Abstract
Introduction
The glucocorticoid receptor (GR) is a key receptor involved in inflammatory responses and is influenced by sex steroids. This study measured GR expression on circulating leukocyte subtypes in males and females.
Methods
A total of 23 healthy adults (12 female) participated in this study. GR expression was measured in leukocyte subtypes using flow cytometry. Peripheral blood mononuclear cell (PBMC) gene expression of GR (NR3C1), GR β, TGF-β1 and 2, and glucocorticoid-induced leucine zipper (GILZ) were determined by real-time polymerase chain reaction.
Results
Leukocyte GR was lower in females, particularly in granulocytes, natural killer cells, and peripheral blood mononuclear cells (p≤0.01). GR protein expression was different across leukocyte subtypes, with higher expression in eosinophils compared with granulocytes, T lymphocytes, and natural killer cells (p<0.05). There was higher gene expression of GR β in males (p=0.03).
Conclusions
This is the first study to identify sexual dimorphism in GR expression in healthy adults using flow cytometry. These results may begin to explain the sexual dimorphism seen in many diseases and sex differences in glucocorticoid responsiveness.
Key words: Glucocorticoid receptor, healthy adults, sex differences
Introduction
Sexual dimorphism in immune and inflammatory function has long been recognized [1–3], but substantial gaps remain in our understanding of the cellular mechanisms of this phenomenon. One possible mechanism is the role of the glucocorticoid receptor (GR), an intracellular receptor, which is found in almost all tissues and which modulates many inflammatory and growth responses [2]. GR expression and regulation vary considerably between tissues [4, 5]. Given the substantial evidence for sexual dimorphism in immune responses, it is somewhat surprising that little is known about the relative magnitude of GR in circulating leukocytes, essential systemic mediators of immune response to trauma or stress, and whether leukocyte GRs are affected by sex. The aim of this study was to compare GR expression on circulating leukocyte subtypes in healthy males and females using flow cytometry. We also wanted to measure gene expression of GR and a few selected genes known to affect GR expression and/or function using real-time polymerase chain reaction (RT-PCR) [6–8].
Sexual dimorphism in a number of diseases (e.g., asthma in adolescents, stress-related psychiatric diseases, autoimmune disorders, etc.) [9, 10] has been observed, highlighting the potential mechanistic importance of GR magnitude (and function) in leukocytes. Further, decreased sensitivity to anti-inflammatory glucocorticoid therapy (glucocorticoid resistance) can complicate the management of diseases such as asthma [11]. Previous studies have demonstrated that sex steroids can modify the GR and lead to alterations in glucocorticoid sensitivity [12–14]. Moreover, inflammatory cytokines such as tumor necrosis factor alpha (TNF)-α or Interleukin-6 (IL-6) are sexually dimorphic and can also influence GR [15]. Thus, in the present study, we determined GR protein expression levels on granulocytes, monocytes, T lymphocytes, eosinophils, basophils, natural killer (NK) cells, and natural killer T (NKT) cells using flow cytometry and examined for relationships between these values and circulating levels of testosterone, estradiol, IL-6, and TNF-α. Gene expression levels in peripheral blood mononuclear cells (PBMCs) of GR (NR3C1), GR β, transforming growth factor (TGF)-β1 and 2, and glucocorticoid-induced leucine zipper (GILZ) were measured using RT-PCR. All studies were performed in healthy, young adults with no known history of immune or inflammatory diseases.
Methods
Subjects
Eleven healthy males and 12 healthy females participated in this study. None of the participants were taking any medications, and none of the women were pregnant.
Ethics, Consent, and Permissions
This study was approved by the Institutional Review Board for human research at the University of California Irvine (UCI), and informed consent was obtained from all subjects. Samples were obtained from the Normal Volunteer Blood Drawing program sponsored by the UCI Institute for Clinical and Translational Science.
Sample Collection
Ten-mL EDTA-treated blood was obtained by venipuncture between 8 and 10 am, and was processed within 1 hour after draw. Complete blood count with differential was performed at the UCI-Clinical Pathology Laboratory. Whole blood was used immediately for leukocyte immune-phenotyping and intracellular staining of the GR. Plasma was separated by centrifugation, and stored in aliquots at −80°C until assayed for cortisol and cytokines. A second 10-mL EDTA-treated peripheral blood sample was collected for PBMCs isolation from a subset of healthy male and female adults.
Flow Cytometry
Simultaneous leukocyte immunophenotyping and intracellular GR staining were performed. Whole blood was used for surface immunophenotyping using surface-marker antibodies and intracellular GR determination.
The following surface antigen-specific, fluorescent-conjugated monoclonal antibodies were used in multiparameter flow cytometry: CD14 PerCP from BD Biosciences (clone MΦP9) and CD3 APC (clone UCHTI), CD16 Pacific Blue (clone 3G8), CD56 PECy5 (clone MEM 188), CD193 APC (clone 5E8), and CD203 APC (clone NP4D6) from BioLegend. Within 1 hour of blood collection, 100 μL of blood from each sample was added to 12×75-mm tubes with specific surface antigen fluorescent-conjugated monoclonal antibodies, mixed well, and incubated in the dark at room temperature for 15 minutes. Next, 2 mL of lysing solution (BD FACS™ Lysing Solution, no. 349202, BD Biosciences) was added to lyse red blood cells, mixed gently, and incubated for 8 minutes at room temperature in the dark; the mixture was centrifuged at 300g for 8 minutes. Lysis solution was decanted off the pelleted cells, and PBS_FCS 1% wash buffer (1×2 mL) was added. The cells were washed by vortex and then centrifuged at 300g for 8 minutes. The wash solution was decanted off the cell pellet. Cells were resuspended in permeabilization buffer (no. 00-833-56, eBioScience) containing FITC-conjugated GR monoclonal antibody (clone5E4, AbD Serotec) or fluorescein isothiocyanate (FITC)-immunoglobulin G (IgG) isotype (clone MOP_21, no. 400138, BioLegend) and incubated on ice for 20 minutes in the dark. After washing with 2×2-mL wash buffer to remove any unbound antibodies from the cytoplasm, the cell pellets were resuspended in 300-μL 1×PBS containing 2% formaldehyde as a fixative. Spherotec FITC Beads (no. ECFP FITC, Spherotech Inc.) were used as a positive control for FITC MFI.
