Abstract
IMPORTANCE
Wild-type (WT) gastrointestinal stromal tumors (GISTs), which lack KIT and PDGFRA gene mutations, are the primary form of GIST in children and occasionally occur in adults. They respond poorly to standard targeted therapy. Better molecular and clinical characterization could improve management.
OBJECTIVE
To evaluate the clinical and tumor genomic features of WT GIST.
DESIGN, SETTING, AND PARTICIPANTS
Patients enrolled in an observational study at the National Institutes of Health starting in 2008 and were evaluated in a GIST clinic held once or twice yearly. Patients provided access to existing medical records and tumor specimens. Self-referred or physician-referred patients younger than 19 years with GIST or 19 years or older with known WT GIST (no mutations in KIT or PDGFRA) were recruited; 116 patients with WT GIST were enrolled, and 95 had adequate tumor specimen available. Tumors were characterized by immunohistochemical analysis (IHC) for succinate dehydrogenase (SDH) subunit B, sequencing of SDH genes, and determination of SDHC promoter methylation. Testing of germline SDH genes was offered to consenting patients and families.
MAIN OUTCOMES AND MEASURES
For classification, tumors were characterized by SDHA, B, C, or D (SDHX) mutations and other genetic and epigenetic alterations, including presence of mutations in germline. Clinical characteristics were categorized.
RESULTS
Wild-type GIST specimens from 95 patients (median age, 23 [range, 7–78] years; 70% female) were classified into 3 molecular subtypes: SDH-competent (n = 11), defined by detection of SDHB by IHC; and 2 types of SDH-deficient GIST (n = 84). Of SDH-deficient tumors, 63 (67%) had SDH mutations, and in 31 of 38 (82%), the SDHX mutation was also present in germline. Twenty-one (22%) SDH-deficient tumors had methylation of the SDHC promoter leading to silencing of expression. Mutations in known cancer-associated pathways were identified in 9 of 11 SDH-competent tumors. Among patients with SDH-mutant tumors, 62% were female (39 of 63), median (range) age was 23 (7–58) years, and approximately 30% presented with metastases (liver [12 of 58], peritoneal [6 of 58], lymph node [15 of 23]). SDHC-epimutant tumors mostly affected young females (20 of 21; median [range] age, 15 [8–50] years), and approximately 40% presented with metastases (liver [7 of 19], peritoneal [1 of 19], lymph node [3 of 8]). SDH-deficient tumors occurred only in the stomach and had an indolent course.
CONCLUSIONS AND RELEVANCE
An observational study of WT GIST permitted the evaluation of a large number of patients with this rare disease. Three molecular subtypes with implications for prognosis and clinical management were identified.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract, but they are uncommon tumors, with incidence estimated to be between 6.8 and 20 per million population.1–4 Most GISTs occurring in adults are driven by activating mutations in the KIT or PDGFRA genes,5,6 but 85% of GISTs in children and 10% to 15% of GISTs in adults are negative for KIT and PDGFRA mutations (wild-type [WT] GIST).7,8 The rarity of these malignant neoplasms has made it difficult to determine their natural history and response to treatment; however, WT GIST is known to be generally unresponsive to the kinase inhibitor therapies used for non-WT GIST. Isolated institutional series and case reports have suggested that WT GIST primarily affects young females, is multifocal but indolent, and the primary tumor is generally gastric in location.9 WT GIST, along with paraganglioma, is a component of the Carney-Stratakis syndrome, an inherited predisposition syndrome caused by germline mutations in the succinyl dehydrogenase (SDH) B, C, or D subunit.10 Based on this association, we and others have previously identified SDHA, SDHB, SDHC, and SDHD mutations (together referred to as SDHX mutations) in some but not all WT GIST.11,12 Wild-type GIST is also associated with a nonfamilial multitumor syndrome known as Carney triad (WT GIST, paraganglioma, and pulmonary chondroma) that is not associated with SDH germline mutations.13
The National Cancer Institute (NCI) of the National Institutes of Health (NIH) instituted a WT GIST clinic to study this rare tumor. Patient assessment along with testing of archived tumor samples allowed us to develop a molecular classification of these tumors that has implications for prognosis and treatment.
