrosis (Blaisdell, 2002; Honda et al., 2005).
Histopathology and large functional deficits persist in the skeletal muscle of rodents following TK-induced I/R (Hammers et al., 2008; Walters et al., 2008). In response to I/R, local up-regulation of the pro-regenerative growth factor, insulin-like growth factor-I (IGF-I) occurs (Edwall et al., 1989; Hammers et al., 2008, 2011). The actions of IGF-I following muscle injury include the stimulation of myoblast proliferation, promotion of myoblast differentiation, and improved survival of affected cells (Adams, 2002; Kooijman, 2006). Over-expression of IGF-I does facilitate skeletal muscle recovery following cardiotoxin-induced injury (Pelosi et al., 2007), therefore we hypothesized that increasing the local concentration of IGF-I would be a potential therapeutic method for the treatment of skeletal muscle I/R. Since the beneficial effects of IGF-I are mediated largely by the Ras/Raf-1/ERK MAP kinase and PI3K/Akt pathways (Adams, 2002), we also hypothesized that one or both of these pathways must be involved in any measurable IGF-I mediated improvements in muscle recovery.
The controlled release of growth factors from an intramuscular (IM), biodegradable matrix over time is an attractive alternative to multiple bolus injections of growth factor, and is more clinically feasible than genetic over-expression treatments. In previous experiments, a polyethylene glycol (PEG)-ylated fibrin gel (PEG-Fib) matrix containing covalently bound growth factor has effectively improved left ventricular function following myocardial infarction (Zhang et al., 2007, 2008). Following injection and subsequent polymerization, the covalently bound growth factor is released from the PEG-Fib matrix into the local environment as degradation occurs, thereby allowing for the controlled release of growth factor from a single injection. This property makes PEG-Fib an attractive tool for growth factor therapy following muscular injuries.
In the present study, we tested our hypothesis that PEG-Fib-mediated delivery of IGF-I will improve the functional recovery of skeletal muscle following I/R. A bi-functional succinimidylglutarate PEG (SG-PEG-SG) was used to successfully conjugate fibrinogen and IGF-I, leading to a matrix capable of releasing supra-physiological amounts (>10 ng/mL) of IGF-I out to at least 4 days, in vitro. The PEG-Fib-IGF matrix was utilized as an IM-delivered treatment for TK-induced I/R of skeletal muscle. Functional and histological evaluations were performed following 14 days of reperfusion. Four-day reperfusion groups were used to assess signaling and histology.
Methods
Animals
Male Sprague–Dawley rats (6–9 months; Charles River) were used for this study. Rats were housed individually, maintained on a 12-h light/dark cycle, and were allowed ad libitum access to food and water. All experimental procedures were approved and conducted in accordance with guidelines set by the University of Texas at Austin Institutional Animal Care and Use Committee.
TK Application
The 2-h TK-induced I/R reperfusion model of muscle injury was performed as previously described (Hammers et al., 2008). Briefly, a single, randomly selected hind limb was elevated, and a pneumatic TK (D.E. Hokanson, Inc.; Bellevue, WA) was placed proximal to the knee. The TK was inflated to 250 mm Hg using the Portable Tourniquet System (Delfi Medical Innovations Inc.; Vancouver, BC, Canada) for a 2-h duration. During the course of this procedure, rats were anesthetized with 2% isoflurane, and body heat was maintained with the use of a heat lamp.
PEGylated Fibrin Gel Delivery of IGF-I
Growth factor conjugated PEGylated fibrin gel was prepared essentially as previously described (Drinnan et al., 2010; Zhang et al., 2007, 2008). Briefly, bifunctional SG-PEG-SG (NOF America Corp, Irvine, CA) was reacted with reconstituted porcine fibrinogen (Sigma-Aldrich Co.; St. Louis, MO; 5:1 PEG:fibrinogen molar ratio; pH 7.8) and hIGF-I (PeproTech Inc.; Rocky Hill, NJ). Polymerization was induced by the addition of 25 U/mL hThrombin (Sigma). The final concentrations of fibrinogen and IGF-I were 10 mg/mL and 25 μg/mL, respectively.
Twenty-four hours following release of the TK, 0.25 mL of either sterile PBS (saline; n = 8), bolus IGF-I (bIGF; 25 μg/mL; n = 4) empty PEGylated fibrin gel (PEG-Fib; n = 6), or IGF-I conjugated PEGylated fibrin gel (PEG-Fib-IGF; n = 6) was injected into the lateral gastrocnemius (LGAS) muscle of the TK-injured limb. PEG-Fib-containing treatments were injected as a fluid and polymerized in situ. Functional assessments were performed at 14 days of reperfusion.
