Abstract
Background
Interstitial lung disease (ILD) frequently complicates systemic autoimmune disorders resulting in considerable morbidity and mortality. The connective tissue diseases (CTDs) most frequently resulting in ILD include: systemic sclerosis, idiopathic inflammatory myositis (including dermatomyositis, polymyositis and anti-synthetase syndrome) and mixed connective tissue disease. Despite the development, over the last two decades, of a range of biological therapies which have resulted in significant improvements in the treatment of the systemic manifestations of CTD, the management of CTD-associated ILD has changed little. At present there are no approved therapies for CTD-ILD. Following trials in scleroderma-ILD, cyclophosphamide is the accepted standard of care for individuals with severe or progressive CTD-related ILD. Observational studies have suggested that the anti-CD20 monoclonal antibody, rituximab, is an effective rescue therapy in the treatment of refractory CTD-ILD. However, before now, there have been no randomised controlled trials assessing the efficacy of rituximab in this treatment population.
Methods/design
RECITAL is a UK, multicentre, prospective, randomised, double-blind, double-dummy, controlled trial funded by the Efficacy and Mechanism Evaluation Programme of the Medical Research Council and National Institute for Health Research. The trial will compare rituximab 1 g given intravenously, twice at an interval of 2 weeks, with intravenously administered cyclophosphamide given monthly at a dose of 600 mg/m2 body surface area in individuals with ILD due to systemic sclerosis, idiopathic inflammatory myositis (including anti-synthetase syndrome) or mixed connective tissue disease. A total of 116 individuals will be randomised 1:1 to each of the two treatment arms, with stratification based on underlying CTD, and will be followed for a total of 48 weeks from first dose. The primary endpoint for the study will be change in forced vital capacity (FVC) at 24 weeks. Key secondary endpoints include: safety, change in FVC at 48 weeks as well as survival, change in oxygen requirements, total 48-week corticosteroid exposure and utilisation of health care resources.
Discussion
This is the first randomised control trial to study the efficacy of rituximab as first-line treatment in CTD-associated ILD. The results generated should provide important information on the treatment of a life-threatening complication affecting a rare group of CTDs.
Trial registration
ClinicalTrials.gov, NCT01862926. Registered on 22 May 2013.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2016-2) contains supplementary material, which is available to authorized users.
Keywords: Myositis, Scleroderma, Mixed connective tissue disease, Pulmonary fibrosis, Respiratory failure, Biomarkers
Background
Interstitial lung disease (ILD) is characterised by inflammation and/or fibrosis that results in thickening and distortion of the alveolar wall with consequent impairment of gas exchange. Affected individuals typically present with progressive breathlessness which frequently causes respiratory failure and death. There are many described causes of ILD; however, one of the commonest is that resulting from lung involvement by systemic autoimmune diseases [1]. This group of conditions, the connective tissue diseases (CTDs), is an important cause of disability and death in the working-age population. Over the last decade, improvements in therapy for the CTDs have seen the prognosis for individuals with these conditions dramatically improve. Despite these improvements in care there has been little, if any, change in therapy for ILD occurring as a consequence of CTD, with respiratory disease growing in importance in these patients. For many CTD sufferers, disease-associated ILD is now the major cause of disability and exercise limitation, whilst in systemic sclerosis it is now the principal cause of mortality [2].
The pathogenesis of CTD-ILD is complex and poorly understood. It is, however, generally accepted that underlying immune system dysfunction and immune-mediated pulmonary inflammation are critical to CTD-ILD development and progression. Abnormalities of cellular and humoral immune function have been described in ILD associated with systemic sclerosis (SSc) [3–5], idiopathic inflammatory myopathy and several other CTDs. The mechanisms leading on to fibrosis remain poorly understood, as do the factors that determine which individuals with CTD develop ILD. Nonetheless, evidence from treatment trials suggests that the modulation of inflammation with immunosuppressant therapies, particularly cyclophosphamide, results in some regression of ILD and prevents the development of further fibrosis.
Different CTDs manifest varying forms of ILD. Individuals with scleroderma and mixed connective tissue disease (MCTD) most commonly develop the histological lesion of nonspecific interstitial pneumonia (NSIP). Those with idiopathic inflammatory myositis typically have combined organising pneumonia and nonspecific interstitial pneumonia (NSIP). By contrast, individuals with rheumatoid disease frequently have fibrosis with the histological pattern of usual interstitial pneumonia (UIP) and tend to be resistant to therapy with high-dose immunosuppression.