Acquisition and Analysis
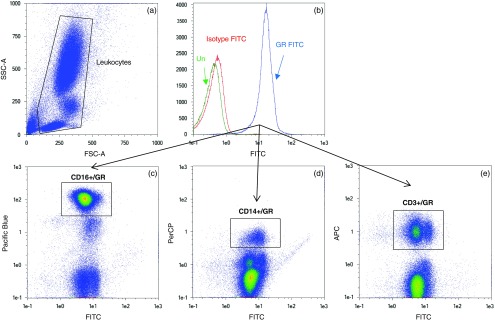
The cytometer was calibrated before data acquisition each day using calibration beads. Using side-scattered (SSC) versus forward-scattered (FSC) light as a primary trigger, a minimum of 100,000 cellular events were acquired on a 3-laser flow cytometer, MacsQuant Analyser (Miltenyi Biotec). Data were analyzed using MACSQuantify software. Intracellular GR expression levels were calculated using IgG as an intracellular negative control to determine background (nonspecific) staining. Leukocyte subpopulations were identified by FSC and SSC and separated by antigenic expressions of CD3+ (T lymphocytes), CD14+ (monocytes), CD16+ (granulocytes), CD3−CD56+ (NK cells), CD3+CD56+ (NKT cells), CD193low SSC (eosinophils), and CD203+ (activated basophils) (Fig. 1). Data are presented as median fluorescent intensity (MFI) of double positivity for GR and leukocyte subtype of interest. To verify the leukocyte subpopulation, double-positive cells were back-gated using a FSC/SSC dot plot. We used standardized flow cytometry to compare GR among currently accepted classifications of monocyte subsets, using CD14 and CD16: classical (CD14++/CD16−), intermediate (CD14++/CD16+), and non-classical (CD14+/CD16++) monocytes [16]. As a positive control for FITC MFI, FITC beads were run on each day along with sample acquisition in a separate tube to serve as monitoring of FITC MFI from day to day. In fact, the FITC beads’ MFI on the FITC channel was not significantly variable from day to day; over a 3-month period, using the same batch of beads, the coefficient of variation was 3.32%.
Fig. 1.
Representative flow cytometry plots: glucocorticoid receptor (GR) expression in granulocytes, monocytes, and T lymphocytes was determined by staining with GR FITC, CD16 Pacific Blue, CD14 PerCP, and CD3 APC, respectively, and identifying cells that were positive for both GR FITC and leukocyte subtypes of interest. (a) Forward-scattered (FSC) and side-scattered (SSC) plot of leukocytes; (b) histogram of unstained leukocytes (green), and after staining with IgG isotype-FITC (red) and GR FITC (blue); (c) double-positive signals for granulocytes CD16/GR, (d) monocytes CD14/GR, and (e) T lymphocytes CD3/GR, as identified in rectangular boxes.
Cortisol, Sex Steroids, and Cytokines
Plasma cortisol was determined by enzyme immunoassay (ELISA) using a commercial ELISA kit (Rocky Mountain Diagnostics). All samples were analyzed in duplicate following the manufacturer’s protocol. Estradiol, testosterone, IL-6, and TNF-α were analyzed in plasma using enzyme immunoassay with commercial ELISA kits. All samples were analyzed in duplicate following the manufacturer’s protocol.
Gene Expression
On a subset of healthy male and female adults, gene expression of the GR (NR3C1) and a few selected genes known to affect GR expression and/or function (GR β, TGF-β1 and 2, and GILZ) [6–8] were determined in PBMCs.
PBMC Isolation
Total ribonucleic acid (RNA) was extracted from PBMCs using TRIzol (Gibco BRL Life Technologies). For gene expression, the RNA was purified using RNeasy Mini Kit (Qiagen). RNA pellets were resuspended in diethyl pyrocarbonate-treated water, and the RNA concentration was determined using the NanoVue Plus Spectrophotometer (GE Healthcare). RNA integrity was assessed on an Agilent Bioanalyzer 2100 (Agilent Technologies) and showed that all the samples used were of high integrity (RIN>9).
Quantitative RT-PCR
One microgram of total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Reagents kit (Applied Biosystems) according to the manufacturer’s instructions, using random primers in a 20-μL reaction mixture. The quantitative PCR analysis was performed with the ABI 7900HT Sequence Detection System (Applied Biosystems) using TaqMan Universal PCR Master Mix and Taqman Gene Expression probes (Applied Biosystems) (GR total assay ID Hs00353740_m1; GR β assay ID Hs00354508_m1; TGF-β1 assay ID Hs00998133_m1; TGF-β2 assay ID Hs00234244_m1; and TSC22D3 assay ID Hs00608272_m1). β Actin gene was used as an endogenous control (assay ID Hs01060665_g1). All reactions were run in duplicates, and quantitative PCR data were analyzed using SDS software (Applied Biosystems). Results are expressed as relative quantities and based on the 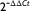 method [17].
method [17].
