Abstract
Objective
To evaluate the diagnostic value of combination detection of serum cancer antigen 125 (CA125), carbohydrate antigen 19-9 (CA199) and carci noembryonic antigen(CEA) in patients with epithelial ovarian cancer by pooling the open published studies according to meta-analysis method.
Methods
Diagnostic studies related to combination detection of serum CA125, CA199 and CEA in patients with epithelial ovarian cancer were electronic searched in the databases of PubMed, Cochrane, Google scholar, EMBASE, ISI Web of Knowledge and CNKI by two independent reviewers. The combined diagnostic sensitivity, specificity, positive likely hood ratio (+LR), negative likely hood ratio (-LR), diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC) were pooled by Med DiSc1.4 software.
Results
Twelve prospective diagnostic publications were finally fulfilled the inclusion criteria and were included in this meta-analysis. The pooled diagnostic sensitivity specificity, positive likely hood ratio, negative likely hood ratio, diagnostic odds ratio, and AUC were 0.90 (95%CI: 0.80 to 0.92), 0.83 (95%CI: 0.80 to 0.86), 5.35(95%CI:3.90 to 7.33), 0.13 (95%CI: 0.10 to 0.16), 48.53 (95%CI: 29.91 to 78.72) and 0.92 (95%C: 0.89 to 0.94) respectively by fixed or random effect model. No publication bias was found according to the funnel plot and line regression test (t=-1.34, P=0.21).
Conclusion
Combination detection serum CA125, CA199 and CEA was a promising biomarker forepithelial ovarian cancer diagnosis with relative high sensitivity and specificity.
Keywords: Epithelial ovarian cancer; Diagnosis; CA125,CA199; CEA; Meta-analysis
1. Introduction
In the past two decades, several serum biomarkers for epithelial ovarian cancer diagnosis have been studied, such as Ca125, Ca199, CEA, HE4 and et al [1]. Ca125 is also known as mucin 16 or MUC16, a protein that in humans is encoded by the MUC16 gene. It was found serum Ca125 had been elevated in patients with specific types of cancers, which could be a potential biomarker [2]. Several studies have discussed the serum Ca125 level in ovarian cancer patients and found about 80% of advanced patients had elevated levels of Ca125 in their blood [3,4]. However, studies also found that serum Ca125 can also elevated in individuals without ovarian cancer which made the diagnostic specificity relative low. Previously studies [5,6] indicated Ca199 can be elevated in gastrointestinal cancer, such as colorectal cancer, esophageal cancer and pancreatic cancer. Published study [7] showed elevated Ca199 also can be detected in ovarian cancer patients in their bloods. CEA was one of the most used serum biomarker for solid malignant carcinoma diagnosis such as lung cancer, esophageal cancer colorectal cancer and epithelial ovarian cancer. However, the diagnostic sensitivity or specificity was not high enough of a single serum biomarker of Ca125, Ca199 or CEA for diagnosis of epithelial ovarian cancer. Then, combination detection several serum biomarkers may increase the diagnostic value [8-10]. Several diagnostic studies had discussed the combination detection serum CA125, CA199 and CEA in epithelial ovarian cancer patients with relative high sensitivity and specificity [11-13]. However, because of the small sample size of each individual study, the conclusion was not consistent. In this meta-analysis, we searched the related databases and included open published studies related to combination detection serum CA125, CA199 and CEA in diagnostic epithelial ovarian cancer in order to provide more evidence for its clinical use.
2. Material and methods
2.1. Publication searching
Diagnostic studies related to combination detection serum CA125, CA199 and CEA in patients with epithelial ovarian cancer were electronic searched in the databases of PubMed, Cochrane, Google scholar, EMBASE, ISI Web of Knowledge and CNKI by two independent reviewers (Guo Junhong&Yu Jiangtao). The searching words were “ epithelial ovarian cancer”, “malignant ovary tumor”, “ca 125 antigen”, “ca 125”, “cancer antigen 125”, “CA199”, “carbohydrate antigen 19-9”, “CEA”, “carcino-embryonic antigen”. All potential relevant studies were assessed in detail and additional and all citations of the included articles were further evaluated in order to identify additional suitable studies.
