Abstract
Background:
Staphylococcus lugdunensis, a member of the coagulase-negative staphylococci (CoNS), has been recognized as a causal organism for infective endocarditis since the 1980s. Although most CoNS have an insidious and chronic nature, they are involved in a variety of systemic infections. S lugdunensis infective endocarditis is a rare entity but is as catastrophic as Staphylococcus aureus infective endocarditis and requires aggressive antibiotic therapy and, typically, valve replacement. S lugdunensis infective endocarditis–induced septic embolic cerebrovascular accident has rarely been reported in the literature.
Case Report:
We present the case of a 63-year-old African American man who presented with sudden-onset aphasia and right-sided hemiplegia and was admitted for the management of cerebrovascular accident. Afterwards, he developed a fever and was found to have S lugdunensis bacteremia, with subsequent imaging revealing vegetations of the mitral valve. Despite being treated with culture-appropriate antibiotics, he remained persistently bacteremic and required surgical mitral valve replacement.
Conclusion:
S lugdunensis infective endocarditis is rare but can have a malignant course and requires early surgical intervention in most cases.
Keywords: Endocarditis, heart valve diseases, Staphylococcus lugdunensis, stroke
INTRODUCTION
Infective endocarditis (IE) is an infection of the endocardial lining of the heart (including native and prosthetic valves and intracardiac and intravascular devices) that can lead to large vegetations, mural thrombi, and abscesses. IE as a disease state was first recognized by Lazare Rivière who in 1674 reported a “cluster of hazelnuts in the opening of the aorta” in a deceased patient with a bizarre constellation of symptoms.1 Two hundred years later, in 1885, William Osler first recognized and presented the physical manifestations of IE in the English literature.1 In 1874, the bacterium, which was later called Staphylococcus, was cultured from wounds by Billroth2 who named it Coccobacteria septica; in 1882, Ogston3 named the bacterium Staphylococcus. In 1977, the first definition of IE was introduced by Pelletier and Petersdorf.4 Subsequently, Von Reyn et al proposed a new, stricter definition of IE 4 years later.5
In 1988, Freney et al first described a new species of CoNS, Staphylococcus lugdunensis, from analysis of 11 strains in Lyon, France.6 S lugdunensis–related IE and cerebrovascular accident (CVA) are rare disease entities, and only a few cases have been reported. According to the nationwide study done in 2009, the annual incidence of IE in the United States was 12.7 per 100,000 population with a mortality rate of 14.5%.7 Staphylococcus aureus was implicated as the causative pathogen in 49% of the cases of IE, followed by streptococci in 24.7% of cases.7 We describe a case of S lugdunensis IE manifesting as a septic embolic stroke.
CASE REPORT
A 63-year-old African American male with multiple comorbidities presented to the emergency department with sudden-onset aphasia and right-sided hemiplegia. Because he remained aphasic, his daughter provided details about what had transpired. Three hours before admission, the patient was found to be minimally responsive with a noticeable facial droop and was brought to the hospital. His family reported that before the onset of symptoms, he had been in a normal state of health. His medical history included hypertension, diabetes mellitus type 2, hyperlipidemia, coronary artery disease, and diverticulitis with recent hospitalization 5 weeks prior for a diverticular gastrointestinal bleed. His daughter denied any alcohol consumption and tobacco usage in the patient's past. The patient's family history was significant only for diabetes mellitus type 2 in his mother with an age of onset at 45 years. A complete review of systems was otherwise negative. His vital signs at the time of admission were unremarkable, including a temperature of 98°F, blood pressure of 146/73 mmHg, and a pulse rate of 105 bpm.
Upon examination, he was alert and able to follow simple commands, but he could not speak. Neurologically, he was noted to have left gaze preference, right-sided facial palsy, decreased muscle tone, and complete right-sided hemiplegia. Cardiovascular examination revealed a regular heart rate, normal first and second heart sounds, and a 3/6 systolic murmur corresponding to the mitral area of the heart with radiation to the axilla, raising suspicion for possible mitral valve involvement. No other significant examination findings were noted, including peripheral stigmata of endocarditis. His National Institutes of Health Stroke Scale score was 18, indicating moderate to severe stroke. Computed tomography (CT) of the head and CT angiogram of the brain demonstrated acute infarct in the left frontal lobe with a thrombus occlusion of the mid-to-distal left M3 branch.