Statistical Analysis
The data were analyzed using SAS 9.4 software (SAS Institute Inc., Cary, NC). Differences in MFI of GR expression in leukocyte subtypes and by sex was assessed using a linear mixed model that took into consideration subjects’ intercorrelation. Models were adjusted for multiple comparisons using a Tukey-Kramer adjustment. Differences in MFI for GR expression in PBMCs by sex was assessed using a nonpaired t-test. Differences in mean relative quantities of selected genes by sex were assessed using a nonpaired t-test. A p value <0.05 was considered significant.
Results
We included 11 males, with mean age 30.8 years (range 24–38), and 12 females, with mean age of 28.2 years (range 21–39). Complete blood counts were within normal limits.
Differences in GR among Leukocyte Subtypes
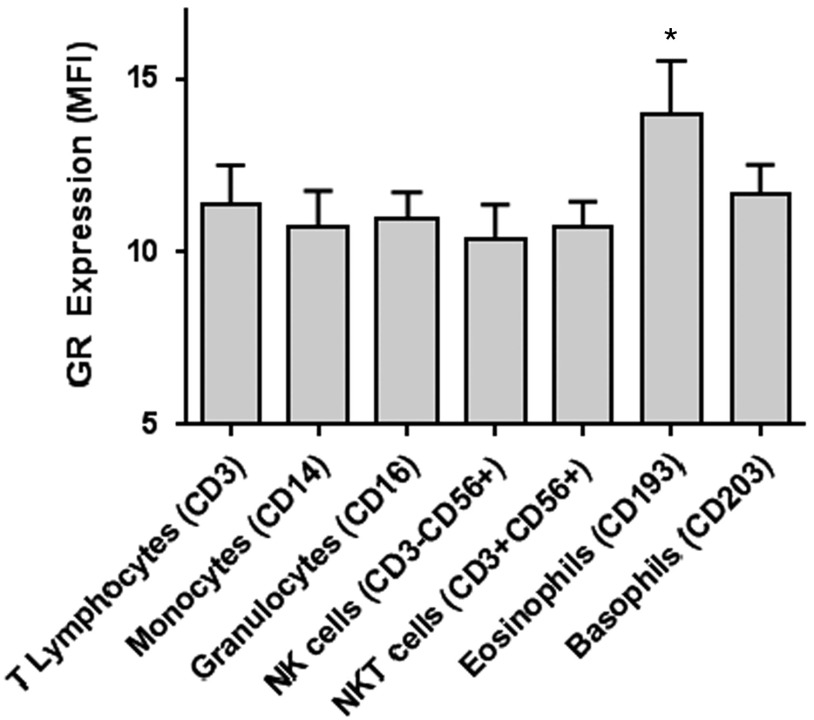
GR MFI was different across leukocyte subtypes in mixed model analysis (p=0.017). The expression of GR in eosinophils (CD193low SSC) was significantly higher compared with granulocytes, T lymphocytes, and NK cells (p<0.05) (Fig. 2).
Fig. 2.
Glucocorticoid receptor (GR) expression by leukocyte subtype in the entire cohort. GR expression represented as median fluorescent intensity (MFI). GR MFI was different across leukocyte subtypes in the mixed model analysis (p=0.017). *GR expression in eosinophils was significantly higher than granulocytes, T lymphocytes, and natural killer (NK) cells (p<0.05). Bars represent means and standard errors. NKT, natural killer T cells.
We examined the expression of GR MFI across monocyte subsets. The expression of GR in classical monocytes (CD14++/CD16−) was significantly lower compared with both non-classical (p=0.01) and intermediate monocytes (p=0.01) (data not shown).