Currently, standard of care for severe, progressive CTD-ILD includes immunosuppression with intravenous cyclophosphamide (600 mg/m2) administered monthly for 6 months, followed by maintenance oral immunosuppression [6, 7]. Occasionally, this intensive immunosuppressive therapy fails to control pulmonary inflammation and alternative therapies may be required. Rituximab, a chimeric (human/mouse) monoclonal antibody with a high affinity for the CD20 surface antigen expressed on pre-B and B-lymphocytes, results in rapid depletion of B-cells from the peripheral circulation for 6 to 9 months [8, 9]. Evidence for the effectiveness of B-cell depletion exists in a number of immune-mediated conditions, including rheumatoid arthritis [10–12], ANCA-associated vasculitis [13, 14] and immune thrombocytopenic purpura [15]. Several case series suggest that rituximab may also be effective in ILD occurring in the context of immunological overactivity, with favourable responses reported in anti-synthetase-associated ILD [16] and scleroderma-ILD [17, 18]. Our own experience has demonstrated rituximab to be an effective, potentially life-saving therapeutic intervention in the treatment of very severe, progressive CTD-ILD unresponsive to conventional immunosuppression [19, 20].
It is hoped that this study will advance the standard of care for those with CTD-ILD. Despite current best treatment, individuals with extensive ILD due to scleroderma have a median survival of less than 5 years and a similar poor prognosis is observed in individuals with inflammatory myositis and MCTD-associated ILD [21]. If rituximab can be shown to improve 6-month and 1-year lung function in this group then it is to be hoped that this will translate in to improvements in longer-term survival and associated reductions in morbidity. The simplified dosing regimen for rituximab when compared to cyclophosphamide also affords the potential for reducing the burden on patients (and their carers) of frequent hospital attendances. Similarly, although drug costs are higher for rituximab, it is hoped that a full economic costing will demonstrate savings based on reduced utilisation of health care resources and fewer hospital visits. There are currently no available biomarkers for assessing response to therapy or risk of disease progression in CTD-ILD. By closely studying patients in each treatment arm and undertaking exploratory biomarker analysis it is hoped that we might identify potential disease- and therapy-specific biomarkers for future development and use in clinical practice.
Against the potential benefits must be balanced the risks of treatment. As noted, rituximab is a well-established therapy for a range of indications and, as such, its safety profile is well known. Potential risks of therapy include; infusion reactions, infection, arthralgia and hypercholesterolaemia. Very rarely, long-term hypogammaglobulinaemia and neutropaenia have been reported. These side effects can be balanced against those known to occur following cyclophosphamide which include haemorrhagic cystitis, nausea and vomiting and ,in the longer term, an increased incidence of bladder malignancy.
Aims and objectives
The overall aims of this study are to:
Demonstrate that intravenously administered rituximab has superior efficacy to current best treatment (intravenous cyclophosphamide) for CTD-ILD
Compare the safety profile of rituximab to intravenously administered cyclophosphamide in individuals with CTD-ILD
Assess the health economic benefits of rituximab compared to current standard of care for CTD-ILD, and
Evaluate a range of exploratory biomarkers for disease severity, prognosis and treatment response in CTD-ILD
Methods/design
The RECITAL study is a UK, multicentre, prospective, randomised, double-blind, double-dummy trial of intravenously administered rituximab compared with intravenously administered cyclophosphamide in patients with severe, progressive CTD-ILD. Patients will be randomised to two groups. Both groups will receive placebo to match the different regimens. The study design is outlined in Table 1 and Fig. 1. More details are available in the RECITAL protocol, version 6.1, dated 15 December 2014.
Table 1.
Outline of planned treatment interventions by visit
| Rituximab group | Cyclophosphamide group | |
|---|---|---|
| Day 0 | IV active rituximab 1000 mg | IV 600 mg mg/m2 body surface area |
| Day 14 | IV active rituximab 1000 mg | Placebo |
| Week 4 | Placebo | IV 600 mg mg/m2 body surface area |
| Week 8 | Placebo | IV 600 mg mg/m2 body surface area |
| Week 12 | Placebo | IV 600 mg mg/m2 body surface area |
| Week 16 | Placebo | IV 600 mg mg/m2 body surface area |
| Week 20 | Placebo | IV 600 mg mg/m2 body surface area |
IV intravenously administered
Fig. 1.
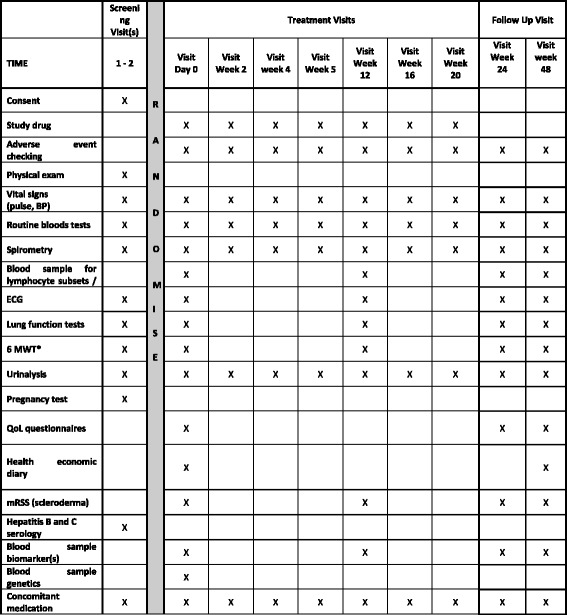
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) figure. BP blood pressure, ECG electrocardiogram, 6MWT 6-min walk test, QoL quality of life, mRSS modified Rodnan Skin Score
Location and setting
RECITAL is sponsored by the Royal Brompton and Harefield NHS Foundation Trust and will recruit subjects from eight to twelve UK centres all with expertise in both ILD and rheumatological disorders.
