Abstract
Background
Persistent expansion of circulating CD4+ effector memory T cells (TEM) in patients with granulomatosis with polyangiitis (GPA) suggests their fundamental role in disease pathogenesis. Recent studies have shown that distinct functional CD4+ TEM cell subsets can be identified based on expression patterns of chemokine receptors. The current study aimed to determine different CD4+ TEM cell subsets based on chemokine receptor expression in peripheral blood of GPA patients. Identification of particular circulating CD4+ TEM cells subsets may reveal distinct contributions of specific CD4+ TEM subsets to the disease pathogenesis in GPA.
Method
Peripheral blood of 63 GPA patients in remission and 42 age- and sex-matched healthy controls was stained immediately after blood withdrawal with fluorochrome-conjugated antibodies for cell surface markers (CD3, CD4, CD45RO) and chemokine receptors (CCR4, CCR6, CCR7, CRTh2, CXCR3) followed by flow cytometry analysis. CD4+ TEM memory cells (CD3+CD4+CD45RO+CCR7-) were gated, and the expression patterns of chemokine receptors CXCR3+CCR4-CCR6-CRTh2-, CXCR3-CCR4+CCR6-CRTh2+, CXCR3-CCR4+CCR6+CRTh2-, and CXCR3+CCR4-CCR6+CRTh2- were used to distinguish TEM1, TEM2, TEM17, and TEM17.1 cells, respectively.
Results
The percentage of CD4+ TEM cells was significantly increased in GPA patients in remission compared to HCs. Chemokine receptor co-expression analysis within the CD4+ TEM cell population demonstrated a significant increase in the proportion of TEM17 cells with a concomitant significant decrease in the TEM1 cells in GPA patients compared to HC. The percentage of TEM17 cells correlated negatively with TEM1 cells in GPA patients. Moreover, the circulating proportion of TEM17 cells showed a positive correlation with the number of organs involved and an association with the tendency to relapse in GPA patients. Interestingly, the aberrant distribution of TEM1 and TEM17 cells is modulated in CMV- seropositive GPA patients.
Conclusions
Our data demonstrates the identification of different CD4+ TEM cell subsets in peripheral blood of GPA patients based on chemokine receptor co-expression analysis. The aberrant balance between TEM1 and TEM17 cells in remission GPA patients, showed to be associated with disease pathogenesis in relation to organ involvement, and tendency to relapse.
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-017-1343-8) contains supplementary material, which is available to authorized users.
Keywords: Vasculitis, Granulomatosis with polyangiitis, TH cells, Effector memory TH cells, Chemokine receptors
Background
Granulomatosis with polyangiitis (GPA) is a severe systemic autoimmune disease of unknown etiology. The hallmark of the disease is the presence of antineutrophil cytoplasmic autoantibodies (ANCAs) mainly directed against protienase-3 (PR3) [1]. GPA is characterized by necrotizing granulomatosis in the respiratory tract, and a systemic vasculitis preferentially affecting pulmonary and renal small- and medium-sized blood vessels. The abundance of T cells in these vasculitic and granulomatous lesions of GPA patients support their involvement in disease pathogenesis [2]. There is substantial evidence of activated T cells and antigen-driven T cell responses in GPA [3–5] In addition, remission has been induced with therapeutics directed against T cells in patients with refractory GPA [6, 7]. These studies strongly indicate a T cell-mediated pathology in this disease.
The involvement of cluster of differentiation (CD)4+ T helper (TH) cells in the pathogenesis of GPA has been suggested to depend on disease activity, and whether the disease is localized, i.e. restricted to the respiratory tract, or generalized. Prior to the discovery of TH17 cells, research in GPA focused on the disturbed balance between TH1 and TH2 cells. It was found that GPA patients with active disease demonstrated a dysregulated cytokine prolife of circulating T cells with increased IFN-γ production versus a normal interleukin (IL)-4 production [8]. Additional studies demonstrated the presence of TH1-associated markers in the circulation as well as in nasal granulomatous lesions of patients with localized disease, while TH2-associated markers were dominant in generalized disease [9–11]. More recently, levels of IL-17A, the TH17-associated cytokine, were found to be elevated in serum of GPA patients irrespective of active or quiescent disease [12]. In addition, a relative increase in autoantigen-specific TH17 cells in GPA patients has been reported [12, 13].
Defects in regulatory T cell (TREG) function in GPA patients may contribute to abnormal skewing in TH cell responses and may result in an expansion of the CD4+ effector memory T (CD4+ TEM) cell population [14]. In addition, altered TH cell distribution in GPA patients may be in part driven by chronic cytomegalovirus (CMV) infection [15]. We have demonstrated previously that circulating CD4+ TEM cells (CCR7-CD45RO+) in GPA patients were proportionally increased during remission [16], but were decreased during renal active disease upon migration to the inflammatory site [17]. However, during active renal disease not all circulating CD4+ TEM cells tend to migrate to the target tissues [17]. It is possible that a subset of circulating CD4+ TEM cells have a distinct migratory capacity and pathogenic function in GPA patients related to distinct clinical manifestations.
The recruitment of the CD4+ TEM cells to inflammatory sites is orchestrated by their chemokine receptors. Analysis of chemokine receptor expression has been instrumental in the characterization of memory TH subsets with distinct cytokine patterns and antigen responses [18]. The expression pattern of four chemokine receptors allows the identification of CD4+ TEM subsets, which are defined as TEM1 [C-C chemokine receptor (CCR)6- CXC chemokine receptor 3 (CXCR3)+CCR4+CRTh2-] TEM2 (CCR6-CXCR3-CCR4+CRTh2+), TEM17 (CCR6+CXCR3-CCR4+CRTh2-) [19, 20], and a subset that exhibits both TH17 and TH1 features, referred to as TEM17.1 (CCR6+CXCR3+CCR4- CRTh2-) [21, 22].
The aim of the present study was to determine the distribution of circulating CD4+ TEM cell subsets based on chemokine receptor expression in GPA patients. Identification of particular circulating CD4+ TEM subsets may reveal distinct associations of specific CD4+ TEM subsets with clinical manifestations or with autoantibodies in GPA patients.
