Abstract
Schizophrenia is an etiologically and clinically heterogeneous disorder. Although neuroimaging studies have revealed brain alterations in schizophrenia, most studies have assumed that the disorder is a single entity, neglecting the diversity of alterations observed in the disorder. The current study sought to explore the distinct patterns of altered cortical thickness in patients with schizophrenia and healthy individuals using a data-driven approach. Unsupervised clustering using self-organizing maps followed by a K-means algorithm was applied to regional cortical thickness data in 108 schizophrenia patients and 121 healthy controls. After clustering, the clinical characteristics and cortical thickness patterns of each cluster were assessed. Unsupervised clustering revealed that a 6-cluster solution was the most appropriate in this sample. There was substantial overlap between the patterns of cortical thickness in schizophrenia patients and healthy controls, although the distributions of the patients and controls differed across the clusters. The patterns of altered cortical thickness in schizophrenia exhibited cluster-specific features; patients within a cluster exhibited the most extensive cortical thinning, particularly in the medial prefrontal and temporal regions, while those in other clusters exhibited reduced cortical thickness in the medial frontal region or temporal lobe. Furthermore, in the schizophrenia group, extensive cortical thinning was correlated with a higher dosage of antipsychotic medication, while preserved cortical thickness appeared to be linked to less negative symptoms. This data-driven neuroimaging approach revealed distinct patterns of cortical thinning in schizophrenia, possibly reflecting the etiological heterogeneity of the disorder.
Keywords: schizophrenia, self-organizing map, unsupervised learning, cortical thickness, heterogeneity
Introduction
Schizophrenia is an etiologically and clinically heterogeneous syndrome.1 Because the pathogenesis of schizophrenia has been linked to multiple factors, including genes, environment, and the interaction of these factors, the underlying pathophysiology in patients with the disorder is likely to vary.2 Furthermore, its clinical manifestation has been found to differ across patients; the age of onset, clinical symptoms, and prognosis can vary substantially from patient to patient.
Neuroimaging techniques, including magnetic resonance imaging (MRI), provide powerful tools for investigating the pathophysiology of schizophrenia.3 Neuroimaging studies have revealed structural and functional alterations in the brains of patients with schizophrenia.4,5 However, the majority of neuroimaging studies published to date, with rare exceptions,6–8 have treated schizophrenia as a singular disorder.6,7 Considering its etiological and clinical heterogeneity, approaches that assume the disorder is a single clinical entity risk overlooking the heterogeneity of brain alterations in schizophrenia.
One possible approach to address the heterogeneity of the disorder in a neuroimaging study is to divide schizophrenia patients into subgroups based on clinical characteristics. For instance, Nenadic et al6 reported different patterns of gray matter reduction and cortical thinning7 in 3 clinically differentiated subgroups, with negative, disorganized, or paranoid symptoms. However, this approach assumes that subgroups based on symptomatology share common underlying pathophysiological mechanisms, neglecting the possibility that a certain phenotype in schizophrenia may be underpinned by different pathophysiological mechanisms in different patients.
Data-driven methods, such as a self-organizing map (SOM) technique,9 provide a possible approach for handling the heterogeneity of schizophrenia. A SOM is a type of artificial neural network, in which unsupervised learning produces low-dimensional views of high-dimensional data. This technique enables the analysis of data without any clinical information such as diagnosis and symptoms. This method has been applied to neuroimaging studies of neurological/psychiatric disorders, including brain tumor,10–12 Parkinson’s disease,13 and autism spectrum disorder.14 Furthermore, a subsequent clustering procedure is able to reveal mathematically defined clusters of data. Here we applied unsupervised clustering procedures, for the first time, to regional cerebral cortical thickness neuroimaging data, to elucidate distinct patterns of cortical thinning in schizophrenia using a data-driven approach.