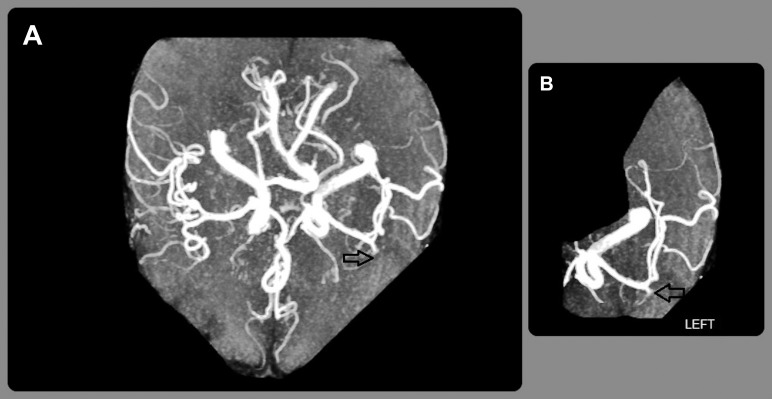
Electrocardiogram was normal. Further blood tests showed the following: hemoglobin 8.2 g/dL (reference range, 11-15.6 g/dL), white blood cell count (WBC) 13 K/μL (reference range, 4.8-10.8 K/μL), platelet count 269 K/μL (reference range, 130-400 K/μL), troponin I 0.12 ng/mL (reference, <0.30 ng/mL), prothrombin time 14.5 s (reference range, 9.8-11.7 s), partial thromboplastin time 24.3 s (reference range, 24.4-33.5 s), international normalized ratio 1.3 (reference range, 0.8-1.2), B-type natriuretic peptide 265 pg/mL (reference, <100 pg/mL), and lactic acid 0.8 mmol/L (reference range, 0.5-2.2 mmol/L). According to the neurologist, the patient was not a candidate for either vascular intervention or thrombolytic treatment because of his recent gastrointestinal bleeding. His symptoms were initially attributed to an embolic stroke, and he was admitted to the stroke unit. Cardiac telemetry monitoring revealed sinus rhythm with infrequent premature ventricular complexes. Magnetic resonance angiography without contrast verified the presence of a left anterior middle cerebral artery territory infarct compatible with left M3 branch occlusion (Figure 1).
Figure 1.
A: Magnetic resonance angiography of the head without contrast shows a paucity of vessels in the left anterior middle cerebral artery territory compared to the right side (arrow). B: A closer left-sided view demonstrates an absence of blood flow that is compatible with left M3 branch occlusion (arrow).
Forty-eight hours after admission, the patient became febrile to 102°F, after which 2 sets of blood cultures, a chest x-ray, and urine studies were obtained with no reported changes in symptoms or physical examination. Chest x-ray and urine studies were unremarkable; repeat complete blood count was significant for a WBC of 11.1 K/μL. Two sets of blood cultures grew coagulase-negative Staphylococcus with species determination of S lugdunensis made on the following day. Because of the patient's recent stroke, fever, and bacteremia, a transthoracic echocardiogram (TTE) was obtained to evaluate for evidence of suspected IE.