Methods
Patients
Patients younger than 19 years with GIST and adults 19 years or older with known WT GIST were recruited to the NIH Pediatric and WT GIST Clinic starting in 2008. Patients were self-referred or referred by their physicians. Information about the clinic was provided to GIST advocacy groups and was available on a website (https://ccr.cancer.gov/gist). All patients were enrolled in a noninterventional natural history protocol that was approved by the NCI institutional review board. All patients or their parents or legal guardians provided written informed consent. Consent for genetic testing was optional. In some cases it was obtained also from family members. Data collected included clinic and hospitalization notes, operation summaries, pathology reports, and imaging studies. Patients underwent a history and physical examination, had standard safety laboratory studies performed, and met with or had their records reviewed by medical specialists as appropriate. Psychological and social work support were available.
Tumor Assessment
Pathologic features, including size and site of origin and of metastases, were collected from previous records. Fresh-frozen tumor samples, formalin-fixed paraffin-embedded archived tumor blocks, or unstained slides were obtained when available and were reviewed by a single pathologist (M.M.M.). All patients included in this report had tumors that were confirmed as WTGIST by documented lack of KIT (exons 9, 11, 13, 17) and PDGFRA (exons 12, 14, 18) mutations. Testing for KIT and PDGFRA were performed as previously described.14 Immunostaining for SDHB was performed using the monoclonal antibody 21A11 (AbCam) diluted 1:1000, including epitope retrieval with Leica retrieval solution (alkaline buffer). Diaminobenzene was used as the chromogen. A positive internal control was required to validate each immunostain. Mitotic index was determined by 1 pathologist (M.M.M.) by counting mitoses in a total area of 5 mm2 from the most mitotically active or most cellular area or until more than 100 mitoses were found.
The OncoVar GIST assay developed at the NCI was performed as previously described.14 Tumor tissue was analyzed by hybrid capture sequencing for variants in genes implicated in GIST tumorigenesis, including the SDH subunit A, B, C, D (SDHX) gene, and kinase pathway genes including KIT, PDGFRA, BRAF, CBL, and NF1. DNA extracted from tumor was used for genomic DNA library construction, in-solution hybridization to customized RNA baits (Agilent SureSelect) targeting the GIST pathogenicity genes, and single-molecule sequencing of the partitioned DNA library (Illumina MiSeq). Reference sequences for SDHA, SDHB, SDHC, SDHD, KIT, BRAF, PDGFRA, CBL, and KRAS are NM_004168, NM_003000, NM_001035511, NM_003002, NM_000222, NM_004333, NM_006206, NM_005188, and NM_004985, respectively. The mean read depth for targeted sequences was more than 100×. The GIST DNA methylation profiles were assayed by Illumina microarrays, and tumors were scored as methyl divergent or methyl centrist as previously described.15 Histologically benign normal tissue and/or fluid (ie, blood or saliva) was used as a reference for somatic mutation status when available.
Statistical Analysis
χ2 Tests were used to examine differences in distributions of categorical variables (eg, sex, focality, location, histologic subtype) among groups. The Kruskal-Wallis test was used to examine differences in age among the 3 groups; pairwise group comparisons were performed using exact Wilcoxon rank sum tests. No adjustments were made for multiple comparisons in this exploratory study.
Results
Molecular Subtypes of WT GIST
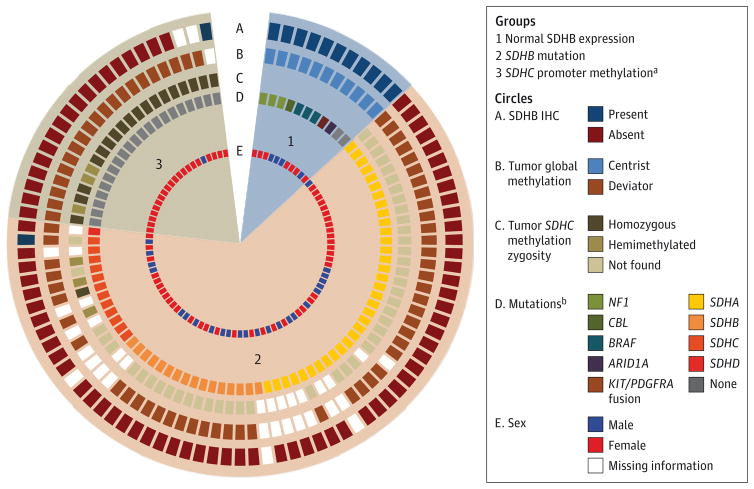
A total of 116 patients with WT GIST were seen in the clinic; of these, 95 had adequate tumor specimen available for molecular analysis and classification by SDHB immunohistochemical analysis (IHC), SDHX gene sequencing, and/or SDHC methylation status. The majority (n = 84 [88%]) of tumors were SDH deficient with absence of SDHB expression (n = 77), and/or presence of SDHX mutation (n = 63) and/or SDHC promoter methylation (n = 25) (Figure; eTable 1 in the Supplement).
