Abstract
Even in predominantly religious societies, there are substantial individual differences in religious commitment. Why is this? One possibility is that differences in social conformity (i.e. the tendency to think and behave as others do) underlie inclination towards religiosity. However, the link between religiosity and conformity has not yet been directly examined. In this study, we tested the notion that non-religious individuals show dampened social conformity, using both self-reported and neural (EEG-based ERPs) measures of sensitivity to others’ influence. Non-religious vs religious undergraduate subjects completed an experimental task that assessed levels of conformity in a domain unrelated to religion (i.e. in judgments of facial attractiveness). Findings showed that, although both groups yielded to conformity pressures at the self-report level, non-religious individuals did not yield to such pressures in their neural responses. These findings highlight a novel link between religiosity and social conformity, and hold implications for prominent theories about the psychological functions of religion.
Keywords: religion, social conformity, late positive potential, EEG/ERP
Approximately 85% of the people in the world identify as religious (Zuckerman, 2005). The influence of religion is pervasive, and in general, religious individuals report being happier and more satisfied with life (Ellison, 1991; Brooks, 2008). Given the widespread acceptance of religion—and the apparent benefits it confers to its adherents—a question of fundamental social relevance is why people differ in religious commitment.
A defining feature of religiosity is that propositions about the nature of reality are accepted on faith (i.e. in the absence of empirical data). Thus, the transmission of religious doctrines likely occurs through mechanisms other than the personal examination of available evidence. An important clue about the factors that promote religiosity involves recognizing that the vast majority of religious individuals adopt the faith of their specific communities and social networks (e.g. families), quite often from childhood (Dawkins, 2016). This raises the possibility that religiosity is supported through conformity to the norms of one’s social networks. If this is true, then a reduced general sensitivity to social conformity could decrease individuals’ proclivity towards religion.
Social psychologists have long understood that individuals adopt the beliefs and behaviors of the surrounding group (Sherif, 1936; Asch, 1951). Conformity has been shown in a broad range of domains, including perceptual decisions (Moscovici et al., 1969), moral judgments (Kundu and Cummins, 2013), and bodily postures and facial expressions (Chartrand and Bargh, 1999). Conforming to the group appears to be intrinsically rewarding as it engages reward-related neural circuitry, particularly the striatum (Klucharev et al., 2009; Mason et al., 2009; Stallen et al., 2013). Indeed, the tendency to conform may be a fundamental human motive arising from evolutionary pressures that favored social learning (Henrich and Boyd, 1998; Henrich and McElreath, 2003). Consistent with this notion, numerous non-human species exhibit social conformity in a wide range of domains as well, such as mate-copying, flee-responses to danger, and foraging decisions (see Danchin et al., 2004 for a review).
One key question about social conformity is whether conformity pressures merely lead individuals to alter what they say they think or believe, or whether they produce a true change in private judgments (Cialdini and Goldstein, 2004). Researchers have held that such changes can arise from distinct motives to conform: individuals may simply alter their outward behavior to be liked by others (normative conformity), or alter their private judgments to incorporate valued information provided by others (informational conformity). Recent neuroimaging research supports this distinction as well. Mason et al. (2009) showed that some brain regions—such as the medial prefrontal cortex (mPFC)—may contribute to normative conformity by merely tracking whether or not an item has been evaluated by others. In contrast, other regions—particularly, the striatum involved in reward—may underlie informational conformity by tracking the actual social value assigned to an item, potentially allowing individuals to alter private appraisals accordingly.
Researchers have also tried to disentangle normative from informational conformity by combining neuroimaging with self-report methods. Since neural measures are fairly insensitive to demand effects, changes to individuals’ neural responses to stimuli under pressures to conform would likely reflect a modification of their private appraisals of these stimuli; alternatively, changes to individuals’ self-reported responses could simply reflect adjustments in their outward behavior (Zaki et al., 2011). As with Mason et al. (2009), Zaki et al. (2011) found that stimuli with acquired high (vs low) social value modulates the striatum.
Methodological considerations
In a recent study (Thiruchselvam et al., 2016), we combined neural (i.e. EEG) and self-report measures to examine how expectations derived from peer-influences shape individuals’ responses to facial attractiveness. Participants viewed high-attractive and low-attractive faces in our paradigm. Prior to each face, they saw a peer-rating that ostensibly reflected the overall attractiveness value assigned to that face by other individuals. Our results showed that participants’ own responses to facial beauty—at both neural and self-report units of analysis—were powerfully altered to be in line with these peer-ratings.
