Abstract
In the DSM5, negative symptoms are 1 of the 5 core dimensions of psychopathology evaluated for schizophrenia. However, negative symptoms are not pathognomonic—they are also part of the diagnostic criteria for other schizophrenia-spectrum disorders, disorders that sometimes have comorbid psychosis, diagnoses not in the schizophrenia-spectrum, and the general “nonclinical” population. Although etiological models of negative symptoms have been developed for chronic schizophrenia, there has been little attention given to whether these models have transdiagnostic applicability. In the current review, we examine areas of commonality and divergence in the clinical presentation and etiology of negative symptoms across diagnostic categories. It was concluded that negative symptoms are relatively frequent across diagnostic categories, but individual disorders may differ in whether their negative symptoms are persistent/transient or primary/secondary. Evidence for separate dimensions of volitional and expressive symptoms exists, and there may be multiple mechanistic pathways to the same symptom phenomenon among DSM-5 disorders within and outside the schizophrenia-spectrum (ie, equifinality). Evidence for a novel transdiagnostic etiological model is presented based on the Research Domain Criteria (RDoC) constructs, which proposes the existence of 2 such pathways—a hedonic pathway and a cognitive pathway—that can both lead to expressive or volitional symptoms. To facilitate treatment breakthroughs, future transdiagnostic studies on negative symptoms are warranted that explore mechanisms underlying volitional and expressive pathology.
Keywords: anhedonia, avolition, asociality, blunted affect, alogia
Introduction
Negative symptoms (ie, reductions in goal-directed activity, social behavior, pleasure, and the outward expression of emotion or speech) have long been considered a core feature of psychotic disorders.1,2 Modern empirical studies confirm these early clinical observations, indicating that negative symptoms are distinct from other domains of psychopathology (eg, psychosis, disorganization)3 and associated with a range of important clinical outcomes (eg, disease liability, quality of life, subjective well-being, recovery).4–7 Unfortunately, psychosocial and pharmacological interventions have yielded limited effectiveness for improving negative symptoms in schizophrenia.8 At present, treatment development is stunted due in large part to a poor understanding of the factors that cause and maintain negative symptoms.9 Thus, candidate mechanisms are unclear and treatment development remains a high priority.
Although traditionally described only in relation to schizophrenia, it has become clear that negative symptoms also occur in other schizophrenia-spectrum disorders, disorders that sometimes have comorbid psychosis, diagnoses not in the schizophrenia-spectrum, certain neurological disorders, and the general “nonclinical” population.10,11 Little attention has been paid to areas of commonality and divergence in the clinical presentation of negative symptoms across diagnostic boundaries. There has also yet to be systematic comparison of mechanisms underlying negative symptoms across diagnostic categories, despite recent advances in etiological models of negative symptoms that have been proposed for schizophrenia. These issues are of critical importance given the potential for research classification systems, such as the RDoC and HiTop, to answer questions related to equifinality and clarify the biological basis of negative symptoms.12
The current manuscript attempts to address these gaps in the literature by achieving 2 primary aims: (1) reviewing qualitative and quantitative differences in the clinical presentation of negative symptoms across DSM-5 diagnostic categories, as well as between clinical and healthy populations; (2) reviewing the extent to which current etiological models of negative symptoms in schizophrenia map onto other diagnostic categories that exhibit negative symptoms.
Do Negative Symptoms Qualitatively Differ Across Diagnostic Categories?
Negative symptoms have been part of the diagnostic criteria for schizophrenia since the DSM-II.13 In the DSM-5,14 negative symptoms are 1 of 5 core dimensions of psychopathology evaluated for schizophrenia. There are 5 commonly agreed upon domains of negative symptoms, which separate into 2 dimensions—volition (anhedonia, avolition, asociality) and expression (blunted affect, alogia)—that are evaluated in the DSM-5 diagnostic criteria for schizophrenia9,15–18 (see table 1 for definitions).
Table 1.
Definitions for the 5 Symptom Domains Identified in the 2005 NIMH Consensus Meeting on Negative Symptoms
| Definition | Consensus Domain |
|---|---|
| A decrease in the outward expression of emotion in terms of facial expression, vocal expression, or body gestures | Blunted affect |
| A reduction in the quantity of speech and amount of spontaneous elaboration | Alogia |
| A reduction in the initiation of and persistence in goal-directed activities and the desire to perform such activities | Avolition |
| A reduction in the intensity of positive emotion and/or a reduction in the frequency of engaging in pleasurable activities | Anhedonia |
| A reduction in the frequency of social interaction and the desire to form close relationships | Asociality |
However, it is clear that negative symptoms are not pathognomonic—they are also part of the diagnostic criteria and associated features for other schizophrenia—spectrum disorders, including schizoaffective disorder, schizophreniform disorder, schizotypal personality disorder, schizoid personality disorder, and paranoid personality disorder. Negative symptoms are not part of the diagnostic criteria for delusional disorder and brief psychotic disorder.
Although not explicitly termed negative symptoms, several psychiatric disorders outside of the schizophrenia-spectrum have negative symptoms within their diagnostic criteria or associated features (eg, bipolar disorder, major depressive disorder, posttraumatic stress disorder), some of which are known to have comorbid psychosis. Other disorders that do not often have comorbid psychosis also include negative symptoms within their diagnostic criteria and associated features (eg, selective mutism, social anxiety disorder, neurocognitive disorders).
Negative symptoms also occur in several DSM-5 neurocognitive disorders, but are commonly referred to as apathy in this context. Neurological disorders where negative symptoms are frequently present include: Alzheimer’s disease, frontotemporal dementia, Huntington’s disease, multiple sclerosis, Parkinson’s disease, progressive supranuclear palsy, and traumatic brain injury.19–23 Apathy described in neurological disorders is qualitatively similar to avolition and asociality in schizophrenia. For example, the Apathy Evaluation Scale,22 a clinical scale commonly used to rate apathy, includes items that focus on the internal experience (eg, interest in activities) and overt behavior (eg, engaging in goal directed behavior) for social and goal-directed activities. Using such clinical rating scales, apathy is relatively frequent among the aforementioned neurological disorders, with prevalence estimates ranging from 32% to 80% depending on the disorder.11
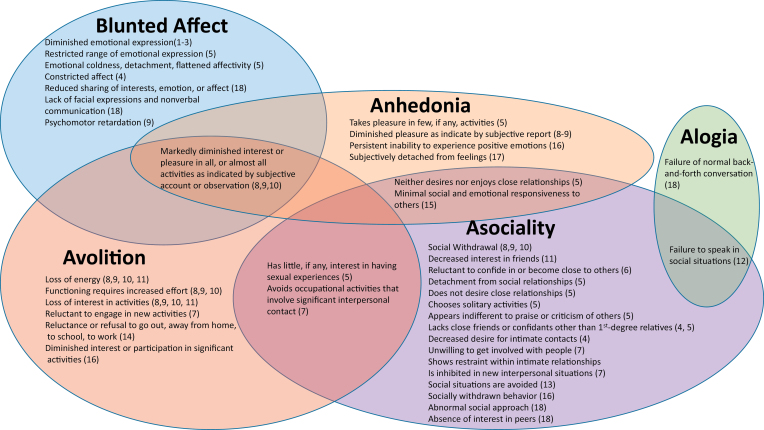
Terminology used to describe phenomenon that can be considered negative symptoms is highly variable across diagnostic categories. The terms blunted affect, alogia, anhedonia, avolition, and asociality9 are rarely used outside the schizophrenia-spectrum, even though symptoms described in other disorders fit definitions for these terms (table 1). Additionally, terminology within the schizophrenia-spectrum is not always consistent. For example, the terms “blunted affect” and “constricted affect” are used to refer to the same phenomenon in schizophrenia and schizotypal personality disorder, respectively. To illustrate these points, we carefully reviewed the “diagnostic criteria” and “associated features supporting diagnosis” sections of disorders included in the 20 DSM-5 sections and recorded terms/phrases that reflected any of the 5 NIMH negative symptom consensus domains. A total of 19 disorders were identified as having blunted affect, alogia, anhedonia, avolition, or asociality within their DSM-5 diagnostic criteria or associated features. Results of this process are presented visually in figure 1. As seen in the figure 1, there is not only a lack of consistency in terminology throughout the DSM-5, but also lack of specificity in descriptions and conflation of symptom domains.
Fig. 1.
Visual representation of negative symptoms throughout DSM-5 diagnoses. Note: Bracketed numbers correspond with diagnoses listed in table 2 (see numbers in far left column). Text included in figure represents excerpted or paraphrased material from DSM-5 diagnostic criteria or associated features supporting diagnoses. Information is organized graphically in relation to the 5 domains of negative symptoms identified in the 2005 NIMH Consensus Conference. The figure illustrates the wide range of terminology used to describe negative symptom phenomenon, as well as conceptual overlap of terminology (text in overlapping circles) and conflation of constructs.
To facilitate qualitative transdiagnostic comparisons, we mapped descriptions used in DSM-5 diagnostic criteria and associated features onto the 5 domains identified in the 2005 NIMH negative symptom consensus conference.9Table 2 depicts the presence/absence of these 5 symptoms across diagnoses. As can be seen in the table 2, asociality and avolition are the negative symptoms that occur most frequently within DSM-5 diagnostic criteria and associated features. Blunted affect and anhedonia occur with moderate frequency, and alogia is least frequent.
Table 2.
Qualitative Comparison of 5 Core Negative Symptom Domains Across Diagnostic Categories
| Negative Symptom Domain | |||||
|---|---|---|---|---|---|
| Asociality | Avolition | Anhedonia | Alogia | Blunted Affect | Disorder |
| X | X | X | X | X | 1. Schizophrenia |
| X | X | X | X | X | 2. Schizoaffective disorder |
| X | X | X | X | X | 3. Schizophreniform disorder |
| X | X | X | 4. Schizotypal personality disorder | ||
| X | X | X | X | 5. Schizoid personality disorder | |
| X | 6. Paranoid personality disorder | ||||
| X | 7. Avoidant personality disorder | ||||
| X | X | X | X | 8. Bipolar disorder (I and II) | |
| X | X | X | X | 9. Major depressive disorder | |
| X | X | 10. Persistent depressive disorder (dysthymia) | |||
| X | X | 11. Premenstrual dysphoric disorder | |||
| X | 12. Selective mutism | ||||
| X | 13. Social anxiety disorder | ||||
| X | 14. Separation anxiety disorder | ||||
| X | X | 15. Reactive attachment disorder | |||
| X | X | X | 16. Posttraumatic stress disorder | ||
| X | 17. Depersonalization/derealization disorder | ||||
| X | X | X | 18. Autism spectrum disorder | ||
| X | X | X | X | X | 19. Neurocognitive disorders |
Note: We reviewed the “diagnostic criteria,” “diagnostic features,” and “associated features supporting diagnosis” sections of disorders included in the 20 DSM-5 sections and recorded terms/phrases that reflected any of the 5 NIMH negative symptom consensus domains. A total of 19 disorders were identified as having blunted affect, alogia, anhedonia, avolition, or asociality within their DSM-5 diagnostic criteria or associated features. Not considered are disorders due to general medical condition, “other specified” disorders, unspecified disorders, and substance induced disorders. Symptoms displayed in neurocognitive disorders vary based on the neurological condition in question.