Methods
Participants and Assessments
A total of 229 subjects were investigated, comprising 108 schizophrenia patients and 121 healthy volunteers. Patients were recruited from hospitals in Kyoto, Japan, and met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV)15 criteria for schizophrenia, confirmed with the patient edition of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID).16 No patients had any comorbid DSM-IV Axis I disorder. The clinical symptoms of all but 2 patients were assessed using the Positive and Negative Syndrome Scale (PANSS).17 The subscales were calculated using the van der Gaag 5-factor model,18 considered one of the most validated models. At the time of scanning, all patients were being treated with antipsychotic medication, with a mean daily dose of 11.6 mg in haloperidol equivalents.19
Healthy age- and sex-matched controls were recruited from the same geographical area. Controls were screened with the non-patient edition of the SCID, confirming no history of psychiatric illness. We also confirmed that controls had no history of psychotic disorders among first-degree relatives. Predicted IQs of all participants were assessed using the Japanese Adult Reading Test.20 Exclusion criteria for all individuals included a history of head trauma, neurological illness, serious medical or surgical illness, or substance abuse. All participants were physically healthy at the time of scanning. After receiving a complete description of the study, all participants gave written informed consent. The study was approved by the Committee on Medical Ethics of Kyoto University.
MRI Data Acquisition
All participants underwent MRI scans using a 3-Tesla whole body scanner equipped with a receiver-only 8-channel phased-array head coil with a 40-mT/m gradient (Trio, Siemens). The scanning parameters of the T1-weighted 3-dimensional magnetization-prepared rapid gradient-echo (3D-MPRAGE) sequences were as follows: echo time (TE) = 4.38 ms; repetition time (TR) = 2000 ms; inversion time = 990 ms; field of view (FOV) = 225 × 240 mm; 240 × 256 matrix; resolution = 0.9375 × 0.9375 × 1.0 mm3; and 208 total axial sections without intersection gaps.
Preprocessing of MRI Data
Cortical thickness analysis in the whole brain was conducted using a surface-based approach using FreeSurfer tools (version 5.0.0; http://surfer.nmr.harvard.edu).21–23 The 3D-MPRAGE images were used to calculate the thickness of the cerebral cortex throughout the cortical mantle. Briefly, the processing stream included a Talairach transform of each of the subject’s native brain, removal of non-brain tissue, and segmentation of grey matter (GM)/white matter (WM) tissue. The GM/WM boundary was tessellated to generate multiple vertices across the whole brain. The cortical surface of each hemisphere was inflated to an average spherical surface to locate the pial surface and the GM/WM boundary. The entire cortex of each subject was visually inspected, and topological defects were corrected manually, blind to subject identities. Cortical thickness was computed as the shortest distance between the pial surface and the GM/WM boundary at each vertex across the cortical mantle. The mean cortical thickness of each of 68 regions was computed using FreeSurfer software.24 The effects of age and sex were regressed out of the data prior to SOM analysis. Finally, the corrected regional mean cortical thickness data (68 components per subject) of the whole sample were extracted as input vectors for SOM analysis.
