Abstract
Introduction
Acute intermittent hypoxia (AIH) enhances lower extremity motor function in humans with chronic incomplete spinal cord injury (SCI). AIH-induced spinal plasticity is inhibited by systemic inflammation in animal models. Since SCI is frequently associated with systemic inflammation in humans, we tested the hypothesis that pretreatment with the anti-inflammatory agent ibuprofen enhances the effects of AIH.
Methods
A randomized, double-blinded, placebo-controlled crossover design was used. Nine adults (mean age 51.1 ± 13.1 years) with chronic motor-incomplete SCI (7.7 ± 6.3 years post-injury) received a single dose of ibuprofen (800 mg) or placebo, 90 minutes prior to AIH. For AIH, 9% O2 for 90 seconds was interspersed with 21% O2 for 60 seconds. Maximal voluntary ankle plantar flexion isometric torque was assessed prior to, and at 0, 30, and 60 minutes post-AIH. Surface electromyography (EMG) of plantar flexor muscles was also recorded.
Results
Torque increased significantly after AIH at 30 (P = 0.007; by ∼20%) and 60 (P < 0.001; by ∼30%) minutes post-AIH versus baseline. Ibuprofen did not augment the effects of AIH. EMG activity did not increase significantly after AIH; however, there was a significant association between increases in torque and EMG in both gastrocnemius (R2 = 0.17, P < 0.005) and soleus (R2 = 0.17, P < 0.005) muscles.
Conclusions
AIH systematically increased lower extremity torque in individuals with chronic incomplete SCI, but there was no significant effect of ibuprofen pretreatment. Our study re-confirms the ability of AIH to enhance leg strength in persons with chronic incomplete SCI.
Keywords: Spinal cord injury, Hypoxia, Ibuprofen, Neuronal plasticity, Muscle strength dynamometer, Rehabilitation, Humans
Introduction
Traumatic spinal cord injuries (SCI) disrupt the descending pathways between the brain and spinal cord, resulting in impairment of motor control and loss of function below the level of the injury. Most SCIs are incomplete,1,2 and spinal plasticity contributes to functional recovery by strengthening spared synaptic pathways and by the formation of new collateral pathways from neurogenic sprouting.3 The extent of spontaneous neurological recovery is often limited,3,4 therefore new strategies to harness and enhance spinal plasticity are needed to promote functional recovery in individuals with chronic incomplete spinal injuries.
Spinal plasticity and functional recovery can be enhanced by exposure to acute intermittent hypoxia (AIH), a novel and non-invasive treatment modality that constitutes brief periods of low oxygen (i.e. ∼10% O2) interrupted by return to normoxia (i.e. ∼21% O2).5–7 This approach was first investigated in animals, whereby AIH elicits robust plasticity within the spinal respiratory networks via a serotonin-dependent mechanism. Specifically, AIH induces episodic serotonin receptor activation, resulting in denovo synthesis of brain-derived neurotropic factor (BDNF) and activation of its high affinity tropomyosin receptor kinase B (TrkB) receptor, thereby strengthening synaptic pathways to phrenic motor neurons.8–12 In animal models of high cervical SCI with respiratory paralysis, AIH promotes recovery of respiratory function by strengthening phrenic motor output through this same mechanism.7,13,14 Lastly, AIH was demonstrated to enhance plasticity within nonrespiratory somatic motor nuclei in these same models of SCI. In particular, repetitive AIH treatment (daily for 1 week) in rats with chronic cervical SCI induced recovery of forelimb function in additional to breathing capacity, lasting for several days.7
These pre-clinical studies prompted two recent landmark reports in humans with chronic SCI. Trumbower et al.5 established that a single, brief AIH presentation (fifteen 1-minute hypoxic episodes, 1-minute intervals) improved ankle strength by more than 80% in individuals with chronic incomplete SCI, and the effects lasted for up to 1 hour post-intervention. In a subsequent study, Hayes et al.6 showed that combined AIH and gait training for 5 days induced prolonged functional improvements in both over-ground walking speed and distance in individuals with chronic incomplete SCI. However, these results demonstrated some variability in responses between participants, which may relate to specific factors that undermine the impact of AIH.
In animal models, multiple factors have been shown to undermine the impact of AIH on spinal motor function.15,16 In particular, systemic low-grade inflammation, induced by injecting a small dose of bacterial endotoxins (lipopolysaccharides), abolishes AIH-induced facilitation of spinal respiratory network.17 Other forms of plasticity within the nervous system, such as hippocampal long-term potentiation and spinal instrumental learning, are also inhibited in the presence of systemic inflammation.18–20 Several studies report that chronic SCI is associated with a low-grade, systemic inflammatory state in the absence of active infections or pressure sores;21–23 suggesting that AIH-induced plasticity may be impaired in individuals with chronic SCI. Accordingly, we tested the hypothesis that pre-treatment with an anti-inflammatory drug will enhance the ability of AIH to induce spinal motor plasticity in humans with chronic SCI. In this pilot study, we administered a single oral dose of ibuprofen, a non-steroidal anti-inflammatory drug (NSAID) that has a low risk profile, to determine if it would enhance the effect of AIH on plantar flexion torque in persons with chronic incomplete SCI.
NSAIDs are simple, safe and effective, and are known to reverse the impact of systemic inflammation on AIH-induced respiratory long-term facilitation (LTF) in rodents.17 Thus, NSAIDs offer the potential to augment the therapeutic benefits of AIH in humans with chronic SCI. The objective of this study was to determine whether pretreatment with a single anti-inflammatory dose of ibuprofen is effective in promoting AIH-induced motor output in a pilot group of individuals with chronic incomplete SCI. We hypothesized that maximal voluntary torque would be increased by AIH and that ibuprofen would enhance this effect compared with placebo. Results would guide a larger clinical trial, or help refine our research methodology for future studies utilizing anti-inflammatory medications.
