Abstract
Objective
To assess the extent to which the trajectories of intellectual, academic achievement, executive functioning, attention, working memory, and emotion recognition tests will be predictive of psychosis in young adults with 22q11.2 deletion syndrome (22q11DS).
Methods
Eighty-two participants with 22q11DS were assessed for psychiatric disorders and neuropsychological functioning with validated instruments. Siblings and community controls were employed as comparison groups.
Results
Individuals with 22q11DS differed significantly from siblings and controls in longitudinal trajectories of visual and auditory working memory as well as academic achievement. Longitudinal trajectories of cognitive set shifting, reading decoding, and emotion recognition predicted the presence of positive symptoms of psychosis in early adulthood. Cognitive set shifting improved at a slower rate for individuals with 22q11DS + psychosis than those without psychosis. Emotion recognition increased steadily in individuals without psychosis, whereas for those with psychosis, scores increased until approximately 15 years of age, at which point they began to decrease rapidly. A similar, but more subtle effect, was seen for reading decoding.
Conclusions
Our data are the first to go beyond IQ assessments in assessing longitudinal neuropsychological outcomes and risk for psychosis in 22q11DS. Individuals with 22q11DS who developed psychotic symptoms improved less appreciably and continued to demonstrate difficulties with cognitive flexibility relative to individuals with 22q11DS who did not have psychotic symptoms. Individuals with 22q11DS who developed psychosis had weaker reading skills in childhood and, after an initial improvement into adolescence, these individuals with psychosis had a decline in reading skills. In 22q11DS, cognitive deficits are both (a) traits that are preexisting and raise the risk for psychosis and (b) associated with the onset of psychotic symptoms. Future research should consider the extent to which cognitive set shifting and reading decoding are related to the Verbal IQ declines observed in the 22q11DS population.
Keywords: 22q11.2 deletion syndrome (22q11DS), psychosis, longitudinal, neuropsychology, developmental
Predicting Cognition and Psychosis in Young Adults With 22q11.2 Deletion Syndrome
22q11.2 deletion syndrome (22q11DS; also known as velo-cardio-facial syndrome) is a relatively common multiple anomaly syndrome, with an estimated population prevalence of 1:1000 live births.1 Caused by a deletion of the q11.2 band of 1 copy of chromosome 22, the syndrome affects roughly 40–50 genes and is associated with a characteristic facial appearance, cardiac and palatal anomalies, hypocalcemia, immune deficiencies, cognitive impairments, and psychiatric disorders.2 Intelligence ranges from intellectual disability to the average range with mean IQ scores generally in the borderline range (low 70s). A longitudinal decline in Full-Scale IQ, especially verbal abilities in adolescence, is predictive of schizophrenia,3 a disorder that affects up to 41% of adults with 22q11DS.4 As a disorder that is identified very early in life and presents such an ultra-high risk for schizophrenia, 22q11DS provides a unique opportunity to study risk factors for psychosis.
Executive functioning, attention, and working memory are cognitive domains that are impaired often in 22q11DS.5 Longitudinal declines in these cognitive domains have also been identified as predictive of psychosis in idiopathic schizophrenia.6–9 While existing research has considered how IQ changes predict psychosis in 22q11DS,3,10–12 most of these follow-up periods have been 2- to 3-year intervals and terminating in adolescence. Likewise, very little research has considered how other domains of cognition beyond IQ predict psychosis. For these reasons, a longitudinal study that (a) follows youth for 9 years and (b) assesses domains other than IQ would fill voids in the literature.
In addition to the novelty of this topic, there is also clear clinical significance to this line of investigation. Executive functioning, attention, and working memory are cognitive abilities that can be improved with intervention both in the 22q11DS13,14 and non-22q11DS15,16 populations and are thought to underlie performance on IQ tests.17 Thus, in addition to understanding how IQ changes are related to psychosis in 22q11DS, it is also important to understand the developmental trajectory of executive functioning, attention, and working memory in individuals with this ultra-high-risk condition for schizophrenia.
Based upon findings in idiopathic schizophrenia, we hypothesize that longitudinal declines in IQ, academic achievement, executive functioning, attention, and working memory will be predictive of psychosis in young adults with 22q11DS. A second and related aim of the study is to compare longitudinal trajectories of IQ, academic achievement, executive functioning, attention, and working memory in 22q11DS relative to siblings and control participants. Based upon longitudinal declines reported in IQ (generally half of a SD),3 we hypothesize that these additional cognitive domains will decline half of a SD in individuals with 22q11DS. Given the stability of these domains in the general population,18–20 we hypothesize that these additional cognitive domains will remain stable in our siblings and community control participants.