In a subsequent experiment aimed at investigating potential mechanisms behind PEG-Fib-IGF-mediated improvements, 0.25 mL of PEG-Fib (n = 4) or PEG-Fib-IGF (n = 4) was injected in the same manner, while animals were allowed 4 days of reperfusion. LGAS muscles were harvested from euthanized animals, embedded in OCT compound, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until further analysis.
Functional Assessment
Following 14 days of reperfusion, evaluation of LGAS force production in situ was performed as previously described (Merritt et al., 2010). Briefly, the GAS muscle was isolated in anesthetized rats, innervation to the medial GAS was removed, and the Achilles tendon was secured to the lever arm of a dual-mode servomotor (Aurora Scientific Model 310B Inc.; Aurora, ON, Canada). The muscle was stimulated using a stimulator (A-M Systems, Carlsborg, WA, Model 2100) with electrodes applied to the tibial nerve. Optimal length (Lo) was determined by finding the length producing the maximal twitch force. Maximal peak tetanic tension (Po) was measured at Lo at a frequency of 150 Hz and the minimal voltage required to elicit a maximal response. Each contraction was followed by 2 min of rest. Muscle temperature was maintained with a heat lamp and mineral oil. Data were stored and analyzed using LabView software. After the completion of contractile measurements, the muscles were dissected free, weighed, embedded in OCT compound, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until further analysis. LGAS measurements of non-injured limbs were used for a non-TK control values (n = 14).
Histology and Immunohistochemistry
Frozen, OCT-embedded muscle samples from 4 and 14 reperfusion groups were sectioned on a cryostat (Leica CM1900; Leica Microsystems Inc.; Buffalo Grove, IL), and prepared on a slide. Hematoxylin & eosin (H&E) staining was performed, as previously described (Merritt et al., 2010), and slides were observed with a light microscope (Nikon Diaphot, Nikon Corp.; Tokyo, Japan) with the 20× objective lens. Images were taken using a mounted digital camera (Optronix Microfire; Optronix; Goleta, CA). Myofiber cross-sectional area (CSA) was measured using ImageJ software (250–600 fibers/group).
Immunohistochemistry (IHC) was performed as previously described (Merritt et al., 2010). Slides were blocked with 5% normal donkey serum and 1% BSA in PBS, and stained with anti-desmin (1:200; Santa Cruz Biotechnology, Inc.; Santa Cruz, CA), anti-neonatal myosin heavy chain (nMHC; 1:200; Santa Cruz), anti-myogenin (1:200; Santa Cruz), or anti-MyoD (1:200; Santa Cruz) antibodies. Sections were detected with donkey anti-goat IgG-FITC fluorescein (1:100; Santa Cruz), donkey anti-rabbit-FITC fluorescein (1:100; Santa Cruz) or a donkey anti-mouse IgG-TRITC fluorescein (1:100; Santa Cruz) and counter-stained with DAPI. Immunofluorescence was visualized with a Leica DM LB2 fluorescence microscope with the 20× objective lens and photographed with a Leica DFC340FX digital camera. For quantification, the total number of nuclei co-expressing DAPI and either MyoD or myogenin were counted using ImageJ and expressed as a percentage of total nuclei present in the image area. The number of intact myofibers expressing desmin or nMHC was counted using ImageJ and expressed as a percentage of the total number of myofibers in the image area. For each measure, three fields of view were evaluated from three muscles per group.
Western Blotting
Western blotting was performed as previously described (Hammers et al., 2008) using anti-IGF-I (Peprotech), anti-pAkt1/2/3 (Ser 473; Santa Cruz Biotechnology), anti-Akt (Cell Signaling Technology, Inc.; Beverly, MA), anti-pmTOR (Ser 2448; Cell Signaling), anti-mTOR (Cell Signaling), anti-p-p70S6K (Thr 389; Santa Cruz Biotechnology), anti-p70S6K (Cell Signaling), and anti-MuRF-1 (ECM Biosciences, Versailles, KY) antibodies. Phosphorylation-specific antibodies were stripped and re-probed with total protein antibodies. Values were quantified relative to those of the PEG-Fib control group. Coomassie blue (CB) staining (Bio-Rad Laboratories, Inc.; Hercules, CA) was performed to verify equal loading.
ELISA
IGF-I release kinetics were performed similar to that previously described (Zhang et al., 2007) using the hIGF-I Quantikine ELISA kit (R&D Systems, Inc.; Minneapolis, MN). Procedures were followed as directed by the manufacturer’s instructions. Release kinetics was quantified as percent of total IGF-I released from the matrix.