Sexual Dimorphism in Leukocyte GR
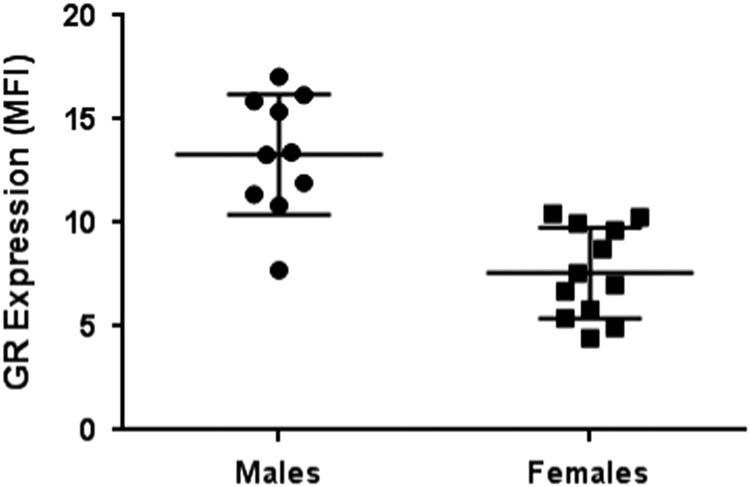
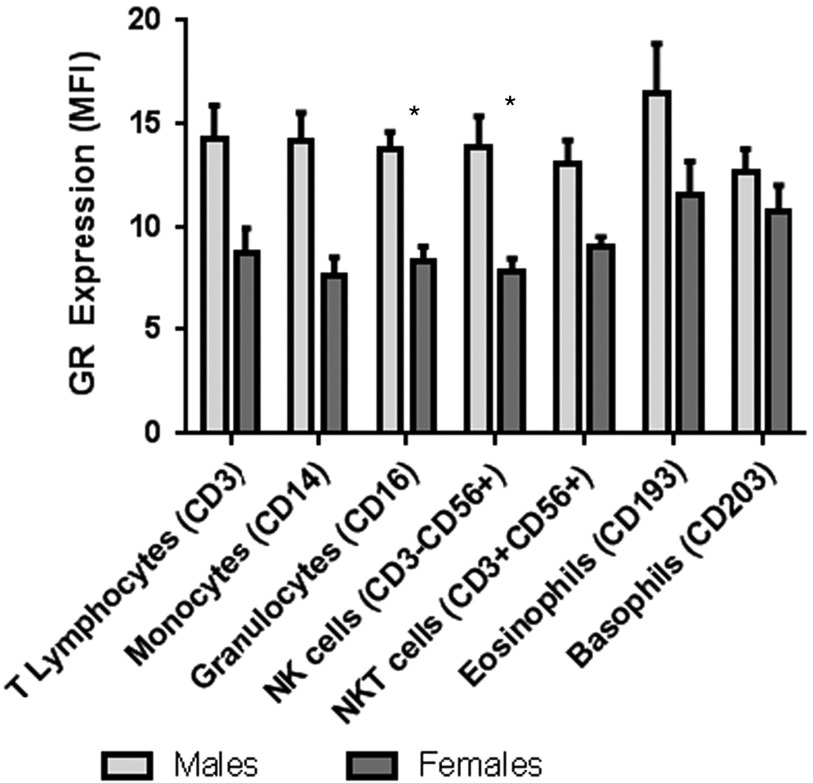
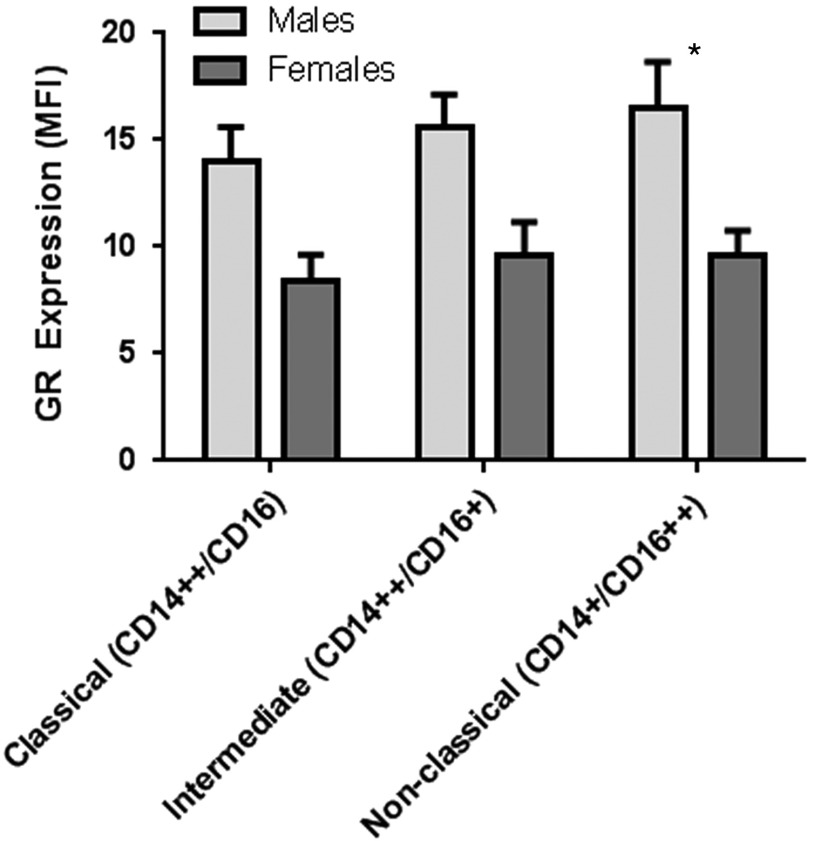
There was a significant sex difference in GR in PBMCs with lower expression of GR among females compared with males (p<0.0001) (Fig. 3). Using mixed model analysis, there was a significant sex effect on GR in leukocyte subpopulations (p=0.0004). After adjustment for multiple comparisons, there were significant differences by sex with lower GR expression in females compared with males in granulocytes and NK cells (Table 1, Fig. 4). There was also a significant sex effect on GR in monocyte subsets with lower expression of GR among females compared with males (p=0.005) in mixed model analyses (Fig. 5). After adjustment for multiple comparisons, there was a significantly lower GR expression in females compared with males in non-classical monocyte subsets (p=0.02).
Fig. 3.
Sex differences in glucocorticoid receptor (GR) expression in peripheral blood mononuclear cells (PBMCs). GR expression represented as median fluorescent intensity (MFI). GR expression in PBMCs was significantly different between males and females (p<0.0001). Bars represent means and standard errors.
Table 1.