Methods
Study population
Peripheral blood was collected from 63 GPA patients in remission (r-GPA) and 42 age- and sex-matched healthy controls (HCs) in a cross-sectional study..The r-GPA patients fulfilled the criteria of the American College of Rheumatology and the Chapel Hill Consensus Conference definition for GPA [23, 24]. Only patients with PR3-ANCA positivity at diagnosis, and complete remission of their disease at the time of sampling, were included in the study. The PR3-ANCA titers were measured by indirect immunofluorescence (IIF) on ethanol-fixed human granulocytes according to the standard procedure as described previously [25]. ANCA titers lower than 1:20 were considered negative. Complete remission was defined as the complete absence of clinical signs and symptoms of active vasculitis, as indicated by a score of zero on the Birmingham Vasculitis Activity Score (BVAS) [26]. According to these criteria, blood samples were taken during a visit to our outpatient clinic.
Disease extent was defined as localized when GPA was restricted to the upper and lower respiratory tract and generalized when systemic disease with vasculitis extended to more clinical manifestations including involvement of kidneys, joints, eye, and nervous system. None of the patients and controls experienced an infection at the time of sampling.
Twenty-nine of 63 r-GPA patients were treated with maintenance immunosuppressive therapy at time of blood sampling. Three r-GPA patients received azathioprine, 12 r-GPA patients received azathioprine in combination with prednisolone, six r-GPA patients were treated with low-dose prednisolone, seven r-GPA patients received low-dose prednisolone in combination with mycophenolate mofetil (MMF), and one r-GPA patient was treated with methotrexate.
Detailed clinical and laboratory characteristics of the patients are summarized in Table 1. All patients and healthy volunteers provided informed consent and the local medical ethics committee approved the study.
Table 1.
Laboratory and clinical characteristics of r-GPA patients and HC
| r-GPA | HC | |
|---|---|---|
| Subjects, n (% male) | 63 (% 44) | 42 (% 40) |
| Age, mean (range) | 62.3 (26.8–85.2) | 57.2 (21.5–86.8) |
| PR3-ANCAa, n (% positive) | 39 (% 62) | |
| PR3-ANCA titer, median (range) | 1:40 (0–1:640) | |
| Creatinine umol/L, median (range) | 86 (52–224) | |
| CRP mg/L, median (range) | 2.7 (0.3–99) | |
| eGFR ml/min*1.73 m2, median (range) | 64 (21–109) | |
| CMV seropositive, n (% positive) (N.D.) | 33 (% 54) (2) | 21 (% 58) (6) |
| S. aureus, n (% positive) (N.D.) | 27 (% 44) (1) | |
| BVAS, mean | 0 | |
| Disease duration in years, median (range) | 9.6 (1.9–42.7) | |
| No. of total relapses, median (range) | 1 (0–7) | |
| Relapserb, n (%) | 43 (% 68) | |
| Disease type, n (% generalized) | 52 (% 83) | |
| Treatment at time of sampling, n (%) | ||
| Azathioprine | 3 (% 5) | |
| Azathioprine + prednisolone | 12 (% 19) | |
| Prednisolone | 6 (% 10) | |
| Mycophenolate mofetil + prednisolone | 7 (% 11) | |
| Methotrexate | 1 (% 2) | |
| No immunosupressive treatment | 34 (% 54) | |
| Co-trimoxazole, high dose/low dose/no dose | 17/15/31 | |
| No. of organs involved, median (range) | 3 (1–7) | |
| Clinical manifestations, n (%) | ||
| Renal | 35 (% 56) | |
| ENT | 45 (% 71) | |
| Joints | 36 (% 57) | |
| Pulmonary | 40 (% 63) | |
| Nervous system | 20 (% 32) | |
| Eyes | 24 (% 38) | |
| Cutaneous | 13 (% 21) | |
| Other | 7 (% 11) | |
Characteristics at sampling time point
BVAS Birmingham Vasculitis Activity Score, CMV cytomegalovirus, CRP C-reactive protein, eGFR estimated glomerular filtration rate, ENT ear, nose and throat, GPA granulomatosis with polyangiitis, HC healthy control, PR3-ANCA antineutrophil cytoplasmic antibodies targeting proteinase 3, r-GPA GPA patient in remission, S. aureus Staphylococcus aureus
aANCA-positive titer ≥1:40, ANCA-negative ≤1:20
bRelapser: GPA patient that had ≥1 relapse after diagnosis until time of sampling
Sample preparation and immunophenotyping by flow cytometry
EDTA-anticoagulated peripheral blood was obtained by venipuncture from r-GPA patients and HCs. Whole blood samples were stained within 4 hours after blood withdrawal with appropriate concentrations of fluorochrome-conjugated monoclonal antibodies for cell surface antigens. The samples were immediately processed to obtain the most sensitive detection for the chemokine receptor expression and to minimize cell manipulation. The peripheral blood was stained using the following monoclonal antibodies for cell surface antigens in combination: Alexa Fluor® 700-conjugated anti-CD3, eFluor® 450 (eF450)-conjugated anti-CD4 (both from eBioscience, San Diego, CA, USA), fluorescein isothiocyanate (FITC)-conjugated anti-CD45RO, phycoerythrin-cyanin7 (PE-C7)-conjugated anti-CCR7 (both from BD Biosciences, Franklin Lakes, NJ, USA), PE-conjugated anti-CRTh2, allophycocyanin-C7 (APC-Cy7)-conjugated anti-CXCR3, peridin chlorophyll α-protein (PerCP-Cy5.5-conjugated anti-CCR4, and Brilliant Violet 605™ (BV605)-conjugated anti-CCR6 (all from BioLegend, San Diego, CA, USA). The appropriated isotype-matched control antibodies of irrelevant specificity were added to a separate tube as negative controls. Samples were incubated for 15 minutes at room temperature. Afterward, cells were treated with 2 mL diluted FACS lysing solution (BD Biosciences) for 10 minutes. Finally, the samples were washed in PBS containing 1% (w/v) bovine serum albumin (BSA), and immediately analyzed by eight-color flow cytometric analyses on BD™ LSR II flow cytometer. Data were collected for 1.0 *106 events for each sample and plotted using Kaluza v1.2 (Beckman Coulter, Brea, CA, USA). Lymphocytes were gated for analysis based on forward and side scatter properties. Positively and negatively stained populations were calculated by quadrant dot-plot analysis or histograms, determined by the appropriate isotype controls. Within the CD4+ TEM cell subset (CD4+CCR7-CD45RO+) the expression pattern of chemokine receptors CCR6-CXCR3+CCR4-CRTh2-, CCR6-CXCR3-CCR4+CRTh2+, CCR6+CXCR3-CCR4+CRTh2-, and CCR6+CXCR3+CCR4-CRTh2- were used to distinguish TEM1, TEM2, TEM17, and TEM17.1 cells, respectively.
