Abstract
Recent emotion dysregulation models of generalized anxiety disorder (GAD) propose chronic worry in GAD functions as a maladaptive attempt to regulate anxiety related to uncertain or unpredictable outcomes. Emotion acceptance is an adaptive emotion regulation strategy increasingly incorporated into newer cognitive behavioral therapy (CBT) approaches to GAD to counter chronic worry. The current study explores the mechanisms of emotion acceptance as an alternate emotion regulation strategy to worry or emotion suppression using functional magnetic resonance imaging. Twenty-one female participants diagnosed with GAD followed counterbalanced instructions to regulate responses to personally relevant worry statements by engaging in either emotion acceptance, worry or emotion suppression. Emotion acceptance resulted in lower ratings of distress than worry and was associated with increased dorsal anterior cingulate cortex (dACC) activation and increased ventrolateral prefrontal cortex (VLPFC)-amygdala functional connectivity. In contrast, worry showed significantly greater distress ratings than acceptance or suppression and was associated with increased precuneus, VLPFC, amygdala and hippocampal activation. Suppression did not significantly differ from acceptance in distress ratings or amygdala recruitment, but resulted in significantly greater insula and VLPFC activation and decreased VLPFC-amygdala functional connectivity. Emotion acceptance closely aligned with activation and connectivity patterns reported in studies of contextual extinction learning and mindful awareness.
Keywords: emotion acceptance, worry, generalized anxiety disorder, emotion regulation, functional magnetic resonance imaging
Introduction
Generalized anxiety disorder (GAD) is defined by its hallmark feature of chronic worry. Newer models of emotion dysregulation in GAD have shifted focus from chronic worry to the function worry subserves. Specifically, worry is seen as a maladaptive attempt to downregulate anxiety triggered by situations that are uncertain, unpredictable, or uncontrollable, which elicit particularly strong negative emotions and anxious reactions in individuals with GAD (Borkovec et al., 2004; Dugas et al., 2007). Worry becomes reinforced as feared outcomes rarely come to fruition, thus providing the illusion that worry facilitated successful aversion of impending catastrophe (Borkovec and Roemer, 1995). Thus, worry in GAD is conceptualized as a maladaptive emotion regulation strategy that develops in response to anxiety over unpredictable or uncontrollable outcomes, and becomes reinforced and well-established over time.
This newer conceptualization of emotion dysregulation in GAD has allowed the target of treatment to shift from directly addressing worries to learning adaptive emotion regulation skills. Emotion acceptance is one such skill that has been increasingly incorporated into newer cognitive behavioral therapy (CBT) treatments to counter worry in GAD, with promising results (Roemer et al., 2008; Barlow et al., 2010). Yet, the mechanism of action of emotion acceptance, and how emotion acceptance might be related to beneficial outcomes, is not well understood. A concise definition of emotion acceptance remains somewhat elusive, but broadly speaking emotion acceptance refers to the process of observing and allowing emotional experiences to occur as they unfold in the context of the present moment, without attempts to control, suppress or alter them in any way. Whereas emotion regulation through more traditional CBT strategies such as cognitive reappraisal focuses on replacing worry thoughts with alternate thinking patterns, emotion regulation with acceptance focuses on allowing the distress related to uncertainty, unpredictability or uncontrollability to occur whilst shifting the focus from internal, self-referent processing to an awareness of the self in context with the external world in the present moment.
This is important, as it allows the possibility for new stimulus-response contingencies to form. For example, using classical conditioning as a framework, the experience of uncertainty (conditioned stimulus) may become aversive because it is continuously paired with catastrophic thoughts and images of future imagined events (unconditioned stimulus). Therefore, as in classic extinction learning, by shifting the focus from future, catastrophic awareness to the present moment—a safe context wherein the forecasted catastrophic event is not actually taking place—engaging in emotion acceptance may serve to break the stimulus-response pattern of uncertainty-worry, promoting the possibility of new learning about the tolerability of uncertainty. Thus, the mechanism of emotion regulation using emotion acceptance may mimic extinction learning of contextual sustained anxiety. In support of this theory, behavioral studies of emotion regulation using acceptance have found greater initial ratings of distress during exposure to an anxiety eliciting stimulus relative to other emotion regulation strategies such as reappraisal or suppression, suggesting increased engagement with the stimulus (exposure). This is followed subsequently by decreased ratings of distress and greater willingness to re-engage with aversive stimuli in the future, suggesting new contingency learning has occurred (extinction; Campbell-Sills et al., 2006). Therefore, one mechanism through which emotion acceptance may exert effects is through exposure and extinction learning.
To help clarify the therapeutic mechanisms of emotion acceptance, the present study examines the neural correlates of acceptance as an emotion regulation strategy in GAD. Investigating the neural correlates of emotion regulation strategies such as acceptance allows for the objective observation of potential unique and overlapping mechanisms of action, thus contributing to the clarification of their potential clinical utility. There have been several fMRI studies of emotion regulation published over the past decade, which have significantly aided our understanding of the neural correlates of emotion regulation more generally (Berkman and Lieberman, 2009). Here, we wished to explore the mechanisms of emotion acceptance as a therapeutic skill aimed at ameliorating distress and countering chronic worry in GAD. Therefore, we felt it was important to understand the mechanisms of emotion acceptance as applied in the most ecologically valid way possible. As a result, in this study we chose to examine the use of emotion acceptance in contrast to the two most commonly used maladaptive emotion regulation strategies in GAD: worry and emotion suppression. Like worry, emotion suppression, which is operationalized here as an attempt to not experience emotions, rather than an attempt not to express emotions (Gross and Levenson, 1997), is an alternate emotion regulation strategy used by individuals with GAD to avoid intense emotional experiences (Mennin et al., 2002). However, suppression has been shown to have ironic effects: whereas suppression leads to decreased emotion experiences in the moment, it also leads to subsequent heightened emotion and emotion avoidance (Campbell-Sills et al., 2006), making this strategy ultimately maladaptive.
Many existing studies of emotion regulation have relied upon standardized stimuli to provoke emotions, such as images or generalized worry topics. However, in order to more closely match the emotion-eliciting experiences of individuals with GAD, and based upon previous findings of the superiority of personally relevant stimuli in the elicitation of strong emotions (Ellard et al., 2012), we chose to use personally relevant worry statements as the emotional stimuli. We focused our investigation on regions of interest (ROIs) previously identified in studies of extinction (Milad et al., 2007; Phelps et al., 2004; Delgado et al., 2008; Schiller et al, 2008; Lang et al., 2009) and acceptance (Smoski et al., 2015), but also cognitive reappraisal (Ochsner et al., 2002, 2004; Ochsner and Gross, 2005; Goldin et al., 2008) and mindfulness (Farb et al., 2007; Herwig et al., 2010; Ives-Deliperi et al., 2011), in order to clarify the mechanisms of emotion acceptance and potential overlap with other emotion regulation processes. These studies converge on a distributed network of ventral and dorsal prefrontal cortex (PFC) regions, as well as posterior parietal, amygdala and hippocampal regions. By comparison to adaptive emotion regulation strategies, fMRI studies of worry have shown compensatory activation of the ventrolateral PFC (VLPFC; Monk et al., 2006; McClure et al., 2007) and reduced amygdala-VLPFC connectivity in GAD (Monk et al., 2008; Etkin et al., 2009), as well as increased connectivity between default mode network structures (dorsomedial PFC; Andreescu et al., 2014). Studies of emotion suppression have implicated increased dorsolateral PFC and insula activation during regulation (Goldin et al., 2008). Given the overlap in regions associated with each of these regulation strategies, we were interested in whether specific strategies resulted in differential temporal patterns of activation within these regions. We therefore examined the temporal course of activation across early, middle and late phases of regulation following procedures used previously in studies of emotion regulation (Goldin et al., 2008; Ziv et al., 2013).