Methods
All experiments were performed at a single rehabilitation center affiliated with a large academic university. All participants gave written informed consent, and our protocol was approved by the university's Institutional Review Board.
Subjects
Adults (N = 10) with chronic ( > 1 year post-injury) motor-incomplete SCI [American Spinal Injury Association Impairment Scale (AIS) score C or D] due to non-progressive etiology were enrolled. We included subjects with injury levels between C2–T12 who had no joint contractures and had some volitional ankle plantar flexion strength in at least one leg.
Study participants were contacted by a physical therapist by telephone for recruitment. The physical therapist screened the participants for inclusion criteria, and obtained a detailed past medical history and list of current medications (both prescription and over-the-counter).
Subjects were excluded if there was ongoing cardiac disease, uncontrolled hypertension, restrictive or obstructive pulmonary disease, suspected or confirmed sleep apnea, tracheostomy, renal disease, or any previous adverse reactions to NSAIDs. To minimize confounding inflammatory conditions, we excluded subjects with uncontrolled diabetes mellitus, active infection, unhealed decubitus ulcers, active heterotopic ossification, presence of deep venous thrombosis, rheumatologic disease, inflammatory arthritis, or cancer. Subjects who met the criteria to participate were required to suspend all NSAIDs and anti-spasticity medications for a minimum of 14 days prior to participation. Twenty-four to forty-eight hours prior to participation, one of the study physicians contacted each subject by telephone to verify the subject's past medical history and to confirm no new acute medical issues had arisen in the interim that would otherwise exclude the subject from participation.
Design
A double-blinded, randomized placebo-controlled, crossover study design was used to compare the effects of ibuprofen and placebo pretreatments. Participants visited the laboratory on two occasions, at least 1 week apart, and orally received either ibuprofen (800 mg) or a matching placebo, in a random order. A research pharmacist performed randomization; and both subjects and investigators were blinded to the study drug administered. Drug administration was followed by a 90-minute period to allow for absorption of the medication, prior to the AIH intervention. Outcome measures were recorded before administration of the drug and at 0, 30, and 60 minutes post-AIH.
Experimental setup and protocol
Upon arrival to the laboratory, absence of joint contractures was confirmed and lower extremity motor score (LEMS) of each subject's stronger leg was assessed by an experienced physical therapist. The subject was then transferred to an adjustable chair. The experimental setup was similar to that used by Trumbower et al.5 The tested foot was secured to the footplate of a custom-built dynamometer (which incorporated a six degree of freedom load cell) with toe and ankle straps. Padded stabilization shoulder and pelvic straps were placed across the upper chest and waist to maintain trunk position. The ankle joint axis was positioned so that it was aligned with the dynamometer's center of rotation (Fig. 1A). The hip was flexed to 80°, the knee was flexed to 10°, and the ankle was plantar flexed to 0° to minimize contributions of other muscle groups. Once seated, surface electromyogram (EMG) electrodes were placed over agonist and antagonist muscles, according to SENIAM guidelines24 and secured with tape.
Figure 1.
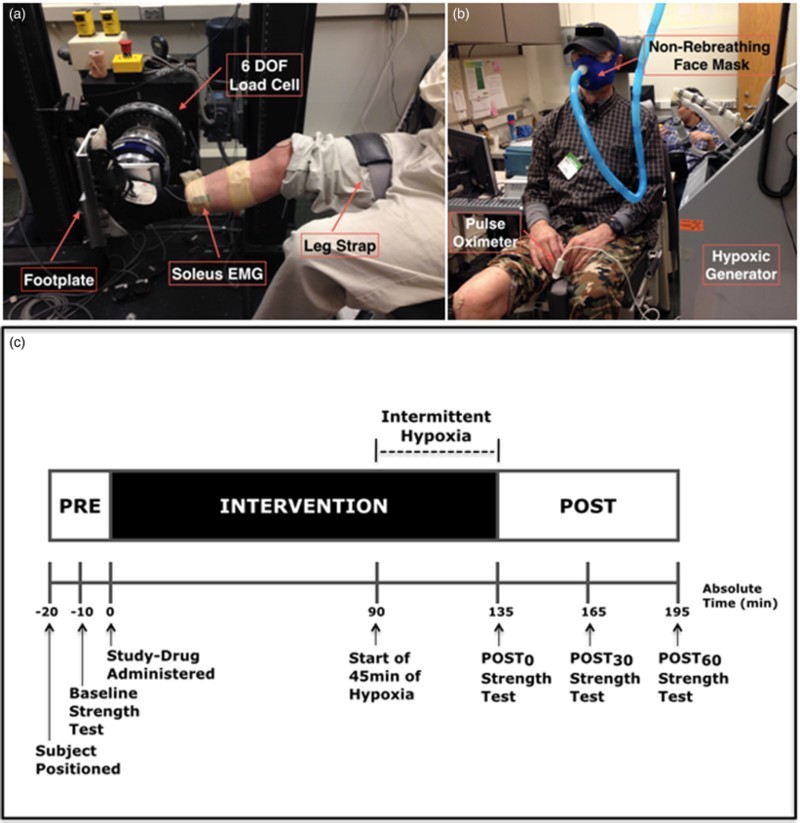
Experimental set-up and protocol. (A) Subject seated in the isokinetic dynamometer, with foot attached to a footplate and aligned with the 6 degrees of freedom (DOF) load cell. Surface EMG electrodes are attached to the soleus, as well as the medial gastrocnemis and tibialis anterior (not in view). (B) The subject is wearing the mask for administration of hypoxia/normoxia and a finger pulse oximeter for measures of heart rate and oxygen saturation. (C) Protocol for baseline measures, the intervention, and post-intervention measures.
After setup was complete, baseline ankle plantar flexion strength and muscle activity were tested (see Outcome measures described below). Ibuprofen or placebo was administered orally, and 90 minutes were allowed for drug absorption and distribution. The subject then received AIH for 45 minutes via a non-rebreathing face mask, connected to an oxygen generator (Model HYP-123, Hypoxico Inc, New York, NY, USA) in which the subject was exposed to 9% O2 for 90 seconds, alternating with 21% O2 for 60 seconds, for a total of 45 minutes.5 Changes in inspired oxygen concentration were continuously monitored (Model OM-25RME; Maxtec Inc, Salt Lake City, UT, USA). Oxyhemoglobin saturation and heart rate were continuously monitored with a finger pulse oximeter for safety, and were recorded at the end of each hypoxic and normoxic episode to document severity of hypoxic episodes (Autocorr Plus; Smith Medical Inc, New York, NY, USA) (Fig. 1B). Plantar flexion strength and EMG were measured again immediately after the AIH intervention (Post0), and 30 minutes and 60 minutes after the intervention (Post30 and Post60 respectively). Fig. 1C summarizes the experimental protocol.
Outcome measures
Isometric torque
The primary outcome measure was ankle plantar flexion strength, as assessed by maximal voluntary isometric torque (MVT) recorded by a custom-built isokinetic dynamometer. For each MVT assessment, the subject was directed to generate maximal plantar flexion force by pushing as hard as possible against the dynamometer footplate for a total of 5 seconds, after the onset of an auditory cue signal. Subjects were provided with vigorous verbal encouragement. The resultant torque was sampled at 1 kHz, and anti-alias filtered on-line at 200 Hz, using an 18-bit analog-to-digital DAQ (NI PCI-6289; National Instruments, Austin, TX, USA) on a single PC. These data were acquired using custom programs in LabVIEW (National Instruments, Inc.) and Matlab (Mathworks, Inc, Natick, MA, USA). Three MVT assessments were obtained at each time point (baseline, Post0, Post30, and Post60), with a minimum of 1 minute between contractions. Maximal torque was taken as the average of ± 50 msec from the peak torque value obtained from each MVT trial. The trial with the highest torque value was used for further analysis.
EMG response
Agonist and antagonist muscle activity during ankle MVT were recorded using surface EMG electrodes placed over the medial gastrocnemius, soleus, and tibialis anterior as our secondary outcome measures. EMG signals were collected using a Bagnoli-16 system (Delsys USA, Inc), sampled at 2000 Hz and synchronized with torque data using the analog-to-digital converter, amplified 1000 times and filtered with a bandwidth of 20–450 Hz. Mean EMG activity and median frequency of the EMG spectrum for each muscle was computed from a period of 150–50 milliseconds preceding the maximum absolute value of the torque signal for each contraction (Fig. 2 is provided as an example). For further analysis of EMG activity, data were taken from the trial with the highest measured torque obtained across the 3 contractions for each time point.
Figure 2.
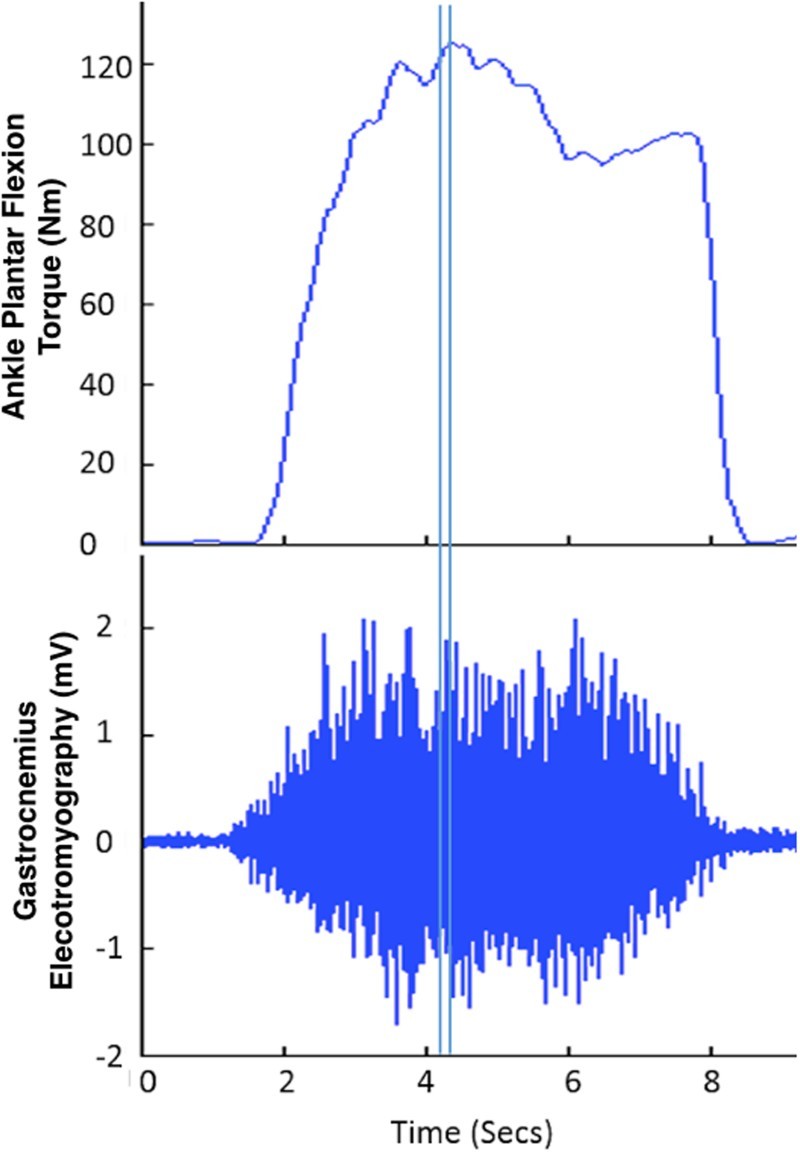
Ankle ankle plantar flexion torque and corresponding EMG response of the gastrocnemius are shown for subject S02 baseline placebo condition. The double vertical lines indicate the 150–50 milliseconds preceding the maximum torque signal. This time frame was used to calculate the mean activity and median frequency of the EMG signal.
Other outcome measures
Blood pressure was measured using an electronic blood pressure cuff, recorded before and immediately after the AIH. The cuff was positioned on the subject's arm, according to the manufacturer's guidelines. We monitored for headaches, pain, lightheadedness, dizziness, respiratory distress, cyanosis, spasms, and autonomic dysreflexia, during and after AIH as reported by the patient or observed by the study administrator.
Statistical analyses
All data are presented as mean ± standard error (SE) unless stated otherwise. Statistical analyses were carried out in SPSS (version 21, IBM Corp, Armonk, NY, USA). Data were tested for normality using the Shapiro-Wilk test. Paired student's t-tests were used to compare blood pressure between ibuprofen and placebo trials, before and after AIH. Repeated-measures analysis of variance (ANOVA) was used to compare the effects of time-point and drug on heart rate, oxygen saturation, torque, and EMG activity. Nonlinear regression analysis was used to examine the relationship between baseline torque and change in torque after AIH. Linear regression was used to correlate the change in torque after AIH with change in EMG activity and median frequency of medial gastrocnemius, soleus, and tibialis anterior muscles.
Results
All subjects completed both sessions with no reported negative effects. No adverse clinical events were recorded. Data for one subject (subject S08) could not be included due to technical difficulties with the equipment, therefore all torque and EMG analyses were carried out on 9 subjects. Demographics, baseline LEMS for the subject's stronger leg, and blood pressure recordings for all subjects are provided in Table 1. Systolic and diastolic blood pressure was significantly reduced after AIH in the ibuprofen condition compared to baseline, but did not differ significantly between the ibuprofen and placebo conditions (Table 1). There were no significant differences in heart rate and oxygen saturation between the two conditions (Fig. 3).
Table 1.
Individual and mean demographics, lower extremity motor scores (LEMS) of the subject's stronger leg, ASIA Impairment Scale (AIS) scores, and blood pressure pre- and post-administration of acute intermittent hypoxia (AIH). Asterisk * indicates significant differences at P < 0.05 compared with pre-AIH
| Blood Pressure |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ibuprofen |
Placebo |
|||||||||
| ID | Sex | Age | Injury level | Time since injury (years) | LEMS | AIS Score | Pre AIH | Post AIH | Pre AIH | Post AIH |
| S01 | M | 51 | C5 | 18 | 19 | D | 112/78 | 113/51 | 116/74 | 103/76 |
| S02 | M | 65 | C4 | 10 | 21 | D | 129/88 | 113/81 | 119/76 | 117/68 |
| S03 | F | 44 | T6 | 14 | 24 | D | 110/81 | 113/83 | 108/80 | 122/86 |
| S04 | M | 42 | C5 | 16 | 22 | D | 112/79 | 93/64 | 106/77 | 94/71 |
| S05 | M | 55 | C2 | 7 | 20 | D | 116/84 | 106/83 | 113/82 | 102/75 |
| S06 | M | 49 | C5 | 2 | 23 | D | 119/85 | 130/84 | 110/77 | 101/73 |
| S07 | M | 66 | T12 | 2 | 20 | D | 144/101 | 113/78 | 121/74 | 129/83 |
| S08 | M | 49 | C5 | 3 | 22 | D | 139/90 | 114/82 | 139/95 | 132/94 |
| S09 | M | 72 | C5 | 2 | 19 | D | 134/80 | 128/76 | 112/67 | 115/66 |
| S10 | M | 28 | C6 | 3 | 18 | D | 122/74 | 105/67 | 148/81 | 131/84 |
| Mean | 52.1 | 7.7 | 20.8 | 124/84 | 113*/75* | 119/78 | 115/78 | |||
| SD | 13.1 | 6.3 | 1.9 | 12/8 | 11/11 | 14/7 | 14/9 | |||
Figure 3.
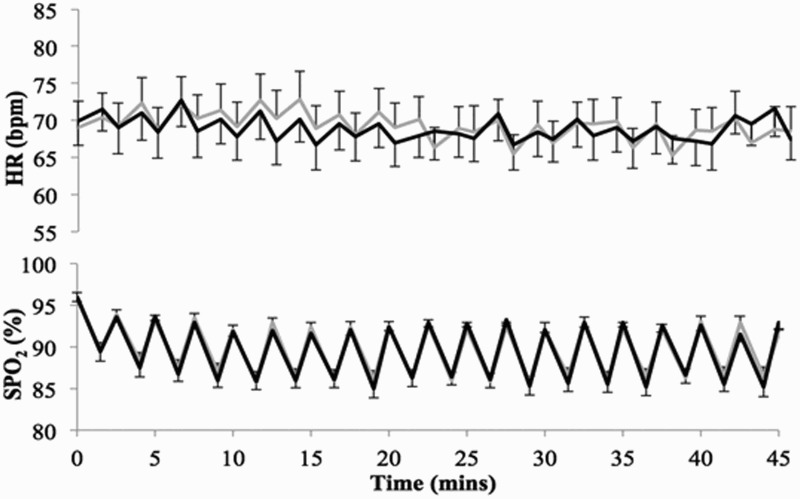
Mean ± 1SE of heart rate (HR; upper panel) and oxygen saturation (SPO2; lower panel) during the 45-minute administration of acute intermittent hypoxia (AIH), for ibuprofen (grey) and placebo (black) conditions.
Isometric torque
Maximal voluntary plantar flexion torque increased with time (P = 0.006). Maximal torque was significantly greater than baseline at Post30 (P = 0.007) and Post60 (P < 0.001) for both conditions. In the ibuprofen condition, post-AIH torque at Post0, Post30, and Post60 was respectively 110.6 ± 8.0%, 117.7 ± 8.3%, and 130.6 ± 12.1% of baseline torque. Equivalent values for the placebo condition were 110.4 ± 7.7%, 121.2 ± 8.9%, and 122.8 ± 8.5%. There were no significant differences in torque values between ibuprofen and placebo conditions at any of the time points (drug: P = 0.231; drug x time: P = 0.819), although the ibuprofen condition appeared to perform better than placebo beginning at 60 minutes post-AIH (Fig. 4A). Our subjects exhibited a wide range of responses to hypoxia with the different drug pre-treatments (Fig. 4B). We also observed a strong correlation (R = 0.7) between baseline torque and the change in torque after AIH (P = 0.003, Fig. 5). Thus, individuals with low baseline torque showed a higher increase in torque following AIH.
Figure 4.
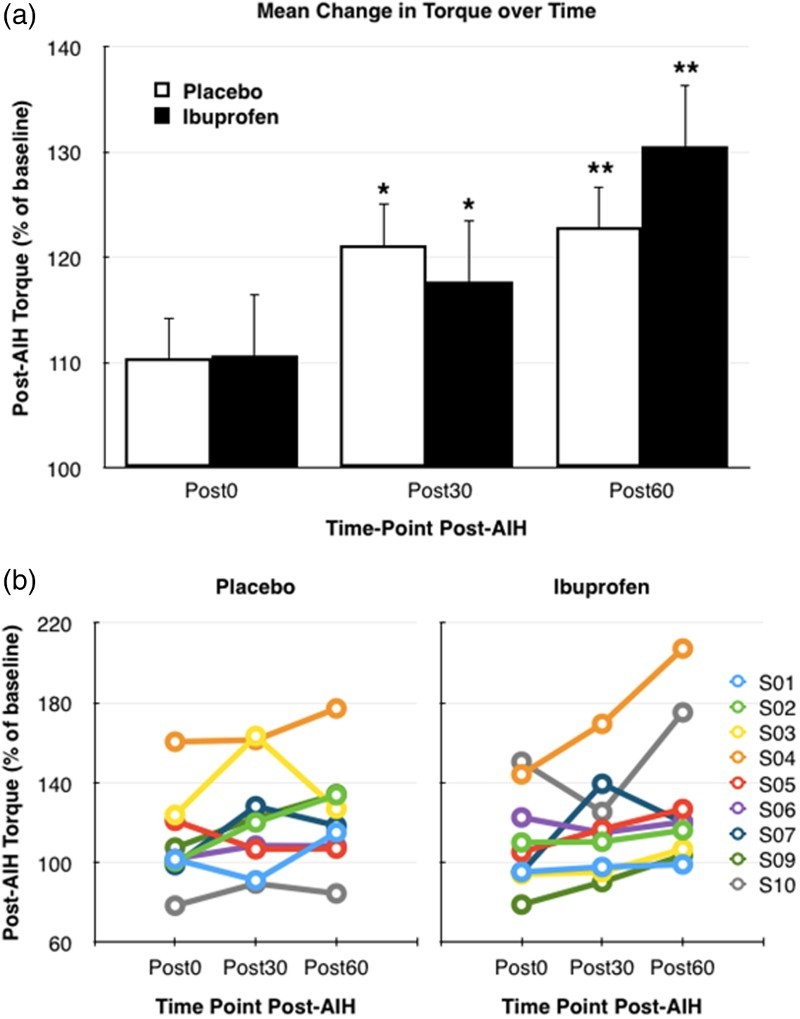
(A) Mean change in torque ±1SE over time after drug and acute intermittent hypoxia (AIH) administration, for placebo (white) and ibuprofen (black) conditions. Asterisks indicate statistical comparison to baseline (pre-AIH) where * indicates significance at P < 0.05 and ** indicates significance at P < 0.005. (B) Individual subject peak torque over time as percent of pre-AIH baseline torque for the placebo trial (left graph) and ibuprofen trial (right graph).
Figure 5.
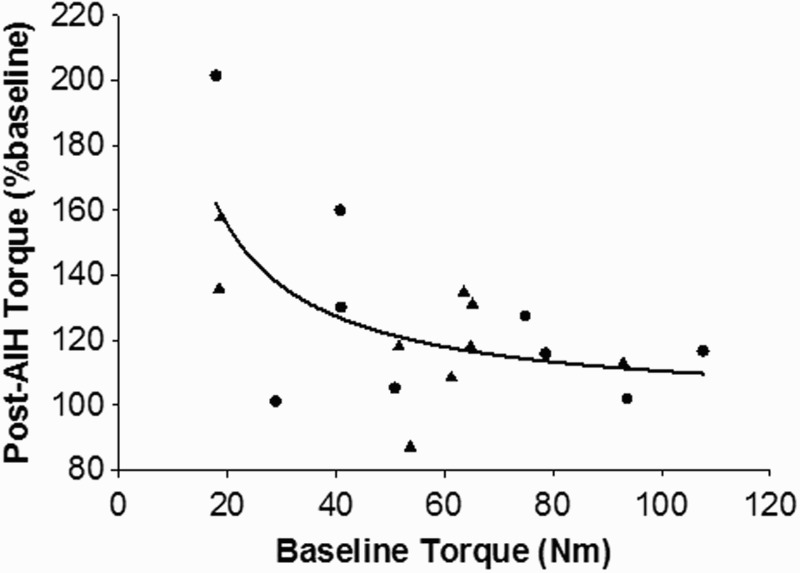
Relationship between baseline torque and percent change in torque following AIH. The ibuprofen (black circles) and placebo (black triangles) data was grouped together, and nonlinear regression analysis was done to show that humans with a lower torque at baseline have a higher increase in torque following the AIH session.
EMG response
There were no significant effects of AIH or drug administration on EMG activity or median power frequency for all muscles measured (Table 2; P > 0.05). However, there was a significant association between the increase in torque and EMG activity of both medial gastrocnemius (R2 = 0.17, P < 0.005) and soleus (R2 = 0.17, P < 0.005) muscles (Fig. 6), and no association with tibialis anterior activity. There was no significant association between the change in maximal torque and median frequency in any muscle measured.
Table 2.
Mean (SD) electromyographic (EMG) activity and median power frequency of the medial gastrocnemius (MG), soleus (Sol) and tibialis anterior (TA) muscles pre (0 min, baseline) and post (135, 165 and 195 mins) study drug administration (respectively Post0, Post30, and Post60 after acute intermittent hypoxia), for ibuprofen and placebo groups
| Time-point (minutes) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 135 Post0 | 165 Post30 | 195 Post60 | 0 | 135 Post0 | 165 Post30 | 195 Post60 | ||
| Muscle | Ibuprofen |
Placebo |
|||||||
| EMG Activity (mV) | MG | 0.33 (0.34) | 0.24 (0.27) | 0.32 (0.36) | 0.35 (0.45) | 0.40 (0.42) | 0.43 (0.48) | 0.41 (0.45) | 0.38 (0.40) |
| Sol | 0.20 (0.26) | 0.20 (0.25) | 0.23 (0.27) | 0.24 (0.31) | 0.18 (0.21) | 0.17 (0.20) | 0.17 (0.21) | 0.20 (0.25) | |
| TA | 0.19 (0.25) | 0.17 (0.30) | 0.16 (0.29) | 0.17 (0.30) | 0.18 (0.14) | 0.16 (0.14) | 0.19 (0.16) | 0.19 (0.16) | |
| Median Frequency | MG | 125.1 (27.7) | 127.5 (25.8) | 127.7 (26.6) | 126.7 (26.3) | 117.9 (28.9) | 120.3 (27.0) | 118.5 (27.6) | 118.4 (26.9) |
| Sol | 124.4 (25.5) | 126.4 (21.0) | 124.4 (23.8) | 129.1 (24.4) | 127.8 (27.2) | 125.8 (19.8) | 124.7 (21.1) | 124.6 (18.5) | |
| TA | 114.2 (28.3) | 119.1 (19.1) | 118.1 (21.4) | 118.8 (21.5) | 107.0 (30.3) | 111.9 (25.0) | 111.1 (23.3) | 111.7 (22.2) | |
Figure 6.
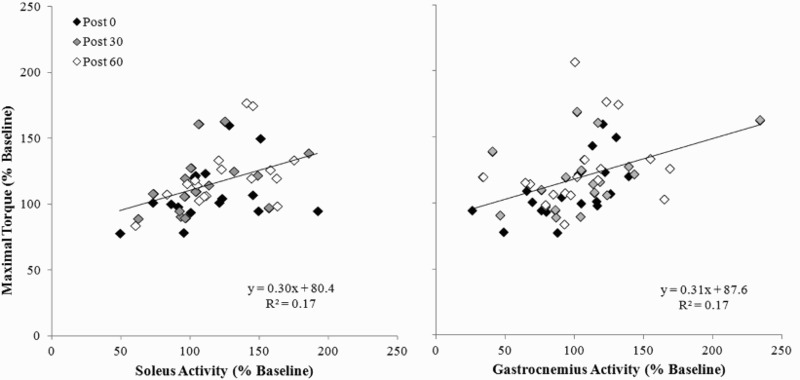
Change in electromyographic activity of soleus and medial gastrocnemius muscles versus change in torque for both conditions post-AIH.
Discussion
AIH is one of the few interventions currently able to bring about immediate increases in motor output in chronic SCI,25,26 with effects demonstrated in subjects injured between 2–18 years ago. In this regard, AIH represents a valuable tool to induce further plasticity in spared neural pathways following incomplete SCI. The present findings show that AIH systematically increased ankle torque generation. Thus we were able to reaffirm the ability of AIH to enhance lower extremity strength in individuals with chronic incomplete SCI.5,6 To our knowledge, this is the first study to investigate the role of anti-inflammatory pretreatment as a means to enhance the impact of AIH in humans.
Phrenic long-term facilitation (pLTF) following AIH is a well-established model of spinal motor plasticity, and it has been studied in a variety of animal models.27–31 In rats, pLTF is adversely affected by low-grade systemic inflammation induced by lipopolysaccharide administration.17,32 Elevated serum concentrations of proinflammatory cytokines (markers of inflammation) have been reported in individuals living with chronic SCI, with or without overt secondary medical complications.33–37 Thus, we suspected that systemic inflammation would be present in our study population even without visible secondary complications, and that ibuprofen administration (an NSAID that mostly functions through cyclooxygenase inhibition at the dose studied) would help augment AIH-induced functional recovery.
This pilot study, however, did not demonstrate significant augmentation in maximal torque with administration of ibuprofen 90 minutes prior to the AIH treatment compared to placebo. There are several possible scenarios that could explain these results. We chose to use a single dose of ibuprofen (i.e. 800 mg) for ease of administration and low risk of adverse effects; however, differential AIH-induced neuro-recovery in ankle plantar flexion strength may have been missed given the limited duration of our experiment. The ibuprofen condition trended towards a higher percent torque output at 195 minutes post-drug administration (60 minutes post-AIH). Differences between conditions may have reached statistical significance if outcome measures were recorded for a longer duration after the intervention. We limited our ibuprofen dose to the highest single administration dose that is FDA-approved in order to maximize the possible effects for each participant; however, this approach does not account for differences in bodyweight between subjects, and this limitation should be acknowledged. It is unclear whether even this maximum allowable dose is sufficient to achieve the systemic anti-inflammatory effects necessary to impact AIH induced functional recovery, or if repeated dosing would be useful. There would be value in investigating the effects of various repeated dosing protocols of ibuprofen over the course of one or several days, alternative NSAIDs, or even corticosteroids to determine the role of inflammation in AIH-induced neuroplasticity.
The true mechanism in which ibuprofen and other NSAIDs may promote AIH-induced LTF is yet to be elucidated. The study by Huxtable et al.17 showed that pLTF impairment in animals outlasted the expression of multiple pro-inflammatory genes, whose expression was initially induced by low-dose lipopolysaccharide administration. Nevertheless, systemic administration of the NSAID ketoprofen restored pLTF following lipopolysaccharide administration. The precise molecules that undermine pLTF through lipopolysaccharide remain unknown,17 although recent studies suggest involvement of spinal p38 mitogen-activated protein (MAP) kinase activity (Huxtable and Mitchell, unpublished observations).
We also observed variability in individual subject response to hypoxia in the placebo and ibuprofen scenarios, such that some subjects demonstrated very high percent increases in torque output following the anti-inflammatory and AIH intervention, whereas other subjects had a minimal response. Although it could not be confirmed statistically, it is possible that ibuprofen may have augmented the effects of AIH in only a subset of our participants, making any mean differences difficult to detect. The level of baseline systemic inflammation in these individuals could be one factor. Alternatively, these differences could be attributed to SCI or demographic characteristics (level and completeness of SCI, time since injury, age, sex, etc.). Future studies should identify a population of individuals with SCI who have elevated serum markers of pro-inflammatory cytokines, to investigate this question further. This protocol may require selecting subjects with known sources of active inflammation (e.g. infection, wounds, heterotopic ossification), rather than excluding such individuals.
Previously Trumbower et al. noted up to an 80% increase in torque output following AIH,5 whereas we observed approximately a 30% increase in torque after our intervention. This may be explained by the fact that the subjects in the study by Trumbower et al. were considerably weaker at baseline compared with our subjects (mean peak torque of approximately 12Nm5 compared with a mean of approximately 60Nm in our study). Indeed, our data indicate that individuals with low baseline torque show a higher increase in torque following AIH (Fig. 5), and that the response to AIH is restricted in individuals with higher initial torque, suggesting perhaps a “ceiling effect.” Therefore, the inclusion of stronger subjects in our study may have caused a comparatively smaller overall effect of AIH on peak torque.
Trumbower et al.5 also noted significant increases in EMG activity in response to AIH in people with incomplete SCI at 30 and 60 minutes post-AIH administration. In the present study we evaluated both EMG amplitude and frequency in order to confirm the findings of Trumbower et al.,5 and to elucidate whether the increases in torque were attributable to increased motor unit recruitment versus rate coding. Surprisingly, there was no significant increase in EMG activity or median firing frequency of the medial gastrocnemius or soleus muscles during ankle MVT at 0, 30 or 60 minutes post-AIH. Since EMG data is inherently more variable than torque data (because it only represents the activity of a subset of neurons in a superficial area of a single plantar flexor muscle), the effect of AIH in the present study may have been insufficient to bring about a statistically significant change in EMG activity. Increased activity may have occurred in deeper plantar flexion muscles, which are not accessible with surface EMG, such as flexor digitorum longus, flexor hallucis longus, and tibialis posterior.
While our pilot study was randomized and blinded, the relatively low number of subjects is a limitation. In addition, since blood samples were not obtained in the present study, we cannot be certain that systemic inflammation was present in our subjects before the drug administration, or whether the drug impacted inflammatory markers. However, as stated previously, we do not know which inflammatory molecules will undermine AIH-induced LTF therefore it is recommended that future studies measure a range of inflammatory markers, before and after drug administration, to establish their specific effects on AIH in humans.
Conclusion
Whereas the extent of neurological recovery after SCI is often limited, AIH holds promise in promoting plasticity and motor output in spared neural pathways. AIH enhances lower extremity maximal torque in individuals with chronic incomplete SCI, but is not affected by a single dose of ibuprofen (800 mg).
Disclaimer statements
Conflicts of interest Gordon Mitchell is a paid consultant for the Christopher and Dana Reeve Foundation. There are no other relevant conflicts of interest to disclose.
Funding Funding provided by the Dr. Ralph and Marian Falk Medical Research Trust.
Scientific meetings The preliminary results of this study were presented in a 10 minute oral presentation at the American Academy of Physical Medicine & Rehabilitation (AAPM&R) Annual Assembly on November 14, 2014 in San Diego, CA.
References
- 1.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 2012;50(5):365–72. doi: 10.1038/sc.2011.178 [DOI] [PubMed] [Google Scholar]
- 2.DeVivo MJ, Chen Y.. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil 2011;92(3):332–8. doi: 10.1016/j.apmr.2010.08.031 [DOI] [PubMed] [Google Scholar]
- 3.Raineteau O, Schwab ME.. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2001;2(4):263–73. doi: 10.1038/35067570 [DOI] [PubMed] [Google Scholar]
- 4.Shin JC, Kim DH, Yu SJ, Yang HE, Yoon SY.. Epidemiologic change of patients with spinal cord injury. Ann Rehabil Med 2013;37(1):50–6. doi: 10.5535/arm.2013.37.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ.. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 2012;26(2):163–72. doi: 10.1177/1545968311412055 [DOI] [PubMed] [Google Scholar]
- 6.Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD.. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 2014;82(2):104–13. doi: 10.1212/01.WNL.0000437416.34298.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, et al. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 2012;32(11):3591–600. doi: 10.1523/JNEUROSCI.2908-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacFarlane PM, Mitchell GS.. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587(Pt 22):5469–81. doi: 10.1113/jphysiol.2009.176982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman MS, Mitchell GS.. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 2011;589(Pt 6):1397–407. doi: 10.1113/jphysiol.2010.201657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker-Herman TL, Mitchell GS.. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 2002;22(14):6239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS.. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol 2001;130(2):207–18. doi: 10.1016/S1095-6433(01)00393-2 [DOI] [PubMed] [Google Scholar]
- 12.Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS.. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 2012;237(1):103–15. doi: 10.1016/j.expneurol.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller DD, Johnson SM, Olson EB Jr, Mitchell GS.. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 2003;23(7):2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golder FJ, Mitchell GS.. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25(11):2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS.. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci 2013;1279:143–53. doi: 10.1111/nyas.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS.. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 2010;669:225–30. doi: 10.1007/978-1-4419-5692-7_45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huxtable AG, Smith SM, Vinit S, Watters JJ, Mitchell GS.. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol (1985) 2013;114(7):879–87. doi: 10.1152/japplphysiol.01347.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vereker E, Campbell V, Roche E, McEntee E, Lynch MA.. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem 2000;275(34):26252–8. doi: 10.1074/jbc.M002226200 [DOI] [PubMed] [Google Scholar]
- 19.Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampà C, et al. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis 2013;52:229–36. doi: 10.1016/j.nbd.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 20.Vichaya EG, Baumbauer KM, Carcoba LM, Grau JW, Meagher MW.. Spinal glia modulate both adaptive and pathological processes. Brain Behav Immun 2009;23(7):969–76. doi: 10.1016/j.bbi.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY.. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc 2007;106(11):919–28. doi: 10.1016/S0929-6646(08)60062-5 [DOI] [PubMed] [Google Scholar]
- 22.da Silva Alves E, de Aquino Lemos V, Ruiz da Silva F, Lira FS, Dos Santos RV, Rosa JP, et al. Low-grade inflammation and spinal cord injury: exercise as therapy? Mediators Inflamm 2013;2013:971841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse LR, Stolzmann K, Nguyen HP, Jain NB, Zayac C, Gagnon DR, et al. Association between mobility mode and C-reactive protein levels in men with chronic spinal cord injury. Arch Phys Med Rehabil 2008;89(4):726–31. doi: 10.1016/j.apmr.2007.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G.. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10(5):361–74. doi: 10.1016/S1050-6411(00)00027-4 [DOI] [PubMed] [Google Scholar]
- 25.Harkema S, Behrman A, Barbeau H.. Evidence-based therapy for recovery of function after spinal cord injury. Handb Clin Neurol 2012;109:259–74. doi: 10.1016/B978-0-444-52137-8.00016-4 [DOI] [PubMed] [Google Scholar]
- 26.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ.. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014;137(Pt 5):1394–409. doi: 10.1093/brain/awu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE.. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol (1985) 1992;73(5):2083–8. [DOI] [PubMed] [Google Scholar]
- 28.Turner DL, Mitchell GS.. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 1997;499(Pt 2):543–50. doi: 10.1113/jphysiol.1997.sp021947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell GS, Powell FL, Hopkins SR, Milsom WK.. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol 2001;124(2):117–28. doi: 10.1016/S0034-5687(00)00197-3 [DOI] [PubMed] [Google Scholar]
- 30.Olson EB Jr., Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, et al. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol (1985). 2001;91(2):709–16. [DOI] [PubMed] [Google Scholar]
- 31.Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, et al. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol (1985). 2008;104(2):499–507. doi: 10.1152/japplphysiol.00919.2007 [DOI] [PubMed] [Google Scholar]
- 32.Vinit S, Windelborn JA, Mitchell GS.. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 2011;176(3):130–5. doi: 10.1016/j.resp.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies AL, Hayes KC, Dekaban GA.. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 2007;88(11):1384–93. doi: 10.1016/j.apmr.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 34.Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma 2002;19(6):753–61. doi: 10.1089/08977150260139129 [DOI] [PubMed] [Google Scholar]
- 35.Manns PJ, McCubbin JA, Williams DP.. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 2005;86(6):1176–81. doi: 10.1016/j.apmr.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 36.Segal JL. Are cytokines pathogenic factors in the physiologic and metabolic sequelae of spinal cord injury? J Am Paraplegia Soc 1992;15(4):209–10. doi: 10.1080/01952307.1992.11761519 [DOI] [PubMed] [Google Scholar]
- 37.Segal JL, Gonzales E, Yousefi S, Jamshidipour L, Brunnemann SR.. Circulating levels of IL-2R, ICAM-1, and IL-6 in spinal cord injuries. Arch Phys Med Rehabil 1997;78(1):44–7. doi: 10.1016/S0003-9993(97)90008-3 [DOI] [PubMed] [Google Scholar]