Detection of S. aureus
From 62 r-GPA patients, S. aureus nasal carriers were determined as described previously [27]. Briefly, S. aureus nasal isolates were sampled by rotating a sterile cotton swab in each anterior nary. Swabs were inoculated on 5% sheep-blood and salt mannitol agar for 72 h at 35 °C. S. aureus was identified by coagulase and DNase positivity. Patients were considered to be chronic nasal carriers when ≥50% of their nasal cultures grew S. aureus.
CMV ELISA
CMV-specific IgG was determined in serum samples using an in-house enzyme-linked immunosorbent assay (ELISA). In brief, 96-well ELISA plates (Greiner, Kremsmünster, Austria) were coated overnight with lysates of CMV-infected fibroblasts. Lysates of non-infected fibroblasts were used as negative controls. Following coating, serial (1:100–1:3200) dilutions of serum samples were incubated for 45 minutes. Next, goat anti-human IgG-HRP (Southern Biotech, Birmingham, AL, USA) was added and incubated for 45 minutes. Samples were incubated with TBE substrate (Sigma-Aldrich, St. Louis, MO, USA) for 15 minutes and the reaction was stopped with sulfuric acid. The plates were scanned on a Versamax reader (Molecular Devices, Sunnyvale, CA, USA). A pool of sera from three CMV-seropositive individuals with known concentrations of CMV-specific IgG was used to quantify levels of CMV-specific IgG in the tested samples.
Statistical analysis
Statistical analysis was performed using SPSS v22 (IBM Corporation, Armonk, NY, USA) and GraphPad prism v5.0 (GraphPad Software, San Diego, CA, USA). Data are presented as median values. Data were analyzed with the D’Agostino-Pearson omnibus normality test for Gaussian distribution. For comparison between r-GPA patients and HCs the unpaired t test was used for data with Gaussian distribution and the Mann-Whitney U test for data without Gaussian distribution. For intra-individual comparison of values at multiple time points during follow-up, repeated measures analysis of variance was used if data were normally distributed and a Friedman test was used if data had a non-Gaussian distribution. The association between clinical parameters and CD4+ TEM cell subsets in inclusion samples of r-GPA patients was investigated using the Spearman’s rank correlation coefficient. In order to account for interactions of CMV and age on the percentage of CD4+T cells subsets and CD4+TEM cell subsets we used a linear (Enter) regression analysis. Non-normally distributed data were log-transformed. Differences were considered statistically significant at two-sided p values equal to or less than 0.05.
Results
Higher frequency of CD4+ TEM cells in peripheral blood of GPA patients in remission
We have previously reported that r-GPA patients have an increased percentage of circulating CD4+ TEM cells compared to HC [16]. Here, we confirm that within the CD4+ T cell population in the peripheral blood of r-GPA patients the frequency of CD4+ TEM cells was significantly higher compared to HCs (Fig. 1b). In addition, the frequency of CD4+ TNaïve cells was significantly lower in r-GPA patients compared to HCs, whereas the proportions of CD4+ TCM cells did not differ between r-GPA patients and HCs. The proportions of CD4+ TTD cells were higher in r-GPA compared to HCs.
Fig. 1.
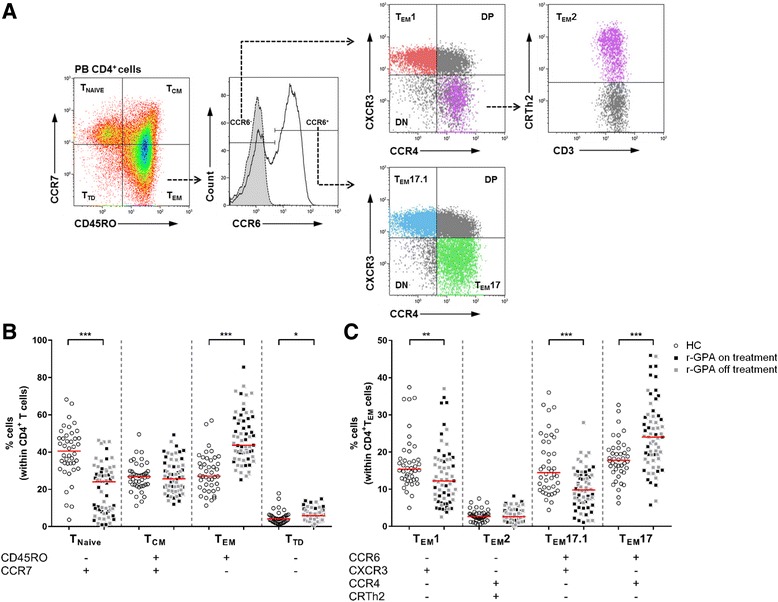
T cell subset distribution between HC and r-GPA patients. a Gating strategy of CD4+ TEM cell subsets using chemokine receptor expression patterns. The flow cytometry plots show sequential gating (dashed arrows) to identify different CD4+ TEM cell subsets within the CD4+TEM cell population. CD4+ T cell subsets from peripheral blood were identified based on the expression of CCR7 and CD45RO. Within CD4+TEM cells CCR6- and CCR6+ cells were identified based on the isotype (grey histogram with dashed line). Within CCR6- CD4+TEM cells expression of CXCR3 and CCR4 was analyzed to identify TEM1 cells (CD4+CD45RO+CCR7-CCR6-CXCR3+CCR4-). Expression of CRTh2 was used to identify lineage-committed TEM2 cells (CD4+CD45RO+CCR7-CCR6-CXCR3-CCR4+CRTh2+) derived from CCR6-CXCR3-CCR4+ CD4+TEM cells. The CXCR3 and CCR4 expression was also analyzed on CCR6+ CD4+TEM cells to identify TEM17 cells (CD4+CD45RO+CCR7-CCR6+CXCR3-CCR4+), and TEM17.1 cells (CD4+CD45RO+CCR7-CCR6+CXCR3+CCR4-). The unclassified populations within the CCR6- and CCR6+ populations were identified, that were double-negative (DN) or double-positive (DP) for CXCR3 and CCR4. Representative flow cytometry plots from one r-GPA patient. b Percentages of CD45RO-CCR7+ (T NAIVE), CD45RO+CCR7+ (T CM), CD45RO+CCR7- (T EM) and CD45RO-CCR7- (T TD) subpopulations within the CD4+ T cell population in peripheral blood of HCs (open circles; n = 42) and r-GPA patients (filled squares; n = 63). c The percentage of CD4+ TEM cell subsets in peripheral blood of HCs (open circles; n = 42) and r-GPA patients (filled squares; n = 63). Black squares indicate r-GPA patients on treatment (n = 29), grey squares indicate r-GPA patients off treatment (n = 34). Horizontal lines represent median percentages. * p < 0.05, ** p < 0.01, and *** p < 0.001. CCR C-C chemokine receptor, CD cluster of differentiation, CXCR3 CXC chemokine receptor 3, HC healthy control, r-GPA GPA patient in remission, T EM effector memory T cell