Materials and methods
Participants
Participants were recruited from the patient waitlist at Boston University Center for Anxiety and Related Disorders (CARD) and through the community via online advertisements. Given the small sample size in the current study, only females were included to control for sex-related differences in responses to affectively negative stimuli (Cahill et al., 2001). Participants recruited through the waitlist at CARD were assessed for a current diagnosis of GAD using the Anxiety Disorders Interview Schedule for DSM-IV—Lifetime Version (ADIS-IV-L; Di Nardo et al., 1994) a semi-structured interview designed to establish reliable diagnoses of DSM-IV (American Psychiatric Association, 2000) anxiety and related mood disorders. Participants recruited from outside advertisements were phone screened for eligibility and for the presence of GAD using the Generalized Anxiety Disorder Questionnaire 4th Edition (GAD-Q-IV; Newman et al., 2002) a nine-item self-report measure, designed for use as an initial screen to diagnose GAD. Participants eligible at the phone screen level were scheduled for further in-person diagnostic assessment using the Mini-ADIS-IV (Brown et al., 1994), a brief version of the ADIS-IV-L that fully assesses current diagnoses and screens for lifetime diagnoses (see Supplementary Material for exclusion criteria).
Twenty-four female participants met eligibility requirements and were recruited into the study. Of these, two had excessive head motion during scanning and were subsequently excluded from data analysis. One participant did not complete the task in the scanner as instructed and was also not included in the analysis. Therefore, the final sample included 21 female participants (age 29.48 ± 8.44 years; 85% Caucasian; see Supplementary Table S1).
Data acquisition
Stimuli
Worry statements used during the scanning session were generated from participant interviews conducted at the time of consent using the Catastrophic Interview Technique (Vasey and Borkovec, 1992). Worry topics were chosen from the GAD section of the ADIS-IV-L or MINI-ADIS. Topics rated at a 5 or higher for excessiveness and controllability on a scale of 0 (No worry/No Difficulty) to 8 (Constantly worried/Extreme difficulty) were included in the interview. Thirty-six statements were created for each participant to be used as stimuli during scanning. To standardize length of text across all participants, all worry statements were edited to be between 6 and 8 syllables long, and were stated as a question beginning with the format ‘What if…?’ Statements were programmed for electronic presentation using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA).
Functional MRI procedure
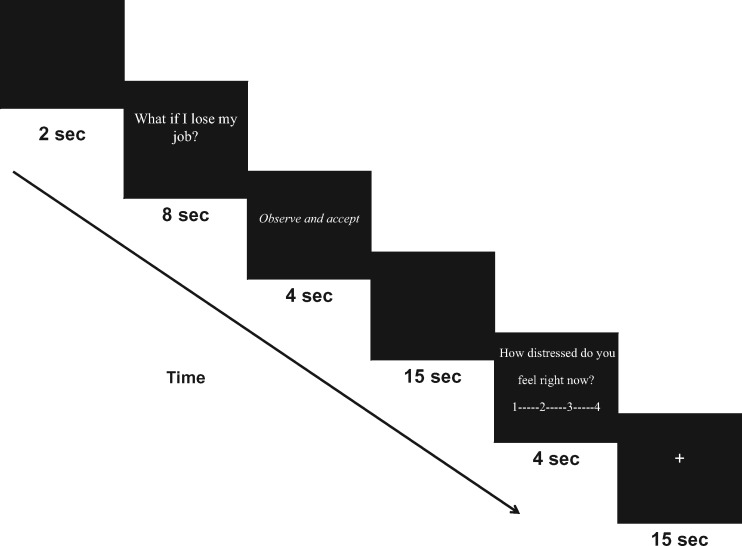
Participants were given typed, detailed instructions explaining each of the three regulation conditions (Accept, Worry, Suppress; see Supplementary Materials). To familiarize participants with the scanner task prior to entering the scanner, all participants were shown an example of the computer task using worry statements unrelated to their own topics of worry. Participants were scanned while viewing the above described personally relevant worry statements and regulating their responses to these statements according to presented regulation instructions over four separate runs (Figure 1). During each run, participants silently read personally relevant worry statements, presented one at a time. Participants were then instructed to regulate their reactions following each of the statement presentations according to three different regulation instructions: ‘Observe and accept’ (Accept condition), ‘Don’t think or feel’ (Suppress condition), or ‘Worry as usual’ (Worry condition). Each run consisted of nine trial blocks counterbalanced by regulation instruction. Following each regulation block, participants were asked to rate the question "How distressed do you feel right now?" by pushing buttons on a button box from 1 (Not at all) to 4 (Extremely). Trial blocks were separated by a 15-s fixation cross. Based upon evidence from previous studies indicating that rapid switching back and forth between regulation instructions is difficult for participants to follow (Deveney and Pizzagalli, 2008), presentation of regulation instructions were grouped by regulation conditions, such that three blocks of each instruction occurred sequentially (e.g. three trials of ‘Accept’ followed by three trials of ‘Suppress’, followed by three trials of ‘Worry’). The order of conditions within runs were randomized and counterbalanced, leaving six possible sets of condition presentation order (e.g. Accept, Suppress, Worry; Worry, Accept, Suppress etc.). Condition sets were then randomized across participants. At the end of the scanning session, participants completed a brief questionnaire rating perceived success at following regulation instructions.
Fig. 1.
Task sequence.
fMRI acquisition
MRI data were acquired using a 3.0-T whole-body scanner (Magnetom TrioTim Syngo; Siemens Medical Solutions) equipped for echo planar imaging (Siemens Medical Systems, Iselin, NJ, USA) with a 32-channel head coil. Head movements were restricted using foam cushions. Following automated scout and shimming procedures, two high-resolution 3D MPRAGE sequences [repetition time (TR) = 2.53 ms, echo time (TE) = 3.47 ms, flip angle = 90º, voxel size = 1.3 × 1.0 × 1.3 mm] were collected for positioning of subsequent scans. Functional blood oxygenation level dependent signal MRI images were acquired using T2*-weighted fast gradient EPI sequence (31 coronal slices, interleaved, aligned at a 0.68 degree rotation perpendicular to the plane intersecting the anterior and posterior commissures, 3 mm thickness, skip 1 mm, TE = 30 ms, TR = 2 s, 90° flip angle, FOV 200 mm, voxel size 2 × 2 × 2mm).
fMRI data processing
Prior to analysis, functional data were processed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Functional MRI images were slice time corrected, realigned and unwarped, and spatially normalized to the standardized space established by the Montreal Neurologic Institute (MNI) (http://www.bic.mni.mcgill.ca), resampled to 2 mm3 voxels and smoothed with a three-dimensional Gaussian kernel of 6-mm width (full width half maximum). Each participant’s data were inspected for excessive head motion and image distortion using Artifact Detection Tools (ARTs; Whitfield-Gabrieli, 2009) Subjects with excessive movement (>2 mm linear movement in the orthogonal planes; >0.5° radians of angular movement) or >20% of timepoints with artifacts within these thresholds were excluded from analysis (n= 2). Movement and artifact parameters derived from ART were saved and included as regressors in first-level fixed-effects analyses.