Unsupervised Clustering
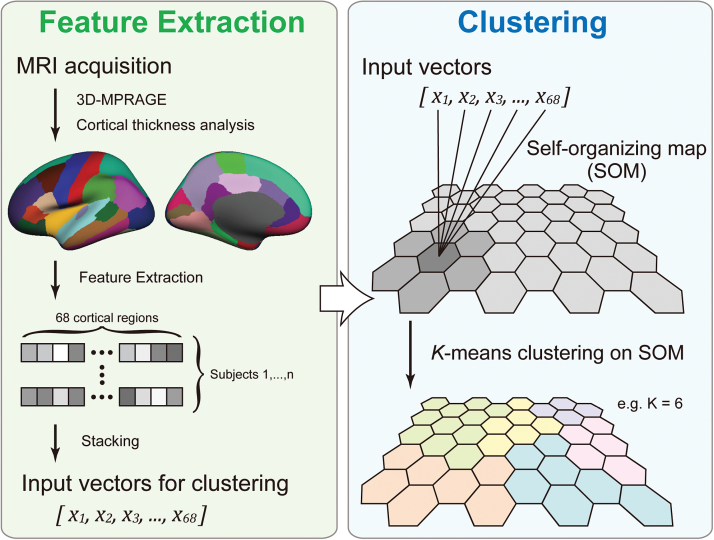
We aimed to reveal distinct patterns of regional cerebral cortical thickness. However, it is unknown how many patterns might exist in a given sample. To achieve the optimal estimation of the number of clusters, we applied a 2-level unsupervised clustering approach using SOM9 and the K-means++ algorithm25 for unsupervised clustering.10,11 First, the input vectors (ie, 68 regional mean cortical thickness per subject) were clustered into a much larger than expected number of clusters, defined as “protoclusters,” using SOM. The protoclusters were then classified into the expected number of clusters, defined as “clusters,” using K-means++. We repeated this step to achieve the best clustering result using cluster numbers from 3 to 9, the number of estimated subgroups of the sample. The clustering validity was examined using the Silhouette Index.26 The procedures are summarized in figure 1, with further details provided in supplementary materials. These algorithms were implemented using in-house software that enables analysis by SOM followed by K-means++.10,11
Fig. 1.
Procedure for magnetic resonance imaging (MRI) processing and unsupervised clustering using Self-organizing Map (SOM) analysis and K-means algorithm.
Statistical Analyses
Chi-square/Fisher’s tests (for categorical variables), and ANOVA or Student’s t tests (for continuous variables) were used to investigate the demographic and clinical differences between clusters. The Bonferroni correction was performed when appropriate. To determine the patterns of altered cortical thickness in schizophrenia patients in each cluster, we performed regional cortical thickness analyses using FreeSurfer. In these analyses, the cortical thickness data of patients in a cluster were compared with those of all the healthy controls, as follows: the thickness value at each vertex for each subject was mapped to the surface of an average brain template, and the cortical map of each subject was smoothed with a Gaussian kernel of 10-mm full-width at half-maximum. The general linear model was applied at each vertex in the whole brain to identify brain regions in which schizophrenia patients exhibited differences in cortical thickness compared with controls.
Results
Demographic and Clinical Characteristics
The demographic and clinical variables of participants are summarized in table 1. The schizophrenia group consisted of mainly chronic patients with a mean duration of illness of 12.2 years (±8.7 [SD]) with relatively mild symptoms.
Table 1.
Demographic and Clinical Characteristics of Participants
| Schizophrenia | Control | P Value | |
|---|---|---|---|
| N | 108 | 121 | |
| Female/male | 54/54 | 49/72 | .10 |
| Age (mean ± SD) | 37.4 ± 9.4 | 35.4 ± 9.0 | .11 |
| Handedness (right/left) | 101/7 | 117/4 | .21 |
| Predicted IQ (mean ± SD) | 103 ± 10.1 | 110 ± 8.3 | <.001 |
| PANSS subscores (mean ± SD) | |||
| Positive symptoms | 11.4 ± 4.6 | N/A | N/A |
| Negative symptoms | 17.7 ± 6.3 | N/A | N/A |
| Disorganization | 9.3 ± 3.1 | N/A | N/A |
| Emotional distress | 8.4 ± 3.0 | N/A | N/A |
| Excitement | 5.8 ± 2.0 | N/A | N/A |
| Duration of illness (y) | 12.2 ± 8.7 | N/A | N/A |
| Medication, HP equivalent | 11.6 ± 8.6 | N/A | N/A |
Note: PANSS, the Positive and Negative Syndrome Scale; HP, haloperidol; N/A, not available.
Clustering with SOM and K-means Algorithm
The map of the initial output is shown in supplementary figure S1A. According to the Silhouette Index, with the number of cluster explorations ranging from 3 to 9, SOM analysis followed by K-means clustering divided the output nodes into 6 subgroups (clusters 1 to 6; supplementary figures S1B and S1C).