Increased frequency of CD4+TEM17 and decreased frequency of CD4+TEM1 in peripheral blood of patients with GPA
Having demonstrated a significant increase in the frequency of CD4+ TEM cells in r-GPA patients we next zoomed in on the phenotypic distribution within this expanded population. As mentioned earlier, CD4+ TEM cell population can be subdivided into four TEM subsets based on their differential expression of the chemokine receptors CCR6, CCR4, CXCR3 and CRTh2. We applied a chemokine receptor gating strategy, as shown in Fig. 1a, to identify the distribution of circulating TEM1 (CCR6-CXCR3+CCR4-CRTh2-), TEM2 (CCR6-CXCR3-CCR4+CRTh2+), TEM17 (CCR6+CXCR3-CCR4+CRTh2-), and TEM17.1 (CCR6+CXCR3+CCR4-CRTh2-) cell subsets among the CD4+ TEM cells. The analysis demonstrated a significant decrease in the frequency of TEM1 and TEM17.1 cells in r-GPA patients compared to HCs (Fig. 1c). The frequencies of TEM17 cells were significant higher in r-GPA compared to HCs. No statistical significant difference was reached for the distribution of TEM2 cells between r-GPA patients and HCs. In addition, no changes were observed in the percentages of both TEM1, and TEM17 subsets in three consecutive samples during 6 months of follow-up in individual r-GPA patients (data not shown). Furthermore, we observed CXCR3+CCR4+ (double positive, DP) and CXCR3-CCR4- (double negative, DN) CCR6+ TEM cells. Although little is known about the function of these cells, it has been described that the DP CCR6+ TEM cells produce low levels of IL-17A and RORC with intermediate IFN-γ and T-bet levels [28]. In our study, we did not observe differences in these unclassified DP and DN population of either CCR6+ or CCR6- CD4+TEM cells between r-GPA patients and HCs (data not shown).
The frequency of TEM17 cells negatively correlates with the frequency of TEM1 cells in peripheral blood of r-GPA patients
In vitro and in vivo studies have provided evidence that TH1 and TH17 cell responses counterregulate each other during disease development in GPA [13, 29]. Therefore, we investigated whether the increased proportion of TEM17 cells correlated with the decreased proportion of TEM1 cells. As shown in Fig. 2, a significant negative correlation between decreased proportions of TEM1 cells and increased proportions of TEM17 cells was observed in r-GPA patients (Spearman’s rho = -0.844, p < 0.0001). However, neither TEM2 cells nor TEM17.1 cells correlated significantly with TEM17 cells (Fig. 2a). Similar observations were found for HCs, although the correlation between TEM1 and TEM17 cells (Spearman’s rho = −0.554) was less pronounced in comparison to r-GPA patients (Spearman’s rho = −0.844) (Fig. 2b).
Fig. 2.
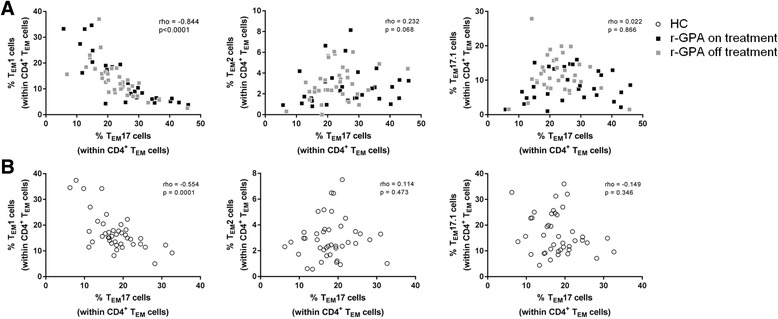
TEM17 cells negatively correlate with TEM1 cells. Correlation between the percentage of TEM17 cells and TEM1 (left), TEM2 (middle) and TEM17.1 (right) cells within the CD4+TEM cell population of a r-GPA patients (filled squares; n = 63), black squares patients on treatment (n = 29) and grey squares patients off treatment (n = 34) and b HC (open circles; n = 42). Correlations were determined by Spearman’s correlation coefficient (rho) and the level of significance is indicated by the p value. CD cluster of differentiation, HC healthy control, r-GPA GPA patient in remission, T EM effector memory T cell
Immunosuppressive therapy and the imbalance in CD4+ TEM cell subsets
To address the question whether current immunosuppressive treatment influences the imbalance in TEM1 and TEM17 cell subsets, r-GPA patients were separated into patients not receiving immunosuppressive treatment (off treatment; n = 34), and patients that did receive immunosuppressive treatment (on treatment; n = 29) (Fig. 1c). No significant differences were observed in the frequencies of TEM1 cells between the untreated and treated r-GPA patients (median 12.3%, interquartile rang 9.5–17.8% vs 9.0%, 5.6–19.2%). In addition, no significant differences were detected in the frequencies of TEM17 cells between untreated and treated r-GPA patients (22.3%, 18.0–26.5% vs 26.5%, 17.2–35.4%). Therefore, immunosuppressive treatment at time of sampling was not responsible for the imbalances observed between the TEM1 and TEM17 cell subsets.
The influence of S. aureus and CMV infection on the frequencies of circulating TEM1 and TEM17 cells in GPA patients
Physiologically, TH17 cells are important in the defense against bacterial infection (e.g. Staphylococcus aureus (S. aureus)), by IL-17-mediated activation of neutrophils. Interestingly, chronic nasal carriage of S. aureus has been suggested to drive the TH17 response in ANCA-associated vasculitis (AAV) [30]. Therefore we investigated whether chronic nasal carriage of S. aureus influenced the proportions of TEM1 and TEM17 cells. No significant differences were found in the proportion of TEM1 and TEM17 cells between r-GPA patients with or without S. aureus nasal carriage (Fig. 3a), even after excluding co-trimoxazole treatment (data not shown).