Statistical Analysis
Functional assessments and IHC values were analyzed using appropriate form of ANOVA (Tukey post-hoc test; α = 0.05), and Western blotting data were analyzed using Student’s t-test (α 0.05). All values are represented as the mean ± SEM, unless noted otherwise.
Results and Discussion
Genetic over-expression studies have demonstrated that IGF-I does facilitate muscle regeneration following traumatic injury (Pelosi et al., 2007), thus making it a promising molecule to be utilized therapeutically for the treatment of muscle injuries. However, its short half-life (10–30 min) and potentially negative systemic effects has limited the molecule’s therapeutic application. The purpose of this study was to evaluate the therapeutic potential of delivering IGF-I in a controlled manner, using an IM-PEG-Fib platform, in the treatment of skeletal muscle I/R.
Conjugation and Release of IGF-I From PEGylated Fibrin Gel
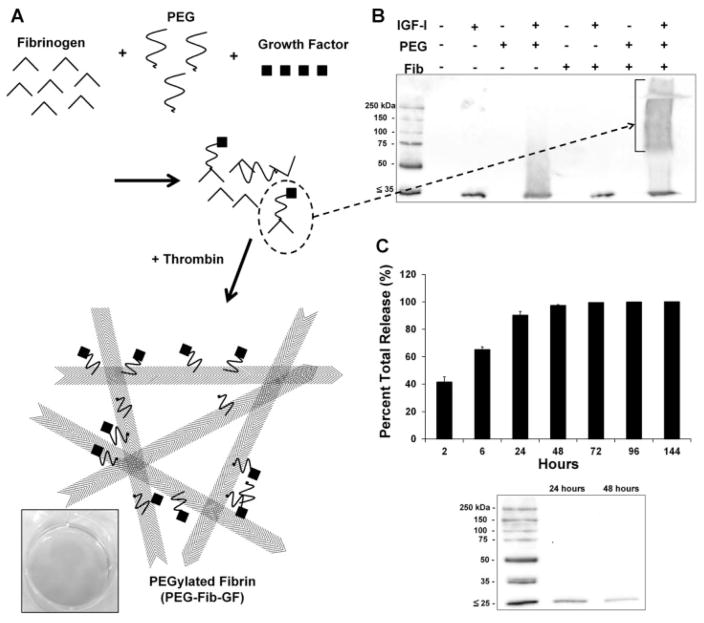
Because covalent bonding of PEG to both fibrinogen and growth factor is essential to achieve controlled release when using the PEG-Fib delivery system (summarized in Fig. 1A), we first had to verify the formation of fibrinogen-PEG-IGF-I complexes. Simultaneous incubation of fibrinogen, SG-PEG-SG, and IGF-I resulted in the formation of large IGF-I immunoreactive complexes, as determined by Western blotting (Fig. 1B). Release kinetics of IGF-I from the PEG-Fib matrix was measured in sequential release samples using ELISA. Though the majority of IGF-I is released from the matrix with the first 24 h (Fig. 1C), a physiologically relevant dose of ~12 ng/mL (Coolican et al., 1997; Davani et al., 2003) was measured at 96 h (Table I). This early release is likely due to the low molecular weight of IGF-I (~7.5 kDa), which limits the number of free amine groups required for reaction with SG-PEG-SG. For example, the slightly larger SDF-1α (~11 kDa) is progressively released across 7 days from PEG-Fib (Zhang et al., 2007). IGF-I immunoreactivity in the release samples was also analyzed by Western blotting to demonstrate that IGF-I was mostly released as free peptides, rather than large complexes that may have impaired bioactivity (Fig. 1C bottom).
Figure 1.
Generation of growth factor-conjugated PEGylated fibrin gel (PEG-Fib-GF) is achieved by the co-incubation of fibrinogen, bi-functional PEG, and growth factor (A). The result is a mixture of various covalently linked products composed of the original components. This mixture is polymerized by the addition of thrombin to generate PEG-Fib-GF. Covalent conjugation of IGF-I to PEGylated fibrinogen (B) is verified by Western blotting using an anti-IGF-I antibody. Release kinetics of IGF-I from the PEGylated fibrin gel delivery system (C, top) was determined by ELISA (% of total IGF-I released). Western blotting confirms that the majority of immunoreactive IGF-I released from PEGylated fibrin gel is consistent that with of IGF-I peptide (C, bottom), rather than large complexes. Values represented are the mean ± SD of three separate trials.
