Abstract
Five commercial broiler chicken flocks were treated with either difloxacin or enrofloxacin for a clinically relevant infection, as instructed by a veterinarian. Campylobacters were isolated from individual fecal samples and from samples associated with the broiler environment before, during, and after treatment. Ciprofloxacin-resistant Campylobacter jejuni and/or C. coli strains were detected pretreatment in four flocks, but they constituted a very small proportion of the campylobacters present. When the broilers were treated with a fluoroquinolone, a rapid increase in the proportion of ciprofloxacin-resistant campylobacters was observed. During treatment nearly 100% of campylobacters were resistant, and in some flocks a high proportion of resistant strains persisted for up to 4 weeks after treatment. Prior to treatment a variety of campylobacter subtypes were present. During and after treatment considerable changes in both species and subtype prevalence were observed, but no single fluoroquinolone-resistant clone became dominant. Instead, resistant C. coli strains or a mixture of resistant C. coli and C. jejuni strains became dominant, whereas susceptible C. jejuni strains had usually been dominant prior to treatment. The resistant subtypes which emerged and became dominant were not always the same as those detected pretreatment. The persistence of resistant strains for up to 4 weeks posttreatment has important implications for any strategy designed to avoid the introduction of such strains into the food chain.
In recent years, the incidence of human campylobacteriosis has risen steadily throughout the world (43). In England and Wales, between 50,000 and 60,000 infections have been reported to the Health Protection Agency Communicable Disease Surveillance Centre each year since 1997 (19). The number of cases is estimated to be nearer half a million, due to mild infections that do not reach the reporting system (41). The majority of human cases of Campylobacter infection in the United Kingdom are acquired from food (1), and a high proportion of these are believed to originate from the farm-animal reservoir. It has been estimated that 50% of poultry flocks in the United Kingdom are colonized with Campylobacter (13), and widespread contamination of carcasses occurs at slaughter (4, 32). The handling of raw poultry (35) and the consumption of undercooked poultry (30) have been identified as risk factors for campylobacteriosis.
Antimicrobial therapy may be necessary for the treatment of severe campylobacteriosis, and erythromycin is the first choice of the clinician (18). Ciprofloxacin is widely used for the empirical treatment of gastroenteritis and is also recommended for the treatment of infections caused by macrolide-resistant campylobacters. However, the rapid emergence of fluoroquinolone resistance is a cause for global concern (10, 20, 29).
In 1991, Endtz et al. (9) proposed that ciprofloxacin-resistant campylobacters emerged in poultry after the flock was treated with a fluoroquinolone and that these bacteria entered the food chain and caused infection in humans. There has since been considerable discussion as to the validity of this hypothesis (11, 42).
Two reports have shown an association between the consumption of poultry and the acquisition of a ciprofloxacin-resistant campylobacter (16, 36). Smith et al. (36) showed that the strain types were similar in domestically acquired human infections and local poultry flocks in Minnesota. Recent studies in the United Kingdom have shown that the distributions of Campylobacter jejuni and C. coli subtypes are similar in retail chickens and humans (12, 25). In a recent extensive survey of retail chickens in the United Kingdom (12), 13% of C. jejuni isolates and 15% of C. coli isolates were resistant to ciprofloxacin. In 2000, 18% of C. jejuni isolates and 26% of C. coli isolates from human infections in England and Wales were resistant to this antibiotic (8). The incidence of resistance among C. jejuni isolates causing infections acquired in the United Kingdom, however, is lower (10%) than that among isolates causing infections associated with foreign travel (53%), and interestingly, travel-associated cases of ciprofloxacin-resistant infections were linked to the consumption of chicken (7). In contrast, in Australia, where fluoroquinolones have never been licensed for use in food animals, only 12 of 370 campylobacters isolated from human infections were fluoroquinolone resistant, and 10 of these were from patients who had recently traveled outside Australia (38).
Treatment with enrofloxacin or sarafloxacin of chickens experimentally infected with susceptible C. jejuni strains rapidly selects for ciprofloxacin-resistant strains (23, 27, 40). This has provided further evidence to support the hypothesis that fluoroquinolone-resistant campylobacters emerge after exposure of commercial poultry flocks to fluoroquinolones.
Commercially reared broiler flocks can be colonized by a wide variety of C. jejuni strains and also by campylobacters other than C. jejuni (33, 39), and it is not known whether these campylobacters respond to fluoroquinolones in the same manner in which they respond in experimental studies. In this study campylobacters were isolated from commercial broiler flocks and their environment before, during, and after treatment with a fluoroquinolone that was administered to treat a clinically relevant infection. The objectives were to determine whether fluoroquinolone-resistant campylobacters were present in the flocks prior to fluoroquinolone exposure, whether fluoroquinolones select for resistant campylobacters in broilers reared in a commercial environment, and whether these bacteria spread throughout the flock due to the selection of one or several resistant clones.
This report presents data on the incidence of fluoroquinolone resistance and the subtypes of campylobacters in relation to the treatment of commercial broiler flocks for an infection with a veterinary fluoroquinolone. This is the first report to show unequivocally that ciprofloxacin-resistant campylobacters emerge in poultry that enter the food chain.
MATERIALS AND METHODS
Chicken flocks.
An alert system was put in place for broiler chicken flocks reared in the United Kingdom, whereby the Food Microbiology Collaborating Unit was informed when a flock was about to be treated with a fluoroquinolone for a clinically relevant infection. The laboratory was alerted that a flock would need treatment with a fluoroquinolone on six occasions when flocks were treated, as instructed by the veterinarian, with difloxacin or enrofloxacin (10 mg per kg of body weight per bird for 5 days administered in the drinking water, as recommended by the manufacturer). Birds in flock 1 (ca. 500 barn-reared birds), flock 3 (a free-range flock of ca. 300 birds), flock 4 (a large broiler flock of ca. 20,000 birds), and flock 5 (ca. 1,250 free-range birds) were all treated with difloxacin (Dicural; Fort Dodge Animal Health); and birds in flock 6 (ca. 5,000 free-range birds) received enrofloxacin (Baytril; Bayer). Flock 2 remained campylobacter free throughout the sampling period.