Although table 2 makes the 5 domains appear qualitatively similar across diagnostic categories, there may be important phenomenological differences. It is possible for 2 different disorders to include the same negative symptom within their diagnostic criteria, but for these symptoms to be caused by very different processes. For example, asociality can occur in paranoid personality disorder because an individual is suspicious and preoccupied with whether others can be trusted, resulting in active social withdrawal to safeguard against the malicious intentions of others. In contrast, in schizophrenia, asociality can sometimes manifest as an apathetic social withdrawal, where an individual does not engage in social interactions because they have little desire to be around others and do not find relationships important or interesting.
The aforementioned example highlights the importance of making the primary/secondary distinction when performing transdiagnostic comparisons. Negative symptoms are considered primary or idiopathic if they are direct manifestations of the illness that cannot be attributed to secondary factors, such as anxiety, depression, disorganization, hallucinations, delusions, or medication effects.24,25 A minority of individuals diagnosed with schizophrenia (~15%–25%) display negative symptoms that are primary and persistent.26 This presentation, termed the “deficit syndrome,” reflects a separate disease within the broader diagnosis of schizophrenia.25 Deficit patients have unique signs and symptoms, course of illness, pathophysiology, neurocognition, functional outcome, risk factors, and treatment response compared to “nondeficit” patients25,26 It is unknown whether negative symptoms that occur in disorders other than schizophrenia also display primary negative symptoms, or whether all of these manifestations can be considered secondary. By definition some could be considered secondary when negative symptoms are driven by depression, anxiety, delusions, or hallucinations.27 Negative symptoms seen in autism spectrum disorders, which have high genetic and diagnostic overlap with schizophrenia, may even be influenced by secondary factors (eg, asociality due to anxiety).28 In neurological disorders, negative symptoms can reflect secondary factors, as indicated by associations between clinical ratings of apathy and depression in Alzheimer’s disease, Parkinson’s disease, temporal lobe epilepsy, and Huntington’s disease19,21,29; however, many neurological patients also display negative symptoms when depression is absent, suggesting that primary negative symptoms may exist.21,23,30,31 Some disorders also display negative symptoms that are more transient and tied to acute exacerbations by secondary causes, which resolve when those symptoms are treated. Such instances are distinct from the more enduring, trait-like presentation of primary negative symptom schizophrenia patients who show little fluctuation in severity across the course of illness despite changes in other symptoms or medication regimen.32
The primary/secondary and persistent/transient distinctions should be carefully considered when conducting research on negative symptoms in transdiagnostic samples. The proportion of individuals whose negative symptoms are primary and persistent differs across diagnostic categories, and the success of research endeavors aiming to examine pathophysiological mechanisms of negative symptoms may depend on how homogeneous samples are in relation to these 2 factors. Similar to Clementz et al,33 a promising approach to reducing heterogeneity could be to identify “biotypes” that cut across diagnostic categories using data-driven methods, and then examine whether those biotypes travel with primary/secondary and persistent/transient clinical classifications.
Do Negative Symptoms Quantitatively Differ Across Diagnostic Categories?
Few studies have made direct quantitative comparisons of negative symptom severity across disorders within the schizophrenia-spectrum. This may be due in part to different scales being used to measure negative symptoms across disorders and phases of illness. For example, negative symptoms are typically assessed in schizotypy using trait self-reports,34 whereas studies examining psychotic disorders tend to use clinical interview-based rating scales.15,16,35,36 The few studies making direct quantitative comparisons suggest the presence of a continuum of severity within phases of illness: chronic (ie, multi-episode with extended illness duration) > first episode >clinical high-risk.37,38 There also appears to be a continuum across diagnostic categories within the schizophrenia-spectrum, with schizophrenia more severe than schizoaffective disorder39–42 which is more severe than schizotypy43; however, this may be more true of blunted affect and alogia than anhedonia, avolition, or asociality.44,45
Results of studies comparing negative symptom severity between schizophrenia and disorders outside the schizophrenia-spectrum are mixed, with some studies reporting greater severity in schizophrenia than bipolar disorder, depression, and anxiety disorders/neuroses44–48 and others reporting comparable severity and frequency.49,50 Although severity may not always differentiate schizophrenia from nonschizophrenia-spectrum disorders, there is evidence that negative symptoms may be more stable and trait-like in schizophrenia than other psychiatric disorders which can be more transient.47,48,51–53 Notably, negative symptoms may not be as transient as one would expect in mood disorders. There is some evidence that depression and bipolar disorder are associated with attenuated negative symptoms that persist between mood episodes.46–48,51,52 When present in neurocognitive disorders, negative symptoms may be relatively severe, even comparable to that observed in chronic schizophrenia.54
Healthy controls with no diagnosable neurological or psychiatric conditions can also display negative symptoms.43,46,55 However, these cases tend to be infrequent and the severity is attenuated. Measures designed for clinical populations may not be ideal for evaluating negative symptoms in healthy populations, as they fail to capture nuances in the lower range of severity.
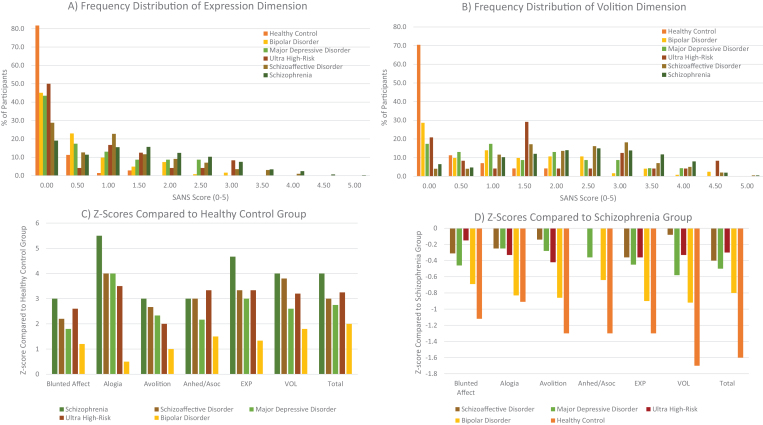
To directly illustrate quantitative differences in negative symptom severity across diagnostic categories and phases of illness, figure 2 and table 3 present archival data7,46,56–72 on the Scale for the Assessment of Negative Symptoms (SANS36) for the volitional dimension, expression dimension, and individual domain global scores. Groups include: chronic schizophrenia (n = 610), schizoaffective disorder (n = 197), bipolar disorder (n = 113), major depressive disorder (n = 23), ultra high-risk (ie, psychosis prodrome: n = 24), and healthy controls (n = 69). The frequency distributions suggest that negative symptoms were quite common across schizophrenia, clinical high-risk, schizoaffective disorder, major depression, and bipolar disorder groups. Moreover, the differences between the schizophrenia group and the schizoaffective, major depression, and ultra-high risk groups were modest. Compared to healthy controls, the magnitude of effect was largest in schizophrenia, followed by clinical high-risk, schizoaffective disorder, major depression, and bipolar disorder (table 3 and figure 2).
Fig. 2.
Transdiagnostic quantitative comparison of the 2 negative symptom dimensions and 5 consensus domains on the SANS. Note: Panels A and B represent frequency distributions of SANS scores on the expressive and volitional dimensions, respectively. Y-axis values reflect cumulative percentages of participants and X-axis scores denote the average SANS dimension score calculated by adding the 2 global items in each factor and dividing by 2. Exp, expression dimension; VOL, volitional dimension; Anhed/Asoc, global anhedonia/asociality item. Panels C and D reflect Z-scores for each group compared to healthy controls and schizophrenia patients, respectively. As can be seen in Panels A and B, there is variability in scores within each group, but both dimensions tend to be positively skewed. Z-scores indicated a continuum of severity scores ranging from: schizophrenia > ultra high-risk > schizoaffective >major depression > bipolar disorder > healthy control.
Table 3.