Figure. Immunohistochemical Analysis (IHC) and Genetic Characteristics of Tumors From 95 Patients With KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors.
Five concentric circles depict succinate dehydrogenase (SDH) B expression by IHC (circle A), global tumor DNA methylation (circle B), presence of tumor SDHC promoter methylation including zygosity (circle C), mutations in NF1, BRAF, CBL, ARID1A, KIT/PDGFRA fusion, SDHA, SDHB, SDHC, or SDHD (circle D), and sex (circle E). Tumors are shown in 3 groups: group 1 tumors have normal SDHB expression (n = 11), group 2 tumors have SDHB mutations (n = 63), and group 3 tumors have SDHC promoter methylation (n = 21).
aOne patient in this group was SDHB positive by IHC.
bSee eTable 1 in the Supplement for details.
Of the 63 (66%) cases of SDH-mutant GIST, 34 had mutations in SDHA, 16 in SDHB, 12 in SDHC, and 1 in SDHD. Additional somatic mutations in KIT, TP53, and KRAS were observed in 1 tumor each (eTable 1 in the Supplement). Nine patients with no tumor DNA had SDHX germline mutations (3 in SDHA, 2 in SDHB, and 4 in SDHC). Of the 38 patients with SDH-mutant GIST who had matching germline and tumor DNA, 31 (82%) had the same mutation detected in germline and tumor. The presence of germline mutations led to genetic counseling and genetic testing of some first-degree relatives. Similar mutations were observed in parents and siblings.
The remainder of the SDH-deficient tumors (n = 21 [22% of total]) had a specific SDHC promoter methylation but no structural mutation. There is no reliable antibody available to test for SDH subunit C by IHC; however, all of these tumors lacked SDHC RNA expression, as previously described.14 This molecular subtype is referred to as SDH epimutant. Eleven patients had tumors that were SDH competent as demonstrated by normal or preserved SDHB expression by IHC and lack of SDHX mutation by sequencing. Three of these SDH-competent GISTs had BRAF p.V600E mutations,16,17 and 3 had NF1 mutations (2 insertion/deletion or frameshift mutations and 1 missense mutation). No matching germline DNA was available for analysis in any patient with an NF1 mutation, but 1 had known neurofibromatosis type 1 disease. One SDH-competent tumor had a mutation in CBL, a gene encoding an E3 ubiquitin ligase, 1 a tandem duplication resulting in fusion between the N-terminal region of KIT and the C-terminal region of PDGFRA, and 1 an ARID1A mutation previously identified as a driver mutation (Figure). The remaining 2 patients with SDH-competent tumors in group 1 had no clearly pathogenic mutations identified in the genes sequenced.
Methylation patterns for the 48 SDH-mutant tumors and the 20 SDH-epimutant tumors with sufficient DNA for methylation analysis showed global tumor hypermethylation. All 11 of the SDH-competent tumors had normal tumor methylation patterns (Figure).
Patient Demographic Characteristics and Clinical Features by WT GIST Molecular Subtype
Demographic Characteristics and Clinical Presentation
The Table presents clinical characteristics of the patients according to molecular tumor subtype. Patients with SDH-mutant GIST were predominantly female with a median age of 23 years (range, 7–78 years); all had a gastric primary tumor; and their tumors were predominantly either epithelioid or mixed epithelioid/spindle cell histologic subtype (50 of 59). Patients with SDH-mutant GIST had a high incidence (15 of 23 [65%]) of nodal involvement, and 26 of 58 (45%) had evidence of lymph node, liver, or peritoneal spread at presentation. Patients with SDH-epimutant GIST were overwhelmingly female (20 of 21 [95%]), with a median (range) age of 15 (8–50) years; all had a gastric primary; and their tumors were predominantly epithelioid or mixed histologic subtype (18 of 20). Many patients presented with liver (7 of 19), lymph node (3 of 8), or peritoneal metastases (1 of 19). Seven patients also had paragangliomas and/or pulmonary chondromas.