Methods
Participants
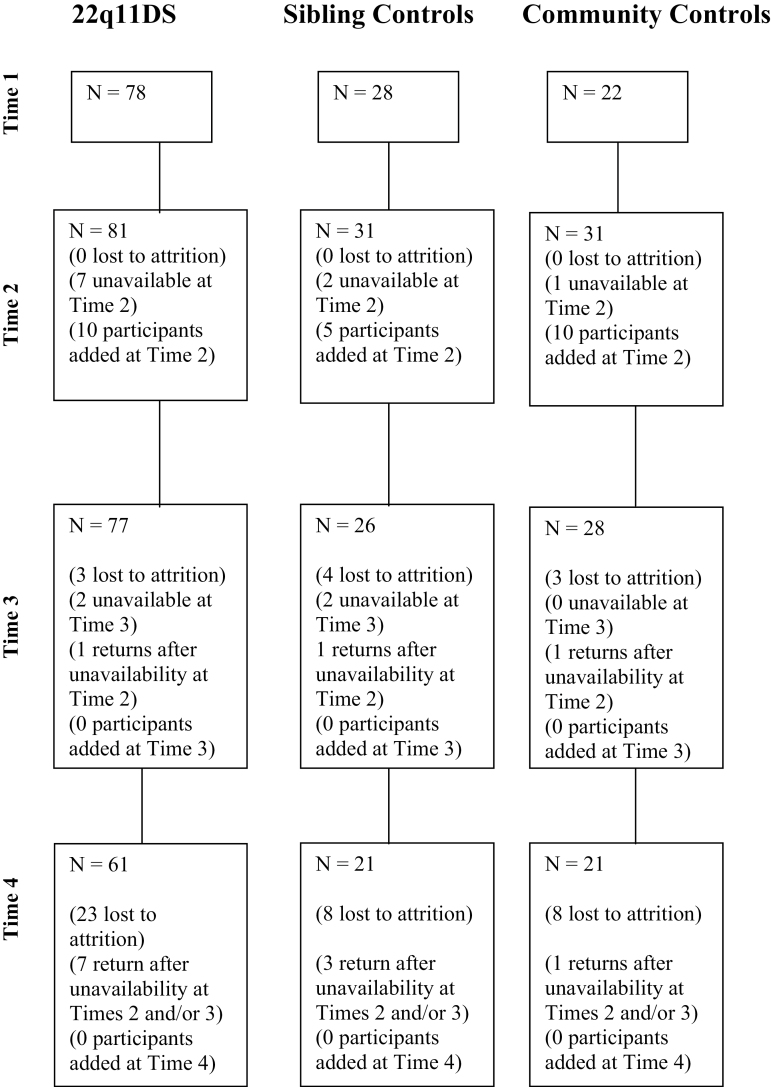
Youth with 22q11DS were recruited from the Center for the Diagnosis, Treatment, and Study of VCFS at the State University of New York Upstate Medical University. Only youth with a fluorescent in situ hybridization-confirmed deletion of 22q11.2 were included in the sample. Our community control participants were recruited from local public schools. Our sibling control group consisted of siblings of participants with 22q11DS. Siblings were included to control for environmental and other inherited factors other than 22q11.2 deletion that may affect cognitive and psychiatric functioning. Youth with an identifiable genetic disorder (other than 22q11DS) or youth with an identifiable neurological condition (eg, traumatic brain injury, preterm birth) that is known to affect cognitive or psychiatric function were excluded from participation. Please see figure 1 for our flow diagram of participants.
Fig. 1.
Flow diagram of participants.
As noted in table 1, there were no differences in age, F(8, 142) = 0.99, P = .442, or gender, χ2 (df = 5) = 0.72, P = .847, between the 3 groups at any of the 4 time points. An independent samples t test indicated that there were no differences in attrition between our 3 groups, t2 = 2.89, P = .307. Furthermore, participants lost to follow-up at Time 4 did not differ from those who did follow-up on any relevant Time 1 sociodemographic measures including participant age, gender, and socioeconomic status. In addition, participants lost to follow-up did not differ from those who did follow-up on any relevant Time 1 psychiatric or cognitive variables. Thus, those participants who completed Time 4 assessments appear representative of the broader Time 1 sample.
Table 1.
Participant Data
| Control | Sibling Control | 22q11DS | |
|---|---|---|---|
| Time 1 N/mean age (SD) | 22/11.6 (2.0) | 28/12.0 (2.0) | 78/11.9 (2.1) |
| Time 2 N/mean age (SD) | 31/15.2 (1.6) | 31/15.3 (1.8) | 81/14.9 (2.2) |
| Time 3 N/mean age (SD) | 28/17.5 (1.4) | 26/18.5 (1.7) | 77/18.0 (2.2) |
| Time 4 N/mean age (SD) | 21/20.6 (1.4) | 21/21.9 (1.7) | 61/21.2 (2.2) |
| Gender (F/M) | 14/18 | 17/16 | 42/46 |
| Mean (SD) | |||
| Time 1 Verbal IQ | 95.5 (13.6) | 103.6 (14.5) | 73.6 (14.6)*** |
| Time 2 Verbal IQ | 99.4 (12.8) | 99.2 (16.3) | 72.7 (14.5)*** |
| Time 3 Verbal IQ | 104.6 (18.6) | 108.9 (16.6) | 75.7 (13.4)*** |
| Time 4 Verbal IQ | 102.8 (14.0) | 106.2 (19.6) | 76.4 (12.0)*** |
| Time 1 Performance IQ | 97.7 (14.8) | 107.9 (15.9) | 71.4 (11.6)*** |
| Time 2 Performance IQ | 102.0 (14.2) | 107.3 (17.2) | 70.5 (14.2)*** |
| Time 3 Performance IQ | 106.5 (18.0) | 112.2 (16.8) | 72.6 (12.5)*** |
| Time 4 Performance IQ | 108.3 (11.4) | 115.2 (18.9) | 75.6 (11.5)*** |
| Time 1 Full-Scale IQ | 96.3 (14.3) | 105.9 (15.5) | 70.2 (13.5)*** |
| Time 2 Full-Scale IQ | 100.4 (12.5) | 103.3 (17.2) | 69.2 (14.6)*** |
| Time 3 Full-Scale IQ | 106.0 (18.2) | 111.4 (16.8) | 72.2 (13.8)*** |
| Time 4 Full-Scale IQ | 105.6 (12.5) | 111.2 (20.9) | 74.2 (12.5)*** |
| Time 1 WIAT-II Reading | 90.3 (11.1) | 100.9 (15.7) | 77.5 (15.9)*** |
| Time 2 WIAT-II Reading | 95.2 (16.1) | 99.1 (14.5) | 80.2 (16.8)*** |
| Time 3 WIAT-II Reading | 99.7 (17.2) | 105.6 (16.9) | 78.5 (17.8)*** |
| Time 1 WIAT-II Math | 89.3 (14.0) | 99.9 (16.6) | 70.9 (17.0)*** |
| Time 2 WIAT-II Math | 91.8 (16.8) | 98.7 (17.5) | 66.8 (19.8)*** |
| Time 3 WIAT-II Math | 93.6 (14.7) | 99.1 (18.2) | 62.9 (19.0)*** |
| Time 1 WIAT-II Total | 88.8 (11.3) | 99.9 (16.2) | 73.2 (15.0)*** |
| Time 2 WIAT-II Total | 93.6 (17.1) | 98.0 (16.4) | 72.9 (16.6)*** |
| Time 3 WIAT-II Total | 98.9 (17.2) | 104.8 (18.8) | 70.9 (17.0)*** |
| Time 1 CVLT List A Total T score | 48.5 (11.0) | 51.7 (12.6) | 37.2 (11.5)*** |
| Time 2 CVLT List A Total T score | 47.6 (13.5) | 45.6 (12.5) | 33.1 (15.0)*** |
| Time 3 CVLT List A Total T score | 45.2 (14.1) | 41.2 (17.7) | 23.3 (14.9)*** |
| Time 4 CVLT List A Total T score | 36.4 (15.4) | 38.4 (17.1) | 15.6 (12.2)*** |
| Time 1 CVLT List A Trial 1 Standard Score | −0.3 (0.8) | 0.0 (0.9) | −0.8 (1.0)*** |
| Time 2 CVLT List A Trial 1 Standard Score | −0.3 (1.0) | −0.5 (0.9) | −1.1 (1.3)*** |