Quantitative Comparison of SANS Global Score Severity Across Diagnostic Categories
| Mean (SD) | Minimum | Maximum | Cohen’s d Compared to Healthy Controls | Cohen’s d Compared to Schizophrenia | |
|---|---|---|---|---|---|
| Schizophrenia (n = 610) | |||||
| Blunted affect | 1.7 (1.3) | 0 | 5 | 1.21 | — |
| Alogia | 1.2 (1.2) | 0 | 5 | 0.97 | — |
| Avolition | 2.1 (1.4) | 0 | 5 | 1.34 | — |
| Anhedonia/asociality | 2.1 (1.4) | 0 | 5 | 1.34 | — |
| EXP dimension | 1.5 (1.1) | 0 | 5 | 1.34 | — |
| AVOL dimension | 2.3 (1.2) | 0 | 5 | 1.74 | — |
| Total | 1.8 (1.0) | 0 | 4.5 | 1.68 | — |
| Schizoaffective disorder (n = 197) | |||||
| Blunted affect | 1.3 (1.3) | 0 | 4 | 0.96 | −0.31 |
| Alogia | 0.9 (1.0) | 0 | 4 | 0.93 | −0.26 |
| Avolition | 1.9 (1.3) | 0 | 5 | 1.38 | −0.15 |
| Anhedonia/asociality | 2.1 (1.4) | 0 | 5 | 1.45 | 0.00 |
| EXP dimension | 1.1 (1.0) | 0 | 4 | 1.15 | −0.37 |
| AVOL dimension | 2.2 (1.1) | 0 | 5 | 1.94 | −0.09 |
| Total | 1.4 (0.9) | 0 | 4.3 | 1.50 | −0.41 |
| Major depressive disorder (n = 23) | |||||
| Blunted affect | 1.1 (1.3) | 1 | 5 | 1.18 | −0.46 |
| Alogia | 0.9 (1.5) | 1 | 5 | 1.06 | −0.25 |
| Avolition | 1.7 (1.4) | 1 | 5 | 1.6 | −0.29 |
| Anhedonia/asociality | 1.6 (1.6) | 1 | 5 | 1.4 | −0.36 |
| EXP dimension | 1.0 (1.4) | 1 | 5 | 1.23 | −0.45 |
| AVOL dimension | 1.6 (1.3) | 1 | 5 | 1.69 | −0.58 |
| Total | 1.3 (1.0) | 1 | 5 | 1.84 | −0.50 |
| Bipolar disorder (n = 113) | |||||
| Blunted affect | 0.8 (1.1) | 0 | 4 | 0.66 | −0.71 |
| Alogia | 0.2 (0.7) | 0 | 4 | 0.18 | −0.88 |
| Avolition | 0.9 (1.2) | 0 | 5 | 0.59 | −0.88 |
| Anhedonia/asociality | 1.2 (1.3) | 0 | 5 | 0.83 | −0.65 |
| EXP dimension | 0.5 (0.7) | 0 | 3.5 | 0.69 | −0.96 |
| AVOL dimension | 1.2 (1.2) | 0 | 4.5 | 0.91 | −0.92 |
| Total | 1.0 (0.8) | 0 | 3.8 | 1.19 | −0.82 |
| Ultra high-risk (n = 24) | |||||
| Blunted affect | 1.5 (1.8) | 0 | 5 | 1.31 | −0.15 |
| Alogia | 0.8 (1.3) | 0 | 4 | 1.05 | −0.33 |
| Avolition | 1.5 (1.8) | 0 | 5 | 1.16 | −0.42 |
| Anhedonia/asociality | 2.3 (1.8) | 0 | 5 | 1.93 | 0.14 |
| EXP dimension | 1.1 (1.4) | 0 | 4.5 | 1.35 | −0.36 |
| AVOL dimension | 1.9 (1.6) | 0 | 5 | 1.78 | −0.33 |
| Total | 1.5 (1.2) | 0 | 4.8 | 1.89 | −0.30 |
| Healthy control (n = 69) | |||||
| Blunted affect | 0.2 (0.5) | 0 | 2 | — | −1.21 |
| Alogia | 0.1 (0.2) | 0 | 1 | — | −0.97 |
| Avolition | 0.3 (0.6) | 0 | 2 | — | −1.34 |
| Anhedonia/asociality | 0.3 (0.6) | 0 | 2 | — | −1.34 |
| EXP dimension | 0.1 (0.3) | 0 | 1.5 | — | −1.34 |
| AVOL dimension | 0.3 (0.5) | 0 | 2 | — | −1.74 |
| Total | 0.2 (0.4) | 0 | 1.8 | — | −1.68 |
The Structure of Negative Symptoms Across Diagnostic Categories
Although figure 2 appears to provide support for a continuous distribution of negative symptoms across diagnostic categories, the structure of negative symptoms remains an open debate. Historically, there has been case for 2 conceptualizations: continuous (ie, individuals differ in degree or amount) and categorical (ie, individuals differ in status or kind). There is support for both perspectives.
Taxometric studies examining the structure of negative symptoms in schizophrenia have provided support for the categorical perspective, indicating the existence of a negative symptom taxon (~28%–36%) that differs from a non-negative symptom taxon on a number of external validators (eg, summer birth, male sex, premorbid adjustment, neurocognition, and social functioning).73,74 However, it may also be that a hybrid categorical-continuous structure best fits negative symptom data in schizophrenia. One article supports this conceptualization, indicating that a distinct class of schizophrenia patients exists when negative symptom scores exceed a certain threshold, and that the magnitude of severity beyond this threshold determines the strength of association with outcome variables.73
In disorders other than schizophrenia, it is unclear whether negative symptoms are best viewed as categorical or continuous. When positive and negative symptoms are entered into taxometric analyses concurrently, evidence for a continuous structure emerges in paranoid, schizoid, and schizotypal personality disorders.75,76 When individual negative symptoms (eg, anhedonia and asociality) are examined in isolation in schizotypy, there is some support for both categorical77,78 and continuous79 structures. In depression, anhedonia has been shown to have a continuous structure in adults.80
It is also important to consider whether the internal structure of negative symptom scales is similar in schizophrenia and other disorders. As previously mentioned, when negative symptom scales are factor analyzed in samples of schizophrenia patients, there is consistent evidence for 2 independent factors reflecting volitional pathology (avolition, asociality, anhedonia) and expressive pathology (blunted affect, alogia).15–18,81 Much like schizophrenia, there is evidence that negative symptoms load on a factor that is separate from positive symptoms in schizotypy,82 and negative symptoms divide into 2 factors akin to expression (constricted affect) and volition (no close friends) on self-report questionnaires.83 In depression, anhedonia loads onto a factor that is distinct from psychomotor symptoms and negative affect, but there is no evidence for separate expression and volition dimensions.84 Factor analysis of apathy scales in neurological disorders most commonly produces one large factor22,85; however, there is some evidence for minor factors representing interest, insight, and social function.85,86 In autism, blunted affect, alogia, and asociality related symptoms load onto a factor separate from motor, sensory, and verbal communication symptoms.87
Future studies should examine the internal structure of negative symptom scales and the continuous vs categorical issue in transdiagnostic samples. It will be important to determine how primary/secondary causes influence the internal structure of negative symptom scales, as well as whether categorical, continuous, or hybrid conceptualizations best fit transdiagnostic samples.
Do Etiological Models of Negative Symptoms Developed for Schizophrenia Apply to Other Schizophrenia-Spectrum Disorders?
Based on evidence for the presence of 2 negative symptom factors,81 the literature on etiology is reviewed separately for volitional and expressive pathology.
Volitional Dimension
Several etiological models have been proposed to explain volitional symptoms of chronic schizophrenia.88–92 However, it is unclear whether the core brain-behavior processes underlying volitional deficits in schizophrenia also underlie volitional pathology displayed in other disorders, or whether there are unique mechanisms that contribute to volitional pathology in disorders other than schizophrenia.
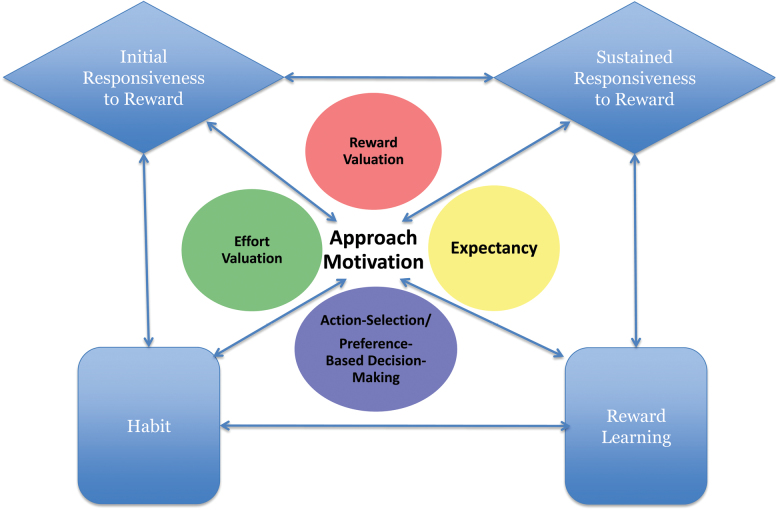
The NIMH RDoC “positive valence system” offers a useful conceptual framework for addressing this question.12 This framework proposes that there are 5 inter-related constructs associated with the initiation of and persistence in goal-directed or reward-seeking activities: initial responsiveness to reward attainment, sustained responsiveness to reward attainment, reward learning, habit, and approach motivation. The approach motivation construct is further divided into 4 subconstructs: reward valuation, effort valuation, expectancy, and action selection. Figure 3 presents a schematic overview of the RDoC positive valence system constructs and how those constructs may interact. Table 4 provides definitions of each construct and their associated biological mechanisms as currently described on the NIMH RDoC website.
Fig. 3.
Graphical representation of the NIMH RDoC positive valence system constructs. Note: Figure depicts our representation of how RDoC positive valence system constructs are hierarchically organized and how they might interact with one another. This figure was not developed or endorsed by NIMH.
Table 4.
RDoC Positive Valence System Constructs and Associated Biological Correlates
| Molecules | Circuits | NIMH Definition | RDoC Construct |
|---|---|---|---|
| CREB, endocannabinoids, FosB, glutamate, Mu and delta opioid, orexin | Anterior insula, dorsal ACC, lateral hypothalamus, medial OFC, nucleus accumbens, ventral pallidum, ventromedial PFC VTA | Mechanisms/processes associated with hedonic responses—as reflected in subjective experiences, behavioral responses, and/or engagement of the neural systems to a positive reinforcer—and culmination of reward seeking | Initial responsiveness to reward attainment |
| Dopamine, endocannabinoids, opioid, orexin, serotonin | Arcuate nucleus BA9/ medial, medial preoptic area, OFC, paraventricular hypothalamus, ventromedial hypothalamus | Mechanisms/processes associated with the termination of reward seeking, eg, satisfaction, satiation, regulation of consummatory behavior | Sustained/longer-term responsiveness to reward attainment |
| Acetylcholine Co-released neuromodular glutamate, CREB, dopamine and dopamine-related molecules, FosB | Amygdala, dorsal striatum, medial prefrontal OFC, ventral striatum, VTA/SN | A process by which organisms acquire information about stimuli, actions, and contexts that predict positive outcomes, and by which behavior is modified when a novel reward occurs or outcomes are better than expected. Reward learning is a type of reinforcement learning, and similar processes may be involved in learning related to negative reinforcement | Reward learning |
| Acetylcholine Co-released neuromodular glutamate, CREB, dopamine and dopamine-related molecules, FosB | Dorsal striatum, medial prefrontal SN/VTA, ventral striatum | Sequential, repetitive, motor, or cognitive behaviors elicited by external or internal triggers that, once initiated, can go to completion without constant conscious oversight. Habits can be adaptive by virtue of freeing up cognitive resources. Habit formation is a frequent consequence of reward learning, but its expression can become resistant to changes in outcome value. Related behaviors could be pathological expression of a process that under normal circumstances subserves adaptive goals. | Habit |
| Approach motivation | |||
| Dopamine, serotonin | Anterior medial OFC, cortico-limbic circuit, ventral limbic striatum (incl. ventral caudate), ventral tegmental area/substantia nigra | Processes by which the probability and benefits of a prospective outcome are computed and calibrated by reference to external information, social context (eg, group input, counterfactual comparisons), and/or prior experience. This calibration is influenced by pre-existing biases, learning, memory, stimulus characteristics, and deprivation states. Reward valuation may involve the assignment of incentive salience to stimuli. | Reward valuation |
| Adenosine, dopamine, GABA | Basolateral amygdale, dorsal ACC, ventral pallidum, ventral striatum (nACC), VTA | Processes by which the cost(s) of obtaining an outcome is computed; tendency to overcome response costs to obtain a reinforcer | Effort valuation |
| Dopamine, serotonin | Amygdala, basal ganglia, dorsal ACC, lateral habenula, orbitofrontal cortex, rostral medial tegmentum, substantia nigra/VTA, ventral striatum | A state triggered by exposure to internal or external stimuli, experiences or contexts that predict the possibility of reward. Reward expectation can alter the experience of an outcome and can influence the use of resources (eg, cognitive resources). | Reward expectancy |
| — | Amygdala | Processes involving an evaluation of costs/ benefits and occuring in the context of multiple potential choices being available for decision-making | Action selection |
Note: Definitions and biological correlates taken verbatim from NIMH website: https://www.nimh.nih.gov/research-priorities/rdoc/constructs/positive-valence-systems.shtml.