Characteristics of Subjects in Each Cluster
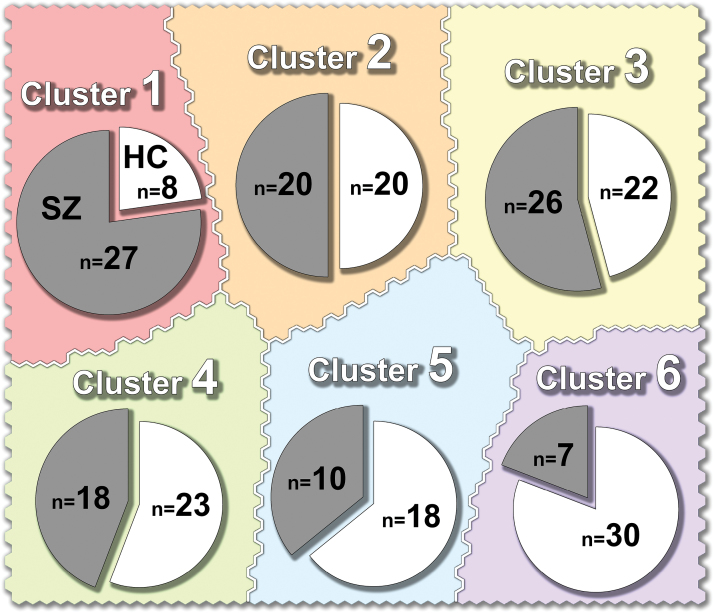
Cluster 1 included significantly more patients with schizophrenia than healthy controls (P = .001) (figure 2). In contrast, cluster 6 included more controls than patients (P < .001). In the control group, there were no significant differences between clusters in age, sex, or predicted IQ, although cluster 6 included more males (supplementary table S1). To increase statistical power, cluster 5 and 6 were combined prior to analysis of the clinical and neuroimaging data in the schizophrenia group (cluster 5/6). There were no significant differences in age, sex, duration of illness, medication, or predicted IQ of the patients in cluster 5/6.
Fig. 2.
Numbers of patients and controls in each cluster. SZ, patients with schizophrenia; HC, healthy controls.
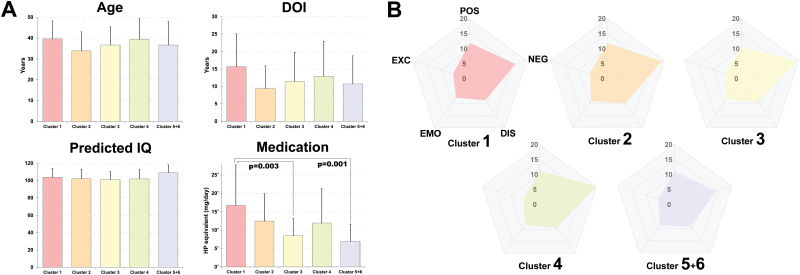
In the schizophrenia group, there were no significant effects of cluster on age, sex, duration of illness, or predicted IQ. However, there was a significant difference in medication between clusters (P = .001) (figure 3A). Post hoc analysis showed that the patients with schizophrenia in cluster 1 were receiving significantly higher doses of antipsychotics compared with those in clusters 3 and 5/6 (P = .003 and .001, respectively). The clinical characteristics of the clusters are summarized in supplementary table S1.
Fig. 3.
Clinical characteristics of the patients with schizophrenia in each cluster. The patients in Cluster 1 received higher doses of antipsychotics (A), while the patients in Cluster 5/6 had milder negative symptoms (B). DOI, duration of illness; HP, haloperidol; POS, positive symptoms; NEG, negative symptoms; DIS, disorganization; EMO, emotional distress; EXC, excitement. Haloperidol equivalents were calculated according to the practice guidelines for the treatment of patients with schizophrenia.19
We investigated the clinical symptoms in each cluster. The results revealed that across the clusters, PANSS subscores showed similar patterns (figure 3B). However, clusters affected the subscore of negative symptoms (P = .023), although this effect did not survive multiple comparisons correction. Thus, the results revealed a tendency towards patients in cluster 5/6 exhibiting lower negative symptoms subscores (supplementary table S1).
