Abstract
The last decade has witnessed a burgeoning interest in studies exploring the link between psychosis spectrum disorders (PSD) and altered immune function. While epidemiological and clinical studies point to evidence for increased peripheral inflammatory markers in PSD, it is not clear whether peripheral inflammation correlates with central inflammation in the brain. Furthermore, these studies are confounded by multiple methodological and disorder-related factors such as antipsychotic medications, smoking, obesity, and metabolic syndrome, all of which independently contribute to altered inflammation. Clinical and animal studies provide encouraging evidence that inflammatory processes can define trans-diagnostic neuropsychiatric domains such as positive/negative valence, affective dysregulation, and cognitive impairment. In this commentary, we speculate on whether inflammation-mediated pathways may serve as a final-common pathway for environmental risk factors of early-childhood adversity, adolescent cannabis use, social exclusion, and on the possible mechanisms mediating the pathophysiology of PSD. We propose an integrative framework and suggest future research strategies that may help disentangle the link between immune dysfunction and PSD.
Keywords: psychosis, schizophrenia, inflammation, immune system, cytokine, environment, cannabis, social defeat, childhood trauma
Schizophrenia has for long been conceptualized as a moderately heritable, heterogeneous syndrome resulting from complex interactions between genetic vulnerability and environmental risk factors. The burgeoning growth in genetic research has revealed the underlying genetic architecture of schizophrenia to be polygenic, with over 100 genetic loci of small effect and thousands more whose effect can only be discerned at the level of the polygenic score.1 Genetic variants, however, account for only a fraction of the variation on the liability scale in contrast to the estimated heritability of 60%–80%. This opens up the possibility that a significant proportion of the heritability may be accounted for by epigenetic mechanisms and gene–environment interactions.
One of the genetic findings that support this possibility is the identification of the C4 gene of the major histocompatibility complex (MHC) as a genetic risk locus for schizophrenia.2 The MHC locus on chromosome 6 is involved in the immune response and had also emerged as a robust signal in the large-scale, genome-wide association studies.2 Pinning it down to the C4 gene, given the well-established role of the complement system in synaptic pruning, opens up an interesting hypothesis that inflammatory pathways may be important in psychosis spectrum disorders (PSD).
In this commentary, we will attempt to: (1) highlight important findings regarding the putative role of aberrant immune regulation in PSD, (2) address methodological issues, (3) discuss the possible link between inflammatory response and environmental risk factors previously associated with PSD, and (4) cogitate on an integrative framework for investigating the interplay between immune system and environment in the etiopathogenesis of PSD.
Evidence From Epidemiological Studies That Converge on Inflammatory Pathways
The link between prenatal infection and schizophrenia is derived from ecological studies that measure historical and serological evidence for gestational viral infection exposure during influenza pandemics. While the early studies showed evidence for an association between second-trimester influenza exposure and psychotic outcome in the offspring, more recent meta-analysis that included 8 ecological studies concluded that evidence was insufficient.3 While there have been some reports pointing to a link between herpes simplex virus, type 1 (HSV-1), anti-N-methyl-D-aspartic acid (anti-NMDA) antibodies, and antineuronal antibodies and schizophrenia, the evidence from epidemiological studies have not been consistent or compelling.
Is There Evidence of Abnormal Inflammatory Processes in PSD?
The evidence for inflammation from postmortem brains is mixed, with studies variably showing an increase, decrease, or no change in inflammatory markers such as glial, astrocytic, microglial markers, and cytokines.4 Despite the variability, there appears to be some consistency in evidence for increased microglial activity and elevated levels of 2 markers: SERPINA3, a serine protein inhibitor, ie, an acute-phase protein and IFITM, an immune-related protein involved in viral replication.4
Studies examining peripheral inflammatory markers in schizophrenia have been relatively more consistent in showing elevated cytokine concentrations, both as a state- and trait-marker. A recent meta-analysis also revealed that some markers such as interleukin 6 (IL-6), tumor necrosis factor α (TNFα), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA) demonstrate transdiagnostic validity across schizophrenia, bipolar disorder (BD), and major depressive disorder (MDD).5 Remarkably, these cytokines are all modulated by the nuclear kappa factor B (NF-κB) signaling pathway that might constitute a point of convergence for therapeutic targeting.6 IL-6, in particular, was found to be significantly increased in acute episodes of schizophrenia, bipolar mania, and MDD; decreased with treatment in schizophrenia and MDD and remained elevated in chronic schizophrenia, euthymic BD, and MDD.5 The elevations in cytokines (IL-1, IL-7, and IL-8) correlated with illness severity, such as positive symptoms, attentional deficits, and affective abnormalities in the North American Prodromal Longitudinal Study study.7 In another longitudinal cohort, the Avon Longitudinal Study of Parents and Children cohort,8 IL-6 levels at age 9 were predictive of depression or psychosis at age 18 years.