| Time 3 CVLT List A Trial 1 Standard Score | −0.4 (1.1) | −1.0 (1.4) | −1.7 (1.4)*** |
| Time 4 CVLT List A Trial 1 Standard Score | −0.8 (1.1) | −0.8 (1.0) | −2.4 (1.1)*** |
| Time 1 CVLT List A Trial 5 Standard Score | −0.1 (1.1) | 0.3 (1.1) | −1.0 (1.3)*** |
| Time 2 CVLT List A Trial 5 Standard Score | −0.4 (1.5) | −0.5 (1.5) | −1.7 (1.7)*** |
| Time 3 CVLT List A Trial 5 Standard Score | −0.9 (1.8) | −1.2 (1.9) | −2.5 (2.1)*** |
| Time 4 CVLT List A Trial 5 Standard Score | −2.1 (1.7) | −1.6 (1.7) | −3.7 (1.7)*** |
| Time 1 CVLT List B Standard Score | −0.2 (0.8) | −0.4 (0.9) | −0.8 (1.1)*** |
| Time 2 CVLT List B Standard Score | −0.7 (1.1) | −0.6 (0.8) | −1.3 (0.9)*** |
| Time 3 CVLT List B Standard Score | −0.5 (1.0) | −1.1 (1.2) | −1.7 (1.0)*** |
| Time 4 CVLT List B Standard Score | −1.1 (0.9) | −1.1 (0.8) | −1.9 (1.0)*** |
| Time 1 GDS Vigilance Task Omission Error z score | −1.5 (3.0) | 0.0 (1.4) | −2.2 (3.7)*** |
| Time 2 GDS Vigilance Task Omission Error z score | 0.0 (1.4) | 0.3 (0.8) | −1.3 (3.2)*** |
| Time 3 GDS Vigilance Task Omission Error z score | 0.0 (0.8) | 0.3 (0.7) | −0.6 (1.7)*** |
| Time 4 GDS Vigilance Task Omission Error z score | 0.5 (0.7) | 0.5 (0.7) | −0.8 (2.0)*** |
| Time 1 GDS Vigilance Task Commission Error z score | −1.9 (3.6) | −0.1 (1.5) | −3.0 (5.5)*** |
| Time 2 GDS Vigilance Task Commission Error z score | −0.6 (1.8) | −0.1 (0.9) | −2.2 (4.3)*** |
| Time 3 GDS Vigilance Task Commission Error z score | −0.1 (0.6) | −0.1 (0.6) | −1.4 (3.2)*** |
| Time 4 GDS Vigilance Task Commission Error z score | 0.1 (0.2) | −0.1 (0.6) | −1.8 (3.2)*** |
| Time 2 GDS Distractibility Task Omission Error z score | −0.1 (1.1) | 0.3 (0.8) | −1.2 (2.0)*** |
| Time 3 GDS Distractibility Task Omission Error z score | −0.4 (0.9) | −0.2 (0.6) | −1.0 (1.4)*** |
| Time 4 GDS Distractibility Task Omission Error z score | 0.0 (0.5) | −0.2 (0.8) | −1.6 (2.0)*** |
| Time 2 GDS Distractibility Task Commission Error z score | −1.9 (6.5) | −0.1 (0.9) | −4.8 (9.1)*** |
| Time 3 GDS Distractibility Task Commission Error z score | −0.5 (0.7) | −0.3 (0.6) | −1.2 (2.0)*** |
| Time 4 GDS Distractibility Task Commission Error z score | 0.0 (0.5) | −0.2 (0.9) | −1.7 (2.9)*** |
| Time 1 WCST Perseverative Error Standard Score | 92.8 (17.3) | 95.4 (14.0) | 71.8 (19.2)*** |
| Time 2 WCST Perseverative Error Standard Score | 100.7 (18.1) | 107.7 (14.8) | 85.8 (16.6)*** |
| Time 3 WCST Perseverative Error Standard Score | 108.4 (16.9) | 111.4 (16.4) | 89.7 (16.8)*** |
| Time 4 WCST Perseverative Error Standard Score | 109.1 (9.8) | 111.4 (17.5) | 86.1 (15.3)*** |
| Time 1 WCST Non-Perseverative Error Standard Score | 91.4 (15.4) | 91.2 (13.7) | 83.8 (15.8)*** |
| Time 2 WCST Non-Perseverative Error Standard Score | 98.3 (18.9) | 100.4 (13.1) | 84.4 (15.1)*** |
| Time 3 WCST Non-Perseverative Error Standard Score | 105.0 (15.3) | 103.0 (10.7) | 92.3 (14.4)*** |
| Time 4 WCST Non-Perseverative Error Standard Score | 99.5 (9.3) | 99.4 (13.3) | 89.1 (9.9)*** |
| Time 1 Tower of London Total Moves | 116.5 (22.6) | 105.4 (23.6) | 138.5 (35.1)*** |
| Time 2 Tower of London Total Moves | 97.8 (15.0) | 92.0 (9.2) | 124.0 (32.1)*** |
| Time 3 Tower of London Total Moves | 94.5 (7.6) | 84.6 (7.5) | 115.5 (24.1)*** |
| Time 4 Tower of London Total Moves | 92.8 (10.5) | 86.3 (8.3) | 105.1 (18.7)*** |
| Time 1 Visual Span Forward z score | −0.2 (0.8) | 0.3 (0.6) | −0.9 (0.8)*** |
| Time 2 Visual Span Forward z score | 0.4 (1.1) | 0.4 (1.2) | −1.0 (1.0)*** |
| Time 3 Visual Span Forward z score | 0.5 (1.2) | 0.7 (1.3) | −0.7 (1.2)*** |
| Time 4 Visual Span Forward z score | 0.4 (1.2) | 0.7 (1.0) | −0.9 (1.1)*** |
| Time 1 Visual Span Backward z score | −0.6 (1.2) | 0.0 (1.1) | −1.3 (1.2)*** |
| Time 2 Visual Span Backward z score | −0.2 (1.2) | 0.1 (0.8) | −1.4 (1.2)*** |
| Time 3 Visual Span Backward z score | 0.4 (1.2) | 0.5 (0.8) | −1.2 (1.3)*** |
| Time 4 Visual Span Backward z score | 0.0 (0.8) | 0.1 (0.9) | −1.2 (1.0)*** |
Note: 22q11DS, 22q11.2 deletion syndrome; CVLT-C, California Verbal Learning Test – Children’s version; GDS, Gordon Diagnostic System; WCST, Wisconsin Card Sorting Test; WIAT-II, Wechsler Individual Achievement Test – second edition. Fifteen additional participants were enrolled at Time 2 to account for attrition.