Our specific neural measure was the Late Positive Potential (LPP), which is particularly valuable in this experimental context. It is an EEG-based event-related potential (ERP) that begins approximately 400ms after stimulus onset and is maximal at parietal areas of the scalp (see Hajcak et al., 2010 for a review). The LPP is reliably enhanced by affective arousal (Cuthbert et al., 2000; Thiruchselvam et al., 2011; 2012), but is insensitive to basic perceptual features such as image size (De Cesarei and Codispoti, 2006) or figure-ground complexity (Bradley et al., 2007). Researchers have argued that the arousal-enhancement of the LPP reflects modulation of extrastriate visual cortices arising from a subcortical (e.g., amygdala) response to affective stimuli (Sabatinelli et al., 2007; de Rover et al., 2012). Recent research has also found a link between LPP responses to pleasant stimuli specifically and fMRI-based BOLD responses within the nucleus accumbens (Liu et al., 2012). Crucially, several studies have shown that the LPP is sensitive not only to facial beauty (Johnston and Oliver-Rodriguez, 1997; van Hooff et al., 2010; Morgan and Kisley, 2014), but also to experimental manipulations that alter the private appraisal of affective stimuli. In particular, when individuals privately alter the construal of affective stimuli via cognitive reappraisal, the LPP is reliably modulated (Foti and Hajcak, 2008; Thiruchselvam et al., 2011). Thus, it is a useful measure to assess how individuals’ private appraisals of facial attractiveness may change due to expectations derived from conformity pressures.
The present study
Non-religious and religious undergraduate subjects completed an experimental task that assessed social conformity in judgments of facial beauty. Importantly, assessing conformity in a domain completely unrelated to individuals’ personal religion allowed us to ask whether differences in religiosity may be characterized by an underlying general inclination towards conformity. In our paradigm, participants viewed and rated faces differing in attractiveness (Thiruchselvam et al., 2016). To induce conformity pressure, each face was preceded by a peer-rating that ostensibly reflected the overall attractiveness value assigned to it by other individuals. For high-attractive faces, peer-ratings were either artificially increased (High-attractive: High peer-rating condition) or decreased (High-attractive: Low peer-rating condition) relative to the actual mean attractiveness value for that face as determined by an independent sample.
Since our reasoning is that privately adopting the beliefs of others (i.e. informational conformity) may be a critical vehicle for the transmission of religious faith, we predicted that non-religious individuals would show reduced sensitivity to peer-ratings in their LPP responses to facial attractiveness. Insofar as the LPP tracks changes in private construal, it should reflect non-religious individuals’ resistance to truly adopting the views of others. However, as self-report responses can largely arise from normative pressures to appear consistent with others, we were agnostic about the link between religiosity and peer-influences at that level of analysis.
Methods
Participants
Forty-nine undergraduate subjects (24 non-religious, 25 religious) from Hamilton College completed the current study. Participant characteristics are shown in Table 1. We first sent emails to the campus community inviting them to complete a short survey to determine eligibility to participate in our study on ‘face perception and the brain’. This survey contained the critical religiosity question (‘What is your level of religiosity in general?’), with responses anchored from 0 (not at all religious) to 10 (very religious).1 Survey respondents were then invited to participate in the study if they responded 0 (for the Non-religious group) or above 6 (for the Religious group); these cutoffs were chosen in order to recruit individuals who identified as non-religious vs at least moderately religious.2 Moreover, since the task stimuli involved opposite-sex faces varying in attractiveness, all participants were heterosexual and had normal or corrected-to-normal vision. Relatedly, we only recruited unattached (i.e. single) individuals, since research shows that being in a committed relationship can dampen one’s responses to attractive faces (Maner et al., 2008). Three participants from the Religious group were excluded from EEG analyses due to excessively noisy data (i.e. more than 60% unusable trials). All participants received $20 in compensation.
Table 1.
Demographic characteristics of the non-religious and religious groups
| Non-religious | Religious | |
|---|---|---|
| M (s.d.) or % | M (s.d.) or % | |
| Age | 19.29 (1.45) | 19.88 (1.42) |
| % Female | 71% | 76% |
| Religious affiliation | ||
| Christian | 72% | |
| Jewish | 8% | |
| Muslim | 8% | |
| Other/unspecified | 12% | |
| Self-reported religiosity (0–10) | 0 (0) | 8.20 (1.22) |
Materials
We obtained facial images displaying fairly neutral expressions from free online sources. Images were grayscaled and cropped to fit 288 × 360 pixel dimensions. In order to determine mean attractiveness levels for the selected faces, we conducted a separate study with an independent sample of undergraduate Hamilton College students (N = 27; 16 females, 11 males) who pre-rated the faces on attractiveness (using a 1–9 scale, anchored from ‘not at all attractive’ to ‘extremely attractive’). We then assigned images to experimental conditions using these ratings.