Sampling.
Samples were collected from the flocks before treatment (1 to 5 days prior to the start of treatment), during treatment (2 to 5 days after the start of treatment), and after treatment (weekly for up to 4 weeks posttreatment) until the flock was slaughtered. Fecal samples were collected and placed into sterile plastic containers. The lip and the lid of the container were used to collect one freshly voided, individual fecal specimen from the broiler house floor. Up to 14 fecal samples were collected on each farm visit. Environmental samples were also collected. Samples of drinking and puddle water were collected in sterile plastic containers, while pooled samples of feed and litter were collected from the broiler house in sterile plastic bags. Surfaces (i.e., the broiler house walls and floor) were sampled as follows. Sterile cotton wool pads (10 g) were placed in a stomacher 400 bag (180 by 300 mm) and moistened by pouring 20 ml of maximum recovery diluent (Oxoid, Basingstoke, United Kingdom) into the bag. The bag was inverted, and the swabs were rubbed over about 0.1 m2 of the surface to be sampled. The swab was returned to the bag and left at the site for a few minutes. The bag was then inverted again and the swabs were rubbed over the same surface. All samples were transported to the laboratory within 3 h of collection.
Detection of campylobacters in fecal samples.
Individual fecal samples from flocks 1 and 2 were examined for the presence of campylobacters by inoculating duplicate charcoal cefoperazone deoxycholate (CCD) agar (Oxoid) plates with a cotton-tipped swab loaded with feces. Campylobacters were detected in samples from flocks 3 to 6 and were enumerated by plating 500 μl of sample, diluted in maximum recovery diluent, onto duplicate CCD agar plates. These CCD agar plates were referred to as the master CCD (mCCD) agar plates. All plates were incubated at 37°C for 48 h in a microaerobic atmosphere (5 to 6% O2, 3 to 7% CO2, and 7% H2 in a balance of nitrogen) (6).
Presumptive campylobacter colonies (identified by colony morphology) were counted, and up to six colonies were subcultured and subsequently confirmed to be Campylobacter spp. (see below). Counting of the colonies on the duplicate plates for each sample provided the number of presumptive Campylobacter spp. per milliliter of the dilution plated. This was multiplied by the dilution factor to calculate the number of presumptive Campylobacter spp. per gram of chicken feces.
Enumeration of ciprofloxacin-resistant campylobacters.
The proportion of ciprofloxacin-resistant campylobacters was initially determined by replica plating (with sterile furniture-grade velvet) the colonies from mCCD agar plates with the fecal samples collected from flock 3 onto Iso-Sensitest agar (Oxoid) containing 1 μg of ciprofloxacin per ml. However, this medium allowed the breakthrough of contaminating growth, which probably originated from the mCCD agar plates (data not shown). Amphotericin (10 μg/ml) and cefoperazone (32 μg/ml) were added to the Iso-Sensitest agar containing ciprofloxacin (hereafter referred to as CIP-agar) at the same concentrations used in the mCCD agar to reduce contaminating growth. This sufficiently improved the selectivity of the agar so that campylobacters could be counted with confidence. For samples from flock 4, there was no difference in the number of colonies recovered from the CIP-agar compared with the number recovered from Mueller-Hinton agar (containing the same concentrations of ciprofloxacin, amphotericin B, and cefoperazone [data not shown]). As a quality control, known ciprofloxacin-resistant and -sensitive C. jejuni strains were also subcultured onto CIP-agar and incubated together with each batch. Replica plates were then incubated at 41.5°C in a microaerobic atmosphere for 24 h, and the colonies were examined together with those on the mCCD agar plate from which they were produced. The number of colonies able to grow on CIP-agar was recorded and was used to calculate the proportion of ciprofloxacin-resistant strains in the fecal samples collected from flocks 4, 5, and 6.
Isolation of Campylobacter spp. from environmental samples.
Campylobacter spp. were isolated from environmental samples by enrichment culture with modified Exeter broth (MEB) (24). MEB, on which a method endorsed by the United Kingdom Health Protection Agency (HPA) is based, has been shown to result in the improved isolation of Campylobacter spp. from chicken samples in comparison with that obtained by the International Standards Organization-recommended Park and Sanders enrichment culture method (22). The HPA method also uses a simple temperature regimen, and appropriate growth conditions are ensured by using screw-cap containers and a small headspace: 225 ml of MEB was added to 25-ml aliquots of drinking and puddle water samples, swab samples in a sterile plastic container, or 25-g samples of feed or litter. Culture for enrichment was performed at 37°C for 48 h. After enrichment, 10 μl of the enrichment broth was streaked onto CCD agar and the plate was incubated as described above.
Identification of presumptive colonies as Campylobacter spp.
Presumptive campylobacter colonies (up to six per fecal sample and three per environmental sample) were subcultured from the CCD agar (some colonies from samples from flocks 4, 5, and 6 were also subcultured from CIP-agar plates) onto Columbia blood agar with 5% defibrinated horse blood (Oxoid), and the plates were incubated at 41.5°C for 24 h in a microaerobic atmosphere. To confirm the identities of the isolates as Campylobacter spp., a wet mount preparation was examined by light microscopy at ×1,000 magnification for motility and cell morphology. A lack of growth in air at 20°C after 48 h was also taken as an indication that the isolate was of the Campylobacter genus. Isolates whose identities were confirmed were sent to the Campylobacter Reference Unit on Amies charcoal transport swabs for species identification and typing.
Identification of species, serotype and phage type, and antibiotic resistance type.