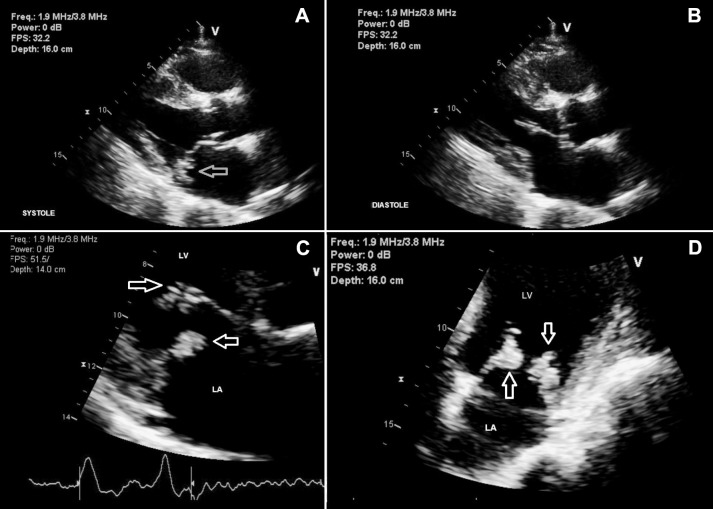
TTE revealed large mobile vegetations on both posterior and anterior mitral valve leaflets, severe mitral regurgitation, and severe left atrial dilatation with an ejection fraction of 61% (Figure 2). Initially, vancomycin 1 gram intravenously (IV) and gentamicin 100 mg IV every 12 hours had been started, pending determination of bacterial species and sensitivities. S lugdunensis sensitivities revealed a pan-sensitive organism after which the patient's antibiotic spectrum was narrowed to nafcillin 2 grams IV every 4 hours and rifampin 300 mg by mouth every 12 hours as he developed mild acute renal failure. Although the patient received culture-appropriate antibiotics, repeated surveillance blood cultures continued to grow S lugdunensis. Because of his persistent bacteremia and TTE findings of large mitral valve leaflet vegetations with severe mitral regurgitation, the patient was transferred to a tertiary care center for mitral valve replacement. He successfully underwent bioprosthesis mitral valve replacement with left atrial appendage closure without any complications. Diagnostic cerebral angiography did not reveal any mycotic aneurysms.
Figure 2.
A and B: Transthoracic echocardiogram shows vegetations at the mitral valve (arrow) in left parasternal views. C and D: Closer views demonstrate vegetations in the anterior and posterior mitral valve (arrows).
After successful mitral valve replacement, daptomycin 500 mg IV was started, and the rest of the patient's hospital stay was uneventful. Fourteen days after surgery, the patient was discharged to a rehabilitation center for physical therapy with a course of IV daptomycin for 4 additional weeks. After 6 weeks, he was examined in the outpatient clinic. He still had some weakness on the right side of his body and was able to ambulate 30-40 feet with a walker. Otherwise, he was feeling well.
DISCUSSION
S lugdunensis, a gram-positive CoNS, is a member of normal skin commensals concentrated in the groin, axilla, and lower extremities, but it can become a life-threatening pathogen as was the case with our patient.8,9 S aureus is the most common causal organism in IE and is usually associated with a destructive pathogenesis that results in large vegetations and native valvular involvement. Thus, differentiating between S lugdunensis and S aureus is important because of the variance in disease etiology, treatment duration, and prognosis. Moreover, S lugdunensis can be falsely identified as S aureus because of the similar colony morphology and clumping factor in these 2 organisms.10 S lugdunensis can produce ornithine decarboxylase, D-mannose acid, and pyrrolidonyl arylamidase, all of which can be used to differentiate it from other CoNS species.11 The initial step of CoNS pathogenesis, including that of S lugdunensis, involves biofilm formation that allows these organisms to attach to the polymer surface of medical devices and also acts as a barrier to host immunity and antibiotics.8,12 Approximately 25% of isolated S lugdunensis organisms can produce extracellular slime or glycocalyx that participates in their colonization and interferes with the neutrophil-induced phagocytosis mechanism.13
S lugdunensis has been implicated in various infections, including native and prosthetic valve endocarditis, prosthetic device infections, osteomyelitis, meningitis, catheter-related infections, systemic embolism, and skin and soft tissue infections. Identifiable sources of infection include the skin, vascular access (eg, central line, dialysis catheter), perineal surgery (eg, vasectomy), and pacemaker insertion sites. However, in up to 67% of S lugdunensis infections, the source of infection is unknown, as with our patient.14 Unlike the indolent nature of other CoNS-related native valve endocarditis cases, S lugdunensis has an aggressive and destructive nature, causing profound valve destruction and vegetations similar to S aureus IE.15,16 Thus, recognizing the disease early is vital. Vandenesch et al reported that S lugdunensis was associated with paravalvular abscess formation in 23% of cases and with systemic embolization in 16.4% of cases.17