Effect of sex on GR expression in leukocyte subtypes
| Leukocyte Subtype | Effect Difference | StandardError | P value | Adj P value* |
|---|---|---|---|---|
| Monocytes (CD14/GR FITC) | –5.666 | 1.708 | 0.001 | 0.067 |
| Granulocytes (CD16/GR FITC) | –6.597 | 1.708 | <0.001 | 0.013 |
| T lymphocytes (CD3/GR FITC) | –5.528 | 1.708 | 0.002 | 0.084 |
| NK cells (CD3-CD56+/GR FITC) | –6.868 | 1.772 | <0.001 | 0.012 |
| NKT cells (CD3+CD56+/GR FITC) | –4.811 | 1.772 | 0.008 | 0.283 |
| Eosinophils (CD193/GR FITC) | –4.748 | 1.731 | 0.007 | 0.269 |
| Basophils (CD203/GR FITC) | –1.956 | 1.708 | 0.254 | 0.997 |
P value adjusted by Tukey-Kramer methods to account for multiple comparisons.
Fig. 4.
Glucocorticoid receptor (GR) expression by leukocyte subtype and sex. GR expression represented as median fluorescent intensity (MFI). Using mixed model analysis, there was a significant sex effect on GR in leukocyte subpopulations (p=0.0004). *GR expression in granulocytes and natural killer (NK) cells was significantly different between males and females. Bars represent means and standard errors. NKT, natural killer T cells.
Fig. 5.
Glucocorticoid receptor (GR) expression among monocyte subtypes by sex. GR expression represented as median fluorescent intensity (MFI). There was a significant sex effect on GR in monocyte subsets with lower expression of GR among females compared with males (p=0.005) in mixed model analyses. After adjustment for multiple comparisons, there was a significantly lower GR expression in females compared with males in non-classical monocyte subsets (p=0.02). Bars represent means and standard errors.
Gene Expression of GR and Related Genes in PBMCs
No sex differences in the gene expression of PBMC total GR, TGF-β1 and 2, and GILZ (see online Supplementary Table S1) were found. However, we did see a significantly higher expression of GR β in males compared with females (p=0.03).
Relationship Between GR and Circulating Cortisol, Sex Steroids, and Cytokines
Serum cortisol did not differ between males and females. There was no correlation between serum cortisol and GR protein expression in leukocyte subpopulations (Table 2). IL-6 and TNF-α did not differ between males and females. No correlations were found between leukocyte GR and IL-6 or TNF-α.
Table 2.
Correlations between glucocorticoid receptor (GR) expression in leukocyte subtypes and circulating cortisol, sex steroids, and cytokines
| Cortisol R (p value) | TNF-α R (p value) | IL-6 R (p value) | Testosterone R (p value) | Estradiol R (p value) | |
|---|---|---|---|---|---|
| Monocytes (CD14/GR FITC) | −0.271 (0.222) | −0.207 (0.368) | 0.008 (0.972) | 0.299 (0.166) | −0.305 (0.178) |
| Granulocytes (CD16/GR FITC) | −0.260 (0.243) | 0.016 (0.944) | 0.039 (0.870) | 0.380 (0.074) | −0.358 (0.111) |
| T lymphocytes (CD3/GR FITC) | −0.291 (0.189) | 0.009 (0.970) | −0.086 (0.718) | 0.505 (0.014)* | −0.445 (0.043)* |
| NK cells (CD3−CD56+/GR FITC) | −0.353 (0.127) | −0.031 (0.901) | −0.003 (992) | 0.467 (0.033)* | −0.397 (0.093) |
| NKT cells (CD3+CD56+/GR FITC) | −0.370 (0.109) | −0.280 (0.236) | −0.144 (0.569) | 0.318 (0.161) | −0.483 (0.036)* |
| Eosinophils (CD193/GR FITC) | −0.305 (0.179) | −0.019 (0.936) | 0.189 (0.439) | −0.045 (0.841) | −0.135 (0.570) |
| Basophils (CD203/GR FITC) | −0.045 (0.841) | −0.034 (0.881) | −0.177 (0.455) | 0.073 (0.740) | −0.041 (0.862) |
NK, natural killer cells; NKT, natural killer T cell.
*Significant correlations p<0.05. GR expression represented as median fluorescent intensity.
As expected, there were significant differences in testosterone and estradiol between male and female participants. Testosterone was positively associated with GR expression in T lymphocytes (R=0.505, p=0.014) and NK cells (R=0.467, p=0.033) across all subjects (see online Supplementary Fig. S1). Estradiol levels were negatively associated with GR expression in T lymphocytes (R=−0.445, p=0.043) and NKT cells (R=−0.483, p=0.036) across all subjects (see online Supplementary Fig. S1). Within males and females separately, only the correlation between estradiol and T lymphocytes remained in males (R=0.410, p=0.03) (see online Supplementary Fig. S2). No correlations were found between testosterone and GR expression in leukocyte subtypes within males and females.
Discussion
This is, we believe, the first study to identify differences in GR among leukocyte subtypes and to demonstrate sexual dimorphism in leukocyte GR in healthy young adults using flow cytometry. In general, leukocyte GR was lower in females, and particularly striking sex differences were found in NK cells and granulocytes. Among the leukocyte subtypes, GR was significantly higher in eosinophils, and within monocyte subsets, GR was lower in the classical subgroup compared with the intermediate and non-classical subgroups.
Sex differences in GR expression in circulating leukocytes of healthy humans have not been well studied, and the bulk of these studies was performed before technologies such as flow cytometry allowed more focused examination of receptors on particular cell types. For example, Tanaka et al. [18] measured GR using radiolabeled ligand binding assays in PBMCs from healthy adults, and, although significant differences were not observed, GR expression was decreased in women. In animal studies, female rats were shown to have fewer GR in the pituitary, thymus, and liver compared with males, as well as lower glucocorticoid binding in the hypothalamus [19–21].