***P < .001.
Measures
Unless otherwise noted, all instruments were administered at all 4 time points.
Cognitive.
Measures of general intellectual functioning were the Wechsler Intelligence Scale for Children – third edition (WISC-III)21 or Wechsler Adult Intelligence Scale – third edition (WAIS-III).22 The WISC-III was administered to all participants at Time 1 and to participants at or under the age of 16 years, 11 months at Times 2, 3, and 4. The WAIS-III was administered to all participants over the age of 16 years 11 months at Times 2, 3 and 4.
Academic achievement was assessed using the Wechsler Individual Achievement Test – second edition (WIAT-II).23 Attention was assessed using a continuous performance test, the Gordon Diagnostic System (GDS).24 Both the Distractibility and Vigilance versions were utilized. Executive functioning was assessed with the Wisconsin Card Sorting Test (WCST).25 Learning and memory was assessed with the California Verbal Learning Test (CVLT)26 and the Visual Span Test.27 The Visual Span is a computer-presented adaptation of the Visual Memory Span subtest of the Wechsler Memory Scale – third edition, produced on the Colorado Assessment Tests. An irregular array of squares is displayed on the screen, a subset of them is illuminated briefly, and the subject must reproduce these sequences of increasing length. Forward and backward span scores are obtained. Please see table 1 for complete cognitive data from all 4 time points.
Emotion Recognition.
The Pennsylvania Emotion Recognition-40 Test28 is a computerized test that assesses the ability to identify facial expressions of emotion. Participants were presented with 40 color photographs of adult faces and are asked to rate each on a 7-point Likert scale from “very unhappy” to “very happy.” The stimuli are balanced by gender and ethnicity with 21 white and 19 non-white faces.29 Correct responses receive a score of 1 and incorrect responses 0, with higher scores indicating better facial emotion recognition. For the present study, responses were scored as correct if it was correct or within 1 point of the correct answer.
Psychiatric.
The Structured Interview for Prodromal Syndromes (SIPS)30 was used to assess prodromal psychotic symptoms. The SIPS was administered to all participants at Time 4 by a doctoral-level clinician during the structured psychiatric interview. The SIPS was also administered separately to the parent(s) of each participant. Interrater reliability, based on 5 SIPS interviews and assessed with the intraclass correlation coefficient, was 0.90. For the current analyses, given that positive symptoms are the most specific to psychosis, only the summary scores for positive prodromal symptoms (eg, grandiosity, hallucinations) were included. Using a method with a precedent in the literature,30 we identified positive prodromal symptoms of psychosis as one or more of the 5 SIPS positive symptom items as being rated a “3” or higher.
Procedure
Informed consent/assent was obtained from parents and youth under protocols approved by the institutional review board. Participants were assessed at 4 time points, with approximately 3 years between time points. Each individual at each time point had a neuropsychological assessment that included standardized tests of IQ, academic achievement, executive function, attention, working memory, and learning. Next, each participant was administered a structured psychiatric interview by a clinical psychologist or a board-certified child psychiatrist. Parents were also administered a structured psychiatric interview about their child by a clinical psychologist or a board-certified child psychiatrist and also completed behavior rating scales and background information questionnaires. The Kiddie-Sads-Present and Lifetime Version (K-SADS-PL)31 was administered at the first 3 time points. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)32 was administered at Time 4. Interrater reliability, which was calculated for 10 interviews, and assessed with the kappa coefficient, was .91.