Study population and eligibility criteria
A total of 116 subjects will be enrolled. Subjects should fulfil the following criteria:
Severe and/or progressive interstitial lung disease (ILD) associated with the underlying CTD
Chest high-resolution computed tomography (HRCT) performed within 12 months of randomisation
Intention of the caring physician to treat the ILD with intravenously administered cyclophosphamide (with treatment indications including: deteriorating symptoms attributable to ILD, deteriorating lung function tests, worsening gas exchange or extent of ILD at first presentation) and where there is a reasonable expectation that immunosuppressive treatment will stabilise or improve CTD-ILD. In individuals with scleroderma it is anticipated that patients will fulfil the criteria for extensive disease defined by Goh et al. [21]
Able to provide written informed consent
Subjects should not enter the study if any of the exclusion criteria listed in Additional file 1 are fulfilled.
Interventions
Cyclophosphamide will be administered by intravenous infusion at a dose of 600 mg/m2 body surface area (BSA). The dose will be repeated every 4 weeks for a total of six doses. If clinically required, individual doses may be delayed by up to 10 days. If longer delay is required the planned dose should be omitted and the next scheduled dose given. Body surface area will be calculated with baseline measurements using the Mosteller method with dose modification for any subjects with a Body Mass Index (BMI) >30 Kg/m2:
Rituximab will be administered by intravenous infusion at a dose of 1000 mg. The dose will be repeated at 14 days. This second dose may be delayed by up to 10 days. If it is not given within this time it should be omitted.
Placebo infusions will be administered in order to maintain the blind and all patients will receive seven infusions in total.
Both cyclophosphamide and rituximab as well as the placebo will have identical appearances (clear, colourless liquid) and will be drawn up in identical volumes so as to avoid unblinding of the study drug.
Non-Investigational Medicinal Product (nIMP) drugs
The following nIMPS will be used in this study and administered to both groups when they receive cyclophosphamide/rituximab/placebo. All nIMPS are open label and generic forms can be used:
Mesna will be administered to patients in both groups at day 0 and monthly until week 20. Mesna 200 mg will be given by intravenous infusion in 100 ml 0.9% sodium chloride over 30 min immediately prior to cyclophosphamide/rituximab/placebo. Additionally, Mesna 400 mg will be administered per os at 2 h and 400 mg at 6 h post cyclophosphamide/rituximab/placebo infusion
Hydrocortisone 100 mg by intravenous injection to be given 30 min prior to cyclophosphamide/rituximab/placebo at day 0 and day 14
Chlorphenamine 10 mg by intravenous injection to be given 30 min prior cyclophosphamide/rituximab/placebo at day 0 and day 14
Paracetamol 1 g to be given per os 30 min prior to cyclophosphamide/rituximab/placebo at day 0 and day 14
Patients will be offered ondansetron an antiemetic as required prior to, and for up to 3 days, following the cyclophosphamide/rituximab/placebo infusions
Concomitant medication
Corticosteroids are frequently used for patients with CTD and it is anticipated that the majority of subjects will be taking concomitant steroid therapy. The choice of dose will rest with the subject-treating clinician but all changes in corticosteroids will be documented in the electronic Case Report Form (eCRF) to permit calculation of cumulative exposure. As general guidance it is anticipated that:
Patients with scleroderma, corticosteroids will be maintained at a stable dose of prednisolone ≤10 mg daily
Individuals with inflammatory myositis will require high-dose orally or intravenously administered corticosteroids at initiation of therapy but that these should be weaned to ≤20 mg prednisolone daily by treatment week 12
Patients with MCTD should be maintained on a stable dose of ≤20 mg prednisolone daily following entry into the study
Immunosuppressants
At week 24, following completion of the treatment phase of the study and after measurement of the primary endpoint, patients will be permitted to commence additional immunosuppressant therapy according to the recommendations of their treating physician
All other disease-specific, nonimmunosuppressant, therapies will be permitted for the duration of the study. Patients may also receive n-acetylcysteine up to 600 mg three times daily (t.d.s.)