Table.
Patient Demographics and Tumor Characteristics
| Characteristic | Group 1: SDH-Competent GIST (n = 11) | Group 2: SDHX-Mutant GIST (n = 63) | Group 3: SDHC-Epimutant GIST (n = 21) | All Patients (n = 95) |
|---|---|---|---|---|
| Age, median (range), ya | 46 (30–78) | 23 (7–58) | 15 (8–50) | 23 (7–78) |
| Female sex, No. (%)b | 7 (64) | 39 (62) | 20 (95) | 66 (70) |
| Tumor size at resection, median (range), cm | 8.9 (4.7–13.5) | 5.6 (1.5–21) | 4.7 (2–16) | 5.6 (1.5–21) |
| Focality, proportion (%)c,d | ||||
| Unifocal | 9/10 (90) | 33/55 (60) | 5/18 (28) | 47/83 (57) |
| Multifocal | 1/10 (10) | 22/55 (40) | 13/18 (72) | 36/83 (43) |
| Primary location, No. (%)e | ||||
| Gastric | 1 (9) | 63 (100) | 21 (100) | 85 (89) |
| Small bowel | 9 (82) | 0 | 0 | 9 (9) |
| Abdominal | 1 (9) | 0 | 0 | 1 (1) |
| Histologic subtype, proportion (%)d,f | ||||
| Epithelioid | 1/11 (9) | 22/59 (37) | 9/20 (45) | 32/90 (36) |
| Spindle | 9/11 (82) | 9/59 (15) | 2/20 (10) | 20/90 (22) |
| Mixed | 1/11 (9) | 28/59 (47) | 9/20 (45) | 38/90 (42) |
| Metastasis at presentation, proportion (%)d | ||||
| Liver | 0/10 | 12/58 (21) | 7/19 (37) | 19/87 (22) |
| Peritoneum | 1/10 (10) | 6/58 (10) | 1/19 (5) | 8/87 (9) |
| Lymph nodes | 0/4 | 15/23 (65) | 3/8 (38) | 18/35 (51) |
| No liver or peritoneal metastases at presentation, proportion (%)d | 9/10 (90) | 41/58 (71) | 12/19 (63) | 62/87 (71) |
Abbreviations: GIST, gastrointestinal stromal tumor; SDH, succinate dehydrogenase.
There was a significant difference in age between the 3 groups (P < .001). Pairwise comparisons were significantly different: group 1 vs 2, P < .001; group 1 vs 3, P < .001; group 2 vs 3, P = .002.
There was a significant difference in distribution of sex by group (P = .02); in pairwise comparisons, there was no difference between groups 1 and 2 (P = .91), but the distribution of sex differed significantly between group 1 and group 3 (P = .02) and between group 2 and group 3 (P = .004).
There was a significant difference between focality of presentation for SDH-competent and SDH-deficient GIST (P = .02).
The number of cases is less than the number of patients because of incomplete information.
There was a significant difference in the distribution of primary location of tumors. All group 2 and 3 patients had gastric tumors while 1 of 11 group 1 patients had a gastric tumor (P < .001).
There was a significant difference in histologic subtype among the groups. Group 1 vs 2: P < .001, group 1 vs 3: P < .001, group 2 vs 3: P = .76.
The 11 patients with SDH-competent GIST were adults, and most (7 of 11 [64%]) were female. Almost all (9 of 11 [82%]) presented with small bowel disease. All but 2 of these 11 tumors had spindle cell histologic subtype. Only 1 of 10 patients with an SDH-competent GIST presented with metastases.
Survival According to GIST Subtype
Of the 63 patients with SDH-mutant GIST seen in clinic, after a median follow-up from diagnosis of 6 (range, 1–44) years, 3 had died (8 to 24 years after initial diagnosis) (eTable 2 in the Supplement). After a median follow-up of 7 (range, 1–32) years, 1 patient with SDH-epimutant GIST died 6 years after diagnosis. Three of 11 (27%) patients with SDH-competent WT GIST in group 1 have died of progressive disease. Median follow-up for patients with this subtype was 8 years (range, 2–17 years).