In addition to strong interrater reliability, the interviewers took notes about the symptoms for each disorder. These notes and the structured interview data were reviewed by the diagnostic committee so that the Committee could make a Best Estimate diagnosis as described by Leckman et al.33 The diagnostic committee was comprised of 3 experienced doctoral-level providers. Definite diagnoses were assigned to subjects who met all diagnostic criteria. Diagnoses were considered definite only if a consensus was achieved that criteria were met to a degree that would be considered clinically meaningful. By “clinically meaningful” we mean that the data collected from the structured interview indicated that the diagnosis should be a clinical concern due to the nature of the symptoms, the associated impairment, and the coherence of the clinical picture. For both the SIPS and the SCID, discrepancies between child and parent rating of child symptoms and functioning were generally resolved by adopting the more severe rating (almost always the parent).
Blind neuropsychological assessment and psychiatric interviews were not possible due to the readily identifiable facial phenotype associated with 22q11DS. Given that participants in our study traveled from across the country, siblings generally were assessed during the same time period. Therefore, it was also not possible to have blinded psychiatric interviews for our control group as sibling controls were assessed during the same visit as the individual with 22q11DS. Despite this lack of blinding, data from the study measures were analyzed without identifiers.
Planned Analyses
Linear mixed model regression analyses were conducted to determine if trajectories of neuropsychological function differed significantly between individuals with 22q11DS, unaffected siblings and community controls. Both linear and curvilinear effects of age, and interactions between age and study groups were included in the models. Wald tests were used to examine differences in intercepts and trajectories between probands and controls/siblings. (Unless specified, results of Wald tests are presented.) When the terms were insignificant, the curvilinear term was dropped and the analysis was repeated with only the linear term. Models controlled for both family relatedness and nonindependence due to assessments of the same individuals over time. Analyses were conducted with and without the addition of Full-Scale IQ scores as a covariate.
We examined the association between trajectories of neuropsychological function and positive symptoms of psychosis within the group of individuals with 22q11DS in 2 ways. First, we conducted a linear mixed model regression, analyzing the association between trajectories of neuropsychological test measures and scores on the Positive Symptoms Subscale of the SIPS. When Time 4 SIPS was not available, Time 3 SIPS was imputed. Second, we divided the group of individuals with 22q11DS into those with and without prodromal/overt psychosis. Individuals who either had a diagnosis of psychosis based on the SCID or whose score on any item of the Positive Symptoms Subscale of the SIPS exceeded 2 were placed in the prodromal/overt psychosis group. This resulted in 18 individuals with, and 64 without, prodromal/overt psychosis. This rate of prodromal/overt psychosis (22%) is generally consistent with what others have reported about psychosis prevalence rates in the 22q11DS young adult population.4
We then conducted mixed model regression analyses to compare their trajectories of neuropsychological function, similar to the earlier analyses. Both sets of analyses were conducted with and without the addition of the presence of Times 1 and 2 prodromal symptoms as a covariate. (Since the SIPS had not been published when we assessed participants at Time 1, we used the presence of past or present symptoms of hallucinations/delusions on the K-SADS-PL at both time points as our measure of Time 1 or Time 2 prodromal symptoms).
Based on a Bonferroni correction that was applied to all analyses, the significance threshold was set at P ≤.003; all results greater than .003 and less than .05 were considered marginally or nominally significant. All analyses were conducted in Stata, version 13.
Results
Trajectories of Neuropsychological Function
Longitudinal trajectories of Performance IQ scores were marginally different between probands and both unaffected siblings (P = .04) and community controls (P = .006). Trajectories of Full-Scale IQ scores also tended to differ between probands and siblings (P = .05) and between probands and controls (P = .04).
Probands with 22q11DS differed significantly from siblings (linear term: z = 3.56; P = .001; curvilinear term: z = −3.41; P = .001) and controls (linear term, z = 3.29; P = .001; curvilinear term: z = −3.05; P = .002) in longitudinal trajectories of visual working memory, as measured by the Visual Span Forward. Probands also differed in longitudinal trajectories of verbal learning, as measured by CVLT List A Trial 1 performance although differences were more robust between probands and controls (P = .008) than probands and siblings (P = .03). Dropping the curvilinear term showed a greater difference in the linear trajectories between probands and controls (z = −3.10, P = .002). Less robust but still marginally significant differences in trajectories were observed in GDS Distractibility Task Commission Scores (22q11DS vs siblings: P = .03). Similarly, less significant differences were noted in the linear trajectories of WISC-III Perceptual Organization Index (POI) scores (between siblings and probands: z = 2.46, P = .01; probands and controls: z = 2.34; P = .02). Probands differed significantly from siblings (linear term: z = −2.79; P = .005; curvilinear term: z = 3.29; P = .001; Wald test: P = .0008) in longitudinal trajectories of overall academic performance as well, as measured by the WIAT-II Total composite score. Nominally significant differences were also observed between probands and siblings (z = 2.33; P = .02) and controls (z = 2.78; P = .005) in the linear trajectories of WIAT-II Math composite scores.
The inclusion of Full-Scale IQ trajectory as a covariate in each model did not alter significant results (except for WIAT-II Math and POI scores, which were no longer significant).