Table I.
IGF-I release from PEGylated fibrin matrix.
| Time (h) | IGF-I (ng/mL) |
|---|---|
| 2 | 1297 ± 198 |
| 6 | 725 ± 240 |
| 24 | 784 ± 327 |
| 48 | 210 ± 57 |
| 72 | 58.5 ± 0.3 |
| 96 | 12.3 ± 2.2 |
| 144 | 3.5 ± 0.1 |
Values indicated are mean ± SD; n = 3 trials.
PEGylated Fibrin Gel Delivery of IGF-I Improves Recovery of Muscle
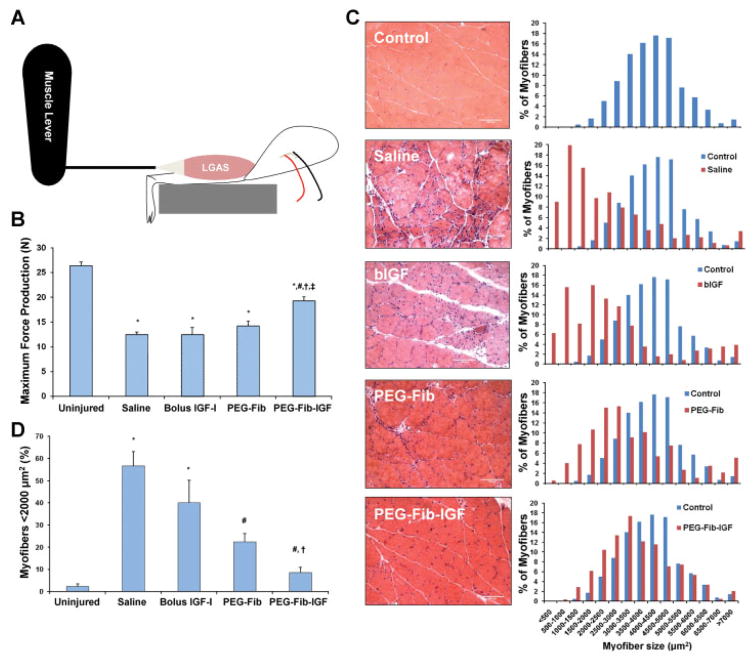
The specific aim of the current study was to determine the efficacy of PEG-Fib-IGF treatment in improving the functional recovery of skeletal muscle from I/R. To accomplish this, an IM injection of saline, bIGF, PEG-Fib, or PEG-Fib-IGF treatment was administered to the LGAS 24 h after TK release. Functional evaluations of the LGAS were performed after 14 days of reperfusion. An illustration depicting in situ functional measurements of the LGAS is found in Figure 2A. We observed no difference in functional recovery with saline, bIGF, or PEG-Fib treatments (Fig. 2B). PEG-Fib-IGF treatment, however, resulted in substantial recovery of force, compared to saline treatment (19.2 ± 1.0 N vs. 12.4 ± 0.6 N; P <0.05). This improvement is dependent on an improvement in specific tension of the muscle, rather than just changes in muscle mass (Table II). Interestingly, there were no significant differences in muscle mass or CSA between the groups, however both control and PEG-Fib-IGF groups strongly trended towards higher values in both parameters than other groups. This lack of statistical difference is likely due to large variations from persisting inflammation and/or edema in groups demonstrating more histological pathology (saline, bIGF, and PEG-Fib) (Friden and Lieber, 1998; Hammers et al., 2008). These results indicate the PEG-Fib-IGF treatment has therapeutic potential for skeletal muscle I/R, achieving comparable results to recovery in muscle-specific IGF-I over-expression models (Pelosi et al., 2007). The lack of benefit from a single bolus injection of IGF-I agrees with the results of previous studies (Borselli et al., 2010), and highlights the importance of covalent bonding of IGF-I to the matrix in order to prolong release and protect the IGF-I molecule from rapid degradation.
Figure 2.
After 14 days of reperfusion, maximum force production of the LGAS was measured in situ (depicted in A, results in B) from the following treatment groups: saline, bolus IGF-I, PEGylated fibrin gel (PEG-Fib), and IGF-I conjugated PEG-Fib (PEG-Fib-IGF). Following functional evaluations, H&E stained sections were prepared (representative slides in C, left) and evaluated for fiber size composition (C, right; 250–600 fibers evaluated per group). Proportions of small muscle fibers (<2,000 μm2; D) were also compared among the different treatment groups. Values represented are the mean ± SEM; *P <0.05 versus uninjured, #P <0.05 versus saline, †P <0.05 versus bolus IGF-I, ‡P <0.05 versus PEG-Fib. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/bit]
Table II.