A major caveat in interpreting this mixed and not entirely consistently replicated data is that measures of peripheral inflammation have not always been shown to correlate well with measures of central inflammation (ie, inflammation in the brain) or with disease severity. However, a recent meta-analysis found support for elevated cerebrospinal fluid (CSF) IL-6 in schizophrenia and BD.9 CSF IL-6 levels have been found to correlate with plasma IL-6 levels in recent-onset schizophrenia10 and with CSF levels of kynurenic acid, a glia-derived NMDA receptor antagonist.11
In vivo positron imaging tomography imaging studies have used radioligands that bind to translocator protein (TSPO), expressed on microglia that are upregulated when activated as part of the inflammatory response. The evidence for an increase in activated microglia from these studies is mixed. While the early studies demonstrated evidence for an increase,12 subsequent studies failed to find any difference, or even a trend toward a decrease in microglial activation.13,14 There are a number of caveats in interpreting these data such as the fact the TSPO is expressed in astrocytes in addition to microglia, the methodological differences in measures of TSPO availability and more recently the suggestion that TSPO may, in fact, be part of an anti-inflammatory response.14 Measures of peripheral inflammation have, however, failed to correlate with measures of activated microglia, measured using TSPO ligands.10,14
In summary, while the evidence for the role of inflammation in PSD is encouraging, it needs to be interpreted with due consideration to extensive heterogeneity between studies, ie, with different cytokines being implicated across different studies. Another caveat in interpreting these data is the potential confounding or moderating effect of factors such as antipsychotic medications, smoking status, body mass index, metabolic syndrome—all of which have independently been shown to alter peripheral inflammatory cytokines—and methodological factors such as the type of assay and fasting status.5
Inflammation and Transdiagnostic Neuropsychiatric Domains Relevant to PSD
Transdiagnostic neuropsychiatric domains that are affected by inflammatory processes include positive and negative valence (fatigue, motor slowing, and reduced motivation), affective domain (anhedonia and depressed mood) and cognitive domain (impaired processing speed, verbal memory, and executive function).15 Of these, cognition represents a transdiagnostic domain that is relevant to PSD. Cognitive impairment is a persistent feature of PSD, associated with poorer treatment response, and a stronger predictor of functional outcomes than positive and negative symptoms.
Cognitive impairment in PSD was associated with elevated serum C-reactive protein (CRP) concentrations,16 and serological evidence of exposure to neurotrophic viruses such as HSV-1 and cytomegalovirus.17 In one study, sustained attention was found to correlate with anti-inflammatory prostaglandin 15d-PGJ2, while executive function correlated with cyclooxygenase-2 expression.18 As central players in the pro-inflammatory state, IL-1 and IL-6 were linked to learning and memory by modulating long-term potentiation.19 Some studies suggest that inflammatory changes may influence cognition via bottom-up information processing evidenced by cytokine changes in the amygdala20 or endotoxin-induced changes in medial temporal lobes.21 There is also emerging interest in the role of tryptophan catabolites (TRYCATs) in the modulation of alpha-7 nicotinic receptors that play an important role in sustained attention.22 While of interest, clearly this work does not yet constitute a body of well-replicated findings.
Is the Inflammatory Pathway a Common Final Pathway for Environmental Risk Factors?
In epidemiological studies, early childhood trauma, adolescent heavy cannabis use, and social defeat have consistently emerged as important risk factors for psychosis expression.23,24 It is noteworthy that these risk factors are relevant at different neurodevelopmental stages, ie, early childhood, adolescence, and early adulthood. Interestingly, immune processes are able to modulate brain development and function at different phases via mechanisms of synaptic pruning, glial priming, and modulation of hypothalamic-pituitary axis.25 Do these risk factors converge onto the inflammatory pathway? If yes, how does the common-final pathway tie in with the neurobiology of PSD?
One possible suggestion for a final common pathway involves stress-induced activation of the inflammatory pathway, microglial activation, and the TRYCATs pathway. It is proposed that cytokines such as IL-6 cause sustained activation of brain indoleamine 2,3-dioxygenase resulting in increased concentration of products of tryptophan catabolism (TRYCATs), such as kynurenine, kynurenic acid, and quinolinic acid which in turn result in increased NMDA antagonism, dopaminergic and serotoninergic dysfunction, excitotoxicity, and oxidative stress. Additionally, microglial activation may lead to excessive synaptic pruning, loss of cortical gray matter in stress-sensitive regions such as the prefrontal cortex and hippocampus and may also lead to disinhibition of subcortical dopamine.26
Animal studies demonstrate that psychosocial stressors such as chronic restraint, social isolation, and repeated social defeat result in increased microglial activation.27 In human studies, psychological stress has been shown to result in increases in IL-6, IL-1, IL-10, and TNFα.28 The difficulty with the social defeat hypothesis, however, is that it is difficult to measure social defeat in humans, making it challenging to disentangle causal effects from reverse causality.