Initial Responsiveness to Reward Attainment.
In chronic schizophrenia, there is consistent evidence for intact initial reward responsiveness at the group level. This evidence comes from laboratory-based studies examining self-reported valence93 and arousal94 to pleasant stimuli, as well as electrophysiological studies and neuroimaging studies examining striatal response to monetary reward outcomes; however, lower initial reward responsiveness has been associated with greater severity of volitional symptoms in some studies, indicating important individual differences and heterogeneity within the broader schizophrenia diagnosis.95–102
At the group level, initial reward responsiveness may be diminished for other schizophrenia-spectrum disorders and earlier phases of illness. For example, high-risk, first episode, and schizotypy samples all show diminished self-reported response to positive stimuli,103,104 potentially driven by high rates of comorbid depression and anxiety. Neural response to reward outcomes may be intact in high-risk, first-episode, and schizotypyal samples at the group level; however, greater severity of negative and depressive symptoms is associated with reduced activation of the ventral striatum and orbitofrontal cortex, respectively.43,105
Outside of the schizophrenia-spectrum, individuals with major depression, anxiety disorders, neurocognitive disorders, and autism also display diminished self-reported and neurophysiological response to reward outcomes in areas like the ventral striatum, caudate, putamen, orbitofrontal cortex, ventromedial prefrontal cortex, anterior cingulate cortex, and insula.106–113 Thus, there is an important discrepancy in the area of initial reward responsiveness, which appears to be intact in chronic schizophrenia, but reduced in other schizophrenia-spectrum disorders and disorders outside the schizophrenia-spectrum that display motivational abnormalities.
Sustained Responsiveness to Reward Attainment.
Although initial reward responsiveness may be intact in chronic schizophrenia, the ability to sustain that response after a reward outcome has terminated may be impaired. For example, while directly viewing pleasant stimuli, the affect modulated startle response is comparable between chronic schizophrenia patients and controls; however, after stimulus offset, only controls continue to show potentiation of this response.114 Similarly, when directly viewing pleasant photographs, chronic schizophrenia patients and controls show similar magnitude of activation in the dorsolateral prefrontal cortex, orbitofrontal cortex, ventromedial prefrontal cortex, basal ganglia, and amygdala; however, in the delay period following stimulus offset, there is reduced activation in the dorsolateral prefrontal cortex that predicts clinically rated anhedonia.115 Diminished ability to sustain affective response after stimulus offset has also been observed in bipolar disorder, but not depression.116,117 Since schizophrenia and bipolar disorder have more severe cognitive control deficits than depression, it is possible that prefrontally mediated deficits in sustaining reward response reflect a cognitive control impairment rather than hedonics.
Habit and Reward Learning.
On implicit learning tasks that measure striatum-mediated habit-based learning that occurs over a number of trials, individuals with chronic schizophrenia typically show normal striatal/model-free learning rates.118–124 In contrast, individuals with major depression and Parkinson’s disease evidence reduced striatal activation and retarded learning rates on such tasks.125–129
In contrast to habit learning, chronic schizophrenia has consistently been associated with impairments in making rapid, trial-by-trial behavioral adjustment on probabilistic reinforcement learning tasks that require forming more explicit top-down representations using the OFC.118,130–132 Greater severity of motivational symptoms is associated with more impairment in rapid/early learning, with some evidence that this association may be more severe for deficits in learning from positive than negative outcome feedback.60,70,92,118,130–135 Mechanisms underlying the deficit in learning from positive outcome feedback are unclear. Some computational modeling and neuroimaging studies implicate OFC-driven deficits in value representation, whereas others implicate positive prediction error signaling in the ventral striatum.67,70,131,132,135–138 Basic working memory impairments may moderate reinforcement learning deficits in chronic schizophrenia.139 Reinforcement learning deficits are also present in high-risk, first episode psychosis, and schizotypal populations, but in milder form than chronic schizophrenia.140,141 Like chronic schizophrenia, there is greater impairment for positive than negative outcome feedback and aberrant fronto-striatal activation during prediction error signaling may underlie reinforcement learning abnormalities in earlier phases of psychosis.140–145 Parkinson’s disease is also associated with intact learning from negative feedback and impaired learning from positive feedback, and this trend is reversed by dopamine agonists.146 In contrast, in depression there is surprisingly intact performance on probabilistic reinforcement learning tasks that require explicit representations.147–150
Important discrepancies therefore exist across disorders in the areas of implicit and explicit reinforcement learning. In disorders with true hedonic deficits, impaired striatal activation may impede implicit reinforcement learning, but intact prefrontal function may overcome this deficit and allow more normal performance on probabilistic learning tasks that require explicit representations. In contrast, disorders with intact hedonics and more severe PFC-driven cognitive control deficits may be impaired at probabilistic reinforcement learning tasks due to trouble forming explicit value representations, but can perform more implicit learning tasks that rely on the basal ganglia normally because they do not rely on top-down representations.
Approach Motivation.
The next 4 sections evaluate subcomponents of the RDoC “approach motivation” construct.
Reward Expectancy.
There is mixed evidence for group differences in ventral striatum activation in response to reward predictive cues among studies comparing schizophrenia and healthy control groups; however, there is consistent evidence for an association between greater blunting of the ventral striatum and volitional pathology.43,102 Similar to chronic schizophrenia, youth at ultra-high-risk for psychosis, first episode psychosis patients, and those with schizotypy show reduced activation of the ventral striatum during reward anticipation that predicts severity of negative symptoms.43,105,151–154 Consistent with a common mechanism for impairments in reward anticipation, reduced striatal activation is also observed during reward anticipation in depression, autism, neurocognitive disorders (eg, Huntington’s), social anxiety disorder, and euthymic bipolar disorder.112,155–161
Reward Valuation.
Schizophrenia patients are impaired at tasks where value representations must be generated, maintained, or updated, even when cognitive demands of tasks are minimal. However, the OFC has not been implicated, as would be expected; other mechanisms appear to contribute, including decreased deactivation of the default network, cognitive control regions, and prediction error signaling.131,136,145 First episode psychosis patients and youth at-risk for psychosis also demonstrate impairments in reversal learning and the Iowa Gambling task that are associated with negative symptoms143,145,162; similar reversal learning deficits are observed in major depression,163 PTSD,164 bipolar disorder,165 and autism spectrum disorders.166 Like schizophrenia, there is evidence that mechanisms other than the OFC contribute to these deficits (eg, striatal response).167
Effort Valuation.
Numerous studies have found impairment in physical or cognitive effort-cost computation in schizophrenia, with most reporting a significant association between willingness to work for rewards and volitional symptoms.168–171 Similar to chronic schizophrenia, effort-cost computation is impaired in individuals with major depressive disorder who show poor integration of probability and magnitude information when deciding whether to work for rewards.172–175 However, effort-cost computation is spared or even superior in schizotypy and autism-spectrum samples that show an increased willingness to work for rewards.176,177 At present, mechanisms underlying effort valuation impairments in disorders are unclear because neuroimaging results have yet to be published.
Action Selection.
Schizophrenia patients display DLPFC-mediated impairment on cognitive control tasks that result from poor maintenance of goal representations.178 Although reward incentives typically increase DLPFC activation and cause a shift toward less reactive and more proactive cognitive control strategies in healthy individuals, incentives fail to shift schizophrenia patients away from reliance on reactive control mechanisms.179 Although schizophrenia patients show similar activation of the DLPFC to controls in the context of sustained reward incentives that can be obtained for successful cognitive control task performance, individual differences in volitional symptoms are associated with reduced DLPFC activation as a function of reward incentives.180 General cognitive control deficits are also observed in depression, bipolar disorder, autism, and neurocognitive disorders; however, magnitude of impairment is typically not as severe as schizophrenia. Incentives modulate cognitive control normally in Parkinson’s disease, despite significant general control deficits.181 In depression, anxiety disorders, and bipolar disorder reward incentives have reduced impact on cognitive control processes182–184; however, neuroimaging has yet to be conducted to determine whether mechanisms underlying action selection impairment are similar to schizophrenia.
Summary.