Muscle parameters following 14 days of reperfusion.
| LGAS mass (mg) | CSA (cm2) | SPo (N/cm2) | |
|---|---|---|---|
| Uninjured | 1689 ± 47 | 1.35 ± 0.04 | 19.6 ± 0.5 |
| Saline | 1461 ± 55 | 1.20 ± 0.04 | 10.5 ± 0.8* |
| Bolus IGF-I | 1491 ± 112 | 1.18 ± 0.10 | 10.5 ± 0.7* |
| PEG-Fib | 1522 ± 93 | 1.12 ± 0.07 | 11.7 ± 1.0* |
| PEG-Fib-IGF | 1643 ± 75 | 1.30 ± 0.06 | 14.8 ± 0.6*,** |
LGAS, lateral gastrocnemius; CSA, cross-sectional area; SPo, specific tension.
Values indicated are mean ± SEM.
P <0.05 versus uninjured.
P <0.05 versus saline, bolus IGF-I, and PEG-Fib groups.
Histological evaluations of H&E stained LGAS cross-sections were also performed at the 14-day reperfusion time point (Fig. 2C). Saline-treated muscle displayed the typical abundance of small myofibers containing central nuclei seen in skeletal muscle following I/R, which is evidence of substantial degeneration/regeneration cycling of injured myofibers. The presence of large myofibers of round morphology in these samples is indicative of focal edema and/or myofiber hypercontraction, which are both hallmarks of muscle pathology. Bolus IGF-I treated muscle displayed similar characteristics as saline treatment in terms of histological pathology and left skewing of distribution of myofiber size. Treating I/R-affected muscles with PEG-Fib alone resulted in modest improvements in the histological morphology and myofiber distribution of the LGAS. This superficial improvement is not surprising, as PEG-Fib strongly binds many important growth factors, including transforming growth factor-β (TGFβ) (Catelas et al., 2008; Giannoni and Hunziker, 2003; Grainger et al., 1995), fibroblast growth factors (Ishii et al., 2007; Sahni et al., 1998), and vascular endothelial growth factor (VEGF) (Sahni and Francis, 2000). Muscles treated with PEG-Fib-IGF displayed almost no signs of pathology and demonstrated a myofiber size distribution comparable to that of control muscle. In addition, the distribution of muscle fibers <2,000 μm2 in size was quantified (Fig. 2D), revealing that PEG-Fib-IGF treatment resulted in significantly less small fibers than other treatment groups and was no different than control muscle. These data indicate improvements in histological morphology accompany the improved functional recovery of skeletal muscle from I/R using PEG-Fib-IGF as a treatment strategy.
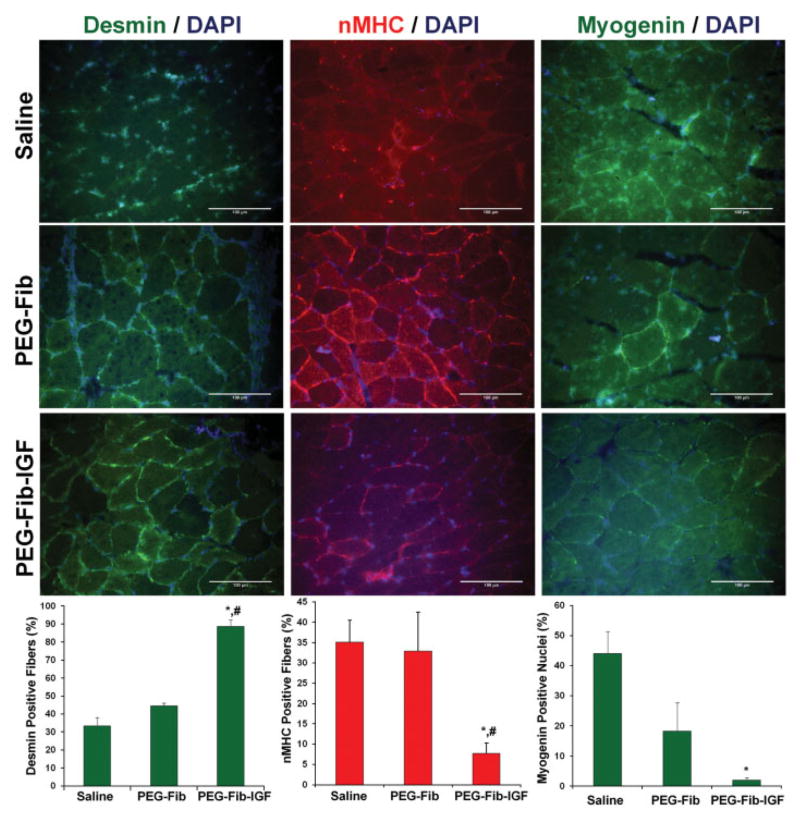