Treatment Response
Treatment data were based only on medical record review, and assessment was not standardized. The documented objective response of SDH-deficient tumors to kinase inhibitors was poor: only 1 of 49 patients treated with imatinib mesylate had a response (partial), and 7 of 38 patients treated with sunitinib malate had an objective response (1 complete, 3 partial, 3 mixed, defined as regression at some sites and progression at others). Stable disease was difficult to ascribe to treatment because protracted periods of stable disease also occurred in untreated patients.
Patients With Syndromic GIST
Of the 95 patients, 18 had syndromic GIST, ie, Carney triad or Carney-Stratakis syndrome, on the basis of the presence of paraganglioma and/or chondroma (eTable 3 in the Supplement). Only 2 patients had complete Carney triad, which is consistent with recent reports in which only 25% of patients with Carney triad had all 3 tumors.18 All tumors in patients with Carney triad or Carney-Stratakis syndrome had SDH abnormalities. Of 11 patients with Carney triad, 5 had SDH-mutant GIST (3 had SDHA and 2 had SDHC germline mutations), and 6 had SDH-epimutant GIST with SDHC promoter–specific methylation. Among the 7 patients with Carney-Stratakis syndrome, 6 had SDH-mutant GIST and 1 had an SDH-epimutant GIST.
Discussion
Patients with uncommon pediatric tumors are as disadvantaged as any population with orphan disease status. Expertise is difficult to develop when only a handful of cases are seen at any single center, and the scientific and financial incentives to develop targeted therapies are limited by the relatively small burden of disease. For cancers, however, there is the possibility of leveraging treatments developed for common tumors if there is an overlap in essential pathways between rare and common tumors.
Based on the knowledge that pediatric GIST, a rare tumor type, responds poorly to tyrosine kinase inhibitors, a pediatric and KIT/PDGFRA WT GIST clinic was established at the NIH. The response from patients was overwhelming, leading to the largest group of patients with WT GIST ever studied. Although we offered a second opinion but not primary care, the patients and their families selflessly donated their time and specimens for the benefit of others.
Initial discoveries made by assessing these patients included aberrant methylation patterns and SDH mutations in sporadic WT GIST.12,15 The size of the cohort has now permitted the development of a new molecular classification. This classification has implications for clinical presentation, prognosis, treatment, and additional cancer risk. In addition, molecular classification clarifies priorities for future research for each molecular subtype.
SDH-competent tumors retain SDHB expression and a normal methylation pattern. They have tumor and patient demographic features similar to those seen in patients with KIT/PDGFRA-mutant tumors: they occur in older patients and have spindle cell histologic subtype, although 82% were of small bowel origin, which may be higher than is observed in KIT/PDGFRA-mutant tumors. Patients with these tumors have more aggressive disease compared with those with SDH-deficient tumors. As pointed out by other groups, these patients should be examined for features of neurofibromatosis and should have their tumors tested for mutations in BRAF.17 Two of the 11 patients seen in our clinic had tumors without identifiable mutations. The identification of a mutation in CBL, known to be downstream of KIT signaling, in a tumor in 1 of our patients raises the possibility that mutations in other proto-oncogenes reported to be downstream of KIT could be driving disease in those tumors in which no mutation was detected.19 Furthermore, our identification of a cryptic fusion between KIT and PDGFRA by RNaseq analysis raises the possibility that additional kinase activations may be identified in this group, which is currently designated “quadruple WT GIST.”20 Identifying tumor mutations might prove useful in determining appropriate treatment. For example, a patient with a GIST harboring a BRAF mutation has responded to dabrafenib, a BRAF inhibitor.21 NF1 mutations are rare in these tumors, but treatments that target MEK may have utility in this rare subgroup.22 Recently, it has also been suggested that ARID1A mutations may be targeted by EZH2 inhibitors that are currently in development.23 Consequently, the first priority for further research in this molecular subtype is more extensive sequencing with methods such as whole-exome sequencing, RNA sequencing, and whole-genome sequencing to discover novel genomic events affecting kinases that could suggest therapeutic vulnerabilities.