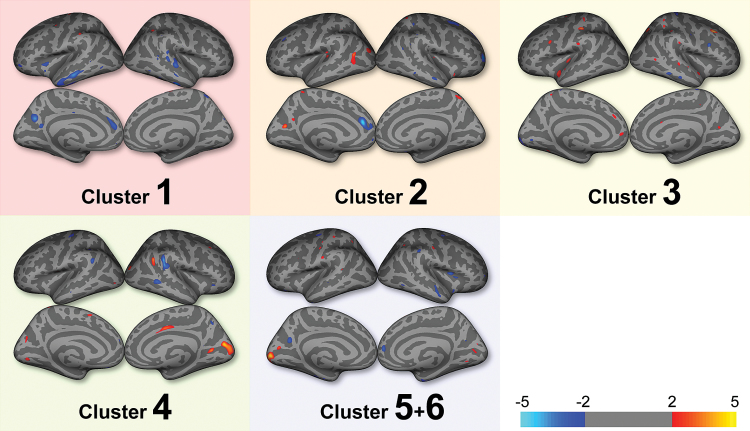
Comparisons of cortical thickness revealed cluster-specific patterns in the patients with schizophrenia compared with the healthy controls (figure 4). The patients in cluster 1 exhibited the most extensive decreases in cortical thickness (supplementary figure S2), especially in the medial prefrontal and temporal cortices (ie, left anterior cingulate gyrus, left orbitofrontal cortex, left middle temporal gyrus, and right superior, middle, and inferior temporal gyri). Reduced cortical thickness in the patients in cluster 2 was evident in the medial frontal region (ie, left anterior cingulate gyrus, and right superior frontal gyrus), and in the temporal lobe (ie, right middle temporal gyrus) of the patients in cluster 4. In contrast, the schizophrenia patients in clusters 3 and 5/6 exhibited little reduction in cortical thickness.
Fig. 4.
Comparisons of cortical thickness between patients with schizophrenia and controls. Color bar indicates −log(10) P value, uncorrected.
Discussion
In the current study, unsupervised clustering using SOM followed by K-means analysis revealed that participants could be divided into 6 subgroups according to observed patterns of cortical thickness. To our knowledge, this is the first neuroimaging study using a data-driven approach to reveal subgroups in schizophrenia independent of clinical information, such as positive and negative symptoms. This approach demonstrated that there are multiple distinct patterns of cortical thinning in schizophrenia. In addition, extensive cortical thinning was found to be related to higher doses of antipsychotic medication, while preserved cortical thickness may be linked to less negative symptoms.
The results revealed substantial overlap in the patterns of cortical thickness between the schizophrenia patients and healthy controls. Our aim was not to explore differences in cortical thickness between groups to discriminate schizophrenia patients from healthy individuals, but to assess the patterns of cortical thinning using a data-driven approach. The finding of a difference between patients with schizophrenia and healthy controls was not surprising, because brain structural alterations in schizophrenia have been reported to be subtle.27 Nevertheless, the distributions of the patients and controls were different across subgroups; the subgroup with the most extensive cortical thinning had the highest patient/control ratio. Furthermore, the subgroup with the most preserved cortical thickness had a low patient/control ratio. This trend suggests that the pathophysiology of schizophrenia is indeed linked to cortical thinning, in accord with previous reports.28,29
The unsupervised clustering of cortical thickness data revealed distinct patterns of cortical thinning in schizophrenia. The patients in cluster 1 (in which the patient/control ratio was highest) exhibited the most extensive cortical thinning in the medial prefrontal and temporal regions, while those in cluster 2 exhibited reduced cortical thickness in the medial frontal region, and those in cluster 4 exhibited reductions in the temporal lobe. Alterations in the medial prefrontal areas have been previously reported in schizophrenia27 and have been associated with positive symptoms5 and altered emotional processing in the disorder.30 Volume reduction in the temporal lobe has been shown in schizophrenia,27 and has been linked to auditory hallucinations.31 However, in the current study, the remaining patients with schizophrenia (ie, clusters 3 and 5/6) exhibited relatively preserved cortical thickness. Despite these cluster-specific patterns of cortical thinning in schizophrenia, the clinical characteristics, except for negative symptoms, did not substantially differ between the clusters. This implies that even if the phenotypes in schizophrenia are similar, the underlying pathophysiology may differ.