Sexual dimorphism in immunological diseases and in response to stress has long been recognized. Women tend to have higher incidence of autoimmune diseases [9], whereas men respond to trauma-induced stress with a more pronounced release of sometimes deleterious inflammatory cytokines such as IL-6 or TNF-α [10]. The mechanisms of these sex differences are not completely understood. Nonetheless, our observation of reduced GR in leukocytes of women is particularly intriguing in light of recent studies examining the response of glucocorticoid treatment in asthma, a common condition that responds favorably to systemic or inhaled glucocorticoids. Several studies suggest that female asthmatics taking inhaled corticosteroids tend to respond less favorably compared with their male counterparts, characterized by more severe asthma than males in general, worse asthma control when treated, increased asthma-related healthcare utilization, and greater deterioration in lung function over time than men [22–26]. Whether these clinical observations are linked to sex-specific reduction in leukocyte GR leading to reduced responsiveness to glucocorticoid therapy is not known. Finally, in two separate studies in patients with systemic lupus erythematosus (SLE) and nephrotic syndrome, disease severity and response to glucocorticoids (a commonly used treatment in both diseases) was correlated with reduced GR in T lymphocytes and monocytes [27, 28]. The incidence of SLE is much greater in women compared with men. Whether this increased disease incidence is related to the generally lower levels of leukocyte GR in females is not yet known.
NK cells and granulocytes are essential cells of innate immunity, and we found the most marked sexual dimorphism in these leukocyte subtypes. The unique role of NK cells in cancer surveillance (breast cancer in particular) [29] and new approaches to modulating these cells to enhance their ability to not only recognize but also kill malignant cells are subjects of much active and promising investigation [30]. Although the ability of glucocorticoids, acting through GR, to enhance lymphoid cell and neutrophil apoptosis is well recognized [31], the role of sexual dimorphism in the GR in enhancing chemotherapy through effects on innate immune cells such as NK cells has not been evaluated. Perhaps related to reduced GR, and subsequent reduced responsiveness to natural levels of cortisol, are the observations that neutrophils from women at term pregnancy and in healthy non-pregnant females have a significant delay in apoptosis [32, 33].
We could not explain our results on the basis of the circulating levels of cortisol. Experimental treatment with dexamethasone, for example, leads to downregulation of GR on lymphocytes and other tissues [34], but in our participants there were no differences in circulating levels of cortisol between men and women. Quinn and Cidlowski recently reviewed the potential mechanisms of glucocorticoid dimorphism and noted that the sex differences likely resulted from sex-related differences in gene expression and transcription factors involved in a number of metabolic pathways [35]. Moreover, inflammatory cytokines (such as IL-6 and TNF-α) are influenced by the leukocyte GR and cortisol [36]. Indeed, Manary et al. [37] demonstrated this complex balance in humans. In our study, circulating IL-6 and TNF-α were not different in men and women despite the differences in leukocyte GR. Consequently, circulating levels of cortisol and leukocyte GR may not sufficiently reveal the key elements of the more classical and expected simple inverse homeostatic relationship between a hormone (cortisol), its cellular receptor (GR), and, ultimately, the dynamics of related inflammatory cytokines known to be produced by leukocytes.
As expected, testosterone and estradiol levels differed substantially between men and women, but overall there were no correlations within groups of men and women between circulating sex steroids and GR except for estradiol levels and GR in T lymphocytes. Very little is known about the specific effect of either testosterone or estradiol on GR expression in immune cells. In the only recent study we could identify, Janele et al. used cultured peripheral leukocytes to test the effect of sex steroids on leukocyte GR. Neither testosterone nor estradiol, even in relatively high concentrations, altered GR in cultured white blood cells [38]. Thus, the effects of testosterone or estradiol do not appear to be directly involved in the mechanism of reduced GR in leukocytes in otherwise healthy females.
We also found intriguing patterns of GR among leukocyte subtypes in both men and women. In monocytes, for example, GR was lower in females, but in both men and women GR was the lowest in the “classical” subgroup of monocytes. Three subsets of monocytes have been identified in circulation in humans—classical, intermediate, and non-classical [39]. Classical monocytes (CD14++CD16−) express high levels of chemokine receptor 2. These monocytes tend to produce higher levels of inflammatory cytokines in response to inflammatory stimuli such as lipopolysaccharides, and some researchers believe that this inflammatory tendency may indicate a more central role for classical monocytes in the development of atherosclerotic heart disease [40, 41]. Glucocorticoids appear to play a role in atherosclerosis, and, although the mechanism of action is not yet clear, it is possible that, in some cases, reduced GR on classical monocytes leads to increased inflammatory profiles and contributes to atherosclerosis pathogenesis [42].
We found that GR expression was significantly higher in eosinophils compared with other leukocyte subtypes. Eosinophilic diseases including nasal polyps and allergic asthma tend to respond favorably to glucocorticoids [43]. For example, in asthma, those with predominantly neutrophilic airway inflammation tend to respond less favorably compared with those with predominantly eosinophilic airway inflammation [44]. Eosinophils appear to play a major role in allergic asthma, but many adults and children are resistant to corticosteroids, a mainstay of therapy [45]. One possibility is that, similar to SLE, the level of GR on eosinophils (or neutrophils) in allergic asthma may help determine which patients will optimally benefit from corticosteroids and/or guide appropriate dosage.