2.2. Data extraction
The data of each included study was extracted by two reviewers Guo JH & Mi HX independently. The general character such as year of publication, first and corresponding author and control type were extracted from each of the included studies. The case number of the true positive (tp), false positive (fp), false negative (fn) and true negative(tn) of the each study were also recorded and cross checked by two reviewers.
2.3. Statistical analysis
Med DiSc1.4 (http://www.biomedsearch.com/nih/Meta-DiSc-software-meta-analysis.) and Stata11.0 (http://www.stata.com; Stata Corporation, College Station, TX) statistical software were used to do all the statistical analysis. Statistical heterogeneity across the included studies was assessed by chi-square/Cochran-Q test and demonstrated by I2 for the effect size of sensitivity, specificity, positive likely hood ratio, negative likely hood ratio and diagnostic odds ratio. Without statistical heterogeneity, the effect size was pooled by fixed effect model, otherwise it was pooled by random effect model. P<0.05 was considered as statistical significant.
3. Results
3.1. Publication searching results
After searching the related databases, 712 papers were initially identified. And 661 articles were excluded after reading the title and abstract. 39 publications were excluded after reading the whole text paper. Finally 12 prospective diagnostic publications were fulfilled the inclusion criteria and were included in this meta-analysis [11-22]. Six studies used benign ovarian tumor as the control group, 3 publications used healthy subjects as the control group and left 3 articles used mixed subjects as the controls. The general characteristics of included 12 papers were demonstrated in Table 1.
Table 1.
General characteristics of included studies
| First author | Year | TP | FP | FN | TN | Sen | Sep | Control type |
|---|---|---|---|---|---|---|---|---|
| Luo XH | 2006 | 26 | 16 | 3 | 36 | 0.90 | 0.69 | Benign ovarian tumor |
| Shao JL | 2007 | 36 | 14 | 7 | 24 | 0.84 | 0.63 | Benign ovarian tumor |
| Song XL | 2007 | 40 | 10 | 2 | 80 | 0.95 | 0.89 | Mixed |
| Pu ZY | 2010 | 50 | 4 | 8 | 46 | 0.86 | 0.92 | Healthy subjects |
| Li L | 2010 | 69 | 3 | 6 | 47 | 0.92 | 0.94 | Healthy subjects |
| Huang F | 2010 | 37 | 13 | 3 | 82 | 0.93 | 0.86 | Mixed |
| Qian M | 2010 | 42 | 4 | 6 | 36 | 0.88 | 0.90 | Benign ovarian tumor |
| Liu L | 2011 | 77 | 7 | 8 | 54 | 0.91 | 0.89 | Benign ovarian tumor |
| Zhang FL | 2011 | 28 | 21 | 2 | 52 | 0.93 | 0.71 | Mixed |
| Yu B | 2011 | 37 | 8 | 4 | 33 | 0.90 | 0.80 | Healthy subjects |
| Jiang J | 2014 | 71 | 16 | 9 | 64 | 0.89 | 0.80 | Benign ovarian tumor |
| Zhang T | 2016 | 59 | 24 | 5 | 135 | 0.92 | 0.85 | Benign ovarian tumor |
3.2. Statistical heterogeneity
Statistical heterogeneity across the included studies was assessed by chi-square/Cochran-Q test and demonstrated by I2 for the effect size of sensitivity, specificity, positive likely hood ratio, negative likely hood ratio and diagnostic odds ratio. Significant heterogeneity was found in the effect size of specificity, positive likely hood ratio and diagnostic odds ratio(P 003<0.05). These effect size were pooled by random effect model, Table 2.
Table 2.
Statisticalheterogeneityevaluationby chi-square/ Cochran-Q test
| Effect size | Chi-square/Cochran-Q | I2 (%) | P |
|---|---|---|---|
| Sensitivity | 5.93 | 0.0 | 0.88 |
| Specificity | 36.92 | 70.2 | 0.0001 |
| Positive likely hood ratio | 43.52 | 74.7 | 0.0000 |
| Negative likely hood ratio | 8.84 | 0.0 | 0.63 |
| Diagnostic odds ratio | 20.62 | 46.6 | 0.03 |
3.3. Pooled sensitivity
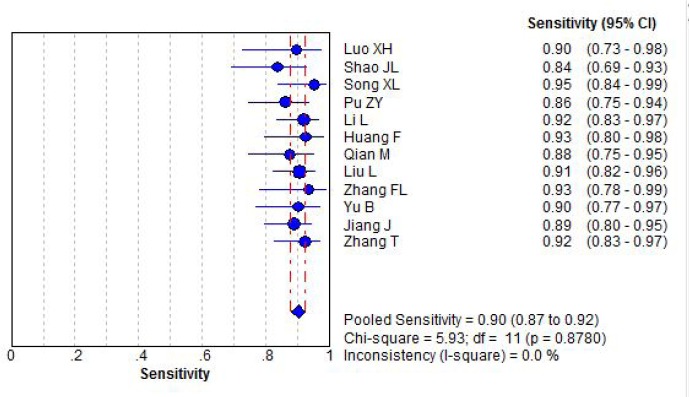
Without significant statistical heterogeneity, diagnostic sensitivity was pooled by fixed effect model. The pooled diagnostic sensitivity of combination detection serum CA125, CA199 and CEA for epithelial ovarian cancer was 0.90 (95%CI: 0.80 to 0.92), Figure 1.
Figure 1.
Forest plot for diagnostic sensitivity of ovarian cancer by combination detection serum CA125 CA199 and CEA.
3.4. Pooled specificity
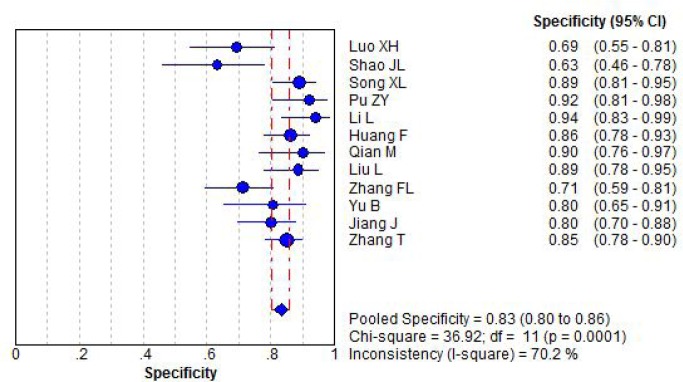
With significant statistical heterogeneity, diagnostic specificity was pooled by random effect model. The pooled diagnostic specificity of combination detection serum CA125, CA199 and CEA for epithelial ovarian cancer was 0.83 (95%CI: 0.80 to 0.86), Figure 2.
Figure 2.
Forest plot for diagnostic specificity of ovarian cancer by combination detection serum CA125 CA199 and CEA.
3.5. Pooled positive likely hood ratio
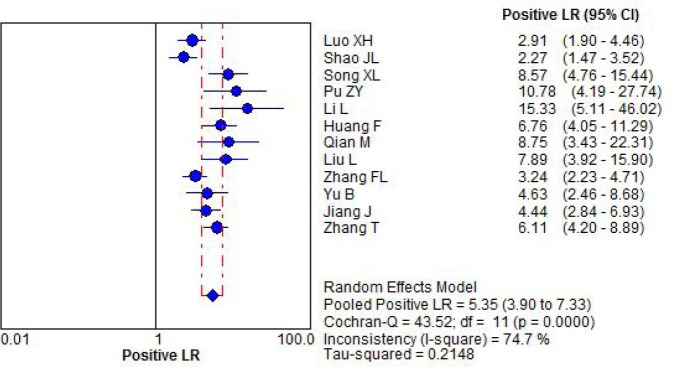
Significant heterogeneity was found in the effect size of positive likely hood ratio. The pooled positive likely hood ratio was 5.35(95%CI:3.90 to 7.33) by random effect model, Figure 3.
Figure 3.
Forest plot for positive likely hood ratio by combination detection serum CA125 CA199 and CEA.
3.6. Pooled negative likely hood ratio
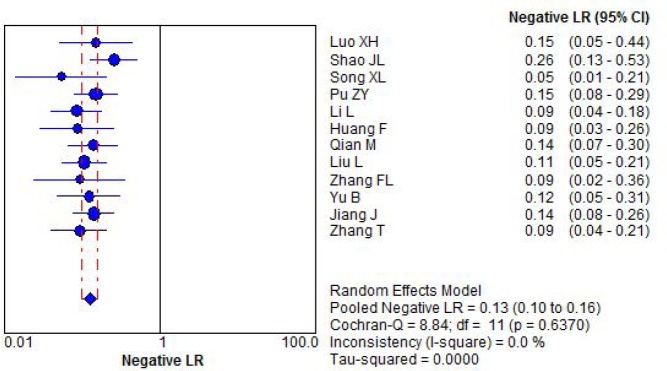
The negative likely hood ratio was pooled by fixed effect model without statistical heterogeneity. It was 0.13 with its 95%CI of 0.10 to 0.16, Figure 4.
Figure 4.
Forest plot for negative likely hood ratio by combination detection serum CA125 CA199 and CEA.
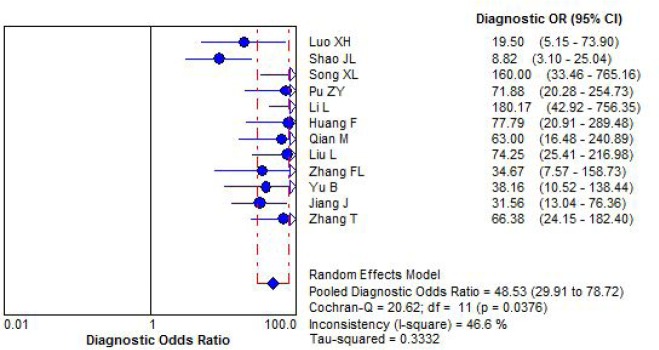
3.7. Pooleddiagnostic odds ratio
The diagnostic odds ratio was pooled by random effect model for significant statistical heterogeneity. The pooled diagnostic odds ratio was 48.53 (95%CI: 29.91 to 78.72), Figure 5.
Figure 5.
Forest plot for diagnostic odds ratio by combination detection serum CA125 CA199 and CEA.
3.8. Pooled SCOR
The pooled area under the receiver operating characteristic curve (ROC) was 0.92 (95%C: 0.89 to 0.94), Figure 6.
Figure 6.
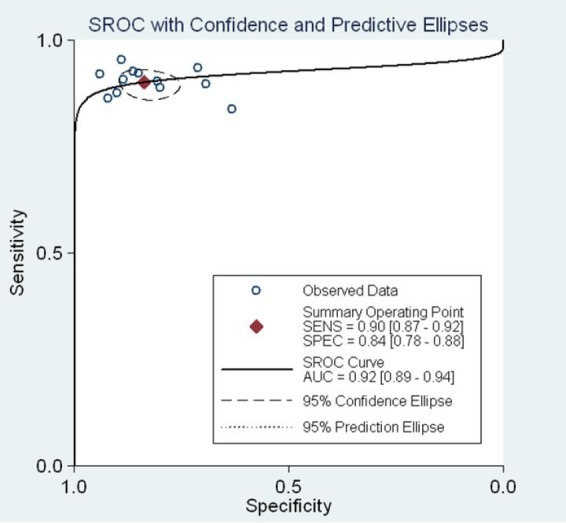
The pooled receiver operating characteristic curve of combination detection serum CA125 CA199 and CEA.
3.9. Subgroup analysis
We further performed subgroup analysis for diagnostic sensitivity, specificity, positive likely hood ratio, negative likely hood ratio, diagnostic odds ratio, and AUC according the control type. The subgroup analysis results were showed in Table 3.
Table 3.
Subgroup analysis according to control type
| Effect size | Benign ovarian tumor | Healthy subjects |
|---|---|---|
| Sen | 0.89 (0.85 to 0.92) | 0.90 (0.84 to 0.94) |
| Sep | 0.81 (0.77 to 0.85) | 0.89 (0.83 to 0.94) |
| +LR | 4.51 (2.94 to 6.90) | 8.37 (3.73 to 18.77) |
| -LR | 0.14 (0.10 to 0.19) | 0.12 (0.08 to 0.18) |
| DOR | 34.37 (17.37 to 67.98) | 75.45 (32.10 to 177.34) |
| AUC | 0.93 (0.89to 0.95) | 0.91 (0.87 to 0.96) |
3.10. Publication bias
No publication bias was found according to the funnel plot (Figure 7) and line regression test (t=-1.34, P=0.21), Figure 7.
Figure 7.
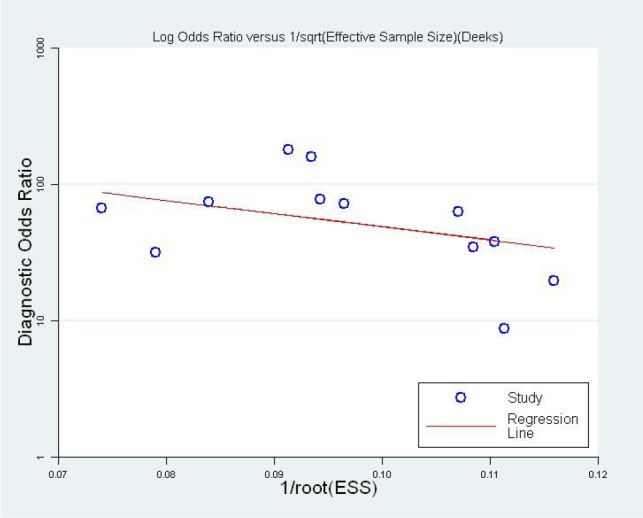
Funnel plot for evaluation publication bias.
4. Discussion
Epithelial ovarian cancer is one of the most diagnosed malignant carcinoma in females [23]. It has been reported that epithelial ovarian cancer was the 2nd most common malignant gynecological carcinoma with an increasing incidence and prevalence [24,25]. Progress has been made for epithelial ovarian cancer early diagnosis; however, most patients were diagnosed in advanced stage with relative poor prognosis (mean 5-year survival rate of 37.6%) [26,27]. The poor 5-year survival rate for epithelial ovarian cancer patients is the result of aggressive biological behavior and a lack of early detection method. It was reported about 70% of epithelial ovarian cancer patients were diagnosed at advanced stage [25]. So, high sensitivity, specificity and accurate methods are need for detection of epithelial ovarian cancer especially for early stage or high-risk subjects. Serum cancer antigen 125 (CA125) are mostly used tumor serum biomarker for several cancers screening including epithelial ovarian cancer, endometrial cancer, cervical cancer, pancreatic cancer, colon cancer, breast cancer and et al. However, the diagnostic sensitivity were not satisfactory. USPSTF stated that detection serum CA125 had almost no effect on reducing ovarian mortality, while instead increasing the risk of harm including diagnostic procedure and decline in quality of life [26]. CA199 is a glucolipid on the cell membrane and a kind of mucin tumor markers, its molecular weight is more than 1 000 Kd. CA199 was another biomarker used for detection of epithelial ovarian cancer. However, it had limited clinical use for epithelial ovarian cancer screening with not satisfactory diagnostic accuracy. CEA is one of the most used tumor markers in clinical application. It has important diagnostic value for gynecology malignant tumor, breast cancer [28], lung cancer [29] and other digestive system malignant tumors. However, single detection serum CA125, CA199 and CEA had little value for epithelial ovarian cancer detection with low sensitivity or specificity. Several published studies has demonstrated that combination detection serum CA125, CA199 and CEA can provide satisfactory diagnostic value for epithelial ovarian cancer [15,16]. However, because of the small sample size of each individual study, the conclusion was not consistent. So, in our present meta-analysis we included 12 prospective diagnostic trials evaluating the diagnostic value of combination detection CA125, CA199 and CEA for epithelial ovarian cancer diagnosis. The results showed pooled diagnostic sensitivity specificity, positive likely hood ratio, negative likely hood ratio, diagnostic odds ratio, and AUC were 0.90 (95%CI: 0.80 to 0.92), 0.83 (95%CI: 0.80 to 0.86), 5.35(95%CI:3.90 to 7.33), 0.13 (95%CI: 0.10 to 0.16), 48.53 (95%CI: 29.91 to 78.72) and 0.92 (95%C: 0.89 to 0.94) respectively. These results indicated that combination detection serum CA125, CA199 and CEA was promising biomarker for epithelial ovarian cancer with relative high sensitivity and specificity.
However, there were several limitations for this meta-analysis. Firstly, only studies published in Chinese or English were searched in the databases and included in this study. This may result in publication searching bias. Secondly, significant statistical heterogeneity was existed in this meta-analysis, which may decrease the statistical power and weaken the conclusion. Thirdly, the original studies included in this manuscript did not use the same cut-off value to determine the serum protein of CA125,CA199 and CEA negative or positive. This is an important clinical heterogeneity across the original studies. Fourthly, for the included12 references, the patients were of the same source population, which may limited, its clinical use.
Footnotes
Conflict of interests: No authors report any conflict of interest.
References
- [1].Komai K, Nishida T. Tumor marker in ovarian cancer. Gan To Kagaku Ryoho. 2002;29:481–486. [PubMed] [Google Scholar]
- [2].Bast RC, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. Bast RC, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. CA 125: the past and the uture. Int J Biol Markers. 1998;13:179–187. doi: 10.1177/172460089801300402. [DOI] [PubMed] [Google Scholar]
- [3].Bocheva Y, Bochev P, Ivanov S. Ca-125 in diagnosis and monitoring of patients with ovarian cancer. Akush Ginekol (Sofiia) 2015;54(1):11–17. [PubMed] [Google Scholar]
- [4].Alexandre J, Brown C, Coeffic D. CA-125 can be part of the tumour evaluation criteria in ovarian cancer trials: experience of the GCIG CALYPSO trial. Br J Cancer. 2012;106(4):633–637. doi: 10.1038/bjc.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shiozawa S, Tsuchiya A, Kim DH, Ogawa K. Prognostic significance of CA19-9 levels in patients with pancreatic cancer. Nihon Rinsho. 2006;64(Suppl 1):297–300. [PubMed] [Google Scholar]
- [6].Liang Y, Wang W, Fang C. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016;7(31):49565–49573. doi: 10.18632/oncotarget.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Canney PA, Wilkinson PM, James RD, Moore M. CA19-9 as a marker for ovarian cancer: alone and in comparison with CA125. Br J Cancer. 1985;52(1):131–133. doi: 10.1038/bjc.1985.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Gao J, Yao H, Wu Z, Wang M, Qi J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol. 2014;35:6127–6138. doi: 10.1007/s13277-014-1811-6. [DOI] [PubMed] [Google Scholar]
- [9].Dayyani F, Uhlig S, Colson B, Simon K, Rolny V, Morgenstern D, Schlumbrecht M. Diagnostic Performance of Risk of Ovarian Malignancy Algorithm Against CA125 and HE4 in Connection With Ovarian Cancer: A Meta-analysis. Int J Gynecol Cancer. 2016;26:1586–1593. doi: 10.1097/IGC.0000000000000804. [DOI] [PubMed] [Google Scholar]
- [10].Lan Z, Fu D, Yu X, Xi M. Diagnostic values of osteopontin combined with CA125 for ovarian cancer: a meta-analysis. Fam Cancer. 2016;15:221–230. doi: 10.1007/s10689-015-9847-3. [DOI] [PubMed] [Google Scholar]
- [11].Yu B. Vol. 34. Jurnal of Binzhou Medical University; 2001. The dinieal value of the combined detection of CEA、CCAl25 and CAl9-9 in ovarian atlrdliOm diagnasis; pp. 294–295. [Google Scholar]
- [12].Jiang J, Deng XY, Hu XH. Clinical significance of the combined detection of CA125, CA199 and CE in diagnosis of ovarian cancer. Chinese Journal of Laboratory Diagnosis. 2014;18:1145–1148. [Google Scholar]
- [13].Zhang T, Cheng XJ, Zhang B. Clinical value of combined detection serum CA125 and CEA for ovarian cancer diagnosis. Guizhou Medical Journal. 2016;40:973–975. [Google Scholar]
- [14].Luo XH, Ch D, Pan KY, Wang Y, Zeng HY. The value of combined serum CA125, CA199 and CEA test for ovarian cancer diagnosis. Journal of Clinical and Experimental Medicine. 2006;5:878–879. [Google Scholar]
- [15].Shao LJ, Xu RL, Hu T. Combination detection serum CA125,CA199 and CEA for ovarian cancer diagnosis. Journal of Radioimmunology . 2007;20:92–93. [Google Scholar]
- [16].Song XL, Wang GS, Luo JM, Zhao ZL. Diagnostic value for combined detection serum CA125, CA199 and CEA in patients with ovarian cancer. Vol. 26. Journal of Henan University(Medical Science); 2007. pp. 70–71. [Google Scholar]
- [17].Huang F. Serum CA125,CA199 and CEA combined detection for ovarian cancer diagnosis. Guangxi Medical Journal. 2010;32:424–425. [Google Scholar]
- [18].Li L, Liao JX. The Clinical Diagnostic Value of joint inspection of Serum CEA、ACA19-9、ACA125 in the Patients with Ovarian Cancer. Journal of Modern Clinical Medicine. 2010;36:122–124. [Google Scholar]
- [19].Pu ZY, Liu FJ, Liu YY. Clinical evaluation serum CA125, CEA and CA19-9 combined detection for ovarian cancer diagnosis. West China Medical Journal. 2010:1879–1880. [Google Scholar]
- [20].Qian M, Yuan JJ, Wang XH, Shu XC. Diagnosis and Prognosis Value of the Serum CA125、ACA199、ACEA Detection in Patients with Epithelinl Ovarian Cancer. China Practical Medical. 2010;05:53–54. [Google Scholar]
- [21].Liu L, Zhang MK, Chen JL, Huang RQ. The significance of serum CA125,199 and CEA test in the diagnosis of ovarian cancer. Chongqing Medicine. 2011;40:2423–2424. 2426. [Google Scholar]
- [22].Zhang FL. Ovarian cancer diagnosis by combination detection serum CEA, CA125and CA199. Chinese Journal of Misdiagnostics. 2011;11:4646–4647. [Google Scholar]
- [23].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- [24].Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- [25].Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2016 doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- [26].Pavlik EJ. Ovarian cancer screening effectiveness: A realization from the UK Collaborative Trial of Ovarian Cancer Screening. Womens Health (Lond) 2016;12:475–479. doi: 10.1177/1745505716666096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Voelker R.. Ovarian Cancer Screening Tests Don’t Pass Muster. JAMA. 2016:316–1538. doi: 10.1001/jama.2016.14614. [DOI] [PubMed] [Google Scholar]
- [28].Tang S, Zhou F, Sun Y, Wei L, Zhu S, Yang R, Huang Y, Yang J. CEA in breast ductal secretions as a promising biomarker for the diagnosis of breast cancer: a systematic review and meta-analysis. Breast Cancer. 2016;23:813–819. doi: 10.1007/s12282-016-0680-9. [DOI] [PubMed] [Google Scholar]
- [29].Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013;80:45–49. doi: 10.1016/j.lungcan.2013.01.002. [DOI] [PubMed] [Google Scholar]