Predictors to Positive Symptoms of Psychosis
Based on our first method of analysis, in which we analyzed SIPS Positive Symptoms Scores as a dimensional variable, we observed that linear longitudinal trajectories of WCST Perseverative Error scores predicted robustly to the presence of positive symptoms of psychosis at Time 4 (z = −3.50; P = .001). Additional predictors included linear longitudinal trajectories of several measures of sustained attention and inhibitory control (Gordon Vigilance Omissions, z = −2.32; P = .02; Gordon Vigilance Commissions, z = −2.16, P = .03; Gordon Distractibility Omissions, z = −2.55; P = .01) as well as verbal learning (CVLT List A Trial 1, z = −2.27; P = .02). The significance levels of these predictors (except for Gordon Vigilance Commissions, which became insignificant) did not change when we covaried by the presence of Time 1 and Time 2 prodromal symptoms.
Based on our second method of analysis, in which we divided the group of individuals with 22q11DS into those with and without prodromal/overt psychosis, and similar to results described above, we found that the trajectory of scores on the WCST Perseverative Errors differed significantly between individuals with 22q11DS with and without prodromal/overt psychosis (linear term: z = −2.76; P = .006; curvilinear term: z = 2.38; P = .017). In addition, we observed significant differences between these groups in score trajectories on the WIAT-II Word Reading subtest (linear term: z = 3.95; P = .0001; curvilinear term: z = −3.91; P = .0001) and the Pennsylvania Emotion Recognition Test (linear term: z = 2.55; P = .011; curvilinear term: z = −2.51; P = .012).
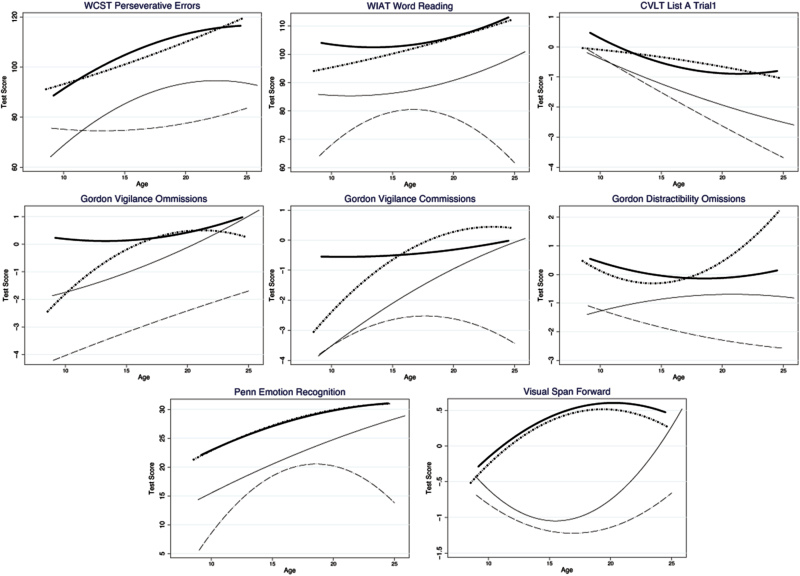
As figure 2 indicates, WCST Perseverative Error scores improved at a slower rate for individuals with 22q11DS + psychosis than those without psychosis. Scores on Pennsylvania Emotion Recognition Test increased steadily in individuals without psychosis, whereas for those with psychosis, scores increased until approximately 15 years of age, at which point they began to decrease rapidly. A similar, but more subtle effect, was seen for scores on the WIAT-II Word Reading. The significance levels of the WCST, Pennsylvania Emotion Recognition Test, and WIAT-II predictors did not change appreciably when we covaried for the presence of T1 and T2 prodromal symptoms.
Fig. 2.
Longitudinal trajectories of neuropsychological test performance. - - - - -: 22q11DS + Psychosis; _______: 22q11DS; *****: Community Controls; _______: Siblings.
Finally, the effect sizes based on Cohen’s f2 for the influence of psychosis on test scores were small to medium with WIAT-II Word Reading having the greatest effect size (0.06) and Pennsylvania Emotion Recognition Test having the lowest (0.02) with WCST Perseverative Error at 0.03. Please see table 2 for the estimated regression coefficients from the 3 significant findings regarding the underlying cognitive processes that distinguish between those that developed psychotic symptoms and those that did not. Based on these coefficients, the predicted difference in average WCST Perseverative Error Standard Scores between individuals with 22q11DS who developed psychosis and those who did not was about 8.44 points, or equivalently about 0.42 SDs. The corresponding numbers for the WIAT-II Word Reading and Pennsylvania Emotion Recognition Test scores were 12 (0.65 SDs) and 3.64 (0.49 SDs), respectively.
Table 2.
Variations in Test Scores Among Individuals With 22q11DS Who Did Not Develop Psychosis (22q11DS − P) as Compared to Individuals Who Developed Psychosis (22q11DS + P)
| 22q11DS − P (Base Omitted Outcome) | 22q11DS + P | Wald Test of Trajectory | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | P Value | Age | P value | Age2 | P Value | Group | P Value | Group Age | P Value | Group Age2 | P Value | P Value | |
| WCST Persev. | 10.88 | .36 | 7.44 | .001 | −0.1656 | .001 | 74.64 | .007 | −9.13 | .006 | .2296 | .02 | .0012 |
| Word Reading | 95.38 | .001 | −1.74 | .11 | 0.0757 | .04 | −91.85 | .001 | 10.95 | .001 | −.3512 | .001 | .0004 |
| PERT | 3.42 | .53 | 1.35 | .07 | −0.0141 | .56 | −39.15 | .005 | 4.71 | .01 | −.1490 | .01 | .04 |
Note: 22q11DS, 22q11.2 deletion syndrome; PERT, Pennsylvania Emotion Recognition Test Total Number of Correct Emotions Identified; WCST Persev., Wisconsin Card Sorting Test Perseverative Error Standard Score; Word Reading, WIAT-II Word Reading Standard Score. Coefficients from mixed model regressions are presented. Intercept is the test score at hypothetical Age 0 as fitted by the regression. Age indicates the change in test score with age. Age2 is the rate of change of test score with age.