Data analysis
fMRI data analysis
First level fixed-effects analyses were conducted using general linear modeling (GLM) applied to the functional time series, convolved with the canonical hemodynamic response function and a 128 s high-pass filter. To examine differential temporal dynamics associated with the three regulation strategies, neural responses during early (0–5 s), middle (6–10 s) and late (11–15 s) phases of each 15-s regulation block were modeled separately, with Statement, Rate and Fixation blocks entered as regressors of no interest (see Supplementary Materials). For each subject, fixed effects for these conditions were estimated at each voxel and statistical parametric maps (SPMs) were produced for each event. Results of fixed effects models were visually inspected for errors in alignment or image distortion.
For group analysis, each subject’s first-level contrast images (SPM) were entered into a second-level flexible factorial model. Random-effects GLMs were modeled using fixed-effects SPMs during each phase of regulation (Early, Middle and Late) and contrasting each of the regulation conditions with each other (e.g. Early Phase Accept >Early Phase Worry). To determine the main effects of regulation conditions during each phase of the regulation block, loci of significant activation were identified for each of these linear contrasts within specified a priori ROIs; specifically, limbic structures (amygdala, hippocampus), regions of the ventral PFC (VLPFC, vmPFC), regions of the dorsal PFC (DLPFC, dmPFC), insular cortex, cingulate cortex and precuneus (see Supplementary Table S2). ROIs were defined and small volume corrected (SVC) using masks provided by the Anatomical Automatic Labeling tool in the Wake Forest University PickAtlas (Tzourio-Mazover et al., 2002; Maldijian et al., 2003, 2004).
Linear contrast analyses were performed on parameter estimates within SVC, a priori ROIs for each modeled event. For the amygdala ROI, an uncorrected threshold of P = 0.01 was used with the added requirement that at least 10 contiguous voxels exceeded this statistical level (i.e. k ≥ 10, volume ≥ 80 mm3). For cortical ROI activations, which are easier to detect than amygdala activations, we employed a more stringent uncorrected threshold of P < 0.005. To correct for multiple comparisons, an additional Monte Carlo simulation correction was applied to each t-test result that met the above significance thresholds using AFNI’s 3dClustSim (Ward, 2002; version May 2015; see Supplementary Materials). Effect sizes were calculated using Cohen’s d, where d = 0.50 represents a moderate effect, and d = 0.80 represents a large effect. Only those max voxels with cluster sizes meeting a corrected probability threshold of P < 0.05 and a moderate to large effect size were pursued for further analysis. Parameter estimates (beta weights) across all contrasts (Early, Middle and Late phase) were extracted from the surviving global maxima voxels (5 mm spherical ROIs) as an index of blood oxygenation level-dependent signal change using MarsBaR (http://marsbar.sourceforge.net). These beta weights were then exported to SPSS (version 18.0) for further analysis.
To ensure differences between regulation conditions in ROI activations were not attributable to differences in activation of these ROIs during the preceding Statement blocks (presentation of worry statements), a series of repeated measures ANOVAs were conducted on the extracted ROI betas to compare activation between Accept, Worry and Suppress conditions during the preceding Statement blocks. Only those ROIs that evidenced no significant differences in activation between regulation conditions during Statement blocks were followed up for further analyses.
Generalized psychophysiological interaction analysis
To explore differences in emotion regulation driven functional connectivity, a generalized psychophysiological interaction (gPPI) analysis (Friston et al., 1997; McLaren et al., 2012) was conducted using SPM8. A deconvolved time course for the amygdala volume of interest resulting from the second level random effects group analysis specified earlier was extracted for each participant. This activity was then regressed against the product of time course and each condition vector, with the physiological and psychological variables and ART motion parameters included as regressors of no interest. The individual subjects results of these analyses were then used in a random-effects group analysis using a flexible factorial model, with a statistical threshold of P < 0.005 uncorrected (see Supplementary Materials for additional methods).
Behavioral data analysis
Analyses of behavioral data were conducted using SPSS (version 18.0). Follow up paired t-tests were conducted on significant three-way ANOVAs. To determine the relationship between patterns of activation in ROIs and baseline worry-related symptom severity as measured by the Penn State Worry Questionnaire (PSWQ; (Meyer et al., 1990) and ratings of distress following regulation blocks, a series of bivariate Pearson correlations were conducted between parameter estimates of linear activation across each regulation condition and self-report measures. To determine the relationship between ROI activation and perceived regulation success in each regulation condition, linear regressions were conducted with ROI activation parameters as the predictor and self-reported regulation success as the dependent variable.
Results
Behavioral results
Significant differences in distress ratings following regulation were found between conditions, F (2, 19) = 17.99, P < 0.001, η2 = 0.65 (Table 1). Follow-up pairwise t-tests revealed significantly higher ratings of distress in the Worry condition relative to Accept [t(20) = 5.60, P < 0.001, Cohen’s d = 2.50] and Suppress [t(20) = 6.01, P < 0.001, Cohen’s d = 2.67]. Ratings between Accept and Suppress approached significance [t(20) = 1.84, P = 0.08, Cohen’s d = 0.82]. We expected high distress ratings to predict greater success at following instructions to Worry and less success at following instructions to Suppress or Accept. As predicted, higher ratings of distress following the Worry condition significantly predicted higher ratings of success at following instructions to Worry [β = 0.63, t(19) = 3.42, P = 0.003] and explained a significant amount of variance in regulation success [R2 = 0.39, F(1, 20) = 11.72, P = 0.003] The same was true in the Suppress condition [β = −0.53, t(19) = −2.67, P = 0.02; R2 = 0.28, F(1, 20) = 7.12, P = 0.02], also in the expected direction (higher ratings of distress following Suppress predicted ‘less’ success following instructions to Suppress). A moderate effect was also found for the Accept condition in the expected direction, but this did not reach statistical significance [β = −0.34, t(19) = −1.54, P = 0.14; R2 = 0.12, F(1, 20) = 2.36, P = 0.14].
Table 1.
Ratings of distress and regulation success
| Accept |
Worry |
Suppress |
F(2,19) | η2 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Distress ratings | 2.19 | 0.53 | 2.92 | 0.57 | 2.03 | 0.62 | 17.99*** | 0.65 |
| Regulation success (%) | 63.00 | 18.09 | 81.50 | 20.07 | 66.00 | 22.10 | 8.15** | 0.30 |
Note. **P < 0.01. ***P < 0.001.