Prohibited concomitant medication
Pre-existing immunosuppression (including azathioprine, mycophenolate mofetil, methotrexate and cyclosporine), as is standard practice prior to cyclophosphamide administration, will be stopped at least 14 days prior to randomisation
Between weeks 0–24, patients will not be permitted to receive additional immunosuppression (including orally administered agents, intravenously administered immunoglobulins or other monoclonal antibody therapies) other than corticosteroids
Outcomes
Primary outcome measure
Absolute rate of change in forced vital capacity (FVC) at week 24
Secondary outcome measures
Change from baseline in diffusing capacity for carbon monoxide (DLco) at 24 weeks
Change from baseline in health-related quality of life scores (St. George’s Respiratory Questionnaire (SGRQ), Short form (36) Health Questionnaire (SF-36), King’s Brief Interstitial Lung Disease (K-BILD))
Change from baseline in global disease activity score
Change in 6-min walk test distance over 48 weeks
Change in FVC and DLco at 48 weeks
- Further analyses on FVC
- ○ Absolute categorical change of %FVC at 24 and 48 weeks (decrease by >5%, increase by >5% and change within <5%)
- ○ Absolute categorical change of %FVC at 24 and 48 weeks (decrease by >10%, increase by >10% and change within <10%)
- ○ 48-week rate of change in FVC
Disease-related mortality (adjudicated by steering committee at close of study)
Overall survival
Progression-free survival (composite endpoint of mortality, transplant, treatment failure or decline in FVC >10% compared to baseline)
Treatment failure (as determined by need for transplant or rescue therapy with either open-label cyclophosphamide or rituximab at any point until 48 weeks)
Total corticosteroid requirement over 48 weeks
Change from baseline in capillary oxygen saturation (SpO2) at 24 and 48 weeks
Health care utilisation during study period (visits to primary care, unscheduled hospital visits, emergency admissions)
Scleroderma-specific endpoints (change in Scleroderma Health Assessment Questionnaire (Scleroderma HAQ), modified Rodnan Skin Score (mRSS)).
Safety and tolerability
Exploratory outcome measures
Change in lymphocyte subsets relative to outcome in the rituximab group
Change in plasma cytokine levels following therapy and in relationship to markers of disease activity (FVC, DLco, quality of life, global disease activity scores)
Change in candidate serum biomarkers of fibrosis (to include KL-6, MMP-1, MMP-7, Sp-A and Sp-D) following therapy
Outcome in relation to underlying CTD
Participant timeline
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) schedule of events for the enrolment, interventions and assessments for participants is shown in Figs. 1 and 2. An indexed SPIRIT Checklist can be found in Additional file 2.
Fig. 2.
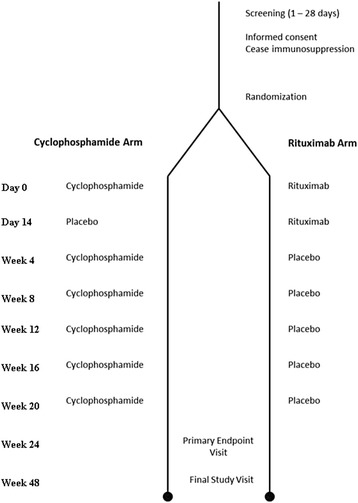
Flowchart of study design
Assignment of interventions
Randomisation allocation will be released using an Interactive Web-based Randomisation System (IWRS: InForm). Patients will be randomised (in a 1:1 double-blind fashion) to receive rituximab or cyclophosphamide. To ensure an equal representation of CTD subtypes in each treatment arm, randomisation will be stratified based upon underlying CTD diagnosis (according to the three diagnostic categories listed in the inclusion criteria of the study protocol). Access to the IWRS at each participating centre will be restricted to authorised study staff.
Sample size estimate
Previous studies of intravenously administered cyclophosphamide in SSc demonstrated a 1% decline in FVC at 12 months, with a coefficient of variation of 7.8% [6, 7]. Our observational data and a previous nonrandomised study of rituximab (used as rescue therapy in those failing treatment with cyclophosphamide) suggest improvements in FVC at 6–12 months of between 9.5 and 20% compared to baseline [19]. Using 1:1 randomisation, a sample size of 52 patients in each group will have a 90% power to detect a 5% difference (approximately 140 ml) between groups at 24 weeks in the change in FVC (as measured in millilitres) with a significance level (alpha) of 0.05 (two-tailed). Anticipating a dropout rate of 10% our target recruitment is, therefore, 58 patients in each arm of the study. On the basis of data derived in other ILD studies, a 5% change in FVC is associated with change in long-term prognosis and can, therefore, be considered a clinically meaningful difference between the two groups [26]. Given the number of individuals treated with cyclophosphamide at our unit and in units that will be participating in the study, this number is feasible to deliver within the planned trial timelines.
Data collection and management
The primary efficacy measurement will be change in FVC. Centres will be asked to undertake clinical trial spirometry on a single, specified spirometer within their clinical physiology department or clinical trials unit. Spirometry will be undertaken by a named individual or individuals who have had training to the standard recommended by the Association for Respiratory Technology and Physiology (ARTP). Usage of spirometers must meet the standards outlined in the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines (P05-12782), including daily calibration of the spirometer, and regular calibration of the calibration pump. Spirometry will be conducted whilst the patient is in a seated position. The test will be done in triplicate and selection of the best result done according to the guidelines. Spirometric results will be filed in the local medical records and will be available for review as required.