To accompany our data depicting improved recovery following PEG-Fib-IGF treatment, IHC staining for desmin, nMHC, and myogenin was performed in saline, PEG-Fib, and PEG-Fib-IGF groups (Fig. 3). Desmin is a sarcomere-associated protein whose immunoreactivity is rapidly lost in perturbed myofibers (Friden and Lieber, 1998). We found a significantly higher incidence of desmin-positive myofibers in PEG-Fib-IGF treated muscles than in saline or PEG-Fib groups, which is in agreement with our H&E depiction of healthier muscle following PEG-Fib-IGF treatment. In addition, we looked at immunoreactivity of nMHC, an immature myosin heavy chain isoform that is found in actively regenerating muscle (Bandman, 1985), and nuclear expression of myogenin, a muscle regulatory factor (MRF; muscle specific transcription factor) expressed in the later stages of myogenesis (Charge and Rudnicki, 2004), as indicators of active muscle regeneration. PEG-Fib-IGF treatment resulted in significantly less nMHC-positive fibers than both saline and PEG-Fib groups, and significantly less myogenin-positive nuclei than saline treatment. These results suggest that the myogenic/regenerative program is near completion in PEG-Fib-IGF treated muscles following 14 days of reperfusion, yet is still prominent in saline and PEG-Fib treatment groups. Taken together, our data indicate that PEG-Fib-IGF mediated therapy hastens both the histological and functional recovery of skeletal muscle from I/R injury, compared to all other tested groups.
Figure 3.
IHC for desmin, nMHC, and myogenin was performed on the LGAS muscles from saline, PEGylated fibrin gel (PEG-Fib), and IGF-I conjugated PEG-Fib (PEG-Fib-IGF) groups following 14 days of reperfusion. Representative slides and marker-specific quantifications are provided. Values represented are the mean ± SEM; *P <0.05 versus saline, #P <0.05 versus PEG-Fib. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/bit]
Hyperactivation of the PI3K/Akt Pathway May Mediate Therapeutic Effect
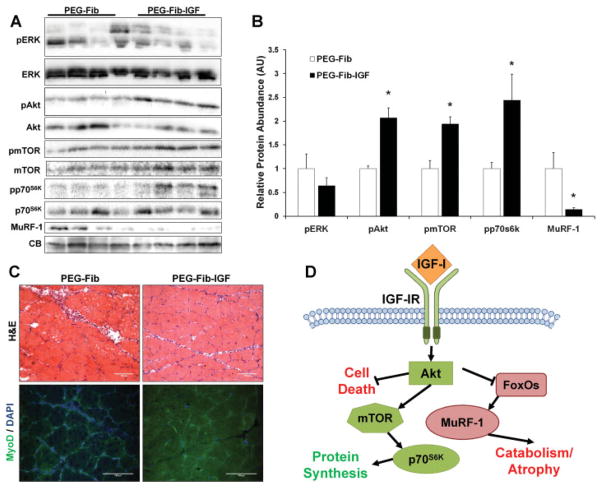
From the data demonstrating PEG-Fib-IGF-mediated improvement in muscle recovery from I/R, we gathered that two potential mechanisms are responsible for our results: (1) PEG-Fib-IGF is facilitating the regenerative actions of myoblasts, thereby speeding up the regenerative time frame, or (2) PEG-Fib-IGF exerts a protective effect on the existing myofibers, thus resulting in less damage from the I/R insult. We therefore repeated PEG-Fib (control) and PEG-Fib-IGF treatment groups with only 4 days of reperfusion to discern which mechanism is likely behind the beneficial effect of PEG-Fib-IGF treatment. The 4-day reperfusion period was chosen because an appreciable amount of IGF-I is still being released from PEG-Fib-IGF (Table I) and it is a time point of maximum myoblast proliferation (Conboy and Rando, 2002; Cornelison and Wold, 1997).