The effects of childhood trauma on inflammation have been better studied. Individuals exposed to childhood trauma have significantly elevated baseline peripheral levels of IL-6, TNFα, and CRP in adulthood.25,29
Cannabis use shows differential associations with inflammatory markers, depending on the relative concentration of its 2 main ingredients: delta-9-tetrahydrocannabinol (THC) (which has pro-inflammatory effects30) and cannabidiol (CBD) (shown to have anti-inflammatory effects31). The endocannabinoid system modulates microglial activation via cannabinoid 1 (CB1R) and cannabinoid 2 (CB2R) receptors,32 opening up the possibility that exogenous cannabis use may also modulate microglial activity.
The role of epigenetic mechanisms such as DNA methylation and histone modification may be impacted by exposure to early childhood adversity and cannabis use.33 How these changes link up with altered inflammation and PSD remains an open question for the field.
Toward an Integrative Framework for Examining the Mediating and Moderating Effects of Environment on Immune Mechanisms in the Etiopathogenesis of PSD
Over the last decades, given advanced technology and rapidly evolving measurement techniques, research has taken major steps toward understanding the cross-talk between the immune system and the central nervous system. Although inadequate to provide a completely consistent story, current data, taken together, present a valid initial hypothesis that underlying immune dysregulation may contribute to the etiopathogenesis of PSD.
Research would benefit from cross-validation studies adopting a multimodal approach that use different platforms (eg, neuroimaging, gene-expression profiling, and circulatory markers) to investigate immune dysregulation within a framework encompassing traditional diagnostic categories, as embedded into the National Institute of Mental Health initiative, the Research Domain Criteria Project. Nevertheless, the storyline ignoring the environmental component—unfortunately often neglected thus far because it is intangible and difficult to capitalize on34—would leave gaping holes in the plot.
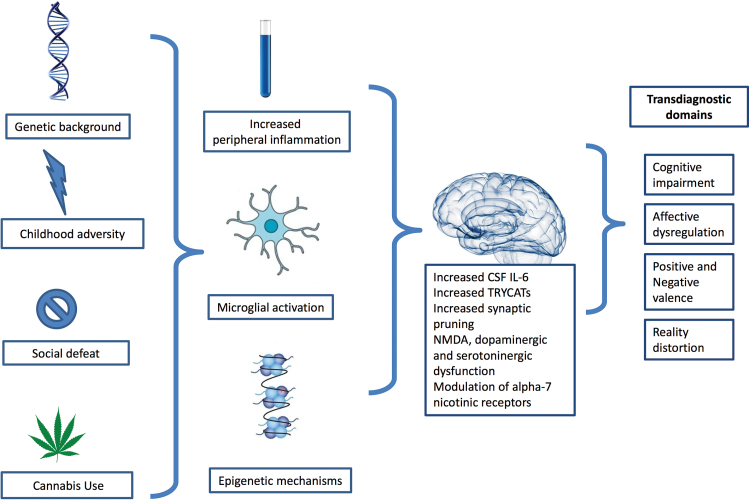
Recent studies show that nongenetic factors explain up to 75% of the immunological variation between healthy individuals.35 Further, as conceptualized in the common conserved transcriptional response to adversity framework, chronic exposure to stress-inducing conditions (eg,, social isolation, low socioeconomic status, care burden, early life adversity, urban environment) might lead to an upregulation of proinflammatory gene expression pathway (including IL-1B, IL-6, and NF-κB) accompanied with a down-regulated antiviral activity, which in turn give rise to immune-mediated diseases.36 In this regard, we draw attention to the necessity of embracing the challenging complexity of immune system–environment interplay in future research if the goal is to move forward with the immune theory of PSD (figure 1). For instance, pathway-based analysis could provide us with the essential tool for hypothesis-driven investigation of the statistical interaction between biologically informative immune pathways and putative environmental factors conferring risk for mental disorders in large epidemiological samples.37 Research may also benefit from advancements in user-friendly mobile and wearable technology that now allow “deep-phenotyping” using real-time data collection of a wide range of health information at a very low cost—actigraphs to track movement and sleep and smartphone applications to capture momentary mental states (eg, sadness, paranoia) dependent on daily life stress. Using the mobile devices together with minimally invasive methods for longitudinal immune profiling, one can design an experimental study to examine the dynamic network involving, eg, sleep, immune system, and mental states over an extended period of time.