For each patient, pulmonary function testing will always start at approximately the same time of the day (with ±60 min maximum difference, time will be recorded). On days of clinic visits (including the screening visit), patients must refrain from strenuous activity for at least 12 h prior to pulmonary function testing.
Smoking will be discouraged throughout the study day (clinic visit) and will not be permitted in the 30-min period prior to spirometry. Patients should also avoid cold temperatures, environmental smoke, dust, or areas with strong odours (e.g. perfumes). Washout of bronchodilator therapies (beta-agonist or anticholinergic drugs) should be undertaken before spirometry: 24 h for long-acting and 8 h for short-acting bronchodilators.
Assessment of safety
The checking for the occurrence of adverse events (AEs) and clinical endpoints will begin from randomisation and will continue for the individual patient until they complete their follow-up at 48 weeks. At each study visit the investigator or designee will make an assessment of safety and will specifically review the clinical history and investigation findings with regard to the occurrence of adverse or serious adverse events (SAEs). Details of adverse and clinical events will be captured on the trial eCRF.
Research blood samples
All biological samples for future research will be collected and handled according to a study-specific procedure, stored anonymously and labelled using a unique study number to permit accurate linkage to clinical data. Samples will be initially processed and stored at study sites in accordance with the study-specific procedure for handling RECITAL biological samples, to facilitate transfer to the Royal Brompton Hospital (RBH) Biological Research Unit (BRU) Royal Brompton Hospital (RBH), Sydney Street, London, SW3 6NP.
The storage of samples and use in future unspecified research will be performed in accordance with the Human Tissue Act 2004, and the RBH policy for ‘the acquisition, storage and use of human biological specimens for research’. Stored samples may be used to assess future biomarkers for the risk of developing lung fibrosis and the prognosis of this condition.
Routine safety blood tests
Routine safety blood tests including full blood count, urea and electrolytes and liver function tests will be undertaken in the clinical laboratories at local sites according to local policies and procedures.
Quality of life assessment
Quality of life will be assessed by self-administered questionnaires. These will be completed at baseline and repeated at the first follow-up visit for primary endpoint at 24 weeks and final follow-up visit at 48 weeks. The instruments used will be the SF-36 and the EuroQol 5 dimensions health survey (EQ5D), the SGRQ, K-BILD and the Scleroderma Health Assessment Questionnaire.
Health economics
The RECITAL study will provide reliable data about the efficacy and safety of rituximab compared to standard therapy. As rituximab is considerably more expensive than cyclophosphamide, the issue of cost-effectiveness and affordability will arise if we show that it is more effective than standard treatments. In a study of this size it may be difficult to perform standard cost-effectiveness analyses (e.g. cost per quality-adjusted life year (QALY)), but we can estimate cost-effectiveness using surrogate clinical outcomes and also reliably estimate costs for care in the two treatment groups including costs of drug, tests and investigations, health care visits (hospital, GP, clinic). We will also have a range of patient-based outcomes using validated questionnaires. Working with the health economic team at the University of East Anglia, the health economic analysis will deliver a high-quality analysis using standard techniques to inform our understanding of the cost-effectiveness of the new treatment.
Discontinuation or withdrawal of study subjects
The study drug will be discontinued if, in the opinion of the local investigator/caring physician, an individual participant’s disease has progressed despite receiving study therapy. The decision regarding progression will rest with the local physician, but indicators of disease progression will include: worsening symptoms, progression of radiological changes and reduction in FVC of >10% or DLco of >15% from baseline or reduction in resting oxygen saturations from baseline. Individuals with progressive disease will be unblinded from the study and, if felt appropriate by their caring physician, may be offered the alternative treatment regimen on an open-label basis (i.e. patients receiving rituximab will be offered cyclophosphamide and those receiving cyclophosphamide will be considered for rituximab). Similarly, in the case of individuals discontinuing treatment because of AEs the option will be open to the local caring physician to initiate open-label treatment with the alternate treatment regimen.
Subjects discontinuing the study drug will be invited to continue with planned monitoring and end-of-study visits. Complete protocol required data will, whenever possible, be collected for all individuals who are randomised into the study whether or not they receive their assigned treatment or discontinue the study prematurely. Subjects discontinuing study treatment will be asked to return for the primary endpoint (week 24) and final (week 48) follow-up visits. Apart from the treatment week-12 visit they will not be required to attend any further treatment phase visits once treatment is discontinued.
Permanent discontinuation of IMP
Permanent discontinuation of study medication should occur in the following circumstances:
Consent withdrawn
Pregnancy
New diagnosis of tuberculosis, infective hepatitis or HIV
Possible temporary discontinuation of IMP
In the following cases withdrawal of study drug is highly recommended:
Episode of severe infection requiring prolonged antibiotic treatment (>14 days) or hospitalisation
New diagnosis of bladder cancer
New occurrence of neurological symptoms suggesting a diagnosis of progressive multifocal leukoencephalopathy (PML)
However, in special circumstances and after review of the clinical data, consultation with the appropriate specialist (urologist, neurologist) and members of the multidisciplinary team, an appropriate risk benefit assessment and consultation with the patient, the investigator may decide not to withdraw the subject. In each case, as this is an intention-to-treat trial, patients will be invited to continue attending study visits to allow for full collection of study data.