fMRI results
Regulation using Worry
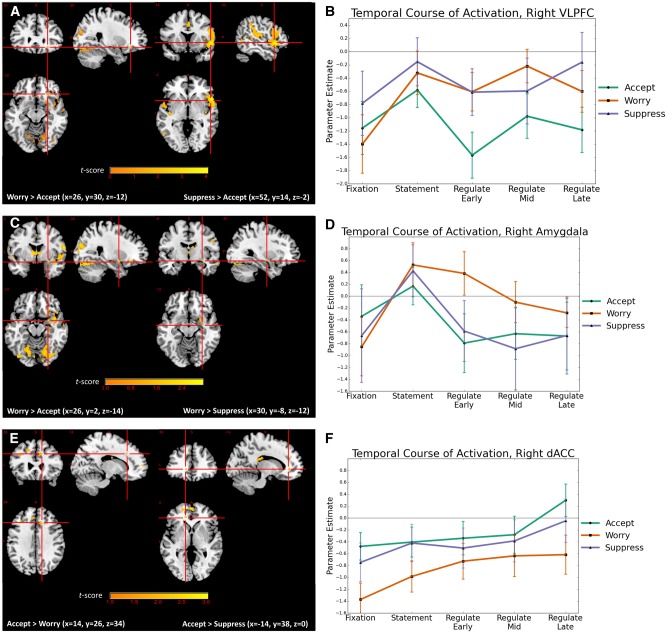
Large effect size (Cohen’s d) differences were found in early phase responses to the Worry regulation instructions relative to Accept regulation instructions in right VLPFC (BA 47, Figure 2A and B) and left precuneus (BA 31), with Worry evidencing significantly greater activation than Accept. Moderate effect size differences following Worry instructions relative to both Accept and Suppress instructions were found in early phase right amygdala (Figure 2C and D) and right hippocampus, with the Worry condition evidencing significantly greater activation. In addition, moderate effect size differences were found following Worry regulation instructions relative to Accept in early phase left dmPFC (BA 6) and early- and mid-phase bilateral precuneus (BA 30, BA 31), with the Worry condition evidencing significantly greater activation. Moderate effect size differences were found between Worry and Suppress during middle phase regulation in right amygdala, right vmPFC (BA 10) and left precuneus (BA 31), with the Worry condition evidencing significantly greater activation (Tables 2 and 3).
Fig. 2.
Differences in peak activation and temporal course of activation by condition. (A) Early phase VLPFC activation in response to Worry or Suppress regulation instructions relative to Accept regulation instructions. (B) Temporal course of VLPFC activation (Suppress > Accept: x = 52, y = 14, z = −2) across early (0–5 s), mid (6–10 s) and late (11–15 s) phases of regulation by condition. (C) Early phase amygdala activation in response to Worry regulation instructions relative to Accept or Suppress regulation instructions and (D) temporal course of amygdala activation (Worry > Accept: x = 26, y = 30, z = −12) across early (0–5 s), mid (6–10 s) and late (11–15 s) phases of regulation by condition. (E) dACC activation in response to Accept regulation instructions relative to Worry (late-phase) or Suppress (early phase) regulation instructions and (F) temporal course of dACC activation (Accept > Worry: x = 14, y = 26, z = 34) across early (0–5 s), mid (6–10 s), and late (11–15 s) phases of regulation by condition. Whole brain results for VLPFC (2A) and dACC (2C) displayed at P < 0.005 uncorrected. Whole brain results for amygdala (2B) displayed at P < 0.01 uncorrected.
Table 2.
Worry vs Emotion Acceptance—early, middle and late-phase regulation
| MNI coordinatesa |
||||||||
|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Vol (mm3) | t-score | P (uncorrected) b | Cohen’s d |
| Worry > Accept | ||||||||
| Early | ||||||||
| R. Amygdala | 26 | 2 | −14 | 216 | 2.90 | 0.002** | 0.63 | |
| R. Hippocampus | 32 | −12 | −16 | 1792 | 3.39 | <0.001** | 0.74 | |
| R. VLPFC | 47 | 26 | 30 | −12 | 760 | 3.99 | <0.001** | 0.87 |
| L. dmPFC | 6 | −4 | −10 | 50 | 1248 | 2.43 | <0.001** | 0.53 |
| L. Precuneus | 30 | −6 | −56 | 8 | 5456 | 4.19 | <0.001** | 0.91 |
| R. Precuneus | 30 | 6 | −52 | 12 | 3.24 | 0.001** | 0.71 | |
| L. Precuneus | 31 | −2 | −64 | 26 | 2.79 | 0.003** | 0.61 | |
| Middle | ||||||||
| L. Precuneus | 31 | −4 | −62 | 28 | 5024 | 2.97 | 0.002** | 0.65 |
| R. Precuneus | 30 | 10 | −54 | 18 | 2.69 | 0.004** | 0.59 | |
| L. Precuneus | 30 | −8 | −56 | 8 | 2.61 | 0.005** | 0.57 | |
| Late | None | |||||||
| Accept > Worry | ||||||||
| Early and Middle | None | |||||||
| Late | ||||||||
| R. dACC | 32 | 14 | 26 | 34 | 3240 | 2.83 | 0.002** | 0.62 |
| L. dmPFC | 9 | −12 | 40 | 26 | 1320 | 2.60 | 0.005** | 0.57 |
Note. aMNI coordinates; x indicates right (+) or left (−); y indications anterior (+) or posterior (−); z indicates superior to the anterior commissure. MNI, Montreal Neurologic Institute.
Results show significant voxels surviving corrections for multiple comparisons using AFNI 3dClustSim Monte Carlo Simulations.
denotes AFNI 3dClustSim corrected P < 0.01.
Table 3.
Worry vs Suppression—early, middle and late-phase regulation
| MNI Coordinatesa |
||||||||
|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Vol (mm3) | t-score | P (uncorrected)b | Cohen’s d |
| Worry > Suppress | ||||||||
| Early | ||||||||
| R. Amygdala | 30 | −8 | −12 | 144 | 2.42 | 0.008** | 0.53 | |
| R. Hippocampus | 36 | −36 | −6 | 1608 | 3.06 | 0.001** | 0.67 | |
| Middle | ||||||||
| R. Amygdala | 28 | −2 | −14 | 104 | 2.11 | 0.01** | 0.46 | |
| vmPFC | 10 | 0 | 56 | −4 | 464 | 3.09 | 0.001** | 0.67 |
| Precuneus | 31 | −6 | −62 | 30 | 5968 | 3.58 | <0.001** | 0.78 |
| Late | None | |||||||
| Suppress > Worry | ||||||||
| Early | ||||||||
| R. anterior insula | 13 | 38 | 18 | 8 | 944 | 2.70 | 0.004** | 0.59 |
| L. anterior insula | 38 | −40 | 6 | −14 | 1000 | 2.70 | 0.004** | 0.59 |
| R. VLPFC | 44 | 58 | 16 | 10 | 3424 | 2.92 | 0.002** | 0.64 |
| R. DLPFC | 8 | 44 | 8 | 40 | 816 | 2.51 | 0.005** | 0.55 |
| Middle | ||||||||
| R. dmPFC | 8 | 8 | 28 | 44 | 728 | 2.94 | 0.002* | 0.64 |
| Late | ||||||||
| R. DLPFC | 6 | 22 | 10 | 50 | 1328 | 2.58 | 0.005** | 0.56 |
Note. aMNI coordinates; x indicates right (+) or left (−); y indications anterior (+) or posterior (−); z indicates superior to the anterior commissure. MNI, Montreal Neurologic Institute.
Results show significant voxels surviving corrections for multiple comparisons using AFNI 3dClustSim Monte Carlo Simulations.
denotes AFNI 3dClustSim corrected P < 0.01.
Denotes AFNI 3dClustSim corrected P < 0.05.
Regulation using Acceptance
Moderate effect size differences were found in responses to Accept regulation instructions relative to Worry regulation instructions during late-phase regulation in right dorsal anterior cingulate cortex (dACC) (BA 32, Figure 2E and F) and left dmPFC (BA 9), with the Accept condition evidencing significantly greater activation. Moderate effect sizes differences in response to Accept relative to Suppress regulation instructions were found in early- and late-phase bilateral dACC (BA 32, Figure 2E and F) and mid-phase right dACC (BA 32), with the Accept condition evidencing significantly greater activation (Tables 2 and 4).
Table 4.