For both the male and female image sets, faces categorized as High-attractive (Males: M = 6.55, s.d. = 0.83; Females: M = 6.99, s.d. = 0.55) had significantly higher ratings than those categorized as Low-attractive (Males: M = 2.85, s.d. = 0.65; Females: M = 3.44, s.d. = 0.57); faces were generally assigned to the High-attractive category if their mean rating was above 5. Within each High-attractive face category, images were randomly divided into two subsets, so that they can be assigned to HighvsLow peer-rating conditions (see Procedure below). We took great care to ensure that the High-attractive faces assigned to each subset were as closely matched as possible in attractiveness ratings: Male faces (Set 1: M = 6.54, s.d. = 0.91; Set 2: M = 6.55, s.d. = 0.76), female faces (Set 1: M = 7.01, s.d. = 0.47; Set 2: M = 6.97, s.d. = 0.63). Pairwise comparisons between the two subsets within each gender category were non-significant (all ps > 0.75).
The experimental task was administered using E-prime 2 (Schneider et al., 2002) in a sound-attenuated EEG chamber. Viewing distance was held constant at approximately 20 inches.
Procedure
Upon arrival, participants completed informed consent. Participants were told that the study concerned judgments of facial attractiveness, and that they would be shown information about how their peers on campus had rated the faces in an earlier study. Participants then completed an experimental task that involved viewing opposite-sex faces and rating their attractiveness.
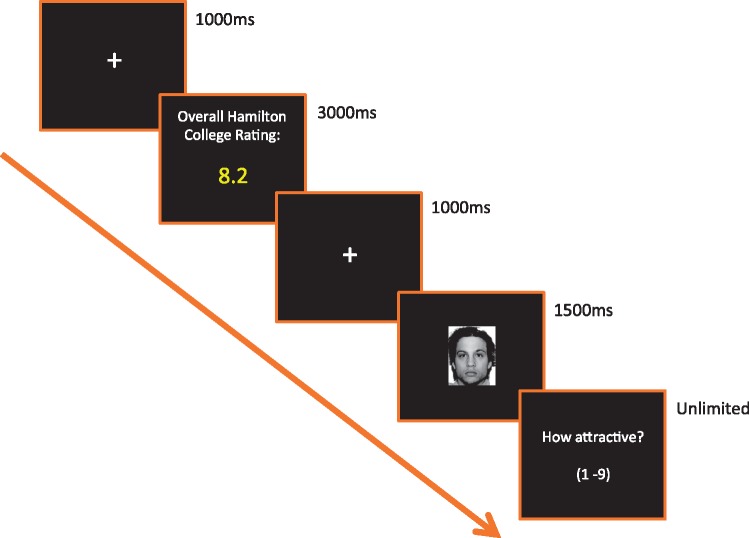
The trial structure is shown in Figure 1. Each trial consisted—in sequential order—of the following: a fixation cross (1000 ms), a peer-rating ostensibly reflecting the overall attractiveness value assigned by campus students to an upcoming face (3000 ms), a second fixation (1000 ms), a face image (1500 ms), and a final screen to rate attractiveness on a 1–9 scale (unlimited duration).
Fig. 1.
Trial structure for the experimental task.
The experiment contained 4 separate blocks, with each block consisting of 28 trials: 14 Low-attractive face trials, and 14 High-attractive face trials. In the High-attractive face trials, 7 were paired with a falsely inflated peer-rating (High-attractive face: High peer-rating), and 7 were paired with a falsely lowered peer rating (High-attractive face: Low peer-rating). In order to derive peer-ratings in the High-attractive face: High peer-ratingvs the High attractive face: Low peer-rating conditions, we added or subtracted 1.8 points from the face’s actual mean attractiveness value obtained from the independent sample.3 The peer-ratings shown in the Low-attractive face condition reflected the actual mean attractiveness value obtained for that face from the independent sample. All trials within each block were presented in a randomized order.
EEG recording, data reduction and analysis
Continuous EEG recordings were made from 32-electrodes using BrainVision’s actiCHamp (Brain Vision, Morrisville, NC). Cz served as the online reference. The EEG signal was recorded in DC mode and sampled at a rate of 500 Hz. Impedance levels were kept below 10 kΩ at all sites.
Offline, preprocessing was conducted using BrainVision’s Analyzer 2 software. To derive the LPP waveform, EEG data were filtered from .1 to 30 Hz and re-referenced to the average of the mastoids. Single-trial EEG epochs were extracted for a period beginning 200 ms before face image onset and continuing for the entire duration of image presentation (1500 ms). Epochs were baseline-corrected using the 200 ms prior to image onset. Trials were discarded due to excessive physiological noise if they contained: (i) an eye-blink, (ii) a voltage step greater than 50 µV/ms between sample points, (iii) a max-min difference greater than 150 µV/ms throughout the epoch and (iv) low activity (i.e. less than 0.5 µV/ms) within a 100 ms window. This resulted in approximately 86% of original trials remaining for analyses (the amount of trials used for analyses did not differ either by Trial Type or Group).4 Consistent with prior research (see Hajcak et al., 2010, for a review), we quantified the LPP as the average signal amplitude at site Pz in the 400–1500 ms time range after image onset.
Results
Self-reported attractiveness ratings
We first submitted self-reported attractiveness scores to a mixed-model repeated measures ANOVA, with Group (Non-religious, Religious) as a between-subjects factor and Trial Type (High-attractive face: High peer-rating, High-attractive face: Low peer-rating, Low-attractive face) as a within-subjects factor. That revealed a main effect of Trial Type [F(2,94) = 723.59, P < 0.001, p2 = 0.94], and an interaction between Group and Trial Type [F(2,94) = 4.20, P = 0.01, p2 = 0.08].
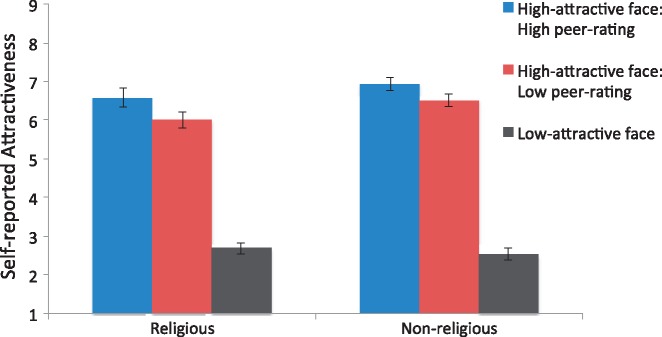
In the Religious group, the peer-ratings altered self-reported attractiveness scores: High-attractive face: High peer-rating (M = 6.58, s.d. = 1.21) produced higher scores than High-attractive face: Low peer-rating (M = 6.00, s.d. = 1.05), [t(24) = 4.59, P < 0.01]. Similarly, in the Non-religious group, self-reported attractiveness scores were also higher in High-attractive face: High peer-rating (M = 6.93, s.d. = 0.78) than in High-attractive face: Low peer-rating (M = 6.51, s.d. = 0.80), [t(23) = 4.90, P < 0.01]. A comparison of the magnitude of the conformity effect (i.e., difference score between High-attractive face: High peer-rating and High-attractive face: Low peer-rating) between the two groups showed that the two groups did not differ [t(47) = 1.06, P = 0.29]. At the self-report level then, both religious and non-religious individuals showed similar levels of conformity. Figure 2 displays the self-reported attractiveness scores in each trial type for the religious and non-religious group.
Fig. 2.
Self-reported attractiveness scores by trial type in the religious and non-religious group. Error bars reflect standard error of the mean.
Late positive potential
We then submitted LPP amplitudes to an ANOVA. That revealed a main effect of Trial Type [F(2,88) = 23.25, P < 0.001, p2 = 0.34], and a significant interaction between Group and Trial Type [F(2,88) = 3.34, P < 0.05, p2 = 0.07].
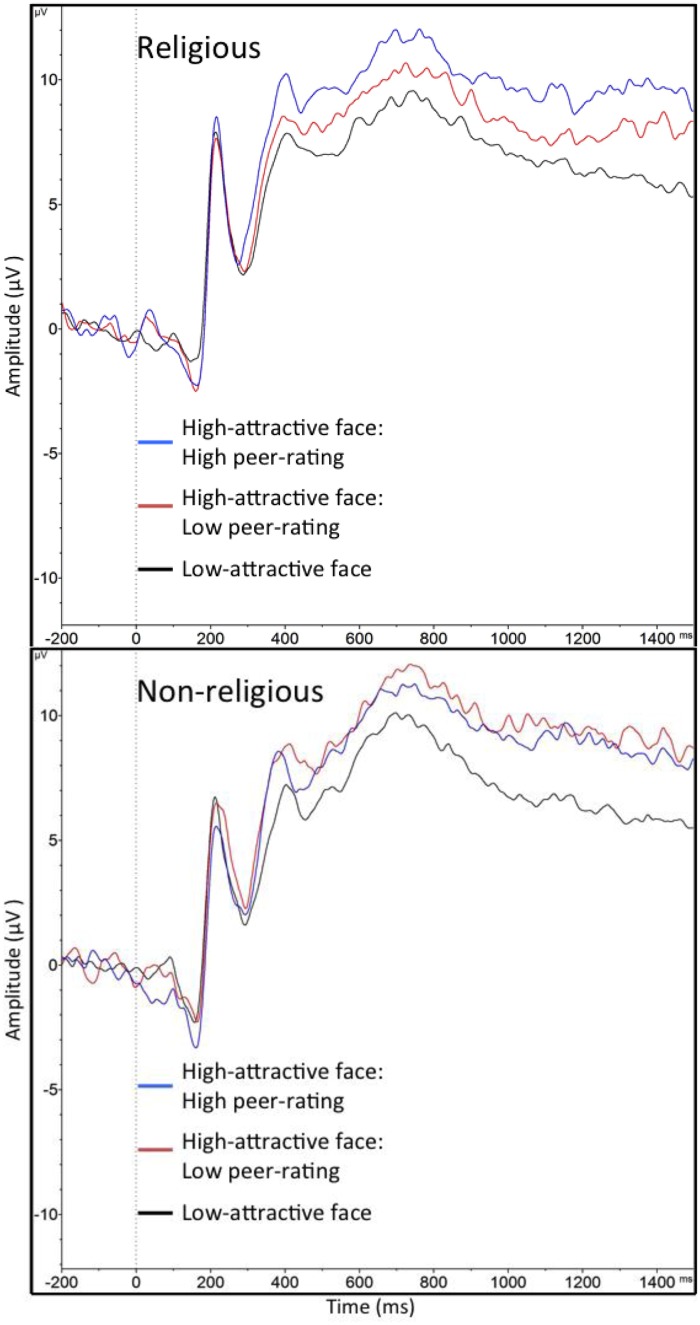
Follow-up pairwise comparisons showed that, in the Religious group, the peer-ratings powerfully altered the LPP: as expected, High-attractive face: High peer-rating (M = 10.00, s.d. = 5.03) produced a significantly larger LPP than High-attractive face: Low peer-rating (M = 8.61, s.d. = 5.24), [t(21) = 2.52, P = 0.01]. This finding successfully replicates prior research on an unselected sample (Thiruchselvam et al., 2016). However, in the Non-religious group, the peer-ratings had no impact on the LPP: High-attractive face: High peer-rating (M = 9.20, s.d. = 4.14) was not statistically different from High-attractive face: Low peer-rating (M = 9.70, s.d. = 4.45), [t < 1]. In addition, a comparison of the magnitude of the conformity effect (i.e. difference score between High-attractive face: High peer-rating and High-attractive face: Low peer-rating) between the two groups confirmed that non-religious individuals indeed exhibited a weaker conformity effect [t(44) = 2.32, P = 0.02]. Thus, in neural responses, non-religious individuals did not yield to conformity pressures, but religious individuals did. As expected, in both groups, the two High-attractive face trial types generally produced a larger LPP than the Low-attractive face type [all ps < 0.02]. The LPP during face presentation for each group is shown in Figure 3.
Fig. 3.
LPP waveforms (at site Pz) to face presentation by trial type in the religious and non-religious group.
Exploratory analyses
In order to investigate whether the Non-religious group’s lower social conformity at the LPP level can be attributed to greater skepticism towards the peer-ratings, we examined the LPP elicited by the peer-ratings themselves. If participants believed the peer-ratings, then High-attractive face: High peer-ratings (producing anticipation of seeing an extremely attractive face) should elicit more arousal than High-attractive face: Low peer-ratings (producing anticipation of seeing a less attractive face); this heightened anticipatory arousal should be reflected in a larger LPP. Conversely, if participants did not believe the peer-ratings and discounted their significance, then peer-ratings should not have altered anticipatory arousal as strongly.5