Serious adverse event (SAE) reporting and adverse event (AE) reporting
AEs and SAEs will be identified according to standard criteria and will be recorded in the eCRF and reported to the sponsor. Given the nature of participants’ underlying disease and also the known profile of the drugs under investigation, a number of expected AEs and SAEs have been defined. Expected adverse reactions are listed as the known side effects of the IMP reported in the Summary of Product Characteristics (SmPCs) of cyclophosphamide, rituximab and sodium chloride. Events that are expected and related to underlying disease will include study endpoints and disease progression or worsening of pre-existing respiratory or rheumatological symptoms.
Data management and data checking
Data will be collected on an eCRF system. The InForm system will be used to develop the eCRF and will be designed in accordance with the requirements of the clinical trial protocol and will comply with regulatory requirements. Local personnel will be trained on the InForm system. Access will be restricted to site personnel, trial managers, trial monitors and the data management team. Personnel will have individual logon and passwords. It will be the investigator’s responsibility to ensure the accuracy of all data entered and recorded in the eCRFs. Trial monitors will check the accuracy of the eCRF data against source documents.
It is anticipated that the majority of source data (medical progress notes and letters, tests and investigations) will be filed in the individual patients’ medical records. Any deviation from source data being present in the medical notes will be identified and documented. The eCRF and source documents must be available at all times for review by the sponsor’s clinical trial monitor, auditors and for inspection by the Medicines Health Regulatory Agency. The accuracy of eCRF data will be verified by review of the source documents and details will be provided in the Trial Monitoring Report.
Statistical analysis
Before starting the data analysis, the level and pattern of the missing data in the baseline variables and outcomes, and any treatment group crossovers, will be established by forming appropriate tables. The likely causes of any missingness and crossovers will be investigated. This information will be used to determine whether the level and type of missing data and the crossover rate have the potential to introduce bias into the analysis results for the proposed statistical methods, or substantially reduce the precision of estimates related to treatment effects.
Primary efficacy analysis
Analysis of the primary outcome will be by intention-to-treat. The data will be analysed according to the initial randomisation groups with no changes made in respect of subsequent withdrawals or crossovers
The hypothesis to be tested is that rituximab is superior to cyclophosphamide. The study will be considered positive if statistical significance at the level of 0.05 (two-tailed) is achieved
- To test the hypothesis above and estimate the difference in FVC at week 24 and its 95% confidence interval, a three-level hierarchical (mixed/multilevel) model will be used: let FVC iw represent the FVC (in millilitres) for patient i at week w and t (i) represent the treatment given to individual i (rituximab or cyclophosphamide). So, we model FVC iw as the sum of four components:
Intercept term: represents the estimate FVC on week 0 (the start of the treatment, first visit after randomisation). This term will comprise an individual-level random effect which will be drawn from a distribution parameterised using the associated centre-level random effect. Hence, the unexplained variation in the FVC scores will be split in to three components corresponding to the three levels of the model, i.e. the variation attributable to the centre (between-centre variation) and the individual (between-individual variation), as well as the residual variation (within-individual variation). CTD diagnosis stratum (categorical) used for randomisation will be added as a covariate. Other baseline covariates might be added if further analysis reveals a substantial imbalance
Change over time term: this represents a coefficient which captures the changes in FVC over time (measured in weeks) and an interaction term between time and treatment. This interaction term will capture the difference in change of FVC between the two treatment groups per week. The magnitude at 24 weeks and its 95% confidence interval will be calculated to answer the research question.
Linear change is assumed over time with different slopes (the interaction term represents the difference in the slope); however, alternatives will be considered if the rate of change is not constant over the 24-week period. Alternatives are to include quadratic and square root terms. This will be assessed before the unblinding.
Residual error term: it is assumed that the residual errors have a normal distribution.
Secondary efficacy analysis
Analysis of secondary efficacy outcomes will also be by intention-to-treat
Change in continuous physiological variables between baseline and 48 weeks will be assessed by similar multilevel modelling as described for the primary outcome
Categorical change in physiological variables will be measured using chi-squared tests under the null hypothesis of no difference between the treatment groups
Mortality, treatment failure and progression-free survival will be measured using Kaplan-Meier estimates. A log-rank test will be used to compare treatment groups and a Cox proportional hazards model will be used to determine hazard ratios for survival analyses
Interim analyses
No formal interim analysis is planned. A regular review of safety data will be conducted to monitor the safety of patients in the trial. A Data Monitoring Committee (DMC) will follow number of deaths, early discontinuation due to AEs and SAEs in an unblinded fashion. A planned DMC meeting will be held to review all available data after the 12th randomised patient has completed the week-24 visit and twice yearly.