Suppression vs Emotion Acceptance—early, middle and late-phase regulation
| MNI Coordinatesa |
||||||||
|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Vol (mm3) | t-score | P (uncorrected)b | Cohen’s d |
| Suppress > Accept | ||||||||
| Early | ||||||||
| L. posterior insula | 13 | −40 | −14 | −4 | 2176 | 4.65 | <0.001** | 1.01 |
| R. anterior insula | 38 | 42 | 8 | −14 | 9480 | 4.21 | <0.001** | 0.92 |
| L. anterior insula | 38 | −40 | 4 | −14 | 176 | 4.27 | <0.001** | 0.93 |
| R. VLPFC | 47 | 52 | 14 | −2 | 6464 | 4.06 | <0.001** | 0.89 |
| dmPFC | 6 | 0 | 6 | 48 | 840 | 2.73 | 0.003* | 0.60 |
| Middle | None | |||||||
| Late | ||||||||
| R. VLPFC | 47 | 26 | 30 | −12 | 6112 | 2.68 | <0.001** | 0.58 |
| Accept > Suppress | ||||||||
| Early | ||||||||
| R. dACC | 32 | 16 | 32 | 18 | 328 | 2.64 | 0.004* | 0.58 |
| L. dACC | 32 | −14 | 38 | 0 | 480 | 3.03 | 0.001* | 0.66 |
| Middle | ||||||||
| R. dACC | 32 | 14 | 42 | 28 | 264 | 2.57 | 0.004* | 0.56 |
| Late | ||||||||
| R. dACC | 32 | 16 | 8 | 28 | 1272 | 2.57 | 0.005** | 0.56 |
| L. dACC | 32 | −18 | 9 | 30 | 576 | 2.62 | 0.005** | 0.57 |
Note. aMNI coordinates; x indicates right (+) or left (−); y indications anterior (+) or posterior (−); z indicates superior to the anterior commissure. MNI, Montreal Neurologic Institute.
Results show significant voxels surviving corrections for multiple comparisons using AFNI 3dClustSim Monte Carlo Simulations.
Denotes AFNI 3dClustSim corrected P < 0.01.
denotes AFNI 3dClustSim corrected P < 0.05.
Regulation using Suppression
Large effect size differences were found in response to Suppress instructions relative to Accept instructions during early phase regulation in bilateral anterior insula (BA 38), left posterior insula (BA 13), and right VLPFC (BA 47, Figure 2A and B), with the Suppress condition evidencing significantly greater activation. Moderate effect size differences between Suppress and Accept conditions were found during early phase regulation in midline dmPFC (BA 6), with Suppress evidencing significantly greater activation. Moderate effect size differences were also found in response to Suppress instructions relative to Worry instructions during early phase regulation in bilateral anterior insula (BA 38) and right VLPFC (BA 47), and during early- and late-phase regulation in right DLPFC (BA 6/8), with Suppress evidencing significantly greater activation. Moderate effect size differences between Suppress and Worry conditions were also found during mid-phase regulation in right dmPFC (BA 8), with Suppress evidencing significantly greater activation (Tables 3 and 4).
Correlations with behavioral ratings
Correlations with symptom severity
Baseline worry symptom severity (PSWQ scores) showed a strong negative correlation with mid-phase dACC/dmPFC (BA 32/9) activation and a strong positive correlation with late-phase vmPFC (BA 10) activation during the Worry condition (see Supplementary Table S3). A moderate positive correlation with amygdala activation was also found during late phase Worry and early phase Suppression, although this reached trend-wise significance for Worry. No other significant correlations with symptom severity were found in any of the remaining ROI comparisons.
Correlations with distress ratings
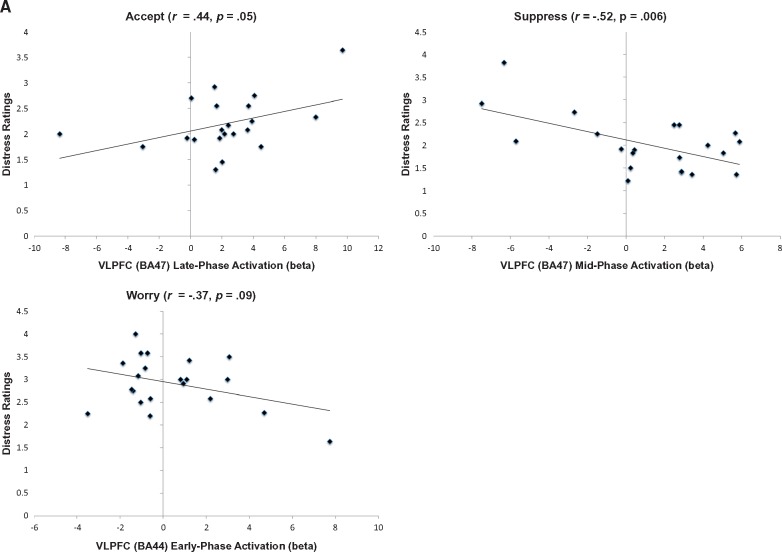
Distress ratings following regulation with Accept showed a moderate positive correlation with late phase VLPFC (BA47) activation during regulation with Accept. In contrast, a strong negative correlation was found between mid-phase VLPFC (BA47) activation during regulation and subsequent distress ratings in the Suppress condition, and a moderate but trend-wise significant negative correlation was found between early phase VLPFC (BA44) activation during regulation and subsequent distress ratings in the Worry condition (Figure 3A, Supplementary Table S3). Following regulation with Suppress or Worry, distress ratings were strongly positively correlated with early phase (Suppress) and mid phase (Worry) vmPFC (BA 10) activation. Distress ratings were also strongly negatively correlated with late phase (Worry) anterior insula/VLPFC (BA 47) activation and moderately positively correlated with early phase (Suppress) anterior insula (BA 38) activation, although this reached trend-wise significance for Suppress. No significant relationships were found following Accept between distress ratings and vmPFC or anterior insula activations. Distress ratings following Worry also showed a strong negative correlation with dACC/dmPFC (BA 32/BA 9) activation, whereas no significant relationships were found following Accept or Suppress between distress ratings and dACC or dmPFC activation. Finally, distress ratings following Worry were moderately positively correlated with early phase hippocampal activation, whereas distress ratings following Suppress and Accept moderately negatively correlated with early (Suppress) and mid phase (Accept) hippocampal activation, but only at trend-wise significance for Accept (Supplementary Table S3).
Fig. 3.
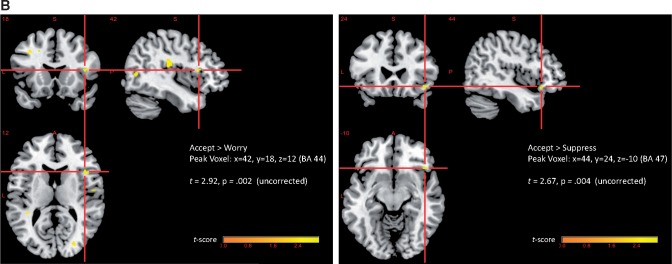
(A) Correlations between VLPFC activation and behavioral ratings of distress by regulation condition. BA 47: x = 52, y = 10, z = −2; BA 44: x = 58, y = 16, z = 10. (B) Right amygdala—right VLPFC functional connectivity (gPPI) during regulation using emotion acceptance relative to worry or emotion suppression. Amygdala seed region: x = 26, y = 2, z = –14. Whole brain results displayed at P < 0.005.