The patients with schizophrenia in cluster 1 (which exhibited the most extensive cortical thinning) received higher doses of medication on average, although the clinical severity in this group did not significantly differ from those of patients in each of the other clusters. This finding is in accord with the findings of recent studies reporting that antipsychotic medication results in brain volume reduction.32–34 However, it should be noted that in our sample, the patients with extensive cortical thinning may have required higher doses of antipsychotic medication to control their symptoms. Meanwhile, the patients in cluster 5/6 had milder negative symptoms and relatively intact cortical thickness. These results appear to be in line with the finding that negative symptoms are associated with extensive cortical thinning7 and the reported link between preserved neuropsychological abilities in schizophrenia and limited cortical thinning.8 Future longitudinal studies are needed to examine the temporal relationships between clinical variables and patterns of cortical thickness.
The current study contained several limitations that should be considered. First, the patients with schizophrenia in this study were all Japanese, and all had mild and stable symptoms. In addition, we did not confirm our findings with an independent sample. Thus, caution should be exercised in generalizing the results to the general population of patients with schizophrenia. Future replication studies with a more widely distributed and larger sample population are warranted to confirm these findings. Second, we did not include any genetic or cognitive function data from the schizophrenia patients in this study. Although we found that some clusters were linked to clinical variables (ie, dose of medication and negative symptoms), the underlying mechanisms of the altered patterns of cortical thickness were not investigated in depth. Finally, we did not assess the specific characteristics of the control subjects in terms of genetics, cognition or life style, all of which can influence cortical thickness. Some individuals, especially in cluster 1, exhibited extensive cortical thinning, but did not develop schizophrenia. Further investigation of these individuals may provide information about protective factors influencing the development of schizophrenia.
The current findings appear to provide support for the hypothesis of etiological heterogeneity in schizophrenia. These results also raise caution against treating a sample of schizophrenia patients as a homogeneous group in neuroimaging studies. Clinically defined subtypes of schizophrenia (eg, paranoid and disorganized types) have been eliminated in DSM-5 because of their limited diagnostic stability, low reliability, and poor validity,35 despite the fact that the disorder is heterogeneous. Thus, novel approaches are needed to investigate the heterogeneity of schizophrenia. The current study illustrates how data-driven neuroimaging approaches can be used to tackle these issues.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This study was supported in part by Grants-in-Aid for Scientific Research C (24591706, 15K09825, 15K09920), B (15H04893), on Innovative Areas (23120009, 16H06572, 16H06402), Grants-in-Aid for Young Scientists B (16K19763) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); and a grant from Takeda Science Foundation and Sumitomo Dainippon Pharma; Impulsing Paradigm Change through Disruptive Technologies Program (ImPACT, 15808865), Japan Science and Technology Agency (JST). Part of this study resulted from research of the Development of BMI Technologies for Clinical Application carried out under the Strategic Research Program for Brain Sciences by MEXT. These agencies had no further role in the study design, collection, analysis or interpretation of the data, the writing of the report, or in the decision to submit the paper for publication.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 2. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Linden DE. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73:8–22. [DOI] [PubMed] [Google Scholar]