Across DSM-5 disorders, there appear to be multiple pathways to the same common endpoints of motivational pathology (ie, equifinality). For example, in chronic schizophrenia, hedonic response is intact and mechanisms contributing to volitional pathology are subsequent to initial reward responsiveness; impairments in cortically mediated cognitive control processes may underlie abnormalities in several aspects of reward processing (eg, effort-cost computation, action selection, reward anticipation, rapid learning) that prevent intact hedonic responses from translating into motivated behavior in chronic schizophrenia.185 In contrast, in disorders other than chronic schizophrenia that have hedonic deficits, diminished initial reward responsiveness may propagate forward, leading to down-stream impairments in other aspects of reward processing (eg, implicit reinforcement learning, reward anticipation) that impede motivated behavior.185
Expressive Dimension
Fewer models have attempted to explain the pathophysiology of expressive symptoms in chronic schizophrenia. When attempting to understand these expressive deficits, it is important to consider that they are more complicated and potentially more subtle than what clinical ratings suggest. A recent review found that clinical ratings of expressive deficits were profoundly different in patients with schizophrenia compared to nonpsychiatric controls (eg, on the order of 4–7 standard deviations), whereas objective analysis of their verbal and nonverbal behavior revealed much more benign anomalies (k = 13 studies, n = 480 patients; d = .80 for alogia, d = .36 for blunted vocal affect).186 In fact, a recent comparison of 309 schizophrenia patients and 117 controls using computationally derived vocal measures during laboratory speaking tasks failed to find any significant group differences, despite there being profound group differences in clinical ratings of negative symptoms.72 We do not mean to imply that clinicians are “wrong” in their ratings. Rather, clinicians have the nearly impossible task of trying to reduce a broad range of expressive functions that naturally fluctuate as a function of time, context, and culture to a single number/category. While clinical ratings may be a valid reflection of global expressive dysfunction, their precision for understanding specific expressive behaviors over extended temporal epochs and varying situations is not established and does not appear to be supported. Below we consider how expressive deficits may wax and wane as a function of hedonic, cognitive, and social systems.
It is worth noting that computational approaches to objectifying expressive behaviors have been applied to disorders outside of schizophrenia, and in many cases, have generally shown modest (at best) levels of expressive deficits. For example, measuring natural vocal expression with acoustic analysis has revealed modest, or at best, inconsistent abnormalities associated with autism, depression, mania, and schizotypal personality traits.71,187–195 On the other hand, there are domains of psychopathology that have yielded relatively consistent findings regarding expressive deficits. First, acoustic vocal anomalies have been consistently observed in neurodegenerative disorders, such as Parkinson’s and Alzheimer’s diseases.196–198 Second, efforts to identify acoustic correlates of depression and dementia using machine learning with large feature sessions comprising hundreds or thousands of variables, have demonstrated promising results.187,199,200 To our knowledge however, no machine learning studies have yielded algorithms that replicate or generalize across studies, samples and speaking tasks, so this remains an emerging field in need of further work.
Hedonic Systems.
Historically, expressive deficits have been considered an extension of hedonic and volitional deficits in schizophrenia.201–203 With increased empirical efforts to isolate and objectify these expressive behaviors in schizophrenia, a more complicated picture is emerging, and it seems unlikely that expressive deficits wholly reflect disruptions in hedonics in chronic schizophrenia. As noted above, factor analysis of “next-generation” negative symptom clinical scales suggests that expressive symptoms are statistically independent of volitional symptoms.16,17 This finding is largely consistent with those from laboratory studies.204 For example, measures of computationally derived verbal production (eg, words and utterances produced) and vocal affect in patients with schizophrenia are largely uncorrelated with measures of affective experience.72,205 In contrast, in disorders where hedonic deficits are prominent (eg, depression, neurocognitive disorders), expression and experience are more tightly coupled.187,189
Cognitive Systems (ie, Attention, Working Memory, Cognitive Control).
If expressivity deficits do not reflect disruptions in positive valence systems in schizophrenia, and they are relatively benign much of the time, what could cause them? There is evidence to suggest that at least some expressive deficits may reflect disruptions in basic cognitive abilities. This is not surprising, in that effective verbal and nonverbal expression require coordinating a host of attentional, memory, language, social cognitive, and other functions,206,207 and that disruption in these abilities due to neuropathy, stress, or attentional distraction correlates with less effective expression in various patient populations.199,200,207 In patients with schizophrenia, measures of natural verbal production have been correlated with more global cognitive deficits.71,190,205 Moreover, using experimental “dual-task” designs, involving participants speaking while simultaneously conducting various working memory tasks, patients show reductions in verbal production at a greater magnitude than for nonpatients.71,208,209 Consider Cohen et al,71 where pause durations nearly doubled in patients with schizophrenia compared to a mild increase in nonpsychiatric patients. Importantly, neither nonverbal expression (eg, vocal prosody) nor speech content (eg, idea density, filler word use) was impacted by the cognitive load manipulation. This suggests that blunted vocal affect may not require attentional resources in the same manner as alogia, or that vocal expression is multi-faceted such that individuals can differentially allocate resources to specific functions to compensate for limitations in cognitive resources.
Cognitive-based mechanisms of expressivity deficits have been examined outside of schizophrenia. In the case of affective disorders (ie, depression, mania), both correlational and experimental studies suggest that pause times are associated with attentional dysfunctions, and that the magnitude of this effect is not statistically different as a function of schizophrenia, depression or mania diagnosis.71,205 In the case of schizotypal personality traits however, the results are more varied. Correlational studies have demonstrated relationships between acoustic speech features and cognitive performance on a standardized battery.194 However, experimental manipulation of attentional load has not led to abnormalities in pause times or any other speech features. This has been demonstrated in 3 studies to date, one of which found that the speech of individuals with schizotypy was abnormally resilient to attentional load.190,195,209 Collectively, these studies complement a large literature examining expressive deficits within the context of neurodevelopmental and neurodegenerative disorders, for which a number of expressive features have been identified and tied to specific motor control and other cognitive mechanisms.210,211 To our knowledge, however, factors potentially contributing to expressivity deficits outside of cognition, for example, involving emotion, social, temporal, diurnal, and other factors, have received limited empirical attention.
Expressivity as a Social Process.
Within the RDoC framework, the constructs of “production of facial communication” and “production of nonfacial communication” overlap conceptually with diminished expressivity, and thus warrant mention here. However, we are aware of little research relating expressivity deficits to specific social-based mechanisms in psychopathology despite the fact that expressive behaviors arise from, and modulate in response to a wide range of social and emotional factors. Behavior that is considered typical in one context (eg, silence while watching TV with a friend) may be considered pathological in another (eg, silence after asked about delusional thought content).207,212,213 Thus, it stands to reason that expressive behavior must be understood within its socio-contextual circumstances, and that consequently, a broad range of contextual factors may potentially contribute to expressive deficits. Within schizophrenia, eg, reduced facial expression could potentially reflect a myriad of “primary” and “secondary” sources, including paranoia, confusion during a social exchange, and trait social anhedonia. Similar behavior could reflect an emotion regulation strategy in depression to attenuate emotional experience during social interactions, or even a social cognition deficit as in autism-spectrum disorders.214,215 Moreover, given comorbidity across these disorders, expressive deficits could reflect different sources within an individual, eg, as an emotion regulation in one context and a social cognitive in another. From this perspective, expressive deficits may be best viewed as an equifinality reflecting a wide range of potential socially-related mechanisms.
Conclusions
Based on the present review, we believe it is clear that negative symptoms are a transdiagnostic construct that occurs within and outside of the schizophrenia-spectrum. There are no domains or features of negative symptoms that are pathognomonic, ie, specific to schizophrenia as the prototypical disorder and not present in other diagnoses. Rather, expressive and volitional negative symptoms are common in several disorders and form a continuous distribution of scores ranging from healthy, to high-risk, to clinical populations. Complicating comparisons across diagnostic categories, there is considerable heterogeneity in terminology used to describe negative symptom phenomenology throughout the DSM-5. Standardization of negative symptom terminology and assessments that can be used across disorders will be an important step to take before progress can be made in determining candidate mechanisms for treatment. The success of transdiagnostic research endeavors may depend on whether diagnostic groups included in a study are similar with respect to the presence of primary and secondary negative symptoms and whether measures are administered to capture the primary/secondary and persistent/transient distinctions.
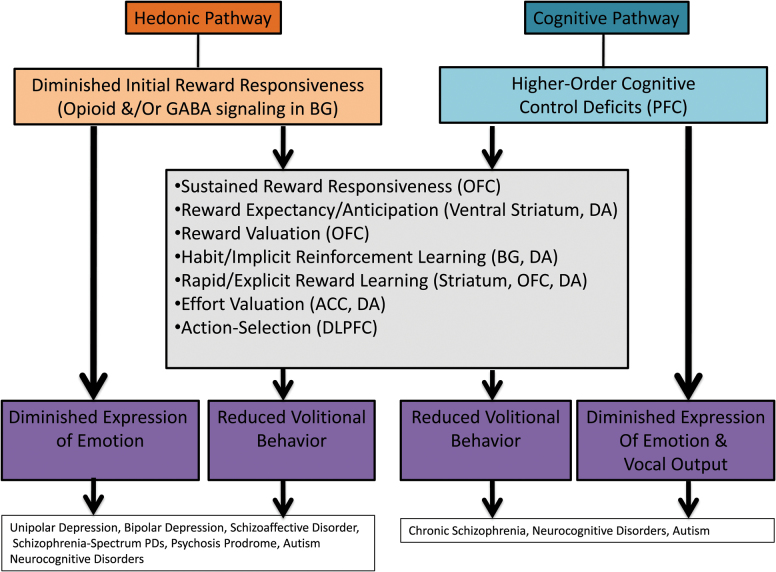
New transdiagnostic models of the etiology of expressive and volitional pathology are also needed for treatment progress to be made. Models developed for schizophrenia have led to significant breakthroughs; however, these models may not apply to other schizophrenia-spectrum disorders or disorders outside of the schizophrenia-spectrum that display negative symptoms. There is clear evidence for equifinality in both volitional and expressive dimensions of negative symptoms across DSM-5 disorders, ie, the same behavioral outcome/symptom is reached via different mechanisms. Figure 4 presents a new model depicting how 2 mechanistic pathways, a hedonic pathway and a cognitive pathway, can lead to both expressive and volitional negative symptoms. In disorders where hedonic deficits are prominent (eg, depression), impaired initial reward responsiveness may propagate forward, leading to reductions in expressing emotion and engaging in goal-directed behaviors. In contrast, in disorders where hedonics are intact (eg, schizophrenia), but cognitive deficits are prominent, expressive deficits may result from difficulty coordinating the many cognitive processes needed to produce contextually appropriate verbal and nonverbal communication. Similarly, motivational deficits may result from higher-order cognitive impairments that impact the ability to generate, update, and maintain explicit value representations needed to make effort-cost decisions and form action plans. Transdiagnostic studies are needed to test hypotheses such as these about common and distinct pathways to motivational and expressive negative symptoms. These studies should take the structure of negative symptoms into account, making study design and analytic decisions based on whether dimensional, categorical, or hybrid conceptualizations best fit the selected sample.