Neural predictors of self-rated regulation success
Early phase right anterior insula/VLPFC (BA 47) activation during regulation strongly negatively predicted rated success at regulation following the Accept or Suppress instructions, and anterior insula (BA 38) activation moderately negatively predicated success following the Worry instructions, although at trend-wise significance for the Worry condition (Supplementary Table S4). Early phase amygdala activation moderately negatively predicted regulation success using Suppress or Accept but not Worry, at trend-wise significance for the Accept condition. Rated success at regulation using Accept was also moderately negatively predicted by late hippocampus activation. No relationship between hippocampus activation and regulation success was found in the Suppress or Worry conditions.
Cortical functional connectivity with amygdala
Generalized PPI analyses were conducted using the amygdala ROI identified above as displaying significantly greater activation during worry as the seed ROI (x = 26, y = 2, z = –14). Results of this analysis showed responses to the Accept instruction resulted in significantly greater amygdala-right VLPFC connectivity relative to Worry (BA 44: t = 2.67, P = 0.004 uncorrected, d = 0.70) or Suppress (BA 47: t = 2.92, P = 0.002 uncorrected, d = 0.76) (Figure 3B). Accept was also associated with greater amygdala-left dmPFC (BA 8) functional connectivity relative to Worry (t = 2.57; P = 0.006 uncorrected, d = 0.67; see Supplementary Figure S1). None of the Worry > Accept or Worry > Suppress contrasts were significant at the P < 0.005 threshold.
Discussion
In this study, we sought to clarify the therapeutic mechanisms of emotion acceptance as an adaptive, alternate emotion regulation strategy to worry or emotion suppression in patients with GAD. In an attempt to understand potential therapeutic mechanisms of emotion acceptance in the most ecologically valid way possible, we examined patterns of neural activation associated with the use of these regulation strategies in response to individualized, personally relevant worry statements. In addition, to better understand the temporal dynamics of each regulation strategy, we examined differential patterns of activation during early, middle and late phases of regulation for all three strategies.
Regulation using emotion acceptance was associated with significantly lower ratings of distress in response to worry statements than allowing oneself to worry, and was characterized by significantly less early phase VLPFC recruitment relative to either worry or emotion suppression, yielding large effect sizes. Additionally, emotion acceptance was associated with significantly decreased early phase amygdala and hippocampal activation relative to worry, yielding moderate effect sizes. Thus, regulation using emotion acceptance resulted in both a dampening down of early limbic and salience-related responses and less recruitment of the VLPFC, a region previously identified as hyperactivated in response to negative emotional stimuli in patients with GAD (Monk et al., 2006; Blair et al., 2008). Additionally, regulation with emotion acceptance resulted in significantly greater late phase dACC and dmPFC (BA9) recruitment than worry and greater early and late bilateral dACC recruitment relative to suppression, yielding moderate effect sizes. The dACC is a region previously identified as important for both extinction learning (Lang et al., 2009; Maier et al., 2012) and emotion regulation through cognitive reappraisal (Ochsner et al., 2002, 2004), and a region previously shown to be hypoactivated during emotion regulation in GAD (Blair et al., 2012). Thus, in this study, regulation using emotion acceptance resulted in significantly less limbic activation early on in the regulation phase, and sustained recruitment of regulatory cortical regions throughout later phases of regulation.
In contrast, worry was associated with higher ratings of distress and significantly greater early engagement of precuneus relative to both emotion acceptance and suppression yielding large effect sizes, and greater dmPFC (in BA 6/8) activation relative to acceptance and vmPFC activation relative to suppression yielding moderate effect sizes, all regions associated with the default mode network. Further, activation of vmPFC regions during worry was strongly positively associated with subsequent ratings of emotional distress. Whereas less is known about the association between worry and default mode network activation, greater recruitment of default network has been associated with increased rumination in depression, suggesting a role for this network in negative self-referent processing (Hamilton et al., 2011; Whitfield-Gabrieli and Ford, 2012). Additionally, worry was associated with significant moderate increases in early phase amygdala and hippocampal activation relative to either acceptance or suppression, and early phase hippocampal activation during worry showed a strong positive correlation to subsequent distress ratings. Thus, worry recruited regions implicated in both negative self-referent processing and the processing of contextual fear early on in the regulation phase, and was the least successful strategy for regulating limbic responses to worry statements and subsequent distress.
Emotion suppression resulted in the lowest ratings of distress and successfully downregulated amygdala activation early in the regulation phase to a similar extent as emotion acceptance; however, suppression recruited different circuitry relative to acceptance to achieve these results. Notably, whereas the explicit regulation instructions for suppression were ‘don’t think and feel’, emotion suppression ironically strongly recruited regions implicated in both interoceptive awareness and the visceral experience of emotion (i.e. ‘feel’), and regions implicated in cognitive processing and control (i.e. ‘think’). Specifically, emotion suppression recruited significantly greater early phase anterior insula and right VLPFC (BA 47) activation relative to both worry and acceptance, yielding large effect sizes relative to acceptance and moderate effect sizes relative to worry. The anterior insula is associated with the integration of interoception and cognition (Menon and Uddin, 2010), and the right anterior VLPFC with reflexive reorienting and response inhibition (Levy and Wagner, 2011). Together, the VLPFC-anterior insula have been shown to form a fronto-insular functional node implicated in switching between salience and executive control networks (Goulden et al., 2014; Sridharan et al., 2008). Relating these functional roles to the findings here, these data suggest emotion suppression is associated with both greater interoceptive processing of and orienting away from salience during early phases of regulation. Orienting away interferes with further processing of stimulus-response associations and precludes the formation of new stimulus-response contingencies, in line with accounts that emotion suppression leads to continued disengagement from (or avoidance of) aversive stimuli (Campbell-Sills et al., 2006). Thus, whereas emotion suppression is effective at reducing subjective distress, it may ultimately serve as a maladaptive strategy through the perpetuation of avoidance.
Greater activation of the VLPFC was associated with ‘lesser’ distress following worry or suppression, yielding moderate to large effect sizes, but greater distress following regulation with emotion acceptance, yielding a moderate effect size. In addition, moderately greater amygdala-right VLPFC functional connectivity was found in emotion acceptance relative to both worry and emotion suppression. The association between VLPFC activation and distress ratings in worry and emotion suppression are consistent with the role of the VLPFC in reflexive reorienting discussed earlier and with existing studies of GAD, in which VLPFC recruitment may represent maladaptive or compensatory activation necessary to regulate worry-related anxiety (Monk et al., 2008; Etkin et al., 2009). However, the increased functional VLPFC-amygdala connectivity in emotion acceptance is in contrast to reduced VLPFC-amygdala connectivity found in GAD more generally (Monk et al., 2008; Etkin et al., 2009). This suggests emotion acceptance may better serve to overcome existing deficits in correlated VLPFC-amygdala activation during emotion regulation than emotion suppression or worry. Additionally, in light of non-significant differences in amygdala activation between emotion acceptance and suppression, these results suggest differential mechanisms through which cortical structures including the VLPFC are recruited to attain similar limbic regulatory goals, with ultimately differential behavioral outcomes, such as continued disengagement from aversive stimuli following suppression vs greater willingness to re-engage with aversive stimuli following acceptance (Campbell-Sills et al., 2006).