As noted, we also examined the expressions of 4 genes known to be involved in the expression and function of GR. We found no sex difference in the gene expressions of total GR, TGF-β1 and 2, and GILZ. We found that there were significantly lower levels of GR β expression in females compared with males. Alternative splicing of the GR gives rise to several isoforms including GR α, the active isoform, and GR β, an isoform that is thought to act as a dominant negative inhibitor. Elevated levels of GR β are associated with glucocorticoid resistance in several conditions including asthma [46, 47]. Along these lines, we speculate that decreased GR β gene expression may be a compensatory mechanism in healthy females with lower levels of GR compared with males. In healthy individuals, the expression of GR β gene is variable, and the protein is sometimes undetectable using a variety of standard techniques [48]. We were unsuccessful at identifying GR β using flow cytometry.
Limitations
There are several limitations to these observations. We did not obtain information regarding female participants and their current phase of the menstrual cycle during blood draw. There is evidence that glucocorticoid feedback regulation of the hypothalamic-pituitary-adrenal axis may differ as phases of the menstrual cycle vary [49]. Although we found significant differences in GR expression, we did not measure GR function or responsiveness using, for example, dexamethasone stimulation. The density or magnitude of GR is only one factor that explains overall glucocorticoid responsiveness [50, 51]. Gene expression analysis was performed on PBMCs for a small number of targeted genes as the genomic control of GR has not been fully elucidated. Sexual dimorphism may exist in the expressions of genes that influence the GR that were untested. Finally, the precise interaction of GR α and β remains unclear, which inhibits our ability to fully explain the meaning of the resulting differences in GRβ gene expression.
Conclusions
This is the first study to identify sexual dimorphism in GR expression in healthy young adults using flow cytometry. In general, leukocyte GR was lower in females, and particularly striking sex differences were found in NK cells and granulocytes. Among the leukocyte subtypes, we also found (independent of sex differences) that GR was significantly higher in eosinophils. Within monocytes, we found that GR was lower in the classical subgroup compared with intermediate and non-classical subgroups. Finally, we found that GR β gene expression was significantly higher in males. These new observations of GR expression on circulating leukocytes may begin to explain sexual dimorphism seen in many disease states as well as known sex differences in response to glucocorticoid therapy.
Acknowledgments
The authors thank Annamarie Stehli, MS, for statistical analyses and Peter Horvath, PhD, for laboratory technical assistance.
Footnotes
Financial Support
NIH P01-HD048721; the UCI Institute for Clinical and Translational Science (CTSA grant) UL1 TR000153; Children’s Hospital of Orange County—University of California Irvine Child Health Research Award.
Declaration of Interest
The authors have no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/cts.2016.20.
click here to view supplementary material
References
- 1. Bangasser DA. Sex differences in stress-related receptors: “micro” differences with “macro” implications for mood and anxiety disorders. Biology of Sex Differences 2013; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Hormones and Behavior 2012; 62: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mirandola L, et al. Sex-driven differences in immunological responses: challenges and opportunities for the immunotherapies of the third millennium. International Reviews of Immunology 2015; 34: 134–142. [DOI] [PubMed] [Google Scholar]
- 4. Kalinyak JE, et al. Tissue-specific regulation of glucocorticoid receptor mRNA by dexamethasone. Journal of Biological Chemistry 1987; 262: 10441–10444. [PubMed] [Google Scholar]
- 5. McCormick JA, et al. 5'-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Molecular Endocrinolgoy 2000; 14: 506–517. [DOI] [PubMed] [Google Scholar]
- 6. Panek M, et al. The NR3C1 glucocorticoid receptor gene polymorphisms may modulate the TGF-beta mRNA expression in asthma patients. Inflammation 2015; 38: 1479–1492. [DOI] [PubMed] [Google Scholar]
- 7. Mittelstadt PR, Ashwell JD. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. Journal of Biological Chemistry 2001; 276: 29603–29610. [DOI] [PubMed] [Google Scholar]
- 8. Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. Journal of Biological Chemistry 1996; 271: 9550–9559. [DOI] [PubMed] [Google Scholar]
- 9. McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Current Molecular Medicine 2009; 9: 1058–1079. [DOI] [PubMed] [Google Scholar]
- 10. Guidry CA, et al. Sex- and diagnosis-dependent differences in mortality and admission cytokine levels among patients admitted for intensive care. Critical Care Medicine 2014; 42: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet 2009; 373: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, et al. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. Journal of Biological Chemistry 2009; 284: 24542–24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krishnan AV, Swami S, Feldman D. Estradiol inhibits glucocorticoid receptor expression and induces glucocorticoid resistance in MCF-7 human breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology 2001; 77: 29–37. [DOI] [PubMed] [Google Scholar]
- 14. Hubler TR, et al. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 2003; 144: 2380–2387. [DOI] [PubMed] [Google Scholar]
- 15. Rohleder N, et al. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic Medicine 2001; 63: 966–972. [DOI] [PubMed] [Google Scholar]
- 16. Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Frontiers in Immunology 2013; 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka H, et al. Glucocorticoid receptors in normal leukocytes: effects of age, gender, season, and plasma cortisol concentrations. Clinical Chemistry 1991; 37(Pt 1): 1715–1719. [PubMed] [Google Scholar]