Fig. 1.
An integrative framework for investigating the role of environmentally shaped immune mechanisms in psychosis spectrum disorders.
In conclusion, substantial evidence suggests that investigating the interplay between environmental exposures and immune system may further accelerate the progress to gaining insight into underlying immune-mediated mechanisms in PSD.
Funding
S.G. would like to acknowledge the European Community’s Seventh Framework Program under grant agreement no. HEALTH-F2-2009–241909 (Project EU-GEI). R.R. is supported by the Dana Foundation David Mahoney Program and Thomas P. Detre Award in Translational Neuroscience Research.
References
- 1. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sekar A, Bialas AR, de Rivera H, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selten JP, Termorshuizen F. The serological evidence for maternal influenza as risk factor for psychosis in offspring is insufficient: critical review and meta-analysis. Schizophr Res. 2016. [DOI] [PubMed] [Google Scholar]
- 4. Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21:1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Altinoz MA, Ince B, Tek C, Srihari VH, Guloksuz S. The NF-kappaB signaling pathway: an important therapeutic target in psychiatric disorders. Mol Psychiatry. 2016. [DOI] [PubMed] [Google Scholar]
- 7. Perkins DO, Jeffries CD, Addington J, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. 2015;41:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexandre K, Wang BJM. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coughlin JM, Wang Y, Ambinder EB, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwieler L, Larsson MK, Skogh E, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia–significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 13. Kenk M, Selvanathan T, Rao N, et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull. 2015;41:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Notter T, Coughlin JM, Gschwind T, et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry. 2017. [DOI] [PubMed] [Google Scholar]
- 15. Capuron L, Castanon N. Role of inflammation in the development of neuropsychiatric symptom domains: evidence and mechanisms. Curr Top Behav Neurosci. 2017;31:31–44. [DOI] [PubMed] [Google Scholar]
- 16. Bulzacka E, Boyer L, Schürhoff F, et al. ; FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) Group Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr Bull. 2016;42:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabrera B, Bioque M, Penadés R, et al. Cognition and psychopathology in first-episode psychosis: are they related to inflammation? Psychol Med. 2016;46:2133–2144. [DOI] [PubMed] [Google Scholar]
- 19. McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. [DOI] [PubMed] [Google Scholar]
- 20. Churchill L, Taishi P, Wang M, et al. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res. 2006;1120:64–73. [DOI] [PubMed] [Google Scholar]
- 21. Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry. 2014;76:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris G, Carvalho AF, Anderson G, Galecki P, Maes M. The many neuroprogressive actions of tryptophan catabolites (TRYCATs) that may be associated with the pathophysiology of neuro-immune disorders. Curr Pharm Des. 2016;22:963–977. [DOI] [PubMed] [Google Scholar]
- 23. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot—a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guloksuz S, van Nierop M, Lieb R, van Winkel R, Wittchen HU, van Os J. Evidence that the presence of psychosis in non-psychotic disorder is environment-dependent and mediated by severity of non-psychotic psychopathology. Psychol Med. 2015;45:2389–2401. [DOI] [PubMed] [Google Scholar]
- 25. Danese A, S JL. Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma? Neuropsyc hopharmacology. 2017;42:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7:e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl). 2016;233:1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zamberletti E, Gabaglio M, Prini P, Rubino T, Parolaro D. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. Eur Neuropsychopharmacol. 2015;25:2404–2415. [DOI] [PubMed] [Google Scholar]
- 31. Kozela E, Juknat A, Gao F, Kaushansky N, Coppola G, Vogel Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suárez-Pinilla P, López-Gil J, Crespo-Facorro B. Immune system: a possible nexus between cannabinoids and psychosis. Brain Behav Immun. 2014;40:269–282. [DOI] [PubMed] [Google Scholar]
- 33. Pishva E, Kenis G, van den Hove D, et al. The epigenome and postnatal environmental influences in psychotic disorders. Soc Psychiatry Psychiatr Epidemiol. 2014;49:337–348. [DOI] [PubMed] [Google Scholar]
- 34. Abbott A. US mental-health chief: psychiatry must get serious about mathematics. Nature. 2016;539:18–19. [DOI] [PubMed] [Google Scholar]
- 35. Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cole SW. Human social genomics. PLoS Genet. 2014;10:e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. European Network of National Networks studying Gene–Environment Interactions in Schizophrenia , van Os J, Rutten BP, et al. Identifying gene–environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]