End of study
The primary endpoint for the trial is at 24 weeks, with a final study visit timed at 48 weeks to determine longer-term efficacy and to ensure that all significant AEs are detected. The trial will formally end when the final subject completes their week-48 visit.
Ethics and dissemination
The study conduct will comply with all relevant laws of the EU and all relevant laws and statutes of the UK including, but not limited to, the Human Rights Act 1998, the Data Protection Act 1998, the Medicines Act 1968, the Medicines for Human Use (Clinical Trial) Regulations 2004, and with all relevant guidance relating to medicines and clinical studies from time to time in force including, but not limited to, the ICH GCP, the World Medical Association Declaration of Helsinki entitled ‘Ethical Principles for Medical Research Involving Human Patients’ (2008 Version), the NHS Research Governance Framework for Health and Social Care (Version 2, April 2005).
The study was approved on 18 October 2013 by the UK Medicines and Healthcare Regulatory Agency in compliance with the European Clinical Trials Directive and the Medicines for Human Use (Clinical Trials) Regulations 2004 and its subsequent amendments. The study was provided with ethical approval by the NRES Committee London Westminster (Ref no.: 13/LO/0968) on 20 August 2013. The approved patient information sheet and consent form are available in Additional files 3 and 4.
Data from the study will be published in abstract form at international meetings and will be submitted for publication in a peer-reviewed journal and will be reported to the study funder.
Discussion
ILD is an important cause of morbidity and mortality in patients with CTD. This trial should better define the optimal management of patients with severe CTD-ILD. Current therapy is limited by side effects and the risk of infection associated with significant immunosuppression. Although rituximab costs more on an individual dose basis, it necessitates fewer visits for administration and may be more convenient for patients and less expensive overall.
Additionally, the RECITAL study will evaluate several novel biomarkers for their ability to predict disease behaviour and response to therapy in CTD-ILD. These biomarkers have already been demonstrated to reflect fibrotic activity in various ILDs and may, therefore, be of value in detecting patients at risk of rapid progression of disease who require aggressive immunomodulation.
Trial status
Recruitment to RECITAL began in November 2014. The study is currently actively recruiting in the UK.
Additional files
Protocol V6.1_15.12.14. (DOC 787 kb)
SPIRIT Checklist. (DOC 125 kb)
Patient Information Sheet. Version 6.0 15.10.2014. (DOC 217 kb)
Informed Consent Form. Version 6.0. 15.10.2014. (DOC 39 kb)
Exclusion criteria. (DOCX 90 kb)
Acknowledgements
Members of the DMC: Andrew Wilson, Clive Kelly and Ashley Jones.
Members of the TSC: Tim Harrison, Bridget Griffiths, Kim Fliglestone, Nik Hirani, Rachel Hoyles, Ira Jakupovic, Alexandra Marler, Daphne Babalis, Vicky Tsipouri, Deborah Ashby, Helen Parfrey and Toby Maher.
We are grateful to the late Mrs. Anne Mawdsley for her input into protocol design and to Ed Waddingham and Matyas Szigeti for their input into the statistical analysis plan. Rick Fordham has provided input into the design of the economic evaluation component of the study.
The funder has no role in the trial design, collection, management, analysis or interpretation of data, writing of reports or submission for publication. Additional infrastructure support for the study is being provided by the Royal Brompton NIHR-funded Biomedical Research Unit. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR.
Funding
The RECITAL study is funded in full by the Efficacy and Mechanism Evaluation Programme of the Medical Research Council and National Institute for Health Research (Ref: 11/116/03). Funding approval was obtained following external peer reviews. Dr. Toby Maher is supported by an NIHR Clinician Scientist Fellowship (NIHR Ref: CS-2013-13-017). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the MRC or the NIHR.
Availability of data and materials
Not applicable, as data not yet available.
Authors’ contributions
All authors meet the ICMJE criteria for authorship. All authors were involved in the study conception and design which was mainly performed by TMM and GJK. VT is involved in trial management and oversight whilst DA has overseen development of the statistical analysis plan. PS is involved in study recruitment. All authors provided final approval to submit the manuscript.
Competing interests
The authors declare that they have no competing interests relating to this trial. TMM and AUW have received consultancy and speakers fees from Roche (the manufacturer of rituximab) in relation to idiopathic pulmonary fibrosis.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study conduct will comply with all relevant laws of the EU and all relevant laws and statutes of the UK including, but not limited to, the Human Rights Act 1998, the Data Protection Act 1998, the Medicines Act 1968, the Medicines for Human Use (Clinical Trial) Regulations 2004, and with all relevant guidance relating to medicines and clinical studies from time to time in force including, but not limited to, the ICH GCP, the World Medical Association Declaration of Helsinki entitled ‘Ethical Principles for Medical Research Involving Human Patients’ (2008 Version), the NHS Research Governance Framework for Health and Social Care (Version 2, April 2005).