- 19. Turner BB. Sex difference in glucocorticoid binding in rat pituitary is estrogen dependent. Life Sciences 1990; 46: 1399–1406. [DOI] [PubMed] [Google Scholar]
- 20. Turner BB, Weaver DA. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Research 1985; 343: 16–23. [DOI] [PubMed] [Google Scholar]
- 21. Endres DB, Milholland RJ, Rosen F. Sex differences in the concentrations of glucocorticoid receptors in rat liver and thymus. Journal of Endocrinology 1979; 80: 21–26. [DOI] [PubMed] [Google Scholar]
- 22. Siroux V, et al. Phenotypic determinants of uncontrolled asthma. Journal of Allergy and Clinical Immunology 2009; 124: 681–687. [DOI] [PubMed] [Google Scholar]
- 23. Convery RP, et al. Effect of inhaled fluticasone propionate on airway responsiveness in treatment-naive individuals—a lesser benefit in females. European Respiratory Journal 2000; 15: 19–24. [DOI] [PubMed] [Google Scholar]
- 24. Dijkstra A, et al. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax 2006; 61: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bacharier LB, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. Journal of Allergy and Clinical Immunology 2009; 123: 1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerald JK, et al. Markers of differential response to inhaled corticosteroid treatment among children with mild persistent asthma. Journal of Allergy and Clinical Immunology. In Practice 2015; 3: 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du J, et al. Flow cytometry analysis of glucocorticoid receptor expression and binding in steroid-sensitive and steroid-resistant patients with systemic lupus erythematosus. Arthritis Research & Therapy 2009; 11: R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hammad A, et al. Low expression of glucocorticoid receptors in children with steroid-resistant nephrotic syndrome. Pediatric Nephrology 2013; 28: 759–763. [DOI] [PubMed] [Google Scholar]
- 29. Markowitz J, et al. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Research and Treatment 2013; 140: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017. Jan; 222(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 31. Pufall MA. Glucocorticoids and cancer. Advances in Experimental Medicine and Biology 2015; 872: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cotton DJ, et al. Selective defect in human neutrophil superoxide anion generation elicited by the chemoattractant N-formylmethionylleucylphenylalanine in pregnancy. Journal of Infectious Diseases 1983; 148: 194–199. [DOI] [PubMed] [Google Scholar]
- 33. Molloy EJ, et al. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 2003; 102: 2653–2659. [DOI] [PubMed] [Google Scholar]
- 34. Odore R, et al. Changes in lymphocyte glucocorticoid and beta-adrenergic receptors in veal calves treated with clenbuterol and steroid hormones for growth-promoting purposes. Journal of Veterinary Pharmacology and Therapeutics 2006; 29: 91–97. [DOI] [PubMed] [Google Scholar]
- 35. Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Annals of the New York Academy of Science 2014; 1317: 1–6. [DOI] [PubMed] [Google Scholar]
- 36. Franchimont D, et al. Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. Journal of Clinical Endocrinology and Metabolism 1999; 84: 2834–2839. [DOI] [PubMed] [Google Scholar]
- 37. Manary MJ, et al. Cortisol and its action on the glucocorticoid receptor in malnutrition and acute infection. Metabolism 2006; 55: 550–554. [DOI] [PubMed] [Google Scholar]
- 38. Janele D, et al. Effects of testosterone, 17beta-estradiol, and downstream estrogens on cytokine secretion from human leukocytes in the presence and absence of cortisol. Annals of New York Academy of Science 2006; 1069: 168–182. [DOI] [PubMed] [Google Scholar]
- 39. Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Frontiers in Immunology 2015; 6: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Radom-Aizik S, et al. Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behavior, and Immunity 2014; 39: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Idzkowska E, et al. The role of different monocyte subsets in the pathogenesis of atherosclerosis and acute coronary syndromes. Scandinavian Journal of Immunology 2015; 82: 163–173. [DOI] [PubMed] [Google Scholar]
- 42. Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutrition & Metabolism (London) 2005; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis 2003; 8: 481–495. [DOI] [PubMed] [Google Scholar]
- 44. Green RH, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002; 57: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boardman C, et al. Mechanisms of glucocorticoid action and insensitivity in airways disease. Pulmonary Pharmacology & Therapeutics 2014; 29: 129–143. [DOI] [PubMed] [Google Scholar]
- 46. Fujishima S, et al. The relationship between the expression of the glucocorticoid receptor in biopsied colonic mucosa and the glucocorticoid responsiveness of ulcerative colitis patients. Clinical Immunology 2009; 133: 208–217. [DOI] [PubMed] [Google Scholar]
- 47. Butler CA, et al. Glucocorticoid receptor beta and histone deacetylase 1 and 2 expression in the airways of severe asthma. Thorax 2012; 67: 392–398. [DOI] [PubMed] [Google Scholar]
- 48. Pujols L, et al. Expression of the human glucocorticoid receptor alpha and beta isoforms in human respiratory epithelial cells and their regulation by dexamethasone. American Journal of Respiratory Cell and Molecular Biology 2001; 24: 49–57. [DOI] [PubMed] [Google Scholar]
- 49. Altemus M, et al. Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor mRNA expression in the luteal phase of the menstrual cycle. Neuropsychopharmacology 1997; 17: 100–109. [DOI] [PubMed] [Google Scholar]
- 50. Shipman GF, et al. Glucocorticoids and lymphocytes. III. Effects of glucocorticoid administration on lymphocyte glucocorticoid receptors. Blood 1983; 61: 1086–1090. [PubMed] [Google Scholar]
- 51. Haarman EG, et al. Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia 2004; 18: 530–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/cts.2016.20.
click here to view supplementary material