- 4. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goghari VM, Sponheim SR, MacDonald AW III. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34:468–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nenadic I, Sauer H, Gaser C. Distinct pattern of brain structural deficits in subsyndromes of schizophrenia delineated by psychopathology. Neuroimage. 2010;49:1153–1160. [DOI] [PubMed] [Google Scholar]
- 7. Nenadic I, Yotter RA, Sauer H, Gaser C. Patterns of cortical thinning in different subgroups of schizophrenia. Br J Psychiatry. 2015;206:479–483. [DOI] [PubMed] [Google Scholar]
- 8. Cobia DJ, Csernansky JG, Wang L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr Res. 2011;133:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohonen TW. Self-organizing Maps. New York, NY: Springer; 1995. [Google Scholar]
- 10. Inano R, Oishi N, Kunieda T et al. Voxel-based clustered imaging by multiparameter diffusion tensor images for glioma grading. Neuroimage Clin. 2014;5:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inano R, Oishi N, Kunieda T et al. Visualization of heterogeneity and regional grading of gliomas by multiple features using magnetic resonance-based clustered images. Sci Rep. 2016;6:30344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mei PA, de Carvalho Carneiro C, Fraser SJ, Min LL, Reis F. Analysis of neoplastic lesions in magnetic resonance imaging using self-organizing maps. J Neurol Sci. 2015;359:78–83. [DOI] [PubMed] [Google Scholar]
- 13. Singh G, Samavedham L. Unsupervised learning based feature extraction for differential diagnosis of neurodegenerative diseases: a case study on early-stage diagnosis of Parkinson disease. J Neurosci Methods. 2015;256:30–40. [DOI] [PubMed] [Google Scholar]
- 14. Wiggins JL, Peltier SJ, Ashinoff S et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011;1380:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, Administration Booklet. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 17. Kay SR. Positive and Negative Syndrome Scale (PANSS): Technical Manual. New York, NY: Multi-Health Systems; 2006. [Google Scholar]
- 18. van der Gaag M, Cuijpers A, Hoffman T et al. The five-factor model of the Positive and Negative Syndrome Scale I: confirmatory factor analysis fails to confirm 25 published five-factor solutions. Schizophr Res. 2006;85:273–279. [DOI] [PubMed] [Google Scholar]
- 19. Lehman AF, Lieberman JA, Dixon LB et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56. [PubMed] [Google Scholar]
- 20. Matsuoka K, Kim Y. Japanese adult reading test (JART). Tokyo: Shinko-Igaku Publishers; 2006. [Google Scholar]
- 21. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 22. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 23. Sasamoto A, Miyata J, Kubota M et al. Global association between cortical thinning and white matter integrity reduction in schizophrenia. Schizophr Bull. 2014;40:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desikan RS, Segonne F, Fischl B et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 25. Arthur D, Vassilvitskii S. k-means plus plus: The Advantages of Careful Seeding. Proceedings of the Eighteenth Annual Acm-Siam Symposium on Discrete Algorithms Philadelphia, PA: Society for Industrial and Applied Mathematics; 2007:1027–1035. [Google Scholar]
- 26. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53–65. [Google Scholar]
- 27. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. [DOI] [PubMed] [Google Scholar]
- 28. Kuperberg GR, Broome MR, McGuire PK et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. [DOI] [PubMed] [Google Scholar]
- 29. Rimol LM, Hartberg CB, Nesvag R et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. [DOI] [PubMed] [Google Scholar]
- 30. Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res. 2012;137:169–173. [DOI] [PubMed] [Google Scholar]
- 32. Lieberman JA, Tollefson GD, Charles C et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. [DOI] [PubMed] [Google Scholar]
- 33. Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Psychiatric Association. Highlights of Changes from DSM-IV-TR to DSM-5 2013. http://www.dsm5.org/Documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf Accessed November 30, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.