Fig. 3.
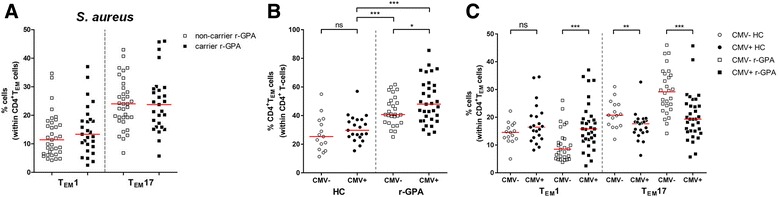
TEM1 and TEM17 cell distribution between r-GPA patients with S. aureus nasal carriage and latent CMV infection. a The circulating percentage of TEM1 and TEM17 cells within the CD4+TEM cell population in peripheral blood of S. aureus-non-carrying r-GPA patients (open squares; n = 35) and carrying r-GPA patients (filled squares; n = 27). b The circulating percentage of CD4+TEM cells within the CD4+ T cell population in peripheral blood, and c the circulating percentage of TEM1 and TEM17 cells within the CD4+TEM cell population in peripheral blood of HC CMV-seronegative (open circles; n = 15), CMV-seropositive (filled circles; n = 21), r-GPA patients CMV-seronegative (open squares; n = 28), and CMV-seropositive (filled squares; n = 33). Horizontal lines represent median percentages. * p < 0.05, ** p < 0.01, and *** p < 0.001. CD cluster of differentiation, CMV cytomegalovirus, HC healthy control, ns not significant, r-GPA GPA patient in remission, S. aureus, Staphylococcus aureus, T EM effector memory T cell
Besides bacterial infections, latent viral infection can also influence the T cell compartment in humans. The expansion in CD4+ TEM cells in GPA patients has been suggested to be driven by latent cytomegalovirus (CMV) [15]. Here, we found a slight increase in the percentage of circulating CD4+TEM cells of CMV-seropositive compared to CMV-seronegative populations. This difference was statistically significant in r-GPA patients (p = 0.044), but not in HCs (p = 0.234) (Fig. 3b). In addition, both CMV-seropositive and CMV-seronegative r-GPA patients showed significantly increased percentages of CD4+TEM cells compared to CMV-seropositive HCs.
To investigate the possibility that the shift from TH1 toward TH17 cells in GPA patients was the result of CMV carriage, we compared the proportions of TEM1 and TEM17 cells between CMV-seropositive and CMV-seronegative r-GPA patients. As shown in Fig. 3c, CMV-seropositive r-GPA patients demonstrated significantly higher frequencies of TEM1 cells and significantly lower frequencies of TEM17 cells compared to CMV-seronegative r-GPA patients. In contrast, CMV serostatus in HCs did not change the proportions of TEM1 cells but a small decrease in the percentage of TEM17 cells in CMV-seropositive HCs compared to CMV-seronegative HCs was observed.
Furthermore, CMV-specific serum IgG levels in r-GPA patients showed a positive correlation with the percentage of TEM1 cells (Spearman’s rho = 0.408, p < 0.001) and a negative correlation with the percentage of TEM17 cells (Spearman’s rho = −0.468, p < 0.0001).
Association of TEM1 and TEM17 frequencies with laboratory and clinical parameters
We next explored whether disturbed frequencies in TEM1 and TEM17 cells correlated with various laboratory and clinical parameters of r-GPA patients (Table 2). Serum ANCA titers in GPA patients have often been related to disease activity and risk of relapse. Therefore, we investigated the relation between ANCA titer at time of sampling and the proportions of TEM1 and TEM17 cells in r-GPA patients. No correlation was observed between ANCA titers and the frequencies of either TEM1 cells or TEM17 cells. In addition, no correlations were found between frequencies of either TEM1 cells or TEM17 cells and any other laboratory parameter measured at the time of inclusion, including creatinine levels, C-reactive protein (CRP) serum levels, and epidermal growth factor receptor (eGFR).
Table 2.
Associations of TEM1 cell and TEM17 cell percentages with clinical parameters
| Clinical parameters | % TEM1 cells | % TEM17 cells | ||
|---|---|---|---|---|
| Spearman’s rho | p value | Spearman’s rho | p value | |
| PR3-ANCA titer | 0.026 | 0.839 | −0.048 | 0.707 |
| Creatinine (umol/L) | −0.039 | 0.759 | 0.164 | 0.198 |
| CRP (mg/L) | 0.047 | 0.715 | 0.015 | 0.909 |
| eGFR (mL/min*1.73 m2) | −0.079 | 0.540 | −0.006 | 0.962 |
| Disease duration (years) | −0.023 | 0.857 | 0.063 | 0.626 |
| No. of total relapses | −0.148 | 0.246 | 0.181 | 0.155 |
| No. of organs involved | −0.264* | 0.037 | 0.390** | 0.002 |
CRP C-reactive protein, eGFR estimated glomerular filtration rate, PR3-ANCA antineutrophil cytoplasmic antibodies targeting proteinase 3, TEM effector memory T cell
* p < 0.05 and ** p < 0.01
Interestingly, the accumulating number of organs involved over the total disease course correlated negatively with the frequency of TEM1 cells (Spearman’s rho = -0.264, p = 0.037) but correlated positively with TEM17 cells (Spearman’s rho = 0.390, p = 0.002). In addition, generalized r-GPA patients showed lower frequencies of TEM1 cells in comparison to localized r-GPA patients (10.7%, 6.3–16.5% vs 17.9%, 12.6–20.4%, p = 0.016). The frequencies of TEM17 cells were higher in generalized r-GPA compared to localized r-GPA patients (24.8%, 19.2–30.8% vs 19.3%, 15.0–21.4%, p = 0.037). This indicates that r-GPA patients with more systemic manifestations have an increased TEM17-mediated immune response, whereas r-GPA patients with more local manifestations have a more TEM1-directed immune response. However, disease duration and total number of relapses did not correlate with either the frequencies of TEM1 cells or TEM17 cells. Of note, we observed that r-GPA patients that did encounter one or more relapses after diagnosis (1 ≥ relapse r-GPA, n = 43) had higher frequencies of circulating TEM17 cells and lower frequencies of circulating TEM1 cells in comparison to r-GPA that experienced no relapse (non-relapse r-GPA, n = 20) since diagnosis (Fig. 4).
Fig. 4.