Fig. 4.
Transdiagnostic equifinality model of negative symptoms. Note: Higher-order cognitive control deficits are defined here as processes that allow information processing and behavior to adaptively adjust from moment-to-moment in response to current goals, facilitating a broad range of cognitive processes such as working memory, attention, long-term memory, emotion processing, and reward processing.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Bleuler E. Dementia praecox or the group of schizophrenias. Vertex. 2010;21:394–400. [PubMed] [Google Scholar]
- 2. Kraepelin E. Dementia Praecox and Paraphrenia (Barclay R. M., Trans.). New York, NY: Krieger; 1919. [Google Scholar]
- 3. Peralta V, Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophr Res. 2001;49:269–285. [DOI] [PubMed] [Google Scholar]
- 4. Fervaha G, Remington G. Validation of an abbreviated quality of life scale for schizophrenia. Eur Neuropsychopharmacol. 2013;23:1072–1077. [DOI] [PubMed] [Google Scholar]
- 5. Piskulic D, Addington J, Cadenhead KS et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophr Bull. 2010;36:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strauss GP, Sandt AR, Catalano LT, Allen DN. Negative symptoms and depression predict lower psychological well-being in individuals with schizophrenia. Compr Psychiatry. 2012;53:1137–1144. [DOI] [PubMed] [Google Scholar]
- 8. Fusar-Poli P, Papanastasiou E, Stahl D et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaiser S, Heekeren K, Simon JJ. The negative symptoms of schizophrenia: category or continuum? Psychopathology. 2011;44:345–353. [DOI] [PubMed] [Google Scholar]
- 11. Foussias G, Agid O, Fervaha G, Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur Neuropsychopharmacol. 2014;24:693–709. [DOI] [PubMed] [Google Scholar]
- 12. Insel T, Cuthbert B, Garvey M et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. [DOI] [PubMed] [Google Scholar]
- 13. American Psychiatric Association CoNaS. DSM-II: Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association; 1975. [Google Scholar]
- 14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 15. Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strauss GP, Hong LE, Gold JM et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strauss GP, Horan WP, Kirkpatrick B et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown RG, Pluck G. Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci. 2000;23:412–417. [DOI] [PubMed] [Google Scholar]
- 20. Geary EK, Seidenberg M, Hermann B. Atrophy of basal ganglia nuclei and negative symptoms in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci. 2009;21:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy ML, Cummings JL, Fairbanks LA et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10:314–319. [DOI] [PubMed] [Google Scholar]
- 22. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 23. Pluck GC, Brown RG. Apathy in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–583. [DOI] [PubMed] [Google Scholar]
- 25. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. [DOI] [PubMed] [Google Scholar]
- 26. Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. 2008;7:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirschner M, Aleman A, Kaiser S. Secondary negative symptoms—a review of mechanisms, assessment and treatment. Schizophr Res 2016. [DOI] [PubMed] [Google Scholar]
- 28. White SW, Roberson-Nay R. Anxiety, social deficits, and loneliness in youth with autism spectrum disorders. J Autism Dev Disord. 2009;39:1006–1013. [DOI] [PubMed] [Google Scholar]
- 29. Ciurli P, Formisano R, Bivona U, Cantagallo A, Angelelli P. Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehabil. 2011;26:116–126. [DOI] [PubMed] [Google Scholar]
- 30. Reichman WE, Coyne AC, Amirneni S, Molino B Jr, Egan S. Negative symptoms in Alzheimer’s disease. Am J Psychiatry. 1996;153:424–426. [DOI] [PubMed] [Google Scholar]
- 31. Getz K, Hermann B, Seidenberg M et al. Negative symptoms in temporal lobe epilepsy. Am J Psychiatry. 2002;159:644–651. [DOI] [PubMed] [Google Scholar]
- 32. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clementz BA, Sweeney JA, Hamm JP et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 35. Kirkpatrick B, Strauss GP, Nguyen L et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andreasen NC, Winokur G. Newer experimental methods for classifying depression. A report from the NIMH collaborative pilot study. Arch Gen Psychiatry. 1979;36:447–452. [DOI] [PubMed] [Google Scholar]
- 37. Brucato G, Masucci MD, Arndt LY et al. Baseline demographics, clinical features and predictors of conversion among 200 individuals in a longitudinal prospective psychosis-risk cohort. Psychol Med 2017:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Álvarez-Jiménez M, Gleeson JF, Henry LP et al. Road to full recovery: longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 years. Psychol Med. 2012;42:595–606. [DOI] [PubMed] [Google Scholar]
- 39. Averill PM, Reas DL, Shack A et al. Is schizoaffective disorder a stable diagnostic category: a retrospective examination. Psychiatr Q. 2004;75:215–227. [DOI] [PubMed] [Google Scholar]
- 40. Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195:475–482. [DOI] [PubMed] [Google Scholar]
- 41. Jäger M, Bottlender R, Strauss A, Möller HJ. On the descriptive validity of ICD-10 schizophrenia: empirical analyses in the spectrum of non-affective functional psychoses. Psychopathology. 2003;36:152–159. [DOI] [PubMed] [Google Scholar]
- 42. Kendler KS, McGuire M, Gruenberg AM, Walsh D. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry. 1995;152:755–764. [DOI] [PubMed] [Google Scholar]
- 43. Kirschner M, Hager OM, Muff L et al. Ventral striatal dysfunction and symptom expression in individuals with schizotypal personality traits and early psychosis. Schizophr Bull 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pini S, de Queiroz V, Dell’Osso L et al. Cross-sectional similarities and differences between schizophrenia, schizoaffective disorder and mania or mixed mania with mood-incongruent psychotic features. Eur Psychiatry. 2004;19:8–14. [DOI] [PubMed] [Google Scholar]
- 45. Fennig S, Bromet EJ, Galambos N, Putnam K. Diagnosis and six-month stability of negative symptoms in psychotic disorders. Eur Arch Psychiatry Clin Neurosci. 1996;246:63–70. [DOI] [PubMed] [Google Scholar]
- 46. Strauss GP, Vertinski M, Vogel SJ, Ringdahl EN, Allen DN. Negative symptoms in bipolar disorder and schizophrenia: a psychometric evaluation of the brief negative symptom scale across diagnostic categories. Schizophr Res. 2016;170:285–289. [DOI] [PubMed] [Google Scholar]
- 47. Herbener ES, Harrow M. Longitudinal assessment of negative symptoms in schizophrenia/schizoaffective patients, other psychotic patients, and depressed patients. Schizophr Bull. 2001;27:527–537. [DOI] [PubMed] [Google Scholar]
- 48. Lewine RR. A discriminant validity study of negative symptoms with a special focus on depression and antipsychotic medication. Am J Psychiatry 1990;147:1463–1466. [DOI] [PubMed] [Google Scholar]
- 49. Gerbaldo H, Helisch A, Schneider B, Philipp M, Benkert O. Subtypes of negative symptoms: the primary subtype in schizophrenic and non-schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:311–320. [DOI] [PubMed] [Google Scholar]
- 50. Klosterkötter J, Albers M, Steinmeyer EM, Hensen A, Sass H. Positive or negative symptoms—which are more appropriate as diagnostic criteria for schizophrenia? Acta Psychiatr Scand. 1995;92:321–326. [DOI] [PubMed] [Google Scholar]
- 51. Bottlender R, Sato T, Groll C, Jäger M, Kunze I, Möller HJ. Negative symptoms in depressed and schizophrenic patients: how do they differ? J Clin Psychiatry. 2003;64:954–958. [DOI] [PubMed] [Google Scholar]
- 52. Bottlender R, Sato T, Jäger M et al. Does considering duration of negative symptoms increase their specificity for schizophrenia? Schizophr Res. 2003;60:321–322. [DOI] [PubMed] [Google Scholar]
- 53. Gerbaldo H, Fickinger MP, Wetzel H, Helisch A, Philipp M, Benkert O. Primary enduring negative symptoms in schizophrenia and major depression. J Psychiatr Res. 1995;29:297–302. [DOI] [PubMed] [Google Scholar]
- 54. Rao V, Spiro JR, Schretlen DJ, Cascella NG. Apathy syndrome after traumatic brain injury compared with deficits in schizophrenia. Psychosomatics. 2007;48:217–222. [DOI] [PubMed] [Google Scholar]
- 55. Emmerson LC, Ben-Zeev D, Granholm E, Tiffany M, Golshan S, Jeste DV. Prevalence and longitudinal stability of negative symptoms in healthy participants. Int J Geriatr Psychiatry. 2009;24:1438–1444. [DOI] [PubMed] [Google Scholar]
- 56. Strauss GP, Allen DN. Emotional Verbal Learning Test: development and psychometric properties. Arch Clin Neuropsychol. 2013;28:435–451. [DOI] [PubMed] [Google Scholar]
- 57. Strauss GP, Allen DN, Duke LA, Ross SA, Schwartz J. Automatic affective processing impairments in patients with deficit syndrome schizophrenia. Schizophr Res. 2008;102:76–87. [DOI] [PubMed] [Google Scholar]
- 58. Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophr Bull. 2010;36:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Strauss GP, Duke LA, Ross SA, Allen DN. Posttraumatic stress disorder and negative symptoms of schizophrenia. Schizophr Bull. 2011;37:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strauss GP, Jetha SS, Ross SA, Duke LA, Allen DN. Impaired facial affect labeling and discrimination in patients with deficit syndrome schizophrenia. Schizophr Res. 2010;118:146–153. [DOI] [PubMed] [Google Scholar]
- 63. Strauss GP, Keller WR, Buchanan RW et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Strauss GP, Lee BG, Waltz JA, Robinson BM, Brown JK, Gold JM. Cognition-emotion interactions are modulated by working memory capacity in individuals with schizophrenia. Schizophr Res. 2012;141:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strauss GP, Llerena K, Gold JM. Attentional disengagement from emotional stimuli in schizophrenia. Schizophr Res. 2011;131:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Strauss GP, Morra LF, Sullivan SK, Gold JM. The role of low cognitive effort and negative symptoms in neuropsychological impairment in schizophrenia. Neuropsychology 2015;29:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Strauss GP, Thaler NS, Matveeva TM et al. Predicting psychosis across diagnostic boundaries: behavioral and computational modeling evidence for impaired reinforcement learning in schizophrenia and bipolar disorder with a history of psychosis. J Abnorm Psychol. 2015;124:697–708. [DOI] [PubMed] [Google Scholar]