The canonical intracellular signaling induced by activation of the IGF-IR in skeletal muscle includes the Ras/Raf-1/ERK MAP kinase and PI3K/Akt pathways (Adams, 2002). Activation of the ERK pathway is generally associated with induction of myoblast proliferation (Coolican et al., 1997), while activation of the PI3K/Akt pathway results in diverse effects, including activation of protein synthesis, abrogation of catabolism, promotion of cell survival, and stimulation of myoblast differentiation (Coolican et al., 1997; Frost and Lang, 2007). To investigate if either of these pathways are differentially expressed between PEG-Fib and PEG-Fib-IGF treated muscles, LGAS muscles were harvested after 4 days of reperfusion and analyzed by Western blotting for phosphorylation of ERK and Akt. Phosphorylation of ERK was not different between the two treatments, however, p-Akt of the PEG-Fib-IGF treatment demonstrated an ~2-fold increase in content over PEG-Fib treated muscles (Fig. 4A and B). IGF-I-induced activation of Akt promotes protein synthesis, which is regulated through the activation of mTOR and p70S6K (Bodine et al., 2001), and negatively regulates muscle atrophy by preventing the expression muscle-specific E3 ubiquitin ligases (components of the ubiquitin-proteasome system) through inactivation of FoxO transcription factors (Sacheck et al., 2004; Stitt et al., 2004). PEG-Fib-IGF treatment also resulted in similar increases in mTOR and p70S6K phosphorylation as those found for Akt, as well as vastly reduced levels of MuRF-1, a cytosolic E3 ubiquitin ligase implicated with sarcomere degradation (Clarke et al., 2007; Cohen et al., 2009), demonstrating hyper-activation of the PI3K/Akt pathway is a feature of PEG-Fib-IGF treatment.
Figure 4.
Western blotting was performed on PEGylated fibrin gel (PEG-Fib) and IGF-I conjugated PEG-Fib (PEG-Fib-IGF) treated LGAS muscles following 4 days of reperfusion (A). Proteins assessed include the phosphorylated and total protein forms of ERK, Akt, mTOR, and p70S6K, as well as the E3 ubiquitin ligase MuRF-1. CB staining verifies equal loading across the samples. Quantified values are found in (B). H&E (C, top) and IHC for the myoblast marker MyoD (C, bottom) were also performed. Portion (D) contains a schematic summarizing events subsequent of IGF-I mediated activation of Akt. Values represented are the mean ± SEM; *P <0.05 versus PEG-Fib. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/bit]
Activation of Akt has been shown to protect cells from apoptotic and/or necrotic fates in a number of models and tissues (Harada et al., 2004; Li et al., 2005; Matheny and Adamo, 2009; Mocanu et al., 2002). In agreement with this, H&E staining revealed dramatically reduced signs of muscle pathology following PEG-Fib-IGF treatment (Fig. 4C top), compared to PEG-Fib treatment, suggesting a lower magnitude of initial muscle injury to correspond with the increased Akt activation. Unexpectedly, we could not detect any difference in frequency of MyoD (Fig. 4C bottom), an early expressed MRF, or myogenin (data not shown) positive nuclei, therefore enhanced activation of myoblasts does not appear to be a mechanism of the improved recovery with PEG-Fib-IGF treatment. These results in conjunction with the signaling data suggest PEG-Fib-IGF treatment improves muscle recovery from I/R by promoting hyper-activation of the PI3K/Akt pathway, thereby limiting the extent of cell death and degeneration that typically accompanies the I/R perturbation (summarized in Fig. 4D). In agreement with this, previous studies in our research group have demonstrated reduced activation of this pathway correlates with a reduction in functional recovery in aged rats from TK-induced I/R (Hammers et al., 2008). To verify this conclusion, however, future investigations to thoroughly quantify differential cellular damage between the two groups would need to be performed.
Conclusion
I/R injury is a considerable perturbation to skeletal muscle, often resulting in substantial and prolonged functional deficits. In the current report, we have demonstrated that the controlled release of IGF-I from an IM, biodegradable PEGylated fibrin gel matrix significantly improves the functional recovery of skeletal muscle from TK-induced I/R. We also identify activation of the PI3K/Akt pathway, and not enhanced myoblast activity, as a potential mechanism mediating this beneficial effect. The results of this study may lead to future investigations to test the effectiveness of PEG-Fib-IGF treatment in other models of muscle injury, including crush injuries and lacerations.
This particular form of growth factor is distinct from purely synthetic polymer delivery systems in the form of monolithic devices, foams, or particles in that the material is both injectable and can retain the delivered agent to the site of injury (Richardson et al., 2001). Since both fibrin-based and PEG-containing products are currently FDA approved, the translation of this form of therapy into the clinical realm is realistic. Additional directions may include the delivery of growth factor combinations to optimize tissue and/or incorporation of stem cells to facilitate the regenerative process of damaged tissue. For example, vasculogenic factors, such as VEGF or HGF, can possibly be used in this system to improve vascular regeneration following injury or other conditions where perfusion is compromised.