Isolates were initially identified phenotypically (6). During the course of the study, phenotypic tests for Campylobacter species identification were replaced by a real-time PCR assay for identification of C. jejuni and C. coli (5). All isolates were typed by standard reference methods, namely, serotyping (14), phage typing (15), and breakpoint screening for drug resistance (37). The breakpoints used for quinolone resistance screening were 1 μg/ml for ciprofloxacin and 16 μg/ml for nalidixic acid. Antimicrobial susceptibility was confirmed for a proportion of the isolates by the agar dilution MIC method recommended by the NCCLS Campylobacter Working Group (26) and is reported in the companion article (17a).
Statistical analysis.
Chi-square tests were used to compare the percentage of ciprofloxacin-resistant organisms (as determined by breakpoint examination of individual strains for resistance) in relation to the treatment phase and flock. The percentage of ciprofloxacin-resistant campylobacters relative to the total number of campylobacters (as determined by replica plating for flocks 4, 5, and 6) was compared by the general linear models procedure in MINITAB statistical software (Release 12.1 standard version). P values of <0.05 were considered significant.
RESULTS
Recovery of Campylobacter spp. from flock samples.
Flock 2 remained campylobacter free throughout the sampling period, so no data are available for this flock. All five remaining flocks investigated were positive for Campylobacter spp. before, during, and after treatment. While some flocks were sampled until 4 weeks posttreatment, others were slaughtered before such sampling could take place. Campylobacter spp. were isolated by direct plating from most (345 of 348) of the fresh fecal samples. Enumeration data from flocks 4, 5, and 6 showed that campylobacters were present in high numbers in the majority of samples (Fig. 1 to 3), suggesting that this sample type was a reliable source of the strains that colonized the flocks. Campylobacter spp. were also isolated by enrichment culture from 20 of 27, 16 of 30, 4 of 12, 8 of 20, and 8 of 23 of environmental samples, e.g., wall swabs, litter, feed, and drinking water collected inside the houses, from flocks 1, 3, 4, 5, and 6, respectively. Campylobacters were also isolated from puddle water samples (3 of 4) collected outside the houses of flocks 1 and 3.
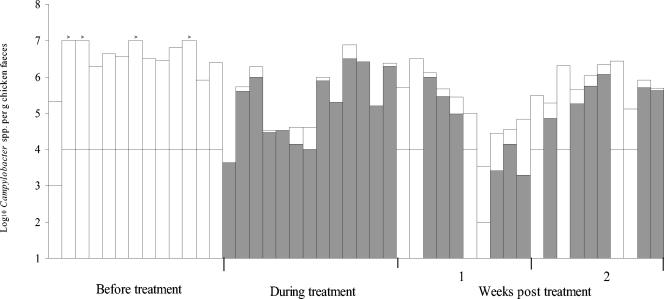
FIG. 1.
Numbers of Campylobacter spp. in chicken feces collected before, during, and after fluoroquinolone treatment from a housed broiler flock on a commercial chicken farm (flock 4). Each bar represents an individual freshly voided fecal sample. Shading indicates the numbers of ciprofloxacin-resistant Campylobacter spp. estimated by replica plating onto Iso-Sensitest agar containing 1 μg of ciprofloxacin per ml, 10 μg of amphotericin B per ml, and 32 μg of cefoperazone per ml (see text). The detection limit for the numbers of resistant strains varied between the samples and is illustrated as a horizontal line through each bar. The > symbol over a bar denotes that the total number of campylobacters exceeded the detection limit indicated by the top of the bar.
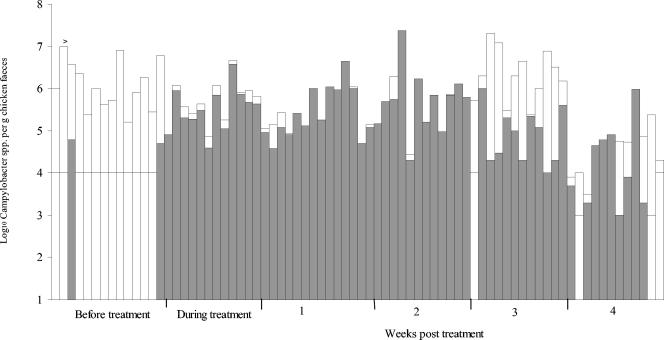
FIG. 3.
Numbers of Campylobacter spp. in chicken feces collected before, during, and after fluoroquinolone treatment from a free-range commercial chicken farm (flock 6). Each bar represents an individual freshly voided fecal sample. Shading indicates the numbers of ciprofloxacin-resistant Campylobacter spp. estimated by replica plating onto Iso-Sensitest agar containing 1 μg of ciprofloxacin per ml, 10 μg of amphotericin B per ml, and 32 μg of cefoperazone per ml (see text). The detection limit for numbers of resistant strains varied between samples and is illustrated as a horizontal line through each bar.
Proportion of ciprofloxacin-resistant strains in relation to treatment phase.
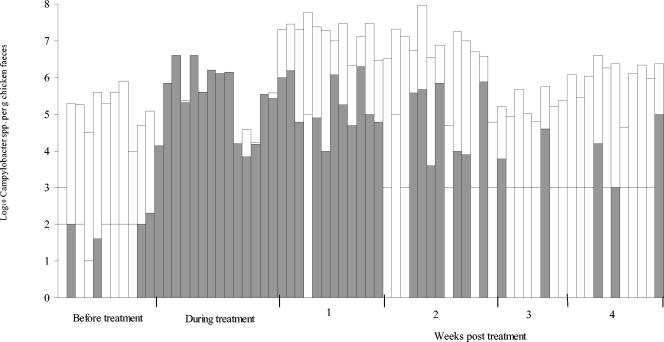
Ciprofloxacin-resistant campylobacters were detected before treatment in some birds in four of the five flocks (Table 1). However, no resistant campylobacters were detected in the majority of the fecal samples collected prior to treatment (the detection limit for the samples ranged from ∼1 to 0.01% when replica plating was performed) (Table 1 and Fig. 1 to 3). When resistant campylobacters were detected, they usually constituted a very small proportion of the total population, although 12% (5 of 42) of the isolates from flock 1 were resistant (Fig. 1 to 3 and Table 1). The percentage of ciprofloxacin-resistant isolates during treatment was significantly (P < 0.001) higher than that before treatment except in flock 3. The proportions of ciprofloxacin-resistant campylobacters in flocks 4, 5, and 6 were determined by replica plating; and during treatment the average log10 number of resistant isolates and the log10 total number of campylobacters in flocks 4, 5, and 6 were 5.2 (standard deviation [SD], 1.0) and 5.4 (SD, 1.0), respectively, 5.5 (SD, 0.5) and 5.7 (SD, 0.5), respectively, and 5.4 (SD, 1.0) and 5.5 (SD, 0.9), respectively.