In our case, the patient presented with an embolic stroke and later developed signs and symptoms of sepsis. He was ultimately diagnosed with S lugdunensis IE with large vegetations on the mitral valve. Because of persistent bacteremia, he underwent early mitral valve replacement surgery. Our case supports the literature regarding the need for early aggressive treatment to achieve good outcomes. Kuzhively et al reported a case of persistent S lugdunensis bacteremia despite appropriate antibiotic therapy.18 The patient had a complicated hospital course with failed extubation and tracheostomy and was not a surgical candidate given his extensive medical history. Since 1998, approximately 85 cases of S lugdunensis IE have been reported in the literature,14,15,18-22 but a septic embolic CVA related to S lugdunensis IE remains rare. An extensive literature search revealed only 14 cases of stroke related to S lugdunensis, and only 12 of the 14 cases were described as S lugdunensis IE–related embolic stroke.18,20-30
Currently, modified Duke criteria are the most widely used scoring system for the diagnosis of IE, with a sensitivity of 80%.31 At a minimum, 3 sets of blood cultures followed by an echocardiogram should be performed in cases with high suspicion of IE.32 It has been noted that only a few cases of IE come with typical clinical features of IE (Osler nodes, Janeway lesions, stroke, and hematuria), but most IE cases have lately presented with limited IE manifestations with an advancement and frequent usage of antibiotics.32
Consequently, it is paramount to think of IE in the presence of fever, new-onset heart murmur associated with an embolic phenomenon, a history of injection drug abuse, and a previous history of IE. Isolation of S lugdunensis from multiple sets of blood cultures is always significant, and it is not considered a contaminant. S lugdunensis is susceptible to penicillin and cephalosporin groups owing to its lack of both the mecA gene (encoding PBP 2a and methicillin resistance) and beta-lactamase. Since Tee et al reported mecA gene–related antibiotic resistance in 2003, it has become paramount not only to make an early diagnosis and determine the species of the organism but also to determine the antibiotic susceptibility for definitive management of S lugdunensis–related infection.33
In Staphylococcus-related IE, a minimum of 6 weeks of treatment is needed to remove the infection, compared to a 4- to 6-week regimen for Streptococcus-associated IE.32 As mentioned earlier, the antibiotic choice in the treatment of S lugdunensis–related infection should be guided by susceptibility testing. Our patient was initially treated with vancomycin and gentamicin; his medication was then switched to nafcillin and oral rifampin, according to the susceptibility testing, followed by IV daptomycin for a total of 4 weeks after valve replacement surgery. According to the American Heart Association (AHA) 2015 guideline, early surgical management in IE is indicated in valve dysfunction with signs of heart failure, infection caused by fungi or highly resistant organisms, presence of IE complications (heart block, annular or aortic abscess, or destructive penetrating lesions), or evidence of persistent infection (persistent bacteremia or fever lasting more than 5-7 days) after the start of appropriate antibiotics, but the timing of surgical intervention in IE complicated by a stroke remains uncertain.32
Although S lugdunensis IE is a rare disease, a moderate amount of evidence suggests early surgical intervention. Liu et al14 published a review stating that S lugdunensis IE was associated with a 38% mortality rate, which was higher than the mortality rate for Staphylococcus epidermidis–related IE (∼20%), and surgery was needed in 68% of cases. In another prospective study, the necessity of surgical intervention was significantly higher in S lugdunensis IE (68%-70%) than in S aureus IE (37%).16 Takahashi et al reported a 70% mortality rate in S lugdunensis–related IE before 1993, which dropped to 18% after 1993, and the only difference was early recognition and timely surgical management.34 Our case has again illustrated the aggressiveness of S lugdunensis IE with neurologic manifestations and persistent bacteremia that eventually required surgical intervention for a favorable outcome.