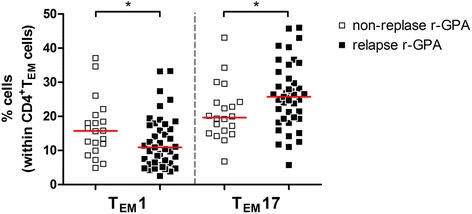
TEM1 and TEM17 cell distribution between relapsing and non-relapsing r-GPA patients. The circulating frequencies of TEM1 and TEM17 cells within the CD4+TEM cell population in peripheral blood of non-relapsing r-GPA patients (open squares; n = 20) and r-GPA patients that experienced ≥ 1 relapse during their disease course until inclusion in this study (filled squares; n = 43). Horizontal lines represent median percentages. * p < 0.05. CD cluster of differentiation, r-GPA GPA patient in remission, T EM effector memory T cell
Interaction of CMV serostatus and age on the different T cell subsets
The results described above regarding the differences between r-GPA patients and HCs in percentage of CD4+TEM cells, TEM1 and TEM17 cells may indicate a possible interaction between CMV and age, as both factors influence the T cell memory compartment. To analyze this interaction a linear regression was performed that included interaction of CMV and age. This analysis demonstrated that differences in CD4+TEM cells, TEM1 cells, and TEM17 cells between r-GPA patients and HCs were not attributed to CMV serostatus and age (see Additional file 1). Moreover, differences in TEM1 between relapse r-GPA patients and non-relapse r-GPA patients were not affected by CMV serostatus and age. The difference in TEM17 cells between relapse r-GPA patients and non-relapse r-GPA patients was minimally influenced by CMV serostatus and age as the differences for both factors were borderline significant.
Thus, CMV and age did not influence the differences in expansion of CD4+TEM cells, TEM1 cells, and TEM17 cells, in both r-GPA patients and HCs.
Discussion
In this study, we aimed to determine the distribution of circulating CD4+ TEM cell subsets based on chemokine receptor expression in GPA patients. We demonstrated a significant increase in the proportion of TEM17 cells with a concomitant decrease in the proportion of TEM1 cells in peripheral blood of patients with r-GPA. Increased proportions of TEM17 cells were more pronounced in r-GPA patients with systemic manifestations, whereas r-GPA patients with local manifestations showed a remarkable increase in TEM1 cells. Interestingly, CMV seropositivity appeared to modulate the disturbed balance of TEM1 and TEM17 cells in r-GPA patients.
The decreased proportions of TEM1 cells in r-GPA patients compared to HCs reflect an aberrant TEM1 response in patients. It has been demonstrated that GPA patients with active or localized disease show a polarization toward a TH1-type response [8, 11, 31]. These studies showed an abundant TH1 cytokine (IFN-γ) and chemokine (CCR5) pattern on circulating T cells, as well as in granulomatous lesions compared to patients in remission or with generalized disease [11, 31]. It has been suggested that the disturbed TH1 response might play a role in the initiation of GPA. The disease can progress into a generalized GPA with a less prominent TH1-type response. The majority of r-GPA patients included in this study present generalized disease with a median disease duration of 9.6 years. This might explain the decreased proportion of circulating TEM1 cells in our r-GPA patients. However, one may also argue that the relative decrease in circulating TEM1 cells is due to an increased tissue migration of these cells. In GPA patients with generalized disease it has been reported that renal lesions show polarization toward TH1 type-responses [32]. However, we did not observe an association of TEM1 cells with renal involvement in r-GPA patients.
Our results regarding the increase in TEM17 response in GPA patients are in line with previous reports on increased TH17-associated activity in these patients. It has been reported that antigen-specific TH17 cells are expanded in GPA patients, irrespective of disease activity and maintenance therapy [13, 33]. In addition, serum IL-17A levels are also found to be elevated in active GPA patients and remained elevated in GPA patients recovering from active disease [12]. In line with this result, we observed a sustained TEM17 expansion over a period of 6 months in our r-GPA patients. Altogether, the involvement of TH17 cells in the immunopathology in GPA appears to be well established, although presently it remains unclear which mechanisms initiate TH17 responses in GPA. Possible explanations for the expanded TEM17 population might be related to the presence of granulomas, or chronic nasal carriage of S. aureus in GPA.
Granulomas are sophisticated and highly organized structures that typically consist of a sphere of highly activated macrophages surround by T lymphocytes. They provide a specialized niche for macrophage-T cell interaction, contributing to the differentiation and maturation of T cells [34]. The pro-inflammatory cytokine environment in granuloma may contribute to the aberrant TH1 and TH17 cell distribution found in the circulation. Since, granulomas are common clinical manifestations in GPA patients they may provide an ideal environment for TEM17 cell expansion. Engagement of CD4+TEM cells with IL-6/TGFβ-producing macrophages may promote CD4+TEM cell differentiation into TEM17 cells. In addition, macrophages also secrete IL-23, which sustains the TH17 population. Indeed, elevated serum levels of TGFβ, IL-6, and IL-23 have been reported in GPA, and, importantly, elevated levels of IL-23 correlated with disease severity in patients with GPA [12].
Chronic carriage of S. aureus constitutes a risk factor for the development of exacerbations in GPA. We have previously shown that the frequency of chronic nasal carriage of S. aureus is higher in GPA patients compared to HC [30]. Moreover, it was shown that nasal S. aureus carriage is associated with increased risk of relapse [30, 35]. Staphylococcal superantigens act as potent immune stimulators for T cells, resulting in polyclonal T cell proliferation and pro-inflammatory cytokine production [36]. In vitro studies demonstrated that stimulating T cells with staphyloccal exotoxins (alpha-toxin and SEB), strongly induced IL-17A-secreting T cells [13, 37]. Therefore, the involvement of TH17 cells in GPA may possibly be driven by chronic nasal carriage of S. aureus. However, we did not observe increased frequencies of TEM17 cells in GPA patients carrying S. aureus. This observation is in line with earlier studies in GPA patients in which no correlation between the presence of staphylococcal superantigens and the expansion of T cell subsets in peripheral blood was found [27].