Acknowledgments
Contract grant sponsor: National Science Foundation
Contract grant number: CBET-0853996 ARRA
LJ Suggs acknowledges funding from the National Science Foundation (CBET-0853996 ARRA).
References
- Adams GR. Invited review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93(3):1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- Bandman E. Continued expression of neonatal myosin heavy chain in adult dystrophic skeletal muscle. Science. 1985;227(4688):780–782. doi: 10.1126/science.3969567. [DOI] [PubMed] [Google Scholar]
- Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10(6):620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107(8):3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelas I, Dwyer JF, Helgerson S. Controlled release of bioactive transforming growth factor beta-1 from fibrin gels in vitro. Tissue Eng Part C Methods. 2008;14(2):119–128. doi: 10.1089/ten.tec.2007.0262. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6(5):376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185(6):1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272(10):6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191(2):270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Davani EY, Brumme Z, Singhera GK, Cote HC, Harrigan PR, Dorscheid DR. Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit Care. 2003;7(6):R176–R183. doi: 10.1186/cc2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnan CT, Zhang G, Alexander MA, Pulido AS, Suggs LJ. Multimodal release of transforming growth factor-beta1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J Control Release. 2010;147(2):180–186. doi: 10.1016/j.jconrel.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Edwall D, Schalling M, Jennische E, Norstedt G. Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology. 1989;124(2):820–825. doi: 10.1210/endo-124-2-820. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Res. 1998;293(1):165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. Protein kinase B/Akt: A nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103(1):378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- Giannoni P, Hunziker EB. Release kinetics of transforming growth factor-beta1 from fibrin clots. Biotechnol Bioeng. 2003;83(1):121–123. doi: 10.1002/bit.10639. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC. Release and activation of platelet latent TGF-beta in blood clots during dissolution with plasmin. Nat Med. 1995;1(9):932–937. doi: 10.1038/nm0995-932. [DOI] [PubMed] [Google Scholar]
- Hammers DW, Merritt EK, Matheny W, Adamo ML, Walters TJ, Estep JS, Farrar RP. Functional deficits and insulin-like growth factor-I gene expression following tourniquet-induced injury of skeletal muscle in young and old rats. J Appl Physiol. 2008;105(4):1274–1281. doi: 10.1152/japplphysiol.90418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers DW, Matheny RW, Jr, Sell C, Adamo ML, Walters TJ, Estep JS, Farrar RP. Impairment of IGF-I expression and anabolic signaling following ischemia/reperfusion in skeletal muscle of old mice. Exp Gerontol. 2011;46(4):265–272. doi: 10.1016/j.exger.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Hatano E, Koizumi N, Nitta T, Yoshida M, Yamamoto N, Brenner DA, Yamaoka Y. Akt activation protects rat liver from ischemia/reperfusion injury. J Surg Res. 2004;121(2):159–170. doi: 10.1016/j.jss.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br. 2007;89(5):693–700. doi: 10.1302/0301-620X.89B5.18450. [DOI] [PubMed] [Google Scholar]
- Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006;17(4):305–323. doi: 10.1016/j.cytogfr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: Role of protein kinase B. Diabetologia. 2005;48(7):1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- Matheny RW, Jr, Adamo ML. Role of Akt isoforms in IGF-I-mediated signaling and survival in myoblasts. Biochem Biophys Res Commun. 2009;389(1):117–121. doi: 10.1016/j.bbrc.2009.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt EK, Hammers DW, Tierney M, Suggs LJ, Walters TJ, Farrar RP. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A. 2010;16(4):1395–1405. doi: 10.1089/ten.TEA.2009.0226. [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34(6):661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21(7):1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287(4):E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96(12):3772–3778. [PubMed] [Google Scholar]
- Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998;273(13):7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Walsh SR, Tang TY, Sadat U, Gaunt ME. Remote ischemic preconditioning in major vascular surgery. J Vasc Surg. 2009;49(1):240–243. doi: 10.1016/j.jvs.2008.07.051. [DOI] [PubMed] [Google Scholar]
- Walters TJ, Kragh JF, Kauvar DS, Baer DG. The combined influence of hemorrhage and tourniquet application on the recovery of muscle function in rats. J Orthop Trauma. 2008;22(1):47–51. doi: 10.1097/BOT.0b013e31815b3591. [DOI] [PubMed] [Google Scholar]
- Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to infarcted heart. Tissue Eng. 2007;13(8):2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14(6):1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]