TABLE 1.
Chicken fecal samples with quinolone-resistant Campylobacter spp.
| Flock | No. of samples with resistant isolates/total no. of samples tested (% quinolone-resistant isolates)a
|
|||||
|---|---|---|---|---|---|---|
| Before treatment | During treatment | Wk posttreatment
|
||||
| 1 | 2 | 3 | 4 | |||
| 1 | 2/14 (12) | 14/14 (95) | 11/14 (64) | 11/13 (69) | —b | — |
| 3 | 1/14 (1.4) | 1/12 (4.6) | 14/14 (70) | 10/12 (71) | 1/13 (2.6) | — |
| 4 | 0/13 (<2) | 13/13 (100) | 12/14 (75) | 10/14 (56) | — | — |
| 5 | 4/14 (2.4) | 12/12 (97) | 14/14 (95) | 13/13 (64) | 13/14 (8.3) | 9/14 (25) |
| 6 | 4/11 (<2) | 14/14 (96) | 11/14 (<1.5) | 8/14 (5.2) | 2/13 (<1.4) | 4/11 (3.5) |
The number of samples with resistant isolates includes samples in which resistant campylobacters were detected by breakpoint screening and/or replica plating. For flocks 4, 5, and 6, the percentages are based on isolates examined by breakpoint screening, although isolates were excluded if they were picked from the replica plates which contained ciprofloxacin.
—, no samples collected.
There was an overall significant (P < 0.001) decrease in the proportion of resistant strains in the weeks after treatment compared to the proportion during treatment. The time taken for the proportion of resistant strains to decrease posttreatment, however, varied between flocks. For example, the percentage of resistant strains in flock 6 was significantly (P < 0.001) lower than the percentage in the other flocks at 2 weeks posttreatment, and this was true for data obtained by examining resistance in individual strains as well as by replica plating. The percentage of resistant campylobacters was significantly lower in flock 3 during treatment; however, at week 1 there was no significant difference in the percentage of resistant campylobacters between the flocks. Relatively high numbers of ciprofloxacin-resistant campylobacters could still be detected 4 weeks after treatment in most birds from flock 5 and in some birds from flock 6 (Fig. 2 and 3), but the overall percentage of resistant campylobacters after treatment was significantly (P < 0.05) lower than that during treatment. There was considerable variation in the proportion of resistant campylobacters in chickens sampled from the same flock on the same occasion (Fig. 1 to 3). Resistance to ciprofloxacin was always accompanied by resistance to nalidixic acid, and 66% of the isolates were also resistant to at least one other antibiotic (usually tetracycline and/or ampicillin), in addition to trimethoprim (17a).
FIG. 2.
Numbers of Campylobacter spp. in chicken feces collected before, during, and after fluoroquinolone treatment on a free-range commercial chicken farm (flock 5). Each bar represents an individual freshly voided fecal sample. Shading indicates the numbers of ciprofloxacin-resistant Campylobacter spp. estimated by replica plating onto Iso-Sensitest agar containing 1 μg of ciprofloxacin per ml, 10 μg of amphotericin B per ml, and 32 μg of cefoperazone per ml (see text). The detection limit for the numbers of resistant strains varied between the samples and is illustrated as a horizontal line through each bar. The > symbol over a bar denotes that the total number of campylobacters exceeded the detection limit indicated by the top of the bar.
Campylobacter subtypes in relation to flock and sampling point.
The species of 1,630 isolates from the five flocks were determined, and the isolates were serotyped and phage typed. Of these, 1,274 (78.2%) were C. jejuni and 353 (21.7%) were C. coli. Three environmental isolates from flock 3 were C. lari serotype HS49, phage type 1 (PT1). More than one distinct campylobacter strain (as defined by species, serotype, and phage type) was isolated from most samples, although there were clearly predominant strains at each sampling time (Table 2). Three to six strains dominated in each flock, and at all stages some strains were isolated from both chicken feces and environmental samples. The ratio of C. jejuni to C. coli changed during and after treatment. Both species were detected before, during, and after treatment in flocks 1 and 3. Only C. jejuni was detected pretreatment in flocks 5 and 6, but C. coli was found in many samples both during and after treatment. C. coli was prevalent in flock 4 before treatment but was much less so during and after treatment. In some flocks a greater variety of strains were present posttreatment than pretreatment (Table 2). While some strains persisted through and beyond the treatment period, strains not detected pretreatment were identified posttreatment in all flocks. In a number of instances these strains were found in both environment and fecal isolates, for example, C. jejuni HS4 PT33, HS50 PT33, and HSUT PT33 in flock 5 and C. jejuni HS13 PT44 in flock 6 (Table 2), with the environmental isolates being found in multiple sources.
TABLE 2.