Remarkably, we observed that the proportion of TEM17 cells in r-GPA patients was highly associated with CMV serostatus with frequencies of TEM17 cells being decreased in CMV-seropositive r-GPA patients as compared to seronegative r-GPA patients. These observations indicate that latent infection with human CMV modulates the distribution of TEM cell subsets, although the underlying mechanisms are unclear. For instance, CMV seropositivity is strongly associated with the presence of memory T cells. It has been demonstrated that only CMV-seropositive individuals possess significant numbers of CD4+CD28- T cells and many of these T cells respond to CMV [38]. In fact, the expansion of CD4+CD28- T cells in GPA is suggested to be driven by CMV infections, and is associated with increased risk of infection and mortality [15]. However, the precise role of CMV infection in TH1 and TH17 responses is poorly understood. Previous studies indicate that T cells expressing CXCR3 (TH1 type) arise during primary CMV infection and are maintained during latency [39]. In line with this study, we observed increased proportions of TEM1 cells in the circulation of CMV-seropositive r-GPA patients. The skewing toward a TEM1 response in CMV-seropositive r-GPA patients could also explain the decrease in the proportion of TEM17 cells since these two TEM cells subsets inversely correlate with each other. Importantly, the difference in TEM1 cells between r-GPA patients and HCs was not influenced by CMV and age. Additionally, CMV serostatus did not influence the proportions of TEM1 cells in HCs whereas in r-GPA patients CMV serostatus had a major impact on both the proportions of TEM1, and TEM17 cells.
TH17 cells may also induce autoimmune responses. Very recently, it was shown that the frequency of TH17 cells (CCR6+) in rheumatoid arthritis (RA) patients is associated with anti-citrullinated protein antibodies (ACPA) status [28]. In particular, CCR6+ TH cell proportions were higher in ACPA-positive RA patients in comparison to ACPA-negative RA patients, and inversely correlated with disease duration in ACPA-negative patients. If this were the case in GPA patients, one may argue that the increase in TEM17 cells might be associated with ANCA status and could be a tool to discriminate ANCA-positive patients from those that are ANCA-negative. In contrast to the data in RA patients, we did not observe any association regarding ANCA status with the frequency of TEM17 cells in r-GPA patients. This is possibly due to the fact that ANCA titers in GPA patients fluctuate during the disease course, whereas ACPA-positive RA patients consistently remain ACPA-positive over time. On the other hand, we found that TEM17 cells in GPA patients showed a positive association with organ involvement, whereas TEM1 cells were negatively associated with organ involvement. This suggests a more severe disease course in individuals with a high frequency of TEM17 cells. Furthermore, we observed that persistent TEM17 expansion is associated with a higher tendency to relapse.
The current study was designed as a cross-sectional study using peripheral blood of quiescent GPA patients and HCs. The main limitations are the lack of absolute lymphocyte counts and study samples from GPA patients with active disease. Therefore, the current data only provides observational information of proportions of circulating CD4+ TEM cell subsets in r-GPA patients. Further studies are warranted to assess blood samples from patients during active disease and to study the distribution of infiltrated TEM cell subsets in nasal and renal biopsies to elucidate distinct migratory capacities of TEM1 and TEM17 cells and to confirm their role in inflamed target tissues in GPA. Since TEM cells also appear in the urine during active renal GPA disease, analysis of urine samples might aid in demonstrating which distinct TEM subsets are possibly involved in renal injury.
Conclusions
This study describes the distribution of circulating CD4+ TEM cell subsets identified based on chemokine receptor expression in r-GPA patients without any in vitro manipulation. It demonstrates an aberrant balance between TEM1 and TEM17 cells in r-GPA patients, which is shown to be associated with severity of the disease in terms of organ involvement, and tendency to relapse. Interestingly, the imbalance between TEM1 and TEM17 cells is modulated in CMV-seropositive r-GPA patients. Accordingly, future T cell phenotype studies should take into account chronic viral infections (i.e. CMV) for CD4+TEM subset characterization.
Acknowledgements
We thank all patients and healthy volunteers for kindly providing blood samples for this study. We thank Minke Huitema for her technical assistance with the anti-CMV ELISA, Dr. Susanne Arends for her statistical assistance, and Dr. Liesbeth Brouwer for inclusion of healthy volunteers.
Funding
This work was supported by funding from the Dutch Arthritis Foundation (Reumafonds project number 12-2-407), WHA is supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement number 668036 (project RELENT).
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its Additional files).
Authors’ contributions
LLL performed the experiments, performed statistical analysis, drafted the manuscript, and contributed to concept and design. WHA and PH contributed to concept and design, interpretation of the data, and critically revised the manuscript. AR and CAS contributed to concept and design, inclusion of patients with GPA, analysis and interpretation of clinical data, and critical revision of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Written informed consent was obtained from all study participants. The study was approved by the institutional Medical Ethics Review Board of the University Medical Center Groningen (METc2010/057). All procedures were in accordance with the Declaration of Helsinki.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACPA
Anti-citrullinated protein antibodies
- BVAS
Birmingham Vasculitis Activity Score
- CCR
C-C chemokine receptor
- CD
Cluster of differentiation
- CMV
Cytomegalovirus
- CRP
C-Reactive protein
- CXCR3
CXC chemokine receptor 3
- eGFR
Estimated glomerular filtration rate
- ELISA
Enzyme-linked immunosorbent assay
- ENT
Ear, nose and throat
- GPA
Granulomatosis with polyangiitis
- HC
Healthy control
- IL
Interleukin
- PR3-ANCA
Antineutrophil cytoplasmic antibodies targeting proteinase 3
- RA
Rheumatoid arthritis
- r-GPA
GPA patient in remission
- S. aureus
Staphylococcus aureus
- TEM
Effector memory T cell
- TH
T helper
- TREG
regulatory T cell
Additional file
Linear regression analysis for percentages of CD4 + TEM cells, TEM1, and TEM17 cells between r-GPA patients and HCs. (PDF 208 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-017-1343-8) contains supplementary material, which is available to authorized users.
Contributor Information
Lucas L. Lintermans, Email: l.l.lintermans@umcg.nl
Abraham Rutgers, Email: a.rutgers@umcg.nl.
Coen A. Stegeman, Email: c.a.stegeman@umcg.nl
Peter Heeringa, Email: p.heeringa@umcg.nl.