The study was approved on by the UK Medicines and Healthcare Regulatory Agency (Ref: 21268/0217/001-0001 – EUDRACT 2012-003633-42 – Protocol Number RBHIPF004) on 18 October 2013, in compliance with the European Clinical Trials Directive and the Medicines for Human Use (Clinical Trials) Regulations 2004 and its subsequent amendments. The study was provided with ethical approval by the NRES Committee London Westminster (Ref: 13/LO/0968) on 1 July 2013.
Consent to enter this study will be obtained after a full account has been provided of its nature, purpose, risks, burdens and potential benefits, with verbal and written explanation (Additional files 4 and 5) and after the patient has had the opportunity to consider whether to join the study.
Trial sponsor
Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London, SW3 6NP, UK.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CTD
Connective tissue disease
- DLco
Diffusing capacity of the lung for carbon monoxide
- FVC
Forced vital capacity
- ILD
Interstitial lung disease
- NSIP
Nonspecific interstitial pneumonia
- SF-36
Short form (36) Health Questionnaire
- SGRQ
St. George’s Respiratory Questionnaire
- UIP
Usual interstitial pneumonia
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2016-2) contains supplementary material, which is available to authorized users.
References
- 1.Maher TM. Immunosuppression for connective tissue disease-related pulmonary disease. Semin Respir Crit Care Med. 2014;35:265–73. doi: 10.1055/s-0034-1371531. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, Bancel DF, Allanore Y, Muller-Ladner U, Distler O, Iannone F, Pellerito R, Pileckyte M, Miniati I, Ananieva L, Gurman AB, Damjanov N, Mueller A, Valentini G, Riemekasten G, Tikly M, Hummers L, Henriques MJ, Caramaschi P, Scheja A, Rozman B, Ton E, Kumanovics G, Coleiro B, Feierl E, Szucs G, Von Muhlen CA, Riccieri V, Novak S, Chizzolini C, Kotulska A, Denton C, Coelho PC, Kotter I, Simsek I, de la Pena Lefebvre PG, Hachulla E, Seibold JR, Rednic S, Stork J, Morovic-Vergles J, Walker UA. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 3.Luzina IG, Atamas SP, Wise R, Wigley FM, Xiao HQ, White B. Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am J Respir Cell Mol Biol. 2002;26:549–57. doi: 10.1165/ajrcmb.26.5.4683. [DOI] [PubMed] [Google Scholar]
- 4.Lafyatis R, O’Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56:3167–8. doi: 10.1002/art.22847. [DOI] [PubMed] [Google Scholar]
- 5.Bosello S, De Santis M, Lama G, Spano C, Angelucci C, Tolusso B, Sica G, Ferraccioli G. B cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trial. Arthritis Res Ther. 2010;12:R54. doi: 10.1186/ar2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, Roberts C, Desai S, Herrick AL, McHugh NJ, Foley NM, Pearson SB, Emery P, Veale DJ, Denton CP, Wells AU, Black CM, du Bois RM. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 8.Perosa F, Prete M, Racanelli V, Dammacco F. CD20-depleting therapy in autoimmune diseases: from basic research to the clinic. J Intern Med. 2010;267:260–77. doi: 10.1111/j.1365-2796.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 9.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–20. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 10.Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:393–403. doi: 10.1016/j.rdc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 13.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh D, Walsh M, Westman K, Jayne DR. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 14.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, Fraser GA, Lim W, Kelton JG. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 16.Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series. Rheumatology. 2009;48:968–71. doi: 10.1093/rheumatology/kep157. [DOI] [PubMed] [Google Scholar]
- 17.Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Korfiatis P, Yiannopoulos G, Andonopoulos AP. Is there a role for B-cell depletion as therapy for scleroderma? A case report and review of the literature. Semin Arthritis Rheum. 2010;40:127–36. doi: 10.1016/j.semarthrit.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, Karampetsou M, Yiannopoulos G, Andonopoulos AP. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49:271–80. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Renzoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Respir J. 2012;40:641–8. [DOI] [PubMed]
- 20.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–9. [DOI] [PubMed]
- 21.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, Corte TJ, Sander CR, Ratoff J, Devaraj A, Bozovic G, Denton CP, Black CM, du Bois RM, Wells AU. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 22.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 23.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 24.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 25.Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol. 1989;16:328–34. [PubMed] [Google Scholar]
- 26.Zappala CJ, Latsi PI, Nicholson AG, Colby TV, Cramer D, Renzoni EA, Hansell DM, du Bois RM, Wells AU. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:830–6. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol V6.1_15.12.14. (DOC 787 kb)
SPIRIT Checklist. (DOC 125 kb)
Patient Information Sheet. Version 6.0 15.10.2014. (DOC 217 kb)
Informed Consent Form. Version 6.0. 15.10.2014. (DOC 39 kb)
Exclusion criteria. (DOCX 90 kb)
Data Availability Statement
Not applicable, as data not yet available.