Discussion
Compared to both siblings and community control participants, individuals with 22q11DS demonstrated different longitudinal trajectories of visual working memory, auditory/verbal working memory, and overall academic attainment. Consistent with prior research in typically developing populations,18–20 both control groups showed somewhat more stability in these domains than the 22q11DS cohort. These are the first longitudinal data following youth with 22q11DS into adulthood that have been published on these cognitive domains. This information could be useful to those who work clinically and educationally with children and adolescents with 22q11DS.
WCST Perseverative Error score trajectories were robust predictors to dimensional levels of prodromal/overt psychotic symptoms in adulthood. As can be seen in figure 2, individuals with 22q11DS who developed prodromal/overt psychotic symptoms improved less appreciably and continued to demonstrate difficulties with cognitive flexibility relative to individuals with 22q11DS who did not have prodromal/overt psychotic symptoms.
When viewing psychosis categorically, WIAT-II Word Reading trajectories were strongly predictive of prodromal/overt psychosis in adulthood. As seen in figure 2, individuals with 22q11DS who developed prodromal/overt psychosis had weaker reading skills in childhood and, after an initial improvement into adolescence, these individuals with psychosis had a decline in reading skills. WCST Perseverative Error and Emotion Recognition trajectories were marginally significant predictors (after applying Bonferroni correction) of prodromal/overt psychosis assessed categorically.
A central, yet unresolved, issue in the psychosis literature is whether cognitive deficits are traits that are preexisting and raise the risk for psychosis or alternatively, whether cognitive deficits are associated with the onset of psychotic symptoms.34 Our data indicate both may be occurring in the 22q11DS population. For example, reading decoding abilities in childhood were lower in those who eventually developed prodromal/overt psychosis in adulthood and the gap widened over time. Conversely, cognitive set shifting in childhood (measured via the WCST) was slightly stronger in those who developed prodromal/overt psychosis in adulthood yet failed to improve relative to those who did not develop psychosis. Others in the idiopathic high-risk psychosis literature35 have likewise demonstrated that cognitive set shifting trajectories discriminate those who transition into psychosis.
In addition to set shifting and reading decoding, emotion recognition also discriminated between the 2 22q11DS groups. In the idiopathic schizophrenia literature, facial emotion recognition deficits are present in those at high risk for psychosis36 and decline during the transition to psychosis.37 Deficits in emotion recognition, like word decoding, were present before the transition to prodromal/overt psychosis. This suggests that both may be considered a vulnerability indicator for psychosis in 22q11DS worthy of further exploration.
These results are consistent with a cross-sectional study of adults with 22q11DS with and without schizophrenia that compared the premorbid adjustment in childhood through late adolescence (studied retrospectively) and its association with psychosis.38 Those authors found that deterioration of social and academic functioning from childhood to adolescence was associated with an increased risk for psychosis in adulthood. Previous longitudinal studies in 22q11DS indicate that Verbal IQ declines presage psychotic symptoms.3,12 The largest study to date, by the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome,3 reported on IQ trajectories. Previous research has indicated that Verbal IQ is negatively correlated with WCST Perseverative Errors, r = −.37.39 Likewise, word decoding is positively correlated with Verbal IQ.40 Future research should consider the extent to which cognitive set shifting and reading decoding are mechanistically related to the Verbal IQ declines observed in the 22q11DS population. Improving reading decoding and set shifting are both likely more modifiable than Verbal IQ and may represent treatment targets.
Efforts like these to understand the course of cognitive functioning in 22q11DS are important toward developing and testing prevention models.41 For example, emotion recognition is an aspect of social cognition,42 a domain that has been identified as a primary feature that underlies functional impairments in schizophrenia.43 Social cognitive training in schizophrenia has been reported to be an efficacious intervention with large effect sizes in idiopathic schizophrenia.44,45 Similarly, previous data from our group46 has indicated that auditory/verbal learning moderated the risk for psychosis. Future research should study the extent to which other cognitive strengths/weaknesses may moderate this risk via testing cognitive remediation interventions as a way to reduce the risk for psychosis in individuals with 22q11DS.
These data need to be considered in the context of our methodological weaknesses. First, assessments were conducted every 3 years. There is a chance that changes occurred between the 3-year intervals that were not recorded yet were relevant to the developmental progression of positive psychosis symptoms. Second, medication histories were not controlled for in the present analyses. It may be that treatment effects attenuated some of the predictions from neuropsychological test results to psychosis by reducing psychosis symptoms.
Despite these methodological weaknesses, our data are the first to go beyond IQ assessments in assessing longitudinal neuropsychological outcomes and risk for prodromal/overt psychosis in 22q11DS. These data suggest that cognitive set shifting, reading decoding, and emotion recognition are variables of interest for further investigation as possible mechanisms associated with the development of psychosis in individuals with 22q11DS.
Funding
This work was funded by the National Institutes of Health (5R01MH064824-14; PI: W.R.K.).