The most common molecular subtype in our clinic was SDH-mutant GIST with SDHX (most commonly SDHA) mutations detected in the tumor, germline, or both. This extends our previous observation of the possible hereditary nature of SDH-deficient GIST.12 Although mutations in SDHB appear to be most common in paragangliomas, in our patients, SDHA was more common.24 While the mechanisms for tumor selectivity are unclear, the minority of GISTs with SDHA mutations (9 of 25 available samples) had loss of heterozygosity as the second hit, while most tumors with SDHB mutations (12 of 13 available samples) had loss of heterozygosity as the second hit. Patients with SDH-epimutant tumors with SDHC promoter–specific methylation were younger, overwhelmingly female, had disease of gastric origin, and often (40%) presented with metastases. Seven group 3 patients had syndromic GIST: 5 with incomplete and 1 with complete Carney triad, and 1 with Carney-Stratakis syndrome; 6 of 7 were female. The distinction between Carney triad and Carney-Stratakis syndrome may be better described by the presence of SDHC promoter hypermethylation (nonheritable syndrome) vs SDHX mutation (heritable syndrome) rather than the presence or absence of chondromas. The homogeneity of patients with SDHC promoter–specific methylation is a tantalizing clue to the potential interaction between sex, SDHC promotor–specific methylation, and oncogenesis. We propose to refer to both the SDHX-mutant and the SDH-epimutant tumors (groups 2 and 3 in Figure) as SDH-deficient GIST.
Patients with SDH-deficient GIST, for whom there is no clearly effective systemic therapy, commonly present with metastatic disease that progresses rapidly; nonetheless, their life expectancy is measured in years. Our survival analysis, however, is limited by bias because GIST clinic follow-up did not start at diagnosis.
This study highlights compelling clinical reasons for determining the molecular subtype of the tumor in all patients with WT GIST. Most patients will have either SDH-deficient GIST or definable mutations in known cancer-associated pathways. In fact, of the 95 patients described in this report whose tumor had no KIT or PDGFRA mutations, only 2 currently have unidentified alterations.
Our data suggest that a diagnosis of SDH-deficient GIST should be considered in patients with gastric GIST when routine diagnostic evaluation does not identify KIT or PDGFRA mutations, particularly if the patient is younger than 30 years. The SDH status of the tumor should first be determined to separate SDH-competent from SDH-deficient GIST, which can be accomplished easily and cheaply using SDHB IHC. If a tumor is SDH deficient by IHC, sequencing of SDHX in tumor and germline should be performed. If no SDH mutation is identified, then the presence or absence of SDHC promoter methylation should be determined. This subtyping is clinically useful because patients with SDH-mutant GIST should be referred to a cancer predisposition clinic, because any germline mutation in SDHX leads to an increased risk of paraganglioma, pheochromocytoma, or other tumors.24 Screening these patients with annual whole-body rapid magnetic resonance imaging and measurement of plasma catecholamines or urinary metanephrine levels may be appropriate.25 Furthermore, patients with SDHX mutations require germline testing to determine whether the mutation is sporadic or germline, and if a germline mutation is found, genetic counseling is indicated. In contrast, those patients found to have SDHC promoter hypermethylation do not require genetic counseling, as these are not germline alterations. However, these patients do still require screening for paragangliomas as noted, as they are often associated with syndromic GIST.
Finally, knowledge of the molecular subtype has implications for treatment strategies. In the SDH-competent subtype, a search for kinase mutations that might predict response to targeted therapy should be undertaken. Because multifocal presentation is common in SDH-deficient subtypes, disease is often unresectable, so that a conservative approach to surgery is recommended, with surgical intervention reserved for symptomatic relief (bleeding, obstruction, pain), or to address risk to important anatomic structures.26 Because of limited response to sunitinib therapy in SDH-deficient GIST, use should be reserved for advanced or progressive disease. Imatinib had almost no activity in our patients.