- 68. Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gold JM, Waltz JA, Matveeva TM et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cohen AS, McGovern JE, Dinzeo TJ, Covington MA. Speech deficits in serious mental illness: a cognitive resource issue? Schizophr Res. 2014;160:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cohen AS, Mitchell KR, Docherty NM, Horan WP. Vocal expression in schizophrenia: less than meets the ear. J Abnorm Psychol. 2016;125:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ahmed AO, Strauss GP, Buchanan RW, Kirkpatrick B, Carpenter WT. Are negative symptoms dimensional or categorical? Detection and validation of deficit schizophrenia with taxometric and latent variable mixture models. Schizophr Bull. 2015;41:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blanchard JJ, Horan WP, Collins LM. Examining the latent structure of negative symptoms: is there a distinct subtype of negative symptom schizophrenia? Schizophr Res. 2005;77:151–165. [DOI] [PubMed] [Google Scholar]
- 75. Ahmed AO, Green BA, Buckley PF, McFarland ME. Taxometric analyses of paranoid and schizoid personality disorders. Psychiatry Res. 2012;196:123–132. [DOI] [PubMed] [Google Scholar]
- 76. Ahmed AO, Green BA, Goodrum NM, Doane NJ, Birgenheir D, Buckley PF. Does a latent class underlie schizotypal personality disorder? Implications for schizophrenia. J Abnorm Psychol. 2013;122:475–491. [DOI] [PubMed] [Google Scholar]
- 77. Blanchard JJ, Gangestad SW, Brown SA, Horan WP. Hedonic capacity and schizotypy revisited: a taxometric analysis of social anhedonia. J Abnorm Psychol. 2000;109:87–95. [DOI] [PubMed] [Google Scholar]
- 78. Horan WP, Blanchard JJ, Gangestad SW, Kwapil TR. The psychometric detection of schizotypy: do putative schizotypy indicators identify the same latent class? J Abnorm Psychol. 2004;113:339–357. [DOI] [PubMed] [Google Scholar]
- 79. Linscott RJ. The latent structure and coincidence of hypohedonia and schizotypy and their validity as indices of psychometric risk for schizophrenia. J Pers Disord. 2007;21:225–242. [DOI] [PubMed] [Google Scholar]
- 80. Prisciandaro JJ, Roberts JE. A taxometric investigation of unipolar depression in the national comorbidity survey. J Abnorm Psychol. 2005;114:718–728. [DOI] [PubMed] [Google Scholar]
- 81. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kwapil TR, Barrantes-Vidal N, Silvia PJ. The dimensional structure of the Wisconsin Schizotypy Scales: factor identification and construct validity. Schizophr Bull. 2008;34:444–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Davidson CA, Hoffman L, Spaulding WD. Schizotypal personality questionnaire–brief revised (updated): an update of norms, factor structure, and item content in a large non-clinical young adult sample. Psychiatry Res. 2016;238:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vrieze E, Demyttenaere K, Bruffaerts R et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord. 2014;155:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hsieh CJ, Chu H, Cheng JJ, Shen WW, Lin CC. Validation of apathy evaluation scale and assessment of severity of apathy in Alzheimer’s disease. Psychiatry Clin Neurosci. 2012;66:227–234. [DOI] [PubMed] [Google Scholar]
- 86. Clarke DE, Reekum RV, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci. 2007;19:57–64. [DOI] [PubMed] [Google Scholar]
- 87. Guthrie W, Swineford LB, Wetherby AM, Lord C. Comparison of DSM-IV and DSM-5 factor structure models for toddlers with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2013;52:797.e2–805.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. 2013;9:409–433. [DOI] [PubMed] [Google Scholar]
- 90. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Waltz JA, Gold JM. Motivational deficits in schizophrenia and the representation of expected value. Curr Top Behav Neurosci. 2016;27:375–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr Res. 2012;142:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. J Abnorm Psychol. 2010;119:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40:196–198. [DOI] [PubMed] [Google Scholar]
- 97. Simon JJ, Biller A, Walther S et al. Neural correlates of reward processing in schizophrenia—relationship to apathy and depression. Schizophr Res. 2010;118:154–161. [DOI] [PubMed] [Google Scholar]
- 98. Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl). 2009;206:121–132. [DOI] [PubMed] [Google Scholar]
- 99. Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7:e35622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Morris RW, Vercammen A, Lenroot R et al. Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol Psychiatry. 2012;17:235: 280–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gilleen J, Shergill SS, Kapur S. Impaired subjective well-being in schizophrenia is associated with reduced anterior cingulate activity during reward processing. Psychol Med. 2015;45:589–600. [DOI] [PubMed] [Google Scholar]
- 102. Mucci A, Dima D, Soricelli A et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol Med. 2015;45:1765–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cohen AS, Callaway DA, Najolia GM, Larsen JT, Strauss GP. On “risk” and reward: investigating state anhedonia in psychometrically defined schizotypy and schizophrenia. J Abnorm Psychol. 2012;121:407–415. [DOI] [PubMed] [Google Scholar]
- 104. Yee CM, Mathis KI, Sun JC et al. Integrity of emotional and motivational states during the prodromal, first-episode, and chronic phases of schizophrenia. J Abnorm Psychol. 2010;119:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wotruba D, Heekeren K, Michels L et al. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front Behav Neurosci. 2014;8:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28:676–691. [DOI] [PubMed] [Google Scholar]
- 107. McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl). 2009;205:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol. 2012;89:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. [DOI] [PubMed] [Google Scholar]
- 110. Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. [DOI] [PubMed] [Google Scholar]
- 111. Delmonte S, Balsters JH, McGrath J et al. Social and monetary reward processing in autism spectrum disorders. Mol Autism. 2012;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mikita N, Simonoff E, Pine DS et al. Disentangling the autism-anxiety overlap: fMRI of reward processing in a community-based longitudinal study. Transl Psychiatry. 2016;6:e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. J Abnorm Psychol. 2011;120:79–87. [DOI] [PubMed] [Google Scholar]
- 115. Ursu S, Kring AM, Gard MG et al. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psychiatry. 2011;168:276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M. Affect-modulated startle in adults with childhood-onset depression: relations to bipolar course and number of lifetime depressive episodes. Psychiatry Res. 2005;134:11–25. [DOI] [PubMed] [Google Scholar]
- 117. Lemaire M, El-Hage W, Frangou S. Increased affective reactivity to neutral stimuli and decreased maintenance of affective responses in bipolar disorder. Eur Psychiatry. 2015;30:852–860. [DOI] [PubMed] [Google Scholar]
- 118. Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kéri S, Kelemen O, Szekeres G et al. Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia. Psychol Med. 2000;30:149–155. [DOI] [PubMed] [Google Scholar]
- 120. Kéri S, Juhász A, Rimanóczy A et al. Habit learning and the genetics of the dopamine D3 receptor: evidence from patients with schizophrenia and healthy controls. Behav Neurosci. 2005;119:687–693. [DOI] [PubMed] [Google Scholar]
- 121. Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia: impact of distractors. Schizophr Res. 2007;89:339–349. [DOI] [PubMed] [Google Scholar]
- 123. Somlai Z, Moustafa AA, Kéri S, Myers CE, Gluck MA. General functioning predicts reward and punishment learning in schizophrenia. Schizophr Res. 2011;127:131–136. [DOI] [PubMed] [Google Scholar]
- 124. Weickert TW, Goldberg TE, Callicott JH et al. Neural correlates of probabilistic category learning in patients with schizophrenia. J Neurosci. 2009;29:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103:460–466. [DOI] [PubMed] [Google Scholar]
- 126. Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vrieze E, Pizzagalli DA, Demyttenaere K et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Herzallah MM, Moustafa AA, Natsheh JY et al. Depression impairs learning, whereas the selective serotonin reuptake inhibitor, paroxetine, impairs generalization in patients with major depressive disorder. J Affect Disord. 2013;151:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. [DOI] [PubMed] [Google Scholar]
- 130. Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Waltz JA, Kasanova Z, Ross TJ et al. The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PLoS One. 2013;8:e57257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Waltz JA, Schweitzer JB, Ross TJ et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40Suppl 2:S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Waltz JA, Schweitzer JB, Gold JM et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Culbreth AJ, Gold JM, Cools R, Barch DM. Impaired activation in cognitive control regions predicts reversal learning in schizophrenia. Schizophr Bull. 2016;42:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Culbreth AJ, Westbrook A, Daw ND, Botvinick M, Barch DM. Reduced model-based decision-making in schizophrenia. J Abnorm Psychol. 2016;125:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Culbreth AJ, Westbrook A, Xu Z, Barch DM, Waltz JA. Intact ventral striatal prediction error signaling in medicated schizophrenia patients. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Collins AG, Brown JK, Gold JM, Waltz JA, Frank MJ. Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci. 2014;34:13747–13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Waltz JA, Demro C, Schiffman J et al. Reinforcement learning performance and risk for psychosis in youth. J Nerv Ment Dis. 2015;203:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chang WC, Waltz JA, Gold JM, Chan TC, Chen EY. Mild reinforcement learning deficits in patients with first-episode psychosis. Schizophr Bull. 2016;42:1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Murray GK, Corlett PR, Clark L et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:239, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Murray GK, Cheng F, Clark L et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Murray GK, Clark L, Corlett PR et al. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry. 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schlagenhauf F, Huys QJ, Deserno L et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. [DOI] [PubMed] [Google Scholar]
- 147. Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychol Med. 2010;40:433–440. [DOI] [PubMed] [Google Scholar]
- 148. Anderson IM, Shippen C, Juhasz G et al. State-dependent alteration in face emotion recognition in depression. Br J Psychiatry. 2011;198:302–308. [DOI] [PubMed] [Google Scholar]
- 149. Cavanagh JF, Bismark AJ, Frank MJ, Allen JJ. Larger error signals in major depression are associated with better avoidance learning. Front Psychol. 2011;2:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Whitmer AJ, Frank MJ, Gotlib IH. Sensitivity to reward and punishment in major depressive disorder: effects of rumination and of single versus multiple experiences. Cogn Emot. 2012;26:1475–1485. [DOI] [PubMed] [Google Scholar]