Acknowledgments
The authors would like to thank Amy Olszewski, Jo-Anna Botti, Lauren Kelchner, and Carlie Thompson for their assistance with the project. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Grati FR, Molina Gomes D, Ferreira JC, et al. Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenat Diagn. 2015;35:801–809. [DOI] [PubMed] [Google Scholar]
- 2. Bassett AS, McDonald-McGinn DM, Devriendt K, et al. ; International 22q11.2 Deletion Syndrome Consortium. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159:332–339.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vorstman JA, Breetvelt EJ, Duijff SN, et al. ; International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schneider M, Debbané M, Bassett AS, et al. ; International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171:627–639–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro HM, Tassone F, Choudhary NS, Simon TJ. The development of cognitive control in children with chromosome 22q11.2 deletion syndrome. Front Psychol. 2014;5:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol. 1999;11:487–508. [DOI] [PubMed] [Google Scholar]
- 7. Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88:26–35. [DOI] [PubMed] [Google Scholar]
- 8. Reichenberg A, Weiser M, Rapp MA, et al. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. [DOI] [PubMed] [Google Scholar]
- 9. Meier MH, Caspi A, Reichenberg A, et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green T, Gothelf D, Glaser B, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–1068. [DOI] [PubMed] [Google Scholar]
- 11. Gothelf D, Eliez S, Thompson T, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. [DOI] [PubMed] [Google Scholar]
- 12. Gothelf D, Schneider M, Green T, et al. Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. J Am Acad Child Adolesc Psychiatry. 2013;52:1192–1203.e3. [DOI] [PubMed] [Google Scholar]
- 13. Mariano MA, Tang K, Kurtz M, Kates WR. Cognitive remediation for adolescents with 22q11 deletion syndrome (22q11DS): a preliminary study examining effectiveness, feasibility, and fidelity of a hybrid strategy, remote and computer-based intervention. Schizophr Res. 2015;166:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrell W, Eack S, Hooper SR, et al. Feasibility and preliminary efficacy data from a computerized cognitive intervention in children with chromosome 22q11.2 deletion syndrome. Res Dev Disabil. 2013;34:2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sonuga-Barke EJ, Koerting J, Smith E, McCann DC, Thompson M. Early detection and intervention for attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2011;11:557–563. [DOI] [PubMed] [Google Scholar]
- 16. Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Nat Acad Sci USA. 2011;108:10081–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: the same or different constructs? Psychol Bull. 2005;131:30–60. [DOI] [PubMed] [Google Scholar]
- 18. Canivez GL, Watkins MW. Long-term stability of the Wechsler Intelligence Scale for Children – third edition. Psychol Assess. 1998;10:285–291. [DOI] [PubMed] [Google Scholar]
- 19. Gutman LM, Sameroff AJ, Cole R. Academic growth curve trajectories from 1st grade to 12th grade: effects of multiple social risk factors and preschool child factors. Dev Psychol. 2003;39:777–790. [DOI] [PubMed] [Google Scholar]
- 20. Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Weintraub S. II. NIH Toolbox Cognition Battery (CB): measuring executive function and attention. Monogr Soc Res Child Dev. 2013;78:16–33. [DOI] [PubMed] [Google Scholar]
- 21. Wechsler D. Wechsler Intelligence Scale for Children – Third Edition. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 22. Wechsler D. Wechsler Adult Intelligence Scale – Third Edition. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 23. Wechsler D. Wechsler Individual Achievement Test – Second Edition. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- 24. Gordon M. The Gordon Diagnostic System. DeWitt, NY: Gordon Systems; 1983. [Google Scholar]
- 25. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 26. Delis D, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – Children’s Version. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- 27. Davis HR. Colorado Assessment Tests – Visual Span Test. Boulder, CO: Colorado Assessment Tests; 1998. [Google Scholar]
- 28. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. [DOI] [PubMed] [Google Scholar]
- 29. Weiss EM, Stadelmann E, Kohler CG, et al. Differential effect of catechol-O-methyltransferase Val158Met genotype on emotional recognition abilities in healthy men and women. J Int Neuropsychol Soc. 2007;13:881–887. [DOI] [PubMed] [Google Scholar]
- 30. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 31. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 32. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV-Clinical Version (SCID-CV) (User’s Guide and Interview). Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 33. Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. [DOI] [PubMed] [Google Scholar]
- 34. Green MF, Harvey PD. Cognition in schizophrenia: Past, present, and future. Schizophr Res Cogn. 2014;1:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wood SJ, Brewer WJ, Koutsouradis P, et al. Cognitive decline following psychosis onset: data from the PACE clinic. Br J Psychiatry Suppl. 2007;51:s52–s57. [DOI] [PubMed] [Google Scholar]
- 36. Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192:67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. [DOI] [PubMed] [Google Scholar]
- 38. Yuen T, Chow EW, Silversides CK, Bassett AS. Premorbid adjustment and schizophrenia in individuals with 22q11.2 deletion syndrome. Schizophr Res. 2013;151:221–225. [DOI] [PubMed] [Google Scholar]
- 39. Ardila A, Pineda D, Rosselli M. Correlation between intelligence test scores and executive function measures. Arch Clin Neuropsychol. 2000;15:31–36. [PubMed] [Google Scholar]
- 40. Ferrer E, McArdle JJ, Shaywitz BA, Holahan JM, Marchione K, Shaywitz SE. Longitudinal models of developmental dynamics between reading and cognition from childhood to adolescence. Dev Psychol. 2007;43:1460–1473. [DOI] [PubMed] [Google Scholar]
- 41. Michel C, Ruhrmann S, Schimmelmann BG, Klosterkötter J, Schultze-Lutter F. A stratified model for psychosis prediction in clinical practice. Schizophr Bull. 2014;40:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fiszdon JM, Reddy LF. Review of social cognitive treatments for psychosis. Clin Psychol Rev. 2012;32:724–740. [DOI] [PubMed] [Google Scholar]
- 45. Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kates WR, Russo N, Wood WM, Antshel KM, Faraone SV, Fremont WP. Neurocognitive and familial moderators of psychiatric risk in velocardiofacial (22q11.2 deletion) syndrome: a longitudinal study. Psychol Med. 2015;45:1629–1639. [DOI] [PubMed] [Google Scholar]