The identification of SDH dysfunction as the primary alteration in the majority of WT GIST provides clues for the development of more effective systemic therapy. SDH (also referred to as mitochondrial complex II) is a heterotetrameric tricarboxylic acid (TCA) (Krebs) cycle enzyme consisting of SDHA and SDHB, which encode the catalytic enzymatic component, and SDHC and SDHD, which anchor the entire SDH complex to the inner mitochondrial membrane. In addition to SDH mutations in GIST and in paragangliomas, mutations in fumarate hydratase (FH), just downstream of SDH in the TCA cycle, have been reported in renal cell cancers and leiomyomas.27 Another TCA cycle enzyme, isocitrate dehydrogenase (IDH), upstream of SDH in the TCA cycle, is mutated in some cartilaginous tumors, gliomas, and leukemias. 28,29 These TCA cycle mutations are associated with tumor hypermethylation, likely due to inhibition of the TET family of 5-methyl cytosine demethylases and the histone lysine family of demethylases, as well as inhibition of other α-ketoglutarate–dependent dioxygenase-catalyzed reactions that generate succinate and carbon dioxide as by-products.30,31 Furthermore, defects in SDH, FH, and IDH lead to upregulation of HIF1a through inhibition of prolyl hydroxylases, other members of the dioxygenase family. This in turn activates angiogenesis in tumors via a “pseudohypoxia” mechanism caused by failure to degrade HIF1a.32 It is tempting to speculate that the better response to sunitinib compared with imatinib observed in patients with pediatric GISTs is due to sunitinib’s activity against VEGFR.7 We are currently studying another potent VEGFR inhibitor, vandetanib, in progressive SDH mutant and SDH-epimutant GIST based on these observations and on reports of vandetanib’s activity in FH-deficient kidney tumors.33 The universal finding of tumor hypermethylation, as well as the specific SDHC promoter methylationwe have observed in SDH-epimutant GIST, suggest the potential utility of DNA methyltransferase inhibitors in these tumors, and a clinical trial of these agents is planned.
Conclusions
The establishment of a clinic for patients with WTGIST has allowed us to begin to identify molecular subtypes that appear to have specific clinical implications. Knowledge gained about the underlying genetic alterations should prove useful in the development of new approaches to systemic therapy. We hope that our efforts will accelerate progress toward better treatment of this disease—or, rather, group of diseases. And we also hope that our experience can encourage similar strategies to benefit patients with other rare malignant neoplasms.
Supplementary Material
Highlights.
Question
What are the clinical and genetic features of wild-type gastrointestinal stromal tumors (GISTs)?
Findings
Of 95 patients in a cohort study whose GIST lacked C-KIT/PDGFRA mutations, 84 had succinate dehydrogenase (SDH)-deficient GIST (75% due to SDH mutations and 25% to SDHC promoter hypermethylation), and 18 had syndromic GIST with chondromas and/or paragangliomas. SDH mutations were often germline.
Meaning
Expanded molecular characterization of wild-type GIST is useful to determine risk of germline mutation (and need for genetic counseling) and of non-GIST tumors, and for patient management.
Acknowledgments
Funding/Support: The study was funded by the intramural National Cancer Institute (NCI), National Institutes of Health (NIH).
Role of the Funder/Sponsor: As a funding entity, the NCI had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The study was reviewed by the NCI institutional review board, and some of the authors are NIH employees.
Footnotes
Conflict of Interest Disclosures: None reported.
Supplemental content at jamaoncology.com
Author Contributions: Dr Helman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Boikos and Pappo contributed equally as first authors. Drs Janeway and Helman contributed equally as last authors.
Study concept and design: Boikos, Pappo, Killian, Weldon, von Mehren, Wright, Schiffman, Meltzer, Stratakis, Helman.
Acquisition, analysis, or interpretation of data: Boikos, Pappo, Killian, LaQuaglia, Weldon, George, Trent, von Mehren, Wright, Raygada, Pacak, Meltzer, Miettinen, Janeway, Helman.
Drafting of the manuscript: Boikos, Pappo, Killian, Stratakis, Janeway, Helman.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Boikos, Wright.
Obtained funding: Stratakis, Helman.
Administrative, technical, or material support: Pappo, Killian, LaQuaglia, George, Trent, von Mehren, Wright, Raygada, Miettinen, Stratakis, Helman.
Study supervision: Boikos, Pappo, Weldon, Trent, Meltzer, Stratakis, Janeway, Helman.
Additional Contributions: We gratefully acknowledge the contributions of the patients and their families. We also thank GIST Support International, the Life Raft Group, and the GIST Cancer Awareness Foundation for their encouragement and recruitment efforts. We also acknowledge the clinical support staff from the NCI, National Institute of Child Health and Human Development, and the NIH Clinical Center.