Number of fecal and environmental samples with ciprofloxacin-susceptible or -resistant Campylobacter sp. subtypes from flocks 1 to 6 in relation to treatment phasea
| Flock |
Campylobacter spp.
|
No. of samplesb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Serotype | Phage type | Before treatment | During treatment | Wk posttreatment
|
||||
| 1 | 2 | 3 | 4 | ||||||
| 1 | C. jejuni | HS4 | 1 | 1 | 1 | (1) | |||
| HS8 | 1 | 4 (1) | 3 (1) | ||||||
| HS27 | 67 | 10 (9) | (1) | (1) | (1) | ||||
| HS31 | 1 | 1 (1), 1 | 1, (7) | (1) | |||||
| C. coli | HS48 | 44 | 3 | 1 (1) | 1 | ||||
| HS56 | 44 | 9 (3) | 9 (1) | ||||||
| HS66 | 2 | 1 (2) | 3 | (1) | |||||
| 3 | C. jejuni | HS2 | 1 | 13 | |||||
| HS5 | 1 | 1, 1 | 2 | ||||||
| HS13 | 1 | 11 (3) | 8 (1), 1 (1) | 1, 3 | |||||
| HS13 | 2 | 3, 7 | |||||||
| UTc | 1 | 12 | 10 (2), 1 | 3 | 1 | 3 | |||
| C. coli | HS56 | 44 | 2 | 1 | 6 (1) | 1, 9 | 2, 1 (1) | ||
| 4 | C. jejuni | HS2 | UT | 4 | 2 | ||||
| HS5 | RDNCd | 1 | 2 | ||||||
| HS37 | 1 | 4 | 1 | ||||||
| HS50 | 1 | 2 | 3, 5 | ||||||
| HS50 | UT | 4 | 1, 6 | ||||||
| UT | 1 | 3 | 1, 2 | 4, 5 | |||||
| UT | RDNC | 1 | 2 | ||||||
| UT | UT | 1, 9 | 2, 3 | ||||||
| C. coli | HS28 | 44 | 9 | 2 | 1 | ||||
| 5 | C. jejuni | HS3 | UT | 12 | 1 | 2, 5 | 1 | ||
| HS4 | 33 | 1 (3) | 2, 1 | 4, 1 | |||||
| HS5 | 33 | 1 | 6, 1 | ||||||
| HS5 | UT | 6 | 1 | 1, 2 | 1 | 2, 1 | |||
| HS50 | 33 | 2 | 1 | 1 | |||||
| UT | 1 | 1 | 2, 1 | ||||||
| UT | 31 | 6, 1 | 1 | ||||||
| UT | UT | 3, 1 | 3, 10 | 2, 7 (1) | 1, 2 | 2 | 1 | ||
| C. coli | HS28 | 2 | 8 (1) | 5 | 3 | 1 | |||
| HS34 | 2 | 2 (1) | 4 | 2 | |||||
| 6 | C. jejuni | HS4 | 19 | 9 (2), 3 | 10 | 1 | 1 | ||
| HS13 | 44 | 13 (2) | 13 (2) | 12 | 11 | ||||
| HS21 | 1 | 4 (2), 2 (1) | 8 | ||||||
| HS42 | 44 | 1 (1) | 3 | 5 | 10, 1 | ||||
| UT | 44 | 2, (1) | 3, (1) | 3 | 3 | ||||
| C. coli | HS25 | 44 | 1 | 2 | 1 | ||||
| UT | 44 | 1, 1 | 4 (1) | 2 | 2 | 2 | |||
Only subtypes detected in three or more samples are included.
The numbers of environmental samples (i.e., surface swabs, drinking and puddle water, and feed) are shown in parentheses. The numbers of samples with ciprofloxacin-resistant isolates (as determined by breakpoint testing) are shown in boldface.
UT, untypeable.
RDNC, reacted but did not conform to a particular phage type.
Fluoroquinolone resistance in relation to Campylobacter subtypes.
The most common resistant Campylobacter subtypes which emerged either during treatment or in the first week after treatment did not necessarily belong to the same HS and PT subtypes which had been prevalent before treatment (Table 2). The dominant resistant campylobacters in flocks 1 and 4, for example, were of different HS and PT subtypes compared to those prevalent before treatment. In contrast, there was selection for the types of campylobacters that were prevalent before treatment in flock 6. In flocks 3 and 5 the prevalent resistant strains that emerged constituted a mixture of subtypes which either had or had not been prevalent before treatment. Three different fluoroquinolone-resistant C. coli strains were found in fecal samples during treatment in flock 5, and two of these were also found in environmental samples (Table 2). These replaced a large proportion of the C. jejuni subtypes which had been prevalent pretreatment. The resistant subtypes which became prevalent during treatment did not always remain dominant in the weeks following treatment. For example, in flock 1, although a resistant C. jejuni isolate was selected for initially, C. coli emerged as the most prevalent resistant type in the weeks after treatment. However, usually, the prevalent resistant subtypes that persisted in the weeks after treatment were a mixture of strains which either had or had not been prevalent during treatment. When resistant isolates were detected pretreatment they could become the most common resistant strains during treatment, as was observed in flock 6, but in the other flocks they either only partly dominated or were not detected during treatment or posttreatment (Table 2).
DISCUSSION
This study has demonstrated that large numbers of ciprofloxacin-resistant campylobacters emerge rapidly in commercial broiler chickens after treatment with a fluoroquinolone. Furthermore, a proportion of such strains can persist for several weeks after treatment has ceased. The present withdrawal times stated for difloxacin (24 h) and enrofloxacin (8 days) (2) would not have precluded the introduction of ciprofloxacin-resistant campylobacters into the food chain. It has been demonstrated that the level of campylobacter contamination on fresh retail carcasses varies (21, 44), but one study (24) found high numbers (≥5.0 log10) in approximately 20% of the chickens examined.