- 151. Nielsen MØ, Rostrup E, Wulff S et al. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71:898–905. [DOI] [PubMed] [Google Scholar]
- 152. Esslinger C, Walter H, Kirsch P et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. [DOI] [PubMed] [Google Scholar]
- 153. Yan C, Wang Y, Su L et al. Differential mesolimbic and prefrontal alterations during reward anticipation and consummation in positive and negative schizotypy. Psychiatry Res. 2016;254:127–136. [DOI] [PubMed] [Google Scholar]
- 154. Radua J, Schmidt A, Borgwardt S et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–1251. [DOI] [PubMed] [Google Scholar]
- 155. Enzi B, Edel MA, Lissek S et al. Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: a functional magnetic resonance study. Exp Neurol. 2012;235:256–264. [DOI] [PubMed] [Google Scholar]
- 156. A Richey J, Ghane M, Valdespino A, Coffman MC, Strege MV, White SW, Ollendick TH. Spatiotemporal dissociation of brain activity underlying threat and reward in social anxiety disorder. Soc Cogn Affect Neurosci. 2017;12:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Schreiter S, Spengler S, Willert A et al. Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychol Med. 2016;46:3187–3198. [DOI] [PubMed] [Google Scholar]
- 158. Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Forbes EE, Hariri AR, Martin SL et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pizzagalli DA, Holmes AJ, Dillon DG et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Smoski MJ, Felder J, Bizzell J et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Leeson VC, Robbins TW, Matheson E et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012;169:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Levy-Gigi E, Richter-Levin G. The hidden price of repeated traumatic exposure. Stress. 2014;17:343–351. [DOI] [PubMed] [Google Scholar]
- 165. Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. D’Cruz AM, Mosconi MW, Ragozzino ME, Cook EH, Sweeney JA. Alterations in the functional neural circuitry supporting flexible choice behavior in autism spectrum disorders. Transl Psychiatry. 2016;6:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hall GB, Milne AM, Macqueen GM. An fMRI study of reward circuitry in patients with minimal or extensive history of major depression. Eur Arch Psychiatry Clin Neurosci. 2014;264:187–198. [DOI] [PubMed] [Google Scholar]
- 168. Green MF, Horan WP. Effort-based decision making in schizophrenia: evaluation of paradigms to measure motivational deficits. Schizophr Bull. 2015;41:1021–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Green MF, Horan WP, Barch DM, Gold JM. Effort-based decision making: a novel approach for assessing motivation in schizophrenia. Schizophr Bull. 2015;41:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Horan WP, Reddy LF, Barch DM et al. Effort-based decision-making paradigms for clinical trials in schizophrenia: part 2—external validity and correlates. Schizophr Bull. 2015;41:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Reddy LF, Horan WP, Barch DM et al. Effort-based decision-making paradigms for clinical trials in schizophrenia: part 1—psychometric characteristics of 5 paradigms. Schizophr Bull. 2015;41:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Treadway MT. The neurobiology of motivational deficits in depression—an update on candidate pathomechanisms. Curr Top Behav Neurosci. 2016;27:337–355. [DOI] [PubMed] [Google Scholar]
- 173. Treadway MT, Waskom ML, Dillon DG et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. McCarthy JM, Treadway MT, Blanchard JJ. Motivation and effort in individuals with social anhedonia. Schizophr Res. 2015;165:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Damiano CR, Aloi J, Treadway M, Bodfish JW, Dichter GS. Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions. J Neurodev Disord. 2012;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lopez-Garcia P, Lesh TA, Salo T et al. The neural circuitry supporting goal maintenance during cognitive control: a comparison of expectancy AX-CPT and dot probe expectancy paradigms. Cogn Affect Behav Neurosci. 2016;16:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Mann CL, Footer O, Chung YS, Driscoll LL, Barch DM. Spared and impaired aspects of motivated cognitive control in schizophrenia. J Abnorm Psychol. 2013;122:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chung YS, Barch DM. Frontal-striatum dysfunction during reward processing: relationships to amotivation in schizophrenia. J Abnorm Psychol. 2016;125:453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Harsay HA, Buitenweg JI, Wijnen JG, Guerreiro MJ, Ridderinkhof KR. Remedial effects of motivational incentive on declining cognitive control in healthy aging and Parkinson’s disease. Front Aging Neurosci. 2010;2:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry. 2005;58:632–639. [DOI] [PubMed] [Google Scholar]
- 183. Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J Child Psychol Psychiatry. 2007;48:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Mueller SC, Ng P, Temple V et al. Perturbed reward processing in pediatric bipolar disorder: an antisaccade study. J Psychopharmacol. 2010;24:1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Barch DM, Pagliaccio D, Luking K. Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. Curr Top Behav Neurosci. 2016;27:411–449. [DOI] [PubMed] [Google Scholar]
- 186. Cohen AS, Mitchell KR, Elvevåg B. What do we really know about blunted vocal affect and alogia? A meta-analysis of objective assessments. Schizophr Res. 2014;159:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Cummins N, Scherer S, Krajewski J, Schnieder S, Epps J, Quatieri TF. A review of depression and suicide risk assessment using speech analysis. Speech Commun. 2015;71:10–49. [Google Scholar]
- 188. Diehl JJ, Paul R. Acoustic differences in the imitation of prosodic patterns in children with autism spectrum disorders. Res Autism Spectr Disord. 2012;6:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cannizzaro M, Harel B, Reilly N, Chappell P, Snyder PJ. Voice acoustical measurement of the severity of major depression. Brain Cogn. 2004;56:30–35. [DOI] [PubMed] [Google Scholar]
- 190. Cohen AS, Auster TL, McGovern JE, MacAulay RK. The normalities and abnormalities associated with speech in psychometrically-defined schizotypy. Schizophr Res. 2014;160:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Karam ZN, Provost EM, Singh S et al. Ecologically valid long-term mood monitoring of individuals with bipolar disorder using speech.Paper presented at: 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Vanello N, Guidi A, Gentili C et al. Speech analysis for mood state characterization in bipolar patients.Paper presented at: Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE, 2012. [DOI] [PubMed] [Google Scholar]
- 193. Bedwell JS, Cohen AS, Trachik BJ, Deptula AE, Mitchell JC. Speech prosody abnormalities and specific dimensional schizotypy features: are relationships limited to male participants? J Nerv Ment Dis. 2014;202:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cohen AS, Iglesias B, Minor KS. The neurocognitive underpinnings of diminished expressivity in schizotypy: what the voice reveals. Schizophr Res. 2009;109:38–45. [DOI] [PubMed] [Google Scholar]
- 195. Cohen AS, Morrison SC, Brown LA, Minor KS. Towards a cognitive resource limitations model of diminished expression in schizotypy. J Abnorm Psychol. 2012;121:109–118. [DOI] [PubMed] [Google Scholar]
- 196. Bocklet T, Nöth E, Stemmer G, Ruzickova H, Rusz J.. Detection of persons with Parkinson’s disease by acoustic, vocal, and prosodic analysis.Paper presented at: 2011 IEEE Workshop on Automatic Speech Recognition and Understanding (ASRU), 2011. [Google Scholar]
- 197. Kim YB. Patterns of speech abnormality in a large dysarthria database: Interactions between severity, acoustic features, and dysarthria type. US: Dissertation Abstracts International: Section B: The Sciences and Engineering, ProQuest Information & Learning; 2008. [Google Scholar]
- 198. Satt A, Hoory R, König A, Aalten P, Robert PH. Speech-based automatic and robust detection of very early dementia. Studies 2014;3:6. [Google Scholar]
- 199. Jarrold W, Peintner B, Wilkins D et al. Aided diagnosis of dementia type through computer-based analysis of spontaneous speech.Paper presented at: Proceedings of the ACL Workshop on Computational Linguistics and Clinical Psychology, 2014. [Google Scholar]
- 200. Sriram TV, Rao MV, Narayana GS, Kaladhar D, Vital TPR. Intelligent parkinson disease prediction using machine learning algorithms. Int J Eng Innov Technol. 2013;3:212–215. [Google Scholar]
- 201. Berrios GE. Positive and negative symptoms and Jackson. A conceptual history. Arch Gen Psychiatry. 1985;42:95–97. [DOI] [PubMed] [Google Scholar]
- 202. Crow TJ. The two-syndrome concept: origins and current status. Schizophr Bull. 1985;11:471–486. [DOI] [PubMed] [Google Scholar]
- 203. Koehler K, Sauer H. Huber’s basic symptoms: another approach to negative psychopathology in schizophrenia. Compr Psychiatry. 1984;25:174–182. [DOI] [PubMed] [Google Scholar]
- 204. Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol. 1993;102:507–517. [DOI] [PubMed] [Google Scholar]
- 205. Cohen AS, Kim Y, Najolia GM. Psychiatric symptom versus neurocognitive correlates of diminished expressivity in schizophrenia and mood disorders. Schizophr Res. 2013;146:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Poeppel D, Emmorey K, Hickok G, Pylkkänen L. Towards a new neurobiology of language. J Neurosci. 2012;32:14125–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Cohen AS, Dinzeo TJ, Donovan NJ, Brown CE, Morrison SC. Vocal acoustic analysis as a biometric indicator of information processing: implications for neurological and psychiatric disorders. Psychiatry Res. 2015;226:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Barch DM, Berenbaum H. Language production and thought disorder in schizophrenia. J Abnorm Psychol. 1996;105:81–88. [DOI] [PubMed] [Google Scholar]
- 209. Melinder MR, Barch DM. The influence of a working memory load manipulation on language production in schizophrenia. Schizophr Bull. 2003;29:473–485. [DOI] [PubMed] [Google Scholar]
- 210. Cohen AS, Hong SL. Understanding constricted affect in schizotypy through computerized prosodic analysis. J Pers Disord. 2011;25:478–491. [DOI] [PubMed] [Google Scholar]
- 211. Lowit A, Kent RD. Assessment of Motor Speech Disorders. San Diego, CA: Plural Publishing; 2010. [Google Scholar]
- 212. Scherer KR. Vocal communication of emotion: a review of research paradigms. Speech Commun. 2003;40:227–256. [Google Scholar]
- 213. Sobin C, Alpert M. Emotion in speech: the acoustic attributes of fear, anger, sadness, and joy. J Psycholinguist Res. 1999;28:167–365. [DOI] [PubMed] [Google Scholar]
- 214. Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106:95–103. [DOI] [PubMed] [Google Scholar]
- 215. Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–1282. [DOI] [PubMed] [Google Scholar]