The extent to which the dACC was recruited during worry was significantly related to baseline worry symptom severity yielding a large effect size, such that greater severity was associated with less recruitment of this region. In addition, dACC was significantly more activated during late phase regulation with emotion acceptance than either worry or suppression, although with moderate effect sizes, and was strongly negatively correlated with distress ratings following worry. This suggests a potential key role for dACC in the adaptive regulation of worry-related anxiety in GAD, and a potential mechanism through which emotion acceptance, as a therapeutic strategy, may be helpful.
This finding is intriguing in light of a recent meta-analysis investigating structural and functional abnormalities across psychopathology (Goodkind et al., 2015). Using data from nearly 200 studies and over 7000 individuals with a range of diagnoses including schizophrenia, bipolar disorder, depression, obsessive compulsive disorder, generalized anxiety and addiction, converging grey matter loss was found across all diagnoses in the dACC and bilateral anterior insula. This suggests deficits in adaptive recruitment of dACC and anterior insula may represent specific transdiagnostic biomarkers of pathology. Indeed, in this study, both worry and emotion suppression were associated with significantly greater early anterior insula recruitment than emotion acceptance, but significantly less late dACC recruitment than emotion acceptance. Further, greater early activation was associated with reduced regulatory success across all three conditions. Thus, one mechanism by which emotion acceptance may exert ameliorating effects in the treatment of GAD is through normalization of activation along these nodes, an area for further research.
Increased late-phase dACC activation in the acceptance condition was found in regions that directly overlap with those reported in the extinction of contextual fear, and in particular the early stages of within-session extinction (Lang et al., 2009; Etkin et al., 2011). Although an emphasis has been placed on the role of subgenual and rostral ACC in the modulation of amygdala activation during extinction of cue conditioned fear in studies of human fear conditioning (Delgado et al., 2008; Milad et al., 2007; Phelps et al., 2004), studies examining the extinction of contextual fear have implicated a larger role for the dACC (Lang et al., 2009; Sehlmeyer et al., 2011; Maier et al., 2012). Contextual conditioning differs from classic cue conditioning in that the conditioned cue (CS) is unpaired with the unconditioned stimulus (US). Thus, unlike in cued conditioning where the US and CS are predictably paired, the onset of the US is unpredictable. This unpredictability leads to a chronic expectation of threat, and uncertainty about when or how that threat will come. Thus, whereas cued conditioning paradigms serve as strong analogs to fear states, contextual fear conditioning paradigms tap more closely into sustained anxiety states and are more aligned with the experience of generalized anxiety (Vansteenwegen et al., 2008). Although speculative given the small sample and only moderate effect sizes reported here, the results of the current study may provide some preliminary support for the overlap between emotion acceptance and extinction learning of this type of contextual fear.
The finding of increased dACC/dmPFC activation associated with emotion acceptance relative to worry or suppression also aligns somewhat with findings for cognitive reappraisal, which have shown a strong correlation between dACC activation during reappraisal and reductions in distress ratings, indicating reappraisal success (Berkman and Lieberman, 2009; Buhle et al., 2014). Existing studies of cognitive reappraisal additionally implicate DLPFC, VLPFC and dmPFC activation, coupled with decreased medial orbitofrontal and amygdala activation (Ochsner et al., 2002; Goldin et al., 2008; Buhle et al., 2014). In this study, regulation using emotion acceptance was associated with significantly stronger dmPFC-amygdala and VLPFC-amygdala functional connectivity and significantly less early amygdala activation relative to worry with moderate effect sizes, in line with findings related to cognitive reappraisal. However, regulation using emotion acceptance demonstrated significantly less recruitment of lateral PFC regions than regulation with suppression or worry. A recent study directly comparing emotion acceptance and cognitive reappraisal in a sample of remitted depression patients found the largest peak activation during emotion acceptance was the dACC, consistent with this study, and less DLPFC and frontal pole recruitment than cognitive reappraisal (Smoski et al., 2015). This suggests less reliance upon recruitment of lateral PFC regions during emotion acceptance may differentiate this strategy from cognitive reappraisal.
Emotion acceptance was associated with significantly less dmPFC (BA 6,8) and insula activation relative to worry and suppression and precuneus activation relative to worry, regions supporting self-referent processing and key nodes in the default mode network. These regions overlap with findings from existing studies of mindfulness, which have reported an overall ‘quieting’ effect of mindfulness on default mode regions associated with subjective awareness (Cavanna and Trimble, 2006; Farb et al., 2007; Herwig et al., 2010). This suggests one mechanism of acceptance may be the ‘interruption’ of self-focused, ruminative processing of emotionally salient information, perhaps facilitated by increased mindfulness.
There are several limitations to consider when interpreting these results. First, because the task in this study is asking participants to engage attention and thought in a particular way in the absence of objective measurement, it is impossible to know for sure what participants were doing while responding to the regulation instructions. Additionally, although a manipulation check assessing participants’ perceived success at engaging each regulation strategy was administered post-scan, we did not assess the extent to which participants utilized the target strategy more so than the others in each condition. However, given the significant differences found across conditions, it is likely the three regulation instructions led to distinctly different responses by the participants. Second, we employed relatively liberal statistical thresholds in our fMRI and gPPI analyses. Given that this is the first study to our knowledge that investigates the neural correlates of emotion acceptance as a regulation strategy in GAD, and our focus on a priori, empirically derived ROIs, we elected to take a less conservative approach to thresholding and multiple comparison corrections so as to control for Type-II error, which may have resulted in an inflation of Type-I error. As recently discussed in Eklund et al. (2016), current methods in fMRI research raise the risk of Type-I error to a degree which calls many fMRI results into scrutiny and question. In this study, we took precautions to attempt to limit the potential for erroneous errors that might have lead to Type-I error. However, we nevertheless applied relatively liberal thresholds to our data, in order to control for Type-II error. As such, the results herein should be considered as hypothesis generating, and are in need of further validation through replication. Third, we employed a block design, which inherently introduces issues of collinearity (Mumford et al., 2015). We attempted to minimize these effects through our condition randomization procedure, and by comparing across conditions for each time window rather than within conditions. However, due to the inability to fully control for time effects on collinearity using the current design, these results again should be interpreted with caution and are in need of replication. Additionally, the sample in this study was highly educated, average to high income, and primarily non-Hispanic Caucasian. Therefore, it is unclear whether the results found here would generalize to individuals from other backgrounds. The current study was also limited to female participants only, so it is unclear whether these results would be replicated in a male sample. Finally, it is notable that lower distress ratings following regulation with emotion acceptance only moderately predicted self-reported success at using emotion acceptance as a regulation strategy, whereas distress ratings significantly predicted success at engaging in worry or suppression. This may be related to the novelty of emotion acceptance as a regulation skill relative to worry or suppression. Unlike worry or suppression, which are common maladaptive regulation strategies associated with GAD, emotion acceptance was introduced as a new skill. Thus, the weaker relationship between self-perceived regulation success and levels of distress may reflect the need for more formal training in emotion acceptance to achieve beneficial effects.
In summary, we sought to examine the mechanisms of emotion acceptance as an alternate emotion regulation strategy to worry or emotion suppression in GAD. Regulating worry in GAD through the use of emotion acceptance successfully downregulated amygdala, insula and hippocampal activation and was associated with significantly lower recruitment of default-mode structures and lower ratings of distress relative to worry. The mechanisms of emotion acceptance in this study aligned most closely with mindful awareness and extinction learning, suggesting emotion acceptance may exert ameliorating effects in GAD through exposure to distress and an increase in present moment awareness, perhaps facilitating the updating of contingencies related to distress over uncertainty or unpredictable outcomes. Future laboratory studies are needed to more closely examine the relationship between emotion acceptance and extinction at the neural level, in order to determine if overlapping neural circuitry represents common or distinct mechanisms. This study provides a clinically relevant first step towards examining this potential mechanism. If a mechanism of emotion acceptance is through extinction learning, this lends support for the use of this strategy in a broader CBT framework in which exposure and extinction learning lie at the heart of therapeutic change.