Rapid selection for resistant campylobacters has also been found in experimental studies in which chickens were infected with one or several susceptible C. jejuni strains before treatment (23, 27). Jacobs-Reitsma et al. (23) used a disk diffusion method to examine the susceptibilities of 200 isolates and found that fluoroquinolone-resistant strains emerged in chickens treated with enrofloxacin (but not in chickens treated with flumequine) and persisted for 2 weeks after treatment. In the second study, broilers were treated with enrofloxacin 5 days after inoculation with ciprofloxacin-susceptible C. jejuni strains. The MICs of fluoroquinolones for 50 strains isolated from feces sampled at each collection point were determined. Highly ciprofloxacin-resistant (MIC, 32 μg/ml) C. jejuni strains were isolated from enrofloxacin-treated birds from 1 day and up to 21 days after treatment had commenced (27). In the present study selection for a resistant mutant of the susceptible strain that was dominant before treatment was also observed. However, other resistant strains already present before treatment were also selected for, and sometimes, resistant subtypes which were not detected pretreatment (resistant or not) became dominant.
A recent survey (3) of antimicrobial agent-resistant C. jejuni and C. coli strains isolated from commercial broilers at slaughter investigated the distribution of resistance in relation to production type and antimicrobial usage. One bird from each of the 600 flocks entering the slaughterhouse was sampled, and 393 strains were isolated; of these strains, two-thirds were C. jejuni and one-third were C. coli. Of the C. jejuni strains, 25% were resistant to nalidixic acid and 17% were resistant to enrofloxacin; 43% of the C. coli strains were resistant to nalidixic acid and 40% were resistant to enrofloxacin. Quinolone therapy had been administered to 6.7% of the standard production flocks and 32.5% of the export flocks. However, the variability in species, subtypes, and susceptibilities shown in our study suggests that the sampling of only one bird per flock may not provide a full picture of the prevalence of antimicrobial resistance present among the campylobacter population within a flock.
The replica plating technique used in the present study enabled a confident assessment of the proportion of ciprofloxacin-resistant campylobacters in individual chickens following fluoroquinolone treatment. There was good agreement between the estimate of the proportion of resistant strains in each sample determined by replica plating and the rates of detection of resistance in those strains sent for breakpoint screening. The MIC of ciprofloxacin was later confirmed to be ≥4 μg/ml for 85% of the strains deemed to be resistant by breakpoint testing (17a).
In the present study we found variations in the proportion of resistant campylobacters recovered from individual samples collected at the same time from the same flock. This could reflect differences in the doses received, differences in strain populations, or different gut environments in individual chickens. Differences in the responses of the campylobacter population to fluoroquinolone treatment in individual chickens have also been observed in experimentally infected chickens (40). There was also some variation in the total number of campylobacters recovered from individual chickens sampled on the same occasion. This may relate to differences in the time span during which feces were exposed to the environment prior to collection (some campylobacters may die during this time). However, care was taken to sample only freshly voided feces, and the variation may reflect true differences in the number of campylobacters per gram of feces shed by different birds.
In the present study, a small proportion of strains were ciprofloxacin resistant prior to fluoroquinolone exposure. Ciprofloxacin resistance among Campylobacter spp. was also shown to be present at a low frequency (3%) in a large study of commercial chicken flocks in Northern Ireland (34). The preexistence of resistant strains suggests that they are established in the farm environment and that flocks may be exposed to and colonized by them in the absence of antibiotic exposure. Furthermore, the appearance of strains in the weeks posttreatment which were not detected pretreatment could suggest that flocks are continuously exposed to a changing environmental campylobacter population, which may include resistant strains.
Two chickens were negative for campylobacter during treatment, and this may have been a result of exposure to fluoroquinolone treatment, as has been shown in experimentally infected chickens (40).
The typing methods chosen were those used for isolates from human infections in the United Kingdom Campylobacter Sentinel Surveillance Scheme, namely, serotyping (14) and phage typing (15). Phenotypic methods for campylobacter typing have well-recognized limitations (31), not the least of which are problems in achieving consistent expression, particularly for serotyping. However, the utility of the combination of serotyping and phage typing was proved in this study, as it has been in studies of human infections. Many of the C. jejuni and C. coli subtypes seen in this study have rarely, if ever, been seen in human infections (data from the Campylobacter Reference Unit, Health Protection Agency), suggesting that strains which do not make a major contribution to human infection can be present in chickens. However, similar strain distributions among isolates from United Kingdom retail chickens and humans have been observed (12, 25). This may suggest that differential survival rates also affect the transfer of Campylobacter strains through the food chain.
Each of the flocks differed in the subtypes of campylobacter identified, the patterns of colonization with C. jejuni and C. coli, and the proportions of fluoroquinolone-resistant and -sensitive strains at different treatment phases. The intermittent appearance of some strains suggests that sampling may have been inadequate to detect those present in small numbers at any given time. This may also explain why some resistant strains that were not present during treatment appeared in the posttreatment sampling.
Fluoroquinolone exposure transiently selected for C. coli rather than C. jejuni in some flocks. This correlates with the findings of a number of studies, in which the prevalence of fluoroquinolone resistance was higher in C. coli than C. jejuni (17, 24, 28). Posttreatment sampling showed that the duration of the transient dominance of C. coli over C. jejuni varied between flocks. These data also suggest that certain subtypes of either species were dominant colonizers both during and after treatment, while others only transiently dominated.
In conclusion, the strain variation seen in this study indicates that changes in fluoroquinolone resistance in the Campylobacter population in a poultry flock during and after a course of treatment are not simply due to the replacement of the resident flora with a single fluoroquinolone-resistant strain. In all five flocks studied, resistant C. jejuni and/or C. coli strains were present in the farm environment in the absence of fluoroquinolone treatment. Furthermore, resistant strains persisted for up to 4 weeks posttreatment.
This study has demonstrated that flocks are exposed to fluoroquinolone-resistant campylobacters from the environment in the absence of fluoroquinolone treatment. The increase in the numbers of ciprofloxacin-resistant isolates during treatment declined posttreatment, but a proportion of resistant strains persisted for up to 4 weeks. The observed variations in the incidence and persistence of the two species C. coli and C. jejuni, as well as variations between strains of the same species, merit further study as understanding of the basis for such variations may inform new strategies to eradicate Campylobacter from the food chain.