CONCLUSION
S lugdunensis–related endocarditis is rare but has a unique presentation and prognosis compared to other CoNS strain–associated infections. We describe a rare case of septic embolic stroke secondary to S lugdunensis IE with an unknown site of infection. This case reminds clinicians of the importance of early diagnosis and aggressive treatment, including surgery if necessary, to help ensure an auspicious outcome, as any delay in treatment can lead to catastrophic results. Because awareness of the particular strain can change the treatment decisions, the exact species involved should be promptly identified in all CoNS bacteremia and suspected IE cases. Early consultation with infectious disease specialists and the cardiothoracic surgical team can be extremely helpful in cases of S lugdunensis IE. Even though the current AHA guideline has no organism-specific recommendation, close observation with early surgical intervention should always be considered in cases of S lugdunensis IE.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article. This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1. Grinberg M, Solimene MC. . Historical aspects of infective endocarditis. Rev Assoc Med Bras. 2011. Mar-Apr; 57 2: 228- 233. [DOI] [PubMed] [Google Scholar]
- 2. Billroth T. . Studies on the vegetation types of Coccobacteria septica and the share which they have in the development and dissemination accidentelle wound diseases: attempt at a scientific review of the various methods of antiseptic wound treatment. Berlin, Germany: G Reimer; 1874. [Google Scholar]
- 3. Ogston A. . Micrococcus poisoning. J Anat Physiol. 1882. October; 17 pt 1: 24- 58. [PMC free article] [PubMed] [Google Scholar]
- 4. Pelletier LL Jr, Petersdorf RG. . Infective endocarditis: a review of 125 cases from the University of Washington Hospitals, 1963-72. Medicine (Baltimore). 1977. July; 56 4: 287- 313. [PubMed] [Google Scholar]
- 5. Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. . Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med. 1981. April; 94 4 pt 1: 505- 518. [DOI] [PubMed] [Google Scholar]
- 6. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Evol Microbiol. 1988. April; 38: 168- 172. [Google Scholar]
- 7. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. . Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013. January; 8 3: e60033 10.1371/journal.pone.0060033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker K, Heilmann C, Peters G. . Coagulase-negative staphylococci. Clin Microbiol Rev. 2014. October; 27 4: 870- 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Mee-Marquet N, Achard A, Mereghetti L, Danton A, Minier M, Quentin R. . Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J Clin Microbiol. 2003. April 1; 41 4: 1404- 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leung MJ, Nuttall N, Pryce TM, Coombs GW, Pearman JW. . Colony variation in Staphylococcus lugdunensis. J Clin Microbiol. 1998. October; 36 10: 3096- 3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mateo M, Maestre JR, Aguilar L, et al. Genotypic versus phenotypic characterization, with respect to susceptibility and identification, of 17 clinical isolates of Staphylococcus lugdunensis. J Antimicrob Chemother. 2005. August; 56 2: 287- 291. [DOI] [PubMed] [Google Scholar]
- 12. Götz F. . Staphylococcus and biofilms. Mol Microbiol. 2002. March; 43 6: 1367- 1378. [DOI] [PubMed] [Google Scholar]
- 13. Stout RD, Ferguson KP, Li YN, Lambe DW Jr. . Staphylococcal exopolysaccharides inhibit lymphocyte proliferative responses by activation of monocyte prostaglandin production. Infect Immun. 1992. March; 60 3: 922- 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu PY, Huang YF, Tang CW, et al. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. J Microbiol Immunol Infect. 2010. December; 43 6: 478- 484. 10.1016/S1684-1182(10)60074-6. [DOI] [PubMed] [Google Scholar]
- 15. Sabe M, Shrestha N, Gordon S, Menon V. . Staphylococcus lugdunensis: a rare but destructive cause of infective endocarditis with echocardiographic and clinical features similar to Staphyloccoccus aureus infective endocarditis. J Am Coll Cardiol. 2012. March; 59 13s1: E2030 10.1016/S0735-1097(12)62031-2 [DOI] [Google Scholar]