References
- 1.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Livingstone AS, Franceschi D, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg. 2006;202(4):623–629. doi: 10.1016/j.jamcollsurg.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 103(4):821–829. doi: 10.1002/cncr.20862. 200. [DOI] [PubMed] [Google Scholar]
- 4.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117(2):289–293. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 7.Janeway KA, Albritton KH, Van Den Abbeele AD, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52(7):767–771. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26(33):5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappo AS, Janeway KA. Pediatric gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23(1):15–34. vii. doi: 10.1016/j.hoc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16(1):79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 11.Belinsky MG, Rink L, von Mehren M. Succinate dehydrogenase deficiency in pediatric and adult gastrointestinal stromal tumors. Front Oncol. 2013;3:117. doi: 10.3389/fonc.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janeway KA, Kim SY, Lodish M, et al. NIH Pediatric and Wild-Type GIST Clinic. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108(1):314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matyakhina L, Bei TA, McWhinney SR, et al. Genetics of Carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92(8):2938–2943. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- 14.Killian JK, Miettinen M, Walker RL, et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med. 2014;6(268):268ra177. doi: 10.1126/scitranslmed.3009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killian JK, Kim SY, Miettinen M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3(6):648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostein I, Faur N, Primois C, et al. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol. 2010;133(1):141–148. doi: 10.1309/AJCPPCKGA2QGBJ1R. [DOI] [PubMed] [Google Scholar]
- 17.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47(10):853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocrinol Metab. 2009;94(10):3656–3662. doi: 10.1210/jc.2009-1156. [DOI] [PubMed] [Google Scholar]
- 19.Zhu MJ, Ou WB, Fletcher CD, Cohen PS, Demetri GD, Fletcher JA. KIT oncoprotein interactions in gastrointestinal stromal tumors: therapeutic relevance. Oncogene. 2007;26(44):6386–6395. doi: 10.1038/sj.onc.1210464. [DOI] [PubMed] [Google Scholar]
- 20.Pantaleo MA, Nannini M, Corless CL, Heinrich MC. Quadruple wild-type (WT) GIST: defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med. 2015;4(1):101–103. doi: 10.1002/cam4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falchook GS, Trent JC, Heinrich MC, et al. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget. 2013;4(2):310–315. doi: 10.18632/oncotarget.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widemann BC, Marcus LJ, Fisher MJ, et al. Phase 1 study of the MEK1/2 inhibitor selumetinib (AZD6244) hydrogen sulfate in children and young adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs) J Clin Oncol. 2014;32(5s suppl) abstr 10018. [Google Scholar]
- 23.Takeda T, Banno K, Okawa R, et al. ARID1A gene mutation in ovarian and endometrial cancers. Oncol Rep. 2016;35(2):607–613. doi: 10.3892/or.2015.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evenepoel L, Papathomas TG, Krol N, et al. Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genet Med. 2015;17(8):610–620. doi: 10.1038/gim.2014.162. [DOI] [PubMed] [Google Scholar]
- 25.Jasperson KW, Kohlmann W, Gammon A, et al. Role of rapid sequence whole-body MRI screening in SDH-associated hereditary paraganglioma families. Fam Cancer. 2014;13(2):257–265. doi: 10.1007/s10689-013-9639-6. [DOI] [PubMed] [Google Scholar]
- 26.Janeway KA, Weldon CB. Pediatric gastrointestinal stromal tumor. Semin Pediatr Surg. 2012;21(1):31–43. doi: 10.1053/j.sempedsurg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73(1):95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou Y, Zeng Y, Zhang DF, Zou SH, Cheng YF, Yao YG. IDH1 and IDH2 mutations are frequent in Chinese patients with acute myeloid leukemia but rare in other types of hematological disorders. Biochem Biophys Res Commun. 2010;402(2):378–383. doi: 10.1016/j.bbrc.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Cervera AM, Bayley JP, Devilee P, McCreath KJ. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol Cancer. 2009;8:89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterfall JJ, Killian JK, Meltzer PS. The role of mutation of metabolism-related genes in genomic hypermethylation. Biochem Biophys Res Commun. 2014;455(1–2):16–23. doi: 10.1016/j.bbrc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Sourbier C, Ricketts CJ, Matsumoto S, et al. Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase-deficient cancer. Cancer Cell. 2014;26(6):840–850. doi: 10.1016/j.ccell.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.