Wayel H. Abdulahad, Email: w.abdulahad@umcg.nl
References
- 1.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512–23. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 2.Gephardt GN, Ahmad M, Tubbs RR. Pulmonary vasculitis (Wegener’s granulomatosis). immunohistochemical study of T and B cell markers. Am J Med. 1983;74(4):700–4. doi: 10.1016/0002-9343(83)91030-6. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer E, Stegeman CA, Huitema MG, Limburg PC, Kallenberg CG. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener’s granulomatosis (WG) Clin Exp Immunol. 1994;98(3):448–53. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer E, Tervaert JW, Horst G, et al. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener’s granulomatosis and clinically related disorders. Clin Exp Immunol. 1991;83(3):379–86. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol. 1999;103(5 Pt 1):885–94. doi: 10.1016/S0091-6749(99)70434-3. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood CM, Thiru S, Isaacs JD, Hale G, Waldmann H. Long-term remission of intractable systemic vasculitis with monoclonal antibody therapy. Lancet. 1993;341(8861):1620–2. doi: 10.1016/0140-6736(93)90759-A. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt WH, Hagen EC, Neumann I, et al. Treatment of refractory Wegener’s granulomatosis with antithymocyte globulin (ATG): an open study in 15 patients. Kidney Int. 2004;65(4):1440–8. doi: 10.1111/j.1523-1755.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- 8.Ludviksson BR, Sneller MC, Chua KS, et al. Active Wegener’s granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160(7):3602–9. [PubMed] [Google Scholar]
- 9.Schonermarck U, Csernok E, Trabandt A, Hansen H, Gross WL. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA-associated vasculitides. Clin Exp Rheumatol. 2000;18(4):457–63. [PubMed] [Google Scholar]
- 10.Muller A, Trabandt A, Gloeckner-Hofmann K, et al. Localized Wegener’s granulomatosis: predominance of CD26 and IFN-gamma expression. J Pathol. 2000;192(1):113–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH656>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Lamprecht P, Bruhl H, Erdmann A, et al. Differences in CCR5 expression on peripheral blood CD4 + CD28- T-cells and in granulomatous lesions between localized and generalized Wegener’s granulomatosis. Clin Immunol. 2003;108(1):1–7. doi: 10.1016/S1521-6616(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira E, Hamour S, Sawant D, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2010;25(7):2209–17. doi: 10.1093/ndt/gfp783. [DOI] [PubMed] [Google Scholar]
- 13.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2008;58(7):2196–205. doi: 10.1002/art.23557. [DOI] [PubMed] [Google Scholar]
- 14.Abdulahad WH, Stegeman CA, Van der Geld YM, Doornbos-van der Meer B, Limburg PC, Kallenberg CG. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2007;56(6):2080–91. doi: 10.1002/art.22692. [DOI] [PubMed] [Google Scholar]
- 15.Morgan MD, Pachnio A, Begum J, et al. CD4 + CD28- T cell expansion in granulomatosis with polyangiitis (Wegener’s) is driven by latent cytomegalovirus infection and is associated with an increased risk of infection and mortality. Arthritis Rheum. 2011;63(7):2127–37. doi: 10.1002/art.30366. [DOI] [PubMed] [Google Scholar]
- 16.Abdulahad WH, van der Geld YM, Stegeman CA, Kallenberg CG. Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int. 2006;70(5):938–47. doi: 10.1038/sj.ki.5001670. [DOI] [PubMed] [Google Scholar]
- 17.Abdulahad WH, Kallenberg CG, Limburg PC, Stegeman CA. Urinary CD4+ effector memory T cells reflect renal disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60(9):2830–8. doi: 10.1002/art.24747. [DOI] [PubMed] [Google Scholar]
- 18.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 19.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187(1):129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162(3):1278–86. [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 22.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33(8):1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 24.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 25.Tervaert JW, Mulder L, Stegeman C, et al. Occurrence of autoantibodies to human leucocyte elastase in Wegener’s granulomatosis and other inflammatory disorders. Ann Rheum Dis. 1993;52(2):115–20. doi: 10.1136/ard.52.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87(11):671–8. [PubMed] [Google Scholar]
- 27.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Staphylococcal superantigens and T cell expansions in Wegener’s granulomatosis. Clin Exp Immunol. 2003;132(3):496–504. doi: 10.1046/j.1365-2249.2003.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulissen SM, van Hamburg JP, Davelaar N, et al. CCR6(+) th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther. 2015;17:344. doi: 10.1186/s13075-015-0800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odobasic D, Gan PY, Summers SA, et al. Interleukin-17A promotes early but attenuates established disease in crescentic glomerulonephritis in mice. Am J Pathol. 2011;179(3):1188–98. doi: 10.1016/j.ajpath.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120(1):12–7. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lamprecht P, Erdmann A, Mueller A, et al. Heterogeneity of CD4 and CD8+ memory T cells in localized and generalized wegener’s granulomatosis. Arthritis Res Ther. 2003;5(1):R25–31. doi: 10.1186/ar610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masutani K, Tokumoto M, Nakashima H, et al. Strong polarization toward Th1 immune response in ANCA-associated glomerulonephritis. Clin Nephrol. 2003;59(6):395–405. doi: 10.5414/CNP59395. [DOI] [PubMed] [Google Scholar]
- 33.Wilde B, Thewissen M, Damoiseaux J, et al. Th17 expansion in granulomatosis with polyangiitis (Wegener’s): The role of disease activity, immune regulation and therapy. Arthritis Res Ther. 2012;14(5):R227. doi: 10.1186/ar4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilhorst M, Shirai T, Berry G, Goronzy JJ, Weyand CM. T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol. 2014;5:432. doi: 10.3389/fimmu.2014.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zycinska K, Wardyn KA, Zielonka TM, Demkow U, Traburzynski MS. Chronic crusting, nasal carriage of staphylococcus aureus and relapse rate in pulmonary Wegener’s granulomatosis. J Physiol Pharmacol. 2008;59(Suppl 6):825–31. [PubMed] [Google Scholar]
- 36.Popa ER, Stegeman CA, Kallenberg CG, Tervaert JW. Staphylococcus aureus and wegener’s granulomatosis. Arthritis Res. 2002;4(2):77–9. doi: 10.1186/ar392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niebuhr M, Gathmann M, Scharonow H, et al. Staphylococcal alpha-toxin is a strong inducer of interleukin-17 in humans. Infect Immun. 2011;79(4):1615–22. doi: 10.1128/IAI.00958-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Leeuwen EM, Remmerswaal EB, Vossen MT, et al. Emergence of a CD4 + CD28- granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol. 2004;173(3):1834–41. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 39.van de Berg PJ, Yong SL, Remmerswaal EB, van Lier RA, ten Berge IJ. Cytomegalovirus-induced effector T cells cause endothelial cell damage. Clin Vaccine Immunol. 2012;19(5):772–9. doi: 10.1128/CVI.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Additional files).