- 16. Anguera I, Del Río A, Miró JM, et al. Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005. February; 91 2: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandenesch F, Etienne J, Reverdy ME, Eykyn SJ. . Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin Infect Dis. 1993. November; 17 5: 871- 876. [DOI] [PubMed] [Google Scholar]
- 18. Kuzhively J, Patel SA, Abraham H. . The long CoN(S): a case of Staphylococcus lugdunensis endocarditis with cerebral and coronary embolism. J Med Cases. 2014. October; 5 10: 535- 537. [Google Scholar]
- 19. Choi SH, Chung JW, Lee EJ, et al. Incidence, characteristics, and outcomes of Staphylococcus lugdunensis bacteremia. J Clin Microbiol. 2010. September; 48 9: 3346- 3349. 10.1128/JCM.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pecoraro R, Tuttolomondo A, Parrinello G, Pinto A, Licata G. . Staphylococcus lugdunensis endocarditis complicated by embolism in an 18-year-old woman with mitral valve prolapse. Case Rep Infect Dis. 2013; 2013: 730924 10.1155/2013/730924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai WC, Chen WL, Tsao YT; . Network for Emergency Care of Infectious Disease (NECID). One-and-a-half syndrome: a less appreciated emergency in native valve infective endocarditis. Am J Emerg Med. 2013. February; 31 2: 459.e1- e3. 10.1016/j.ajem.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 22. David M, Loftsgaarden M, Chukwudelunzu F. . Embolic stroke caused by Staphylococcus lugdunensis endocarditis complicating vasectomy in a 36-year-old man. Tex Heart Inst J. 2015. December 1; 42 6: 585- 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgert SJ, LaRocco MT, Wilansky S. . Destructive native valve endocarditis caused by Staphylococcus lugdunensis. South Med J. 1999. August; 92 8: 812- 814. [DOI] [PubMed] [Google Scholar]
- 24. Chatzigeorgiou KS, Ikonomopoulou C, Kalogeropoulou S, et al. Two successfully treated cases of Staphylococcus lugdunensis endocarditis. Diagn Microbiol Infect Dis. 2010. December; 68 4: 445- 448. [DOI] [PubMed] [Google Scholar]
- 25. Al Ebrahim KE. . Successful surgical treatment of mitral valve endocarditis caused by Staphylococcus lugdunensis. J King Abdulaziz University Med Sci. 2007; 14 1: 73- 79. [Google Scholar]
- 26. Koh TW, Brecker SJ, Layton CA. . Successful treatment of Staphylococcus lugdunensis endocarditis complicated by multiple emboli: a case report and review of the literature. Int J Cardiol. 1996. July 26; 55 2: 193- 197. [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez-Gascón M, Roig P, Montagud JB, Merino J. . Acute Staphylococcus lugdunensis endocarditis with septic cerebral and pulmonary emboli, showing favorable evolution [in Spanish]. Enferm Infecc Microbiol Clin. 2003. October; 21 8: 465- 467. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez A, Martínez I, Sanz F, López F, Aguado JM. . Aggressive acute endocarditis caused by Staphylococcus lugdunensis complicated with multiple cerebral septic emboli [in Spanish]. Enferm Infecc Microbiol Clin. 2000. December; 18 10: 526- 527. [PubMed] [Google Scholar]
- 29. Sanchís-Bayarri Vaillant V, Llucian Rambla R, Sanchís-Bayarri Bernal V. 7 cases of Staphylococcus lugdunensis infection [in Spanish]. An Med Interna. 1999. July; 16 7: 361- 362. [PubMed] [Google Scholar]
- 30. Tamdy A, El Louali F, Ounzar M, et al. Multiple mycotic aneurysms reveal Staphylococcus lugdunensis endocarditis in a young patient. Heart Lung. 2011. Jul-Aug; 40 4: 352- 357. [DOI] [PubMed] [Google Scholar]
- 31. Bayer AS, Ward JI, Ginzton LE, Shapiro SM. . Evaluation of new clinical criteria for the diagnosis of infective endocarditis. Am J Med. 1994. March; 96 3: 211- 219. [DOI] [PubMed] [Google Scholar]
- 32. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015; 132: 1435- 1486. [DOI] [PubMed] [Google Scholar]
- 33. Tee WSN, Soh SY, Lin R, Loo LH. . Staphylococcus lugdunensis carrying the mecA gene causes catheter-associated bloodstream infection in premature neonate. J Clin Microbiol. 2003. January; 41 1: 519- 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi N, Shimada T, Ishibashi Y, et al. The pitfall of coagulase-negative staphylococci: a case of Staphylococcus lugdunensis endocarditis. Int J Cardiol. 2009. September 11; 137 1: e15- e17. 10.1016/j.ijcard.2008.05.041. [DOI] [PubMed] [Google Scholar]