Funding
This work was supported by a Predoctoral National Research Service Award from the National Institutes of Mental Health (Grant No. F31 MH084422) to K.E.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
References
- Andreescu C., Sheu L.K., Tudorascu D., Walker S., Aizenstein H. (2014). The ages of anxiety–differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. International Journal of Geriatric Psychiatry, 29(7), 704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, and American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association 75. [Google Scholar]
- Barlow D.H., Ellard K.K., Farchione C.P., Boisseau C.L., Allen L.B., Ehrereich-May J.T. (2010). Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders. New York: Oxford University Press. [Google Scholar]
- Berkman E.T., Lieberman M.D. (2009). Using Neuroscience to Broaden Emotion Regulation: Theoretical and Methodological Considerations. Social and Personality Psychology Compass, 3(4), 475–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Geraci M., Smith B.W., et al. (2012). Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry, 72(6), 476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K., Shaywitz J., Smith B.W., et al. (2008). Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. American Journal of Psychiatry, 165(9), 1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec T.D., Roemer L. (1995). Perceived functions of worry among generalized anxiety disorder subjects: distraction from more emotionally distressing topics?. Journal of Behavioral Therapy and Experimental Psychiatry, 26(1), 25–30. [DOI] [PubMed] [Google Scholar]
- Borkovec T.D., Alcain O., Behar E. (2004). Avoidance theory of worry and generalized anxiety disorder In Heimberg R.G., Turk C.L., Menin D.S., editors. Generalized Anxiety Disorder: Advances in Research and Practice, pp. 77–108. New York: Guilford Press. [Google Scholar]
- Brown T.A., Di Nardo P.A., Barlow D.H. (1994). Mini Anxiety Disorders Interview for DSM-IV (Mini-ADIS). Unpublished assessment interview.
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., Haier R.J., White N.S., et al. (2001). Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory, 75(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Campbell D.W., Sareen J., Paulus M.P., Goldin P.R., Stein M.B., Reiss J.P. (2007). Time-varying amygdala response to emotional faces in generalized social phobia. Biological Psychiatry, 62, 455–63. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L., Barlow D.H., Brown T.A., Hofmann S.G. (2006). Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behaviour Research and Therapy, 44(9), 1251–63. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–83. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nearing K.I., Ledoux J.E., Phelps E.A. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney C.M., Pizzagalli D.A. (2008). The cognitive consequences of emotion regulation: an ERP investigation. Psychophysiology, 45(3), 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo P.A., Brown T.A., Barlow D.H. (1994). Anxiety Disorders Interview Schedule for DSM-IV: Liftime Version (ADIS-IV-L). San Antonio TX: Psychological Corporation. [Google Scholar]
- Dugas M.J., Savard P., Gaudet A., et al. (2007). Can the components of a cognitive model predict the severity of generalized anxiety disorder?. Behavioral Therapy, 38(2), 169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard K.K., Farchione T.J., Barlow D.H. (2012). Relative effectiveness of emotion induction procedures and the role of personal relevance in a clincial sample: A comparison of films, images and music. Journal of Psychopathology and Behavioral Assessment, 34, 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Science, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361–72. [DOI] [PubMed] [Google Scholar]
- Farb N.A., Segal Z.V., Mayberg H., et al. (2007). Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience, 2(4), 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., et al. (2015). Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry, 72(4), 305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N., Khusnulina A., Davis, N. J.,. et al. (2014). The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage, 99, 180–90.. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Levenson R.W. (1997). Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology, 106(1), 95.. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. (2011). Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U., Kaffenberger T., Jancke L., Bruhl A.B. (2010). Self-related awareness and emotion regulation. Neuroimage, 50(2), 734–41. [DOI] [PubMed] [Google Scholar]
- Ives-Deliperi V.L., Solms M., Meintjes E.M. (2011). The neural substrates of mindfulness: an fMRI investigation. Social and Neuroscience, 6(3), 231–42. [DOI] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. (2011). Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224, 40–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S., Kroll A., Lipinski S.J., et al. (2009). Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. European Journal of Neuroscience, 29(4), 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S., Szalkowski A., Kamphausen S., et al. (2012). Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression?. PLoS One, 7(11), e50120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.B., Kraft R.A. (2003). An automated method for neuroanatomic and Cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Maldijian J.A., Laurenti P.J., Burdette J.B. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage, 21, 450–5. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage, 61, 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., et al. (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry, 64(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., Heimberg R.G., Turk C.L., Fresco D.M. (2002). Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychology: Science and Practice, 9(1), 85–90. [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5–6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. (1990). Development and validation of the Penn State Worry Questionnaire. Behavioral Research Therapy, 28(6), 487–95. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Wright C.I., Orr S.P., Pitman R.K., Quirk G.J., Rauch S.L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62(5), 446–54. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E., McClure E.B., et al. (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry, 163(6), 1091–7. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J.A., Poline J.-B., Poldrack R.A. (2015). Orthogonalization of regressors in fMRI models. PLos One, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.G., Zuellig A.R., Kachin K.E., et al. (2002). Preliminary reliability and validity of the Generalized Anxiety Disorder Questionnaire-IV: a revised self-report diagnostic measure of generalized anxiety disorder. Behavioral Therapy, 33, 215–33. [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. (2002). Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., LeDoux J.E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43(6), 897–905. [DOI] [PubMed] [Google Scholar]
- Roemer L., Orsillo S.M., Salters-Pedneault K. (2008). Efficacy of an acceptance-based behavior therapy for generalized anxiety disorder: evaluation in a randomized controlled trial. Journal of Consulting and Clinical Psychology, 76(6), 1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Levy I., Niv Y., LeDoux J.E., Phelps E.A. (2008). From fear to safety and back: reversal of fear in the human brain. Journal of Neuroscience 28(45), 11517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C., Dannlowski U., Schoning S., et al. (2011). Neural correlates of trait anxiety in fear extinction. Psychological Medicine, 41, 789–98. [DOI] [PubMed] [Google Scholar]
- Smoski M.J., Keng S.L., Ji J.L., Moore T., Minkel J., Dichter G.S. (2015). Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Social Cognitive and Affective Neuroscience, 10(9), 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105(34), 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazover N., Landeau B., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D., Iberico C., Vervliet B., Marescau V., Hermans D. (2008). Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biology and Psychology 77(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Vasey M.W., Borkovec T.D. (1992). A catastrophizing assessment of worrisome thoughts. Cognitive Therapy and Research, 16, 505–20. [Google Scholar]
- Ward B.D. (2002). AlphaSim. Bethesda, MD: National Institute of Mental Health. [Google Scholar]
- Whitfield-Gabrieli S. (2009). Artifact Detection Tools.
- Whitfield-Gabrieli S., Ford J.M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Ziv M., Goldin P.R., Jazaieri H., Hahn K.S., Gross J.J. (2013). Emotion regulation in social anxiety disorder: Behavioral and neural responses to three socio-emotional tasks. Biology of Mood and Anxiety Disorders, 3, 2–17. http://www.biolmoodanxietydisord.com/content/3/1/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.