Abstract
Introduction
Bone-borne palatal expansion relies on mini-implant stability for successful orthopedic expansion. The large magnitude of applied force experienced by mini-implants during bone-borne expansion may lead to high failure rates. Use of bicortical mini-implant anchorage rather than monocortical anchorage may improve mini-implant stability. The aim of this study was to analyze and compare the effects of bicortical and monocortical anchorage on stress distribution and displacement during bone-borne palatal expansion using finite element analysis (FEA).
Methods
Two skull models were constructed to represent expansion prior to and after midpalatal suture opening. Three clinical situations with varying mini-implant insertion depths were studied in each skull model: monocortical, 1mm bicortical, and 2.5mm bicortical. FEA simulations were performed for each clinical situation in both skull models. Von Mises stress distribution and transverse displacement was evaluated for all models.
Results
Peri-implant stress was greater in the monocortical anchorage model compared to both bicortical anchorage models. In addition, transverse displacement was greater and more parallel in the coronal plane for both bicortical models compared to the monocortical model. Minimal differences were observed between the 1mm bicortical and 2.5mm bicortical models for both peri-implant stress and transverse displacement.
Conclusions
Bicortical mini-implant anchorage results in improved mini-implant stability, decreased mini-implant deformation and fracture, more parallel expansion in the coronal plane, and increased expansion during bone-borne palatal expansion. However, the depth of bicortical mini-implant anchorage was not significant.
Introduction
Transverse maxillary deficiency has been reported to affect 8–23% of adolescent patients and less than 10% of adult patients.1–5 Rapid palatal expansion (RPE), which typically uses a tooth-borne appliance with a center jackscrew, is a well-established and reliable technique to correct this problem for adolescent patients.6–8 For adults however, nonsurgical RPE with a tooth-borne appliance can result in dentoalveolar tipping that may cause unfavorable periodontal effects due to the interdigitated midpalatal suture and decreased elasticity of bone found in adults.9–11 Therefore, in adults, skeletal orthopedic expansion is necessary to prevent these issues and to correct transverse maxillary deficiency.12–14
Surgically assisted RPE (SARPE) is the conventional treatment of choice to correct transverse maxillary deficiency in adults.9–11,15 However, SARPE is an invasive process that has been found to result in lateral rotation of the two maxillary halves with minimal horizontal translation.9–11 In addition, SARPE may be detrimental to the periodontium and has been shown to result in a large amount of relapse during the postretention period.16,17
Recently, bone-borne palatal expanders have been reported in several case presentations to have the capability to correct transverse maxillary deficiency in adults making it a potential alternative to SARPE.18–21 Bone-borne expanders have also been shown to prevent the dentoalveolar tipping seen in adults when attempting to use traditional tooth-borne RPE appliances.20,22,23 For adolescent patients, bone-borne expansion has been shown to produce greater transverse skeletal expansion while minimizing dental side effects such as dental tipping, alveolar bending, and vertical alveolar bone loss compared to tooth-borne RPE appliances.24 Bone-borne expansion also has been combined with a face mask for maxillary protraction which has been shown to reduce adverse effects such as mesialization of anterior teeth.25
Bone-borne palatal expansion relies on skeletal anchorage obtained through mini-implants to directly apply force to the basal bone. Thus, mini-implant stability is essential for successful skeletal orthopedic expansion. Mini-implant loss and loosening rates for orthodontic tooth movement ranges from 6.9–28.0%, and their success is dependent on several factors which include the magnitude and direction of the applied force; operator experience; insertion site; quality of cortical bone; surface contact area in cortical bone; length, depth, diameter, thread configuration and shape of the mini-implant; and patient’s age.26–35 While there have not been any specific reports analyzing mini-implant failure rates during bone-borne expansion in mature patients, such failure rates are likely to be higher than in orthodontic tooth movement due to the increased magnitude of the applied force necessary to split the interlocking suture. Therefore, new approaches to improve mini-implant stability during bone-borne expansion are needed.
Bicortical mini-implant anchorage has been demonstrated in orthodontic tooth movement applications to be biomechanically more favorable than monocortical anchorage. As such, they should also be considered for clinical situations requiring heavy anchorage.32,36 Bone-borne expanders, which require heavy anchorage, represent a good clinical situation to use bicortical anchorage that has not yet been explored in existing literature. This study will therefore seek to determine the differences between bicortical and monocortical mini-implant anchorage on skeletal orthopedic expansion.
Finite element analysis (FEA) is a numerical approximation technique that is widely used to assess biomechanical problems. FEA has been applied to study different aspects of bone-borne expanders, primarily focusing on stress distribution and displacement of different expander designs as well as its biomechanical effects on craniofacial sutures.37–41 However, there has not been a study that has compared bicortical and monocortical anchorage for bone-borne expanders using FEA. Thus, the aim of this study was to analyze and compare the effects of bicortical and monocortical anchorage on stress distribution and displacement during bone-borne palatal expansion using finite element analysis (FEA).
Material and Methods
A skull finite element model was generated using volumetric data from a cone-beam computed tomography (CBCT) scan (slice thickness of 0.30 mm) of an adult dry skull using Mimics software (version 15.0; Materialise, Leuven, Belgium). Threshold segmentation was performed generating a 3D virtual surface model of the dry skull. Individual masks of sutures of width 1.5–2 mm were manually generated for the midpalatal, median nasal, lateral nasal, pterygomaxillary, zygomaticotemporal, and zygomaticomaxillary sutures.40–42 The thickness of the cortical bone and masticatory mucosa in the hard palate was determined using the studies by Farnsworth et al. and Studer et al. respectively.43,44 Two 3D surface models of the dry skull were generated. The first model contained the interlocking midpalatal suture and represented the skull prior to midpalatal suture opening (Fig. 1A). The second model did not contain the interlocking midpalatal suture and represented the skull after midpalatal suture opening without sutural resistance against expansion force (Fig. 1B). Bicortical and monocortical anchorage was compared in both models using measurements at 3 points (Fig. 1B; points A, B, and C). These 3D skull surface models were imported into 3-matic software (version 7.0; Materialise, Leuven, Belgium) to generate an FE volumetric mesh.
Fig 1.
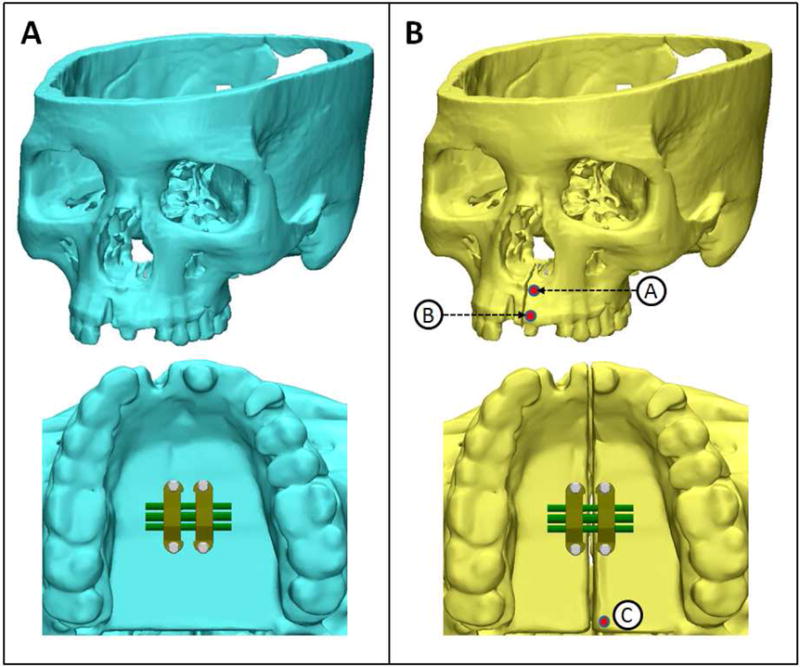
3D virtual models of dry skull with bone-borne expander. A, Model to be used for FEA simulation of expansion prior to midpalatal suture opening. B, Model to be used for FEA simulation of expansion after midpalatal suture opening. Transverse displacement will be measured at points A,B, and C.
The mini-implant (diameter, 1.5mm; length, 11.0mm) (ACR Series; BioMaterials Korea, Inc., Seoul, Korea) and a specific design of bone-borne palatal expander, the maxillary skeletal expander (MSE; BioMaterials Korea, Inc., Seoul, Korea), used in this study were constructed using computer aided design (CAD) software SolidWorks (version 2011; Dassault Systemes, Velizy, France) with the design specifications provided by the manufacturer. These CAD models were exported from SolidWorks as 3D surface stereolithography files. The stereolithography files of the mini-implant and maxillary skeletal expander were then also imported into 3-matic software for FE volumetric mesh generation.
In 3-matic software, the expander was positioned similar to a patient case using clinical photos and CBCT scans as positioning aids (Fig. 1). The mini-implants were positioned, using PA cephalograms as a positioning aid, to have varying insertion depths representative of three different clinical situations: monocortical, 1mm bicortical, and 2.5mm bicortical (Fig. 2). The expander was in the same position for all three clinical situations with only the vertical position of the mini-implants varying between each clinical situation. All three clinical situations were analyzed in both FE skull models.
Fig 2.
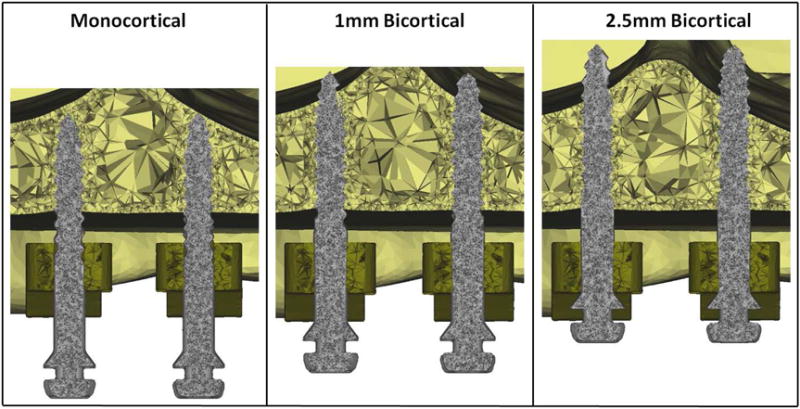
Coronal plane cut view of mini-implants position in three different clinical situations: monocortical, 1mm bicortical, and 2.5mm bicortical. The expander is in the same position for all three clinical situations with only the vertical position of the mini-implants varying between each clinical situation.
Tetrahedral elements were used for volumetric mesh generation. Each skull was composed of roughly 4,500,000 elements and 1,200,000 nodes. For the skull generation, the maxilla and sutures were locally remeshed to contain more fine elements than elsewhere on the skull. Each mini-implant was composed of roughly 85,000 elements and 16,000 nodes. The expander was composed of roughly 30,000 elements and 9,000 nodes.
The FE models of the skull, mini-implants, and expander were imported into Abaqus FEA software (version 6.13; Dassault Systemes, Velizy, France) to perform FEA simulations. The material properties used are shown in Table 1.37,45,46 Each material was considered to be homogeneous and isotropic. The boundary conditions applied were setting the nodes of the foramen magnum to be completely fixed in all degrees of freedom.47
Table I.
Material Properties
| Young’s Modulus (MPa) | Poisson’s Ratio | |
|---|---|---|
| Cortical Bone | 13,700 | 0.30 |
| Cancellous Bone | 1,370 | 0.30 |
| Suture | 10 | 0.49 |
| Masticatory Mucosa | 25 | 0.30 |
| Titanium | 113,000 | 0.33 |
| Stainless Steel | 210,000 | 0.30 |
In the model simulating bone-borne expansion prior to midpalatal suture opening, the expander was activated transversely by 0.5mm in the transverse plane and were unfixed in the sagittal and coronal planes to prevent interference with the resultant movement.37,38 In the model simulating bone-borne expansion after midpalatal suture opening, the expander was activated transversely by 0.25mm for 20 steps resulting in a total of 5mm of expansion. Similar to the model described above, the expansion was also activated in the transverse plane and was unfixed in the sagittal and coronal planes to prevent interference with the resultant movement. In both models, Von Mises stress distribution and transverse displacement were evaluated.
Results
Von Mises stress at the peri-implant site was measured for the skull model containing the interlocking midpalatal suture and was found to be clearly higher in the monocortical anchorage model compared to both bicortical anchorage models (Fig. 3). In all models, the Von Mises stress was localized around the initial cortical bone layer. Minimal difference was observed between the 1mm bicortical and 2.5mm bicortical models. The total Von Mises stress at the bone-implant interface was calculated for each model and was found to be 476,000 MPa for the monocortical model, 234,000 MPa for the 1mm bicortical model, and 227,000 MPa for the 2.5mm bicortical model. The percent difference between the monocortical model and 1mm bicortical model was 68.17%, while the difference between the monocortical and 2.5mm bicortical model was 70.84%, and that between the 1mm and 2.5mm bicortical models was 3.04%.
Fig 3.
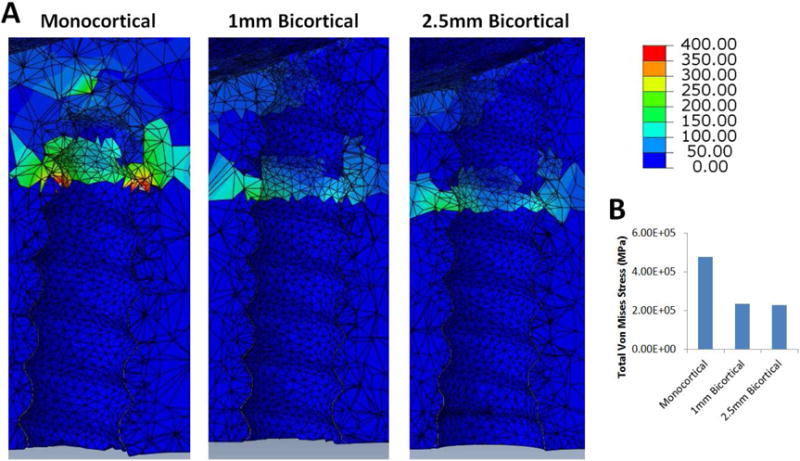
A, Von Mises stress of the peri-implant site for the skull model with midpalatal suture for the monocortical, 1mm bicortical, and 2.5mm bicortical models. B, Bar graph showing total Von Mises Stress in MPa for all 3 anchorage models.
Von Mises stress of the mini-implants was also measured in the skull model containing the interlocking midpalatal suture and was found to be significantly higher in the monocortical model compared to both bicortical anchorage models (Fig 4). In all models, the Von Mises stress on the implant was localized at the bone-implant interface around the initial cortical bone layer. Total Von Mises stress was measured at the bone-implant interface and was determined to be 5,831,000 MPa for the monocortical model, 3,576,000 MPa for the 1mm bicortical model, and 3,845,000 MPa for the 2.5mm bicortical model. The percent difference between the monocortical model and 1mm bicortical model was 47.94%, the difference between the monocortical and 2.5mm bicortical model was 41.05%, and that between the 1mm and 2.5mm bicortical models was 7.25%. For the monocortical model, the maximum principal stress at the bone-implant interface was 664.49 MPa and the minimum principal stress was 229.94 MPa. For the 1mm bicortical model, the maximum principal stress at the bone-implant interface was 270.246 MPa and the minimum principal stress was 53.95 MPa. For the 2.5mm bicortical model, the maximum principal stress at the bone-implant interface was 289.87 MPa and the minimum principal stress was 75.94 MPa. Bending of the mini-implants was clearly evident. The degree of bending in all four mini-implants was measured and the mean degree of bending was calculated to be 4.55° for the monocortical model, 1.94° for the 1mm bicortical model, and 1.71° for the 2.5mm bicortical model.
Fig 4.
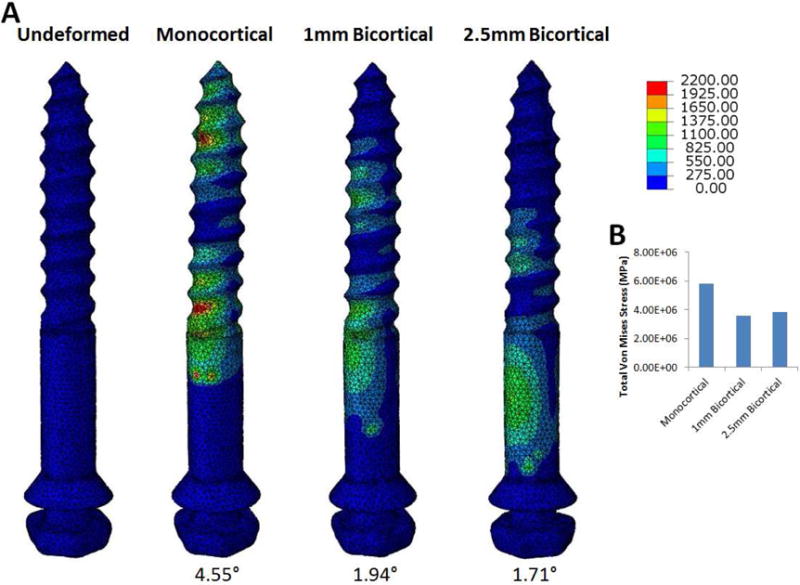
A, Von Mises stress of the mini-implant for the skull model with midpalatal suture for the monocortical, 1mm bicortical, and 2.5mm bicortical models. Degree of bending of mini-implants is reported. B, Bar graph showing total mini-implant Von Mises Stress in MPa for all 3 anchorage models.
Transverse displacement was measured on the left side of the skull model not containing an interlocking midpalatal suture and was determined for each step, twenty steps in total (Fig 5). These twenty steps were equivalent to twenty 0.25mm turns, for a total of 5mm of expansion (2.5mm on each side). Left side transverse displacement was measured at points A, B, and C (Fig 1B) and plotted in Figure 6. The total and mean transverse displacements were recorded in Table II. At point A, the total transverse displacement was 1.608mm for the monocortical model, 1.988mm for the 1mm bicortical model, and 2.067mm for the 2.5mm bicortical model. The percent difference at point A for total transverse displacement between the monocortical model and 1mm bicortical model was 21.13%, the difference between the monocortical and 2.5mm bicortical model was 24.98%, and that between the 1mm and 2.5mm bicortical models was 3.90%. At point B, the total transverse displacement was 2.215mm for the monocortical, 2.744mm for the 1mm bicortical model, and 2.848mm for the 2.5mm bicortical model. The percent difference at point B for total transverse displacement between the monocortical model and 1mm bicortical model was 21.33%, the difference between the monocortical and 2.5mm bicortical model was 25.00%, and that between the 1mm and 2.5mm bicortical models was 3.72%. At point C, the total transverse displacement was 1.141mm for the monocortical model, 1.444mm for the 1mm bicortical model, and 1.442mm for the 2.5mm bicortical model. The percent difference at point A for total transverse displacement between the monocortical model and 1mm bicortical model was 23.44%, the difference between the monocortical and 2.5mm bicortical model was 23.31%, and that between the 1mm and 2.5mm bicortical models was 0.14%.
Fig 5.
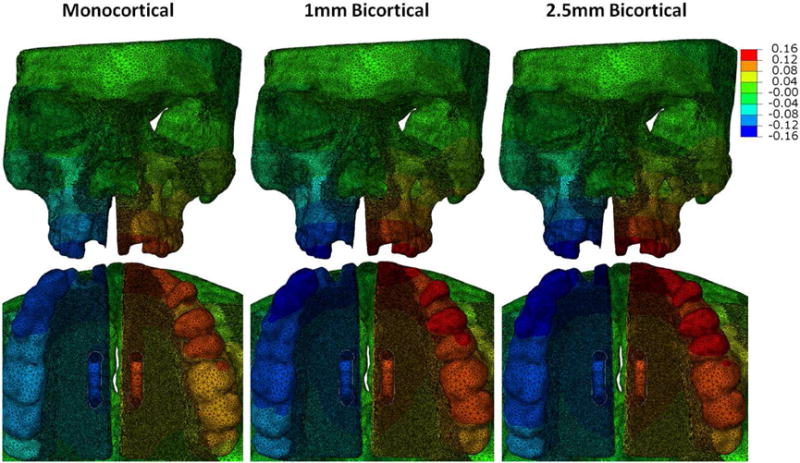
Frontal and Occlusal views of step 20 (5mm of expansion) of skull model simulation after midpalatal suture opening with contour map showing transverse displacement.
Fig 6.
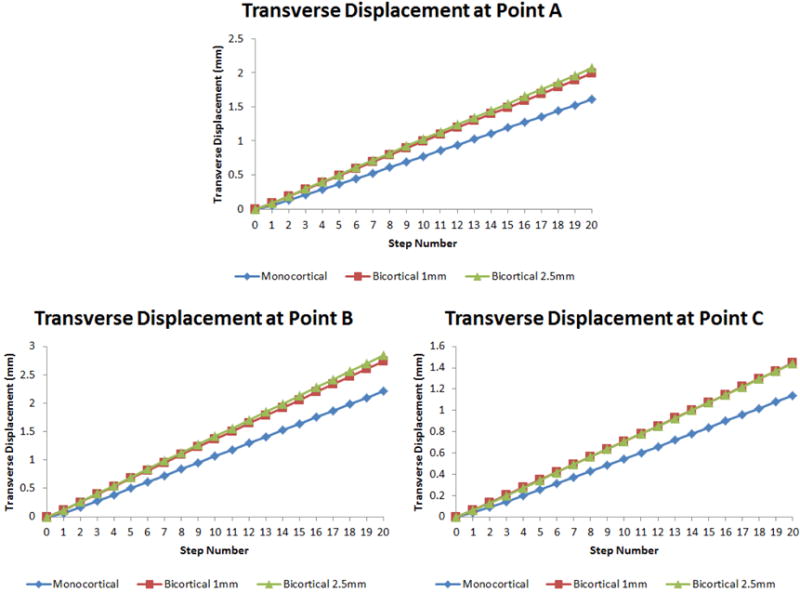
Line graphs showing transverse displacement at each step during expansion.
Table II.
Left side transverse displacement (mm) after midpalatal suture opening
| Monocortical A | Monocortical B | Monocortical C | Bicortical 1mm A | Bicortical 1mm B | Bicortical 1mm C | Bicortical 2.5mm A | Bicortical 2.5mm B | Bicortical 2.5mm C | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 1.608 | 2.215 | 1.141 | 1.988 | 2.744 | 1.444 | 2.067 | 2.848 | 1.442 |
| Mean | 0.080 | 0.111 | 0.057 | 0.099 | 0.137 | 0.072 | 0.103 | 0.142 | 0.072 |
The total transverse displacement at step 20 was measured at levels D and E which were located at the coronal midplane of the bone-borne palatal expander (Fig 7). The ratio between D and E was calculated to compare the amount of displacement measured at levels D and E. The closer the ratio was to 1.000, the more parallel the expansion. The ratio was found to be 0.634 for the monocortical model, 0.692 for the 1mm bicortical model, and 0.701 for the 2.5mm bicortical model. The percent difference between the monocortical and 1mm bicortical model was 8.72%, the difference between the monocortical and 2.5mm bicortical model was 10.06%, and that between the 1mm and 2.5mm bicortical models was 1.34%.
Fig 7.
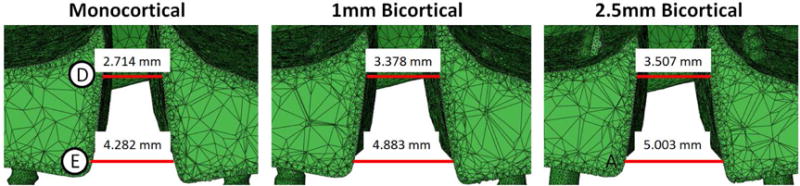
Cut view at the coronal midplane of the bone-borne palatal expander. Total displacement at levels D and E were measured for each model.
Discussion
Bone-borne palatal expanders have been demonstrated to be a viable treatment option to correct transverse maxillary deficiency in adults through several reports showing evidence of clinical success.18–21,48–50 As bone-borne expanders rely on skeletal anchorage obtained by mini-implants applying force directly to the basal bone, mini-implant stability is integral to successful skeletal orthopedic expansion. Bicortical mini-implant anchorage has been demonstrated to be superior compared to monocortical mini-implant anchorage for orthodontic tooth movement but has not been explored for bone-borne palatal expansion.32,36 Therefore, this study was designed to evaluate whether bicortical anchorage likewise increased stability and improved skeletal orthopedic expansion compared to monocortical anchorage.
This study used two skull models to study the effects of bicortical and monocortical anchorage prior to midpalatal suture opening and post midpalatal suture opening. The midpalatal suture was removed in the model that represented post midpalatal suture opening to allow for expansion in the FEA simulation. Three clinical situations of varying mini-implant insertion depth were used for both skull models which included a monocortical model, a 1mm bicortical model, and a 2.5mm bicortical model. In all three clinical situations, the expander was in the same position, and only the mini-implants varied in vertical position. All three of these clinical situations have been observed in patients treated at the UCLA School of Dentistry and were chosen to explore the differences between monocortical and bicortical anchorage as well as to determine whether the depth of bicortical anchorage is significant. Operator experience may also play a role in the varying depths of implantation seen clinically and has been reported to be a factor in mini-implant stability.27,29
Overloading of the peri-implant bone has been demonstrated to lead to loss of primary stability of orthodontic mini-implants.51 In addition, there is decreased risk of mini-implant loosening if the stress in the cervical region of the peri-implant bone region is low.52 In the skull model containing the midpalatal suture, this study demonstrated that there is significantly lower stress at the peri-implant site in the bicortical models compared to the monocortical model, suggesting that mini-implants placed bicortically decrease the risk of mini-implant loosening. Minimal differences were observed between the two different bicortical models. These findings are consistent with previous studies,32,36 finding that in bone-borne expansion, bicortical anchorage is more favorable than monocortical anchorage and that the depth of bicortical anchorage has minimal impact on stability. In addition, this finding is also supported through Wolff’s Law and the maximum principal stress values reported in this study. The monocortical model had an increased maximum principal stress value compared to the bicortical models. A high principal stress value, like in the monocortical model, may place the bone remodeling in the “pathologic overload window” in which stress fractures and bone resorption, not coupled to formation, occur leading to overloaded implants and implant loosening.53
A larger magnitude of force experienced by mini-implants increases the likelihood of deformation and mini-implant fracture.54 This study found that monocortical mini-implants experienced significantly greater stress at the bone-implant interface, specifically around the initial cortical bone layer, compared to bicortical mini-implants. There were minimal differences between the mini-implant stress levels of the two different bicortical models. In addition, the monocortical mini-implants were found to have over double the degree of bending compared to the two bicortical models. Again, there was minimal difference between the bending found in the two bicortical models. These findings suggest that mini-implant fracture is most likely to occur at the initial cortical bone layer and demonstrate that mini-implant deformation and fracture in bone-borne expansion are more likely to occur with monocortical anchorage rather than bicortical anchorage and that the depth of bicortical anchorage has little impact on mini-implant deformation and fracture.
Transverse displacement was measured in the skull model that not containing the interlocking midpalatal suture for twenty steps. Each step was equivalent to a 0.25mm turn of the palatal expander for a total of 5mm of simulated expansion. Analyzing the bone-borne expansion for multiple turns of the expander allowed for more in depth analysis than previous FEA studies that studied expansion using only one static step. Furthermore, this stepwise model was more representative of a clinical situation.
Transverse displacement was found to be significantly lower in the monocortical model at all three points of measurement and after every turn compared to both bicortical models. Minimal differences in transverse displacement were observed between the two bicortical models. The difference in transverse displacement between the monocortical and bicortical models may be due to the greater surface contact area in cortical bone experienced by the bicortical models, which allows for more uniform force transfer. Mini-implant contact surface area in cortical bone has been shown to be a more significant contributor to mini-implant stability than cancellous bone.33,55 In addition, the monocortical model may have experienced less transverse displacement because of its increased degree of bending. This increased amount of bending created a greater discrepancy between the mini-implant orientation and line of applied force. Any discrepancy between mini-implant orientation and line of applied force has been shown to decrease load distribution uniformity leading to disproportionate load distribution at the bone-implant interface which would likely decrease transverse displacement.56 These findings therefore demonstrate that bicortical anchorage leads to increased expansion compared to monocortical anchorage and that the depth of bicortical anchorage has minimal impact on the amount of expansion.
The ratio between levels D and E was significantly greater for both bicortical models compared to the monocortical model. There was minimal difference between the ratios of the two bicortical models. A larger ratio between levels D and E indicates more parallel expansion in the coronal plane. These results demonstrate that bicortical engagement produces more parallel expansion of the maxillary complex in the coronal plane compared to monocortical engagement.
The V-shaped expansion in coronal and occlusal planes with traditional tooth-borne expanders makes it difficult to attain precise width-coordination between maxillary and mandibular basal bones without resulting in excessive dento-alveolar expansion.54,55 The monocortical model, similar to previous bone-borne expansion models, produced significantly more parallel expansion than traditional tooth-borne expanders.11,45,57,59 More parallel expansion is favorable for patients because it improves stability and increases the amount of expansion in the posterior region of the maxilla where expansion is often necessary and difficult to achieve.11,56,58 However, while the monocortical model and previous bone-borne expansion models were better than tooth-borne expanders, they still produced a partial V-shaped expansion indicating that further parallel expansion was needed. The bicortical models meet this need by producing even greater parallel expansion. Even distribution of force on both layers of the cortical bones and less bending of the mini-implants may have played a significant role in producing bodily expansion of the two halves of maxilla. On the other hand, transverse displacement discrepancies between points A and C were not significant, indicating that bone-borne palatal expansion produced relatively parallel expansion in occlusal plane for all three models and suggesting that bicortical engagement plays a more significant role in producing parallel expansion in the coronal versus the occlusal plane.
This study applied FEA, a computational numerical approximation technique, to a dry skull model. Our results and numeric findings may differ from actual clinical results because clinical situations vary in numerous factors such as maturity of the suture, density of the bones, biological considerations, and shape of palate and other anatomical structures which all affect biomechanical systems of maxillary expansion. Therefore, a single FE model will not be representative of every clinical situation. In addition, FE modeling always includes numerous simplifications and assumptions, which decrease the accuracy of the model. In this model, simplifications we applied that decreased the accuracy of the model included: modeling the sutures, material properties, and boundary conditions. Due to the inherent limitations of FEA and the assumptions made in this study which decrease the accuracy of the model, future studies using mechanical tests and conventional clinical model analysis are necessary to confirm our results. Constantly improving software and modeling techniques may allow for future studies to decrease the amount of necessary assumptions leading to more accurate FEA simulations.
Conclusion
Within the limitations of this study, the following conclusions were drawn:
Bicortical mini-implant anchorage results in improved mini-implant stability, decreased mini-implant deformation and fracture, more parallel expansion in the coronal plane, and increased expansion in bone-borne palatal expansion.
The depth of bicortical mini-implant anchorage has little impact on mini-implant stability, deformation, and transverse displacement in bone-borne palatal expansion.
Bicortical and monocortical anchorage during bone-borne expansion were compared.
Bicortical mini-implant anchorage improves mini-implant stability and expansion.
Bicortical mini-implant anchorage decreases mini-implant deformation and fracture.
Bicortical mini-implant anchorage produces more parallel expansion.
The depth of bicortical mini-implant anchorage is not significant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
aConception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
bConception and design, data analysis and interpretation, manuscript writing.
cConception and design, data analysis and interpretation, manuscript writing, administrative support, final approval of manuscript.
References
- 1.da Silva Filho OG, Santamaria M, Capelozza Filho L. Epidemiology of posterior crossbite in the primary dentition. J Clin Pediatr Dent. 2007;32:73–8. doi: 10.17796/jcpd.32.1.h53g027713432102. [DOI] [PubMed] [Google Scholar]
- 2.Egermark-Eriksson I, Carlsson GE, Magnusson T, Thilander B. A longitudinal study on malocclusion in relation to signs and symptoms of cranio-mandibular disorders in children and adolescents. Eur J Orthod. 1990:12399–407. doi: 10.1093/ejo/12.4.399. [DOI] [PubMed] [Google Scholar]
- 3.Heikinheimo K, Salmi K. Need for orthodontic intervention in five-year-old Finnish children. Proc Finn Dent Soc. 1987;8:165–9. [PubMed] [Google Scholar]
- 4.Kutin G, Hawes RR. Posterior cross-bites in the deciduous and mixed dentitions. Am J Orthod. 1969;56:491–504. doi: 10.1016/0002-9416(69)90210-3. [DOI] [PubMed] [Google Scholar]
- 5.Brunelle JA, Bhat M, Lipton JA. Prevalence and distribution of selected occlusal characteristics in the US population, 1988–1991. J Dent Res. 1996;75 doi: 10.1177/002203459607502S10. Spec No:706–13. [DOI] [PubMed] [Google Scholar]
- 6.Garib DG, Henriques JFC, Janson G, Freitas MR, Coelho RA. Rapid maxillary expansion—tooth tissue-borne versus tooth-borne expanders: a computed tomography evaluation of dentoskeletal effects. Angle Orthod. 2005;75:548–57. doi: 10.1043/0003-3219(2005)75[548:RMETVT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Gurel HG, Memili B, Erkan M, Sukurica Y. Long-term effects of rapid maxillary expansion followed by fixed appliances. Angle Orthod. 2010;80:5–9. doi: 10.2319/011209-22.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Long H, Ye N, et al. The effectiveness of non-surgical maxillary expansion: a meta-analysis. Eur J Orthod. 2014;36:233–42. doi: 10.1093/ejo/cjt044. [DOI] [PubMed] [Google Scholar]
- 9.Kokich VG. Age changes in the human frontozygomatic suture from 20 to 95 years. Am J Orthod. 1976;69:411–30. doi: 10.1016/0002-9416(76)90209-8. [DOI] [PubMed] [Google Scholar]
- 10.Harzer W, Schneider M, Gedrange T, Tausche E. Direct bone placement of the hyrax fixation screw for surgically assisted rapid palatal expansion (SARPE) J Oral Maxillofac Surg. 2006;64:1313–7. doi: 10.1016/j.joms.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Tausche E, Hansen L, Hietschold V, Lagravère MO, Harzer W. Three-dimensional evaluation of surgically assisted implant bone-borne rapid maxillary expansion: a pilot study. Am J Orthod Dentofacial Orthop. 2007;131(4 Suppl):S92–9. doi: 10.1016/j.ajodo.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Capelozza Filho L, Cardoso Neto J, da Silva Filho OG, Ursi WJ. Non-surgically assisted rapid maxillary expansion in adults. Int J Adult Orthodon Orthognath Surg. 1996;11:57–66. [PubMed] [Google Scholar]
- 13.Baysal A, Karadede I, Hekimoglu S, Ucar F, Ozzer T, Veli I, Uysal T. Evaluation of root resorption following rapid maxillary expansion using cone-beam computed tomography. Angle Orthod. 2012;82:488–94. doi: 10.2319/060411-367.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halicioğlu K, Kiki A, Yavuz I. Maxillary expansion with the memory screw: a preliminary investigation. Korean J Orthod. 2012;42:73–9. doi: 10.4041/kjod.2012.42.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty V, Caridad JM, Caputo AA, Chaconas SJ. Biomechanical rationale for surgical-orthodontic expansion of the adult maxilla. J Oral Maxillofac Surg. 1994;52:742–9. doi: 10.1016/0278-2391(94)90492-8. [DOI] [PubMed] [Google Scholar]
- 16.Byloff FK, Mossaz CF. Skeletal and dental changes following surgically assisted rapid palatal expansion. Eur J Orthod. 2004;26:403–9. doi: 10.1093/ejo/26.4.403. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier C, Voyer R, Paquette M, Rompré P, Papadakis A. Periodontal effects of surgically assisted rapid palatal expansion evaluated clinically and with cone-beam computerized tomography: 6-month preliminary results. Am J Orthod Dentofacial Orthop. 2011;139(4 Suppl):S117–28. doi: 10.1016/j.ajodo.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Lee KJ, Park YC, Park JY, Hwang WS. Miniscrew-assisted nonsurgical palatal expansion before orthognathic surgery for a patient with severe mandibular prognathism. Am J Orthod Dentofacial Orthop. 2010;137:830–9. doi: 10.1016/j.ajodo.2007.10.065. [DOI] [PubMed] [Google Scholar]
- 19.Kim KB, Helmkamp ME. Miniscrew implant-supported rapid maxillary expansion. J Clin Orthod. 2012;46:608–12. [PubMed] [Google Scholar]
- 20.Garib DG, Navarro RDL, Francischone CE, Oltramari PVP. Rapid maxillary expansion using palatal implants. J Clin Orthod. 2008;42:665–71. [PubMed] [Google Scholar]
- 21.Lagravère MO, Carey J, Heo G, Toogood RW, Major PW. Transverse, vertical, and anteroposterior changes from bone-anchored maxillary expansion vs traditional rapid maxillary expansion: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 2010;137:304.e1–12. doi: 10.1016/j.ajodo.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Tausche E, Hansen L, Schneider M, Harzer W. Bone-supported rapid maxillary expansion with an implant-borne Hyrax screw: the Dresden Distractor. Orthod Fr. 2008;79:127–35. doi: 10.1051/orthodfr:2008008. [DOI] [PubMed] [Google Scholar]
- 23.Carlson C, Sung J, McComb RW, Machado AW, Moon W. The use of a micro-implant assisted rapid palatal expansion (MARPE) appliance to orthopedically correct transverse maxillary deficiency in an adult patient. Am J Orthod Dentofacial Orthop. 2016 doi: 10.1016/j.ajodo.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Ahn HW, Kim SJ, Moon SC, Kim SH, Nelson G. Tooth-borne vs bone-borne rapid maxillary expanders in late adolescence. Angle Orthod. 2015;85:253–62. doi: 10.2319/030514-156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilmes B, Nienkemper M, Drescher D. Application and effectiveness of a mini-implant-and tooth-borne rapid palatal expansion device: the hybrid hyrax. World J Orthod. 2010;11:323–30. [PubMed] [Google Scholar]
- 26.Crismani AG, Bertl MH, Celar AG, Bantleon H-P, Burstone CJ. Miniscrews in orthodontic treatment: review and analysis of published clinical trials. Am J Orthod Dentofacial Orthop. 2010;137:108–13. doi: 10.1016/j.ajodo.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Lim HJ, Choi YJ, Evans CA, Hwang HS. Predictors of initial stability of orthodontic miniscrew implants. Eur J Orthod. 2011;33:528–32. doi: 10.1093/ejo/cjq122. [DOI] [PubMed] [Google Scholar]
- 28.Wu T-Y, Kuang S-H, Wu C-H. Factors associated with the stability of mini-implants for orthodontic anchorage: a study of 414 samples in Taiwan. J Oral Maxillofac Surg. 2009;67:1595–9. doi: 10.1016/j.joms.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Kim YH, Yang SM, Kim S, Lee JY, Kim KE, Gianelly AA, Kyung SH. Midpalatal miniscrews for orthodontic anchorage: factors affecting clinical success. Am J Orthod Dentofacial Orthop. 2010;137:66–72. doi: 10.1016/j.ajodo.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 30.Miyawaki S, Koyama I, Inoue M, Mishima K, Sugahara T, Takano-Yamamoto T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2003;124:373–8. doi: 10.1016/s0889-5406(03)00565-1. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Xu Z, Tan L, Zhao Z, Yang P, Li Y, Tang T, Zhao L. Orthodontic mini-implant stability under continuous or intermittent loading: a histomorphometric and biomechanical analysis. Clin Implant Dent Relat Res. 2015;17:163–72. doi: 10.1111/cid.12090. [DOI] [PubMed] [Google Scholar]
- 32.Holberg C, Winterhalder P, Rudzki-Janson I, Wichelhaus A. Finite element analysis of mono- and bicortical mini-implant stability. Eur J Orthod. 2014;36:550–6. doi: 10.1093/ejo/cjt023. [DOI] [PubMed] [Google Scholar]
- 33.Hong C, Lee H, Webster R, Kwak J, Wu BM, Moon W. Stability comparison between commercially available mini-implants and a novel design: part 1. Angle Orthod. 2011;81:692–9. doi: 10.2319/092410-556.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong C, Truong P, Song HN, Wu BM, Moon W. Mechanical stability assessment of novel orthodontic mini-implant designs: Part 2. Angle Orthod. 2011;81:1001–9. doi: 10.2319/031011-176.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song HN, Hong C, Banh R, et al. Mechanical stability and clinical applicability assessment of novel orthodontic mini-implant design. Angle Orthod. 2013;83:832–41. doi: 10.2319/111412-876.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brettin BT, Grosland NM, Qian F, et al. Bicortical vs monocortical orthodontic skeletal anchorage. Am J Orthod Dentofacial Orthop. 2008;134:625–35. doi: 10.1016/j.ajodo.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Lee SC, Park JH, Bayome M, Kim KB, Araujo EA, Kook Y-A. Effect of bone-borne rapid maxillary expanders with and without surgical assistance on the craniofacial structures using finite element analysis. Am J Orthod Dentofacial Orthop. 2014;145:638–48. doi: 10.1016/j.ajodo.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Lee HK, Bayome M, Ahn CS, et al. Stress distribution and displacement by different bone-borne palatal expanders with micro-implants: a three-dimensional finite-element analysis. Eur J Orthod. 2014;36:531–40. doi: 10.1093/ejo/cjs063. [DOI] [PubMed] [Google Scholar]
- 39.Boryor A, Hohmann A, Wunderlich A, et al. Use of a modified expander during rapid maxillary expansion in adults: an in vitro and finite element study. Int J Oral Maxillofac Implants. 28:e11–6. doi: 10.11607/jomi.2078. [DOI] [PubMed] [Google Scholar]
- 40.MacGinnis M, Chu H, Youssef G, Wu KW, Machado AW, Moon W. The effects of micro-implant assisted rapid palatal expansion (MARPE) on the nasomaxillary complex-a finite element method (FEM) analysis. Prog Orthod. 2014;15:52. doi: 10.1186/s40510-014-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon W, Wu KW, MacGinnis M, Sung J, Chu H, Youssef G, Machado A. The efficacy of maxillary protraction protocols with the micro-implant-assisted rapid palatal expander (MARPE) and the novel N2 mini-implant-a finite element study. Prog Orthod. 2015;16:16. doi: 10.1186/s40510-015-0083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soboleski D, McCloskey D, Mussari B, Sauerbrei E, Clarke M, Fletcher A. Sonography of normal cranial sutures. AJR Am J Roentgenol. 1997;168:819–21. doi: 10.2214/ajr.168.3.9057541. [DOI] [PubMed] [Google Scholar]
- 43.Farnsworth D, Rossouw PE, Ceen RF, Buschang PH. Cortical bone thickness at common miniscrew implant placement sites. Am J Orthod Dentofacial Orthop. 2011;139:495–503. doi: 10.1016/j.ajodo.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 44.Studer SP, Allen EP, Rees TC, Kouba A. The thickness of masticatory mucosa in the human hard palate and tuberosity as potential donor sites for ridge augmentation procedures. J Periodontol. 1997;68:145–51. doi: 10.1902/jop.1997.68.2.145. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig B, Baumgaertel S, Zorkun B, Bonitz L, Glasl B, Wilmes B, Lisson J. Application of a new viscoelastic finite element method model and analysis of miniscrew-supported hybrid hyrax treatment. Am J Orthod Dentofacial Orthop. 2013;143:426–35. doi: 10.1016/j.ajodo.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Goktas S, Dmytryk JJ, McFetridge PS. Biomechanical behavior of oral soft tissues. J Periodontol. 2011;82:1178–86. doi: 10.1902/jop.2011.100573. [DOI] [PubMed] [Google Scholar]
- 47.Gautam P, Valiathan A, Adhikari R. Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: a finite element method study. Am J Orthod Dentofacial Orthop. 2007;132:5e1–11. doi: 10.1016/j.ajodo.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 48.Ngan P, Moon W. Evolution of Class III treatment in orthodontics. Am J Orthod Dentofacial Orthop. 2015;148:22–36. doi: 10.1016/j.ajodo.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Moon W, Khulla R. Class III Orthopedic Treatment with Skeletal Anchorage. Bentham eBooks. 2014:116–150. [Google Scholar]
- 50.Moon W. An interview with Won Moon. By André Wilson Machado, Barry Briss, Greg J Huang, Richard Kulbersh and Sergei Godeiro Fernandes Rabelo Caldas. Dental Press J Orthod. 18:12–28. doi: 10.1590/s2176-94512013000300005. [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Mogra S, Shetty VS, Shetty S, Philip P. Three-dimensional finite element analysis of strength, stability, and stress distribution in orthodontic anchorage: a conical, self-drilling miniscrew implant system. Am J Orthod Dentofacial Orthop. 2012;141:327–36. doi: 10.1016/j.ajodo.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Florvaag B, Kneuertz P, Lazar F, Koebke J, Zoller JE, et al. Biomechanical properties of orthodontic miniscrews. An in-vitro study. J Orofac Orthop. 2010;71:53–67. doi: 10.1007/s00056-010-9933-y. [DOI] [PubMed] [Google Scholar]
- 53.Frost HM. Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64:175–88. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 54.Pithon MM, Figueiredo DSF, Oliveira DD. Mechanical evaluation of orthodontic mini-implants of different lengths. J Oral Maxillofac Surg. 2013;71:479–86. doi: 10.1016/j.joms.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Motoyoshi M, Inaba M, Ono A, Ueno S, Shimizu N. The effect of cortical bone thickness on the stability of orthodontic mini-implants and on the stress distribution in surrounding bone. Int J Oral Maxillofac Surg. 2009;38:13–8. doi: 10.1016/j.ijom.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Pickard MB, Dechow P, Rossouw PE, Buschang PH. Effects of miniscrew orientation on implant stability and resistance to failure. Am J Orthod Dentofacial Orthop. 2010;137:91–9. doi: 10.1016/j.ajodo.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Garrett BJ, Caruso JM, Rungcharassaeng K, Farrage JR, Kim JS, Taylor GD. Skeletal effects to the maxilla after rapid maxillary expansion assessed with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2008;134:8–9. doi: 10.1016/j.ajodo.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Ghoneima A, Abdel-Fattah E, Hartsfield J, El-Bedwehi A, Kamel A, Kula K. Effects of rapid maxillary expansion on the cranial and circummaxillary sutures. Am J Orthod Dentofacial Orthop. 2011;140:510–9. doi: 10.1016/j.ajodo.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, Ting K, Nelson M, Sun N, Sung SJ. Maxillary expansion in customized finite element method models. Am J Orthod Dentofacial Orthop. 2009;136:367–74. doi: 10.1016/j.ajodo.2008.08.023. [DOI] [PubMed] [Google Scholar]