This is the first report to show unequivocally that ciprofloxacin-resistant campylobacters emerge during fluoroquinolone treatment in commercially reared chickens that subsequently enter the food chain.
Acknowledgments
This study was funded by Defra (project code OZ0501). L.J.V.P. is a recipient of a Bristol-Myers Squibb unrestricted grant in infectious diseases.
We thank Karen Martin, Karen Mattick, Marco Siccardi, Paul Hocking, Lisa Williams, Maggie Johnson, and John E. Lee for technical assistance and Toby Knowles for advice on the statistical analysis.
REFERENCES
- 1.Adak, G. K., S. M. Long, and S. J. O'Brien. 2002. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut 51:832-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Compendium of datasheets for veterinary products 2000-2001, p. 68 and 203. National Office of Animal Health Ltd., Enfield, United Kingdom.
- 3.Avrain, L., F. Humbert, R. L'Hospitalier, P. Sanders, C. Vernozy-Rozand, and I. Kempf. 2003. Antimicrobial resistance in Campylobacter from broilers: association with production type and antimicrobial use. Vet. Microbiol. 96:267-276. [DOI] [PubMed] [Google Scholar]
- 4.Berrang, M. E., R. J. Buhr, J. A. Cason, and J. A. Dickens. 2001. Broiler carcass contamination with Campylobacter from feces during defeathering. J. Food Prot. 64:2063-2066. [DOI] [PubMed] [Google Scholar]
- 5.Best, E. L., E. J. Powell, C. Swift, K. A. Grant, and J. A. Frost. 2003. Applicability of a rapid duplex real-time PCR assay for speciation of Campylobacter jejuni and Campylobacter coli directly from culture plates. FEMS Microbiol. Lett. 229:237-241. [DOI] [PubMed] [Google Scholar]
- 6.Bolton, F. J., D. R. A. Wareing, M. B. Skirrow, and D. N. Hutchinson. 1992. Identification and biotyping of campylobacters, p. 151-161. In G. R. Board, D. Jones, and F. A. Skinner (ed.), Identification methods in applied and environmental microbiology. Blackwell Scientific Publications, Oxford, United Kingdom.
- 7.Campylobacter Sentinel Surveillance Scheme Collaborators. 2002. Ciprofloxacin resistance in Campylobacter jejuni: case-case analysis as a tool for elucidating risks at home and abroad. J. Antimicrob. Chemother. 50:561-568. [DOI] [PubMed] [Google Scholar]
- 8.Communicable Disease Surveillance Centre. 2002. Antimicrobial resistance in 2000: England and Wales. [Online.] http://www.hpa.org.uk/infections/topics_az/antimicrobial_resistance/amr.pdf. Accessed 18 December 2003.
- 9.Endtz, H. P., G. J. Ruijs, B. van Klingeren, W. H. Jansen, T. van der Reyden, and R. P. Mouton. 1991. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J. Antimicrob. Chemother. 27:199-208. [DOI] [PubMed] [Google Scholar]
- 10.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. 2002. Enrofloxacin for poultry; notice of hearing. Fed. Regist. 67:7700-7701. [Google Scholar]
- 12.Food Standards Agency. 2003. UK wide survey of salmonella and campylobacter contamination of fresh and frozen chicken on retail sale. [Online.] http://www.foodstandards.gov.uk/multimedia/pdfs/campsalmsurv.pdf. Accessed 27 November 2003.
- 13.Food Standards Agency. 2003. Food Standards Agency strategy for control of campylobacter in chickens. Food Standards Agency Consultation document. [Online.] http://www.foodstandards.gov.uk/multimedia/pdfs/campyloconsult0603e.pdf. Accessed 18 December 2003.
- 14.Frost, J. A., A. N. Oza, R. T. Thwaites, and B. Rowe. 1998. Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of heat-stable antigens. J. Clin. Microbiol. 36:335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost, J. A., J. M. Kramer, and S. A. Gillanders. 1999. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaunt, P. N., and L. J. V. Piddock. 1996. Ciprofloxacin resistant Campylobacter spp. in humans: an epidemiological and laboratory study. J. Antimicrob. Chemother. 37:747-757. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie, I. A., S. J. O'Brien, J. A. Frost, G. K. Adak, P. Horby, A. V. Swan, M. J. Painter, and K. R. Neal. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 8:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Griggs, D. J., M. M. Johnson, J. A. Frost, T. Humphrey, F. Jørgensen, and L. J. V. Piddock. 2005. Incidence and mechanism of ciprofloxacin resistance in Campylobacter spp. isolated from commercial poultry flocks in the United Kingdom before, during, and after fluoroquinolone treatment. Antimicrob. Agents Chemother. 49:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrant, R. L., T. Van Gilder, T. S. Steiner, N. M. Thielman, L. Slutsker, R. V. Tauxe, T. Hennessy, P. M. Griffin, H. DuPont, R. B. Sack, P. Tarr, M. Neill, I. Nachamkin, L. B. Reller, M. T. Osterholm, M. L. Bennish, and L. K. Pickering. 2001. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32:331-351. [DOI] [PubMed] [Google Scholar]
- 19.Health Protection Agency. 2003. Campylobacter spp. Laboratory reports of faecal isolates England & Wales, 1986-2001. [Online.] http://www.hpa.org.uk/infections/topics_az/campy/data_ew.htm. Accessed 18 December 2003.
- 20.Hein, I., C. Schneck, M. Knogler, G. Feierl, P. Plesss, J. Kofer, R. Achmann, and M. Wagner. 2003. Campylobacter jejuni isolated from poultry and humans in Styria, Austria: epidemiology and ciprofloxacin resistance. Epidemiol. Infect. 130:377-386. [PMC free article] [PubMed] [Google Scholar]
- 21.Hong, Y., M. E. Berrang, T. Liu, C. L. Hofacre, S. Sanchez, L. Wang, and J. J. Maurer. 2003. Rapid detection of Campylobacter coli, C. jejuni, and Salmonella enterica on poultry carcasses by using PCR-enzyme-linked immunosorbent assay. Appl. Environ. Microbiol. 69:3492-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey, T. J. 1995. Techniques for the isolation of campylobacters from food and the environment, p. 79-83. In Proceedings of a WHO meeting, April 1994. World Health Organization, Geneva, Switzerland.
- 23.Jacobs-Reitsma, W. F., C. A. Kan, and N. M. Bolder. 1994. The induction of quinolone resistance in Campylobacter bacteria in broilers by quinolone treatment. Lett. Appl. Microbiol. 18:228-231. [Google Scholar]
- 24.Jorgensen, F., R. Bailey, S. Williams, P. Henderson, D. R. Wareing, F. J. Bolton, J. A. Frost, L. Ward, and T. J. Humphrey. 2002. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int. J. Food Microbiol. 76:151-164. [DOI] [PubMed] [Google Scholar]
- 25.Kramer, J. M., J. A. Frost, F. J. Bolton, and D. R. Wareing. 2000. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J. Food Prot. 63:1654-1659. [DOI] [PubMed] [Google Scholar]
- 26.McDermott, P., S. Bodeis, F. Aerestrup, S. Brown, M. Traczewski, P. Fedorka-Cray, M. Wallace, I. Critcheley, C. Thornsberry, S. Graff, R. Flamm, J. Beyer, D. Shortridge, L. J. V. Piddock, and V. Ricci. 2004. Development of a standardized susceptibility test for Campylobacter with quality control ranges for ciprofloxacin, doxifloxacin, erythromycin, gentamicin and meropenem. Microb. Drug Resist. 10:124-131. [DOI] [PubMed] [Google Scholar]
- 27.McDermott, P. F., S. M. Bodeis, L. L. English, D. G. White, R. D. Walker, S. Zhao, S. Simjee, and D. D. Wagner. 2002. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J. Infect. Dis. 185:837-840. [DOI] [PubMed] [Google Scholar]
- 28.Moore, J. E., M. Crowe, N. Heaney, and E. Crothers. 2001. Antibiotic resistance in Campylobacter spp. isolated from human faeces (1980-2000) and foods (1997-2000) in Northern Ireland: an update. J. Antimicrob. Chemother. 48:455-457. [DOI] [PubMed] [Google Scholar]
- 29.Nachamkin, I., H. Ung, and M. Li. 2002. Increasing fluoroquinolone resistance in Campylobacter jejuni, Pennsylvania, USA, 1982-2001. Emerg. Infect. Dis. 8:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neimann, J., J. Engberg, K. Molbak, and H. C. Wegener. 2003. A case-control study of risk factors for sporadic campylobacter infections in Denmark. Epidemiol. Infect. 130:353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell, D. G., J. A. Frost, B. Duim, J. A. Wagenaar, R. H. Madden, and S. L. W. On. 2000. New developments in the subtyping of Campylobacter species, p. 27-44. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. ASM Press, Washington, D.C.
- 32.Newell, D. G., J. E. Shreeve, M. Toszeghy, G. Domingue, S. Bull, T. Humphrey, and G. Mead. 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 67:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oza, A. N., J. P. McKenna, S. W. McDowell, F. D. Menzies, and S. D. Neill. 2003. Antimicrobial susceptibility of Campylobacter spp. isolated from broiler chickens in Northern Ireland. J. Antimicrob. Chemother. 52:220-223. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues, L. C., J. M. Cowden, J. G. Wheeler, D. Sethi, P. G. Wall, P. Cumberland, D. S. Tompkins, M. J. Hudson, J. A. Roberts, and P. J. Roderick. 2001. The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol. Infect. 127:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, M. T. Osterholm, et al. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 37.Thwaites, R. T., and J. A. Frost. 1999. Drug resistance in Campylobacter jejuni, C. coli, and C. lari isolated from humans in north west England and Wales, 1997. J. Clin. Pathol. 52:812-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unicomb, L., J. Ferguson, T. V. Riley, and P. Collignon. 2003. Fluoroquinolone resistance in campylobacter absent from isolates, Australia. Emerg. Infect. Dis. 9:1482-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uyttendaele, M., R. Schukkink, B. van Gemen, and X. Debevere. 1996. Comparison of the nucleic acid amplification system of NASBA and agar isolation for detection of pathogenic campylobacter in naturally contaminated poultry products. J. Food Prod. 59:683-687. [DOI] [PubMed] [Google Scholar]
- 40.van Boven, M., K. T. Veldman, M. C. de Jong, and D. J. Mevius. 2003. Rapid selection of quinolone resistance in Campylobacter jejuni but not in Escherichia coli in individually housed broilers. J. Antimicrob. Chemother. 52:719-723. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler, J. G., D. Sethi, J. M. Cowden, P. G. Wall, L. C. Rodrigues, D. S. Tompkins, M. J. Hudson, P. J. Roderick, et al. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 318:1046-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. 1998. The use of quinolones in food animals and potential impact on human health. Report of a WHO meeting, Geneva, Switzerland, 2 to 5 June 1998. [Online.] http://www.who.int/emc-documents/zoonoses/docs/whoemczdi9810.pdf. Accessed 18 December 2003.
- 43.World Health Organization. 2000. The increasing incidence of human campylobacteriosis. Report and proceedings of a WHO consulation of experts, Copenhagen, Denmark, 21 to 25 November 2000. [Online.] http://www.who.int/emc-documents/zoonoses/docs/whocdscsraph20017.pdf. Accessed 18 December 2003.
- 44.Yang, C., Y. Jiang, K. Huang, C. Zhu, and Y. Yin. 2003. Application of real-time PCR for quantitative detection of Campylobacter jejuni in poultry, milk and environmental water. FEMS Immunol. Med. Microbiol. 38:265-271. [DOI] [PubMed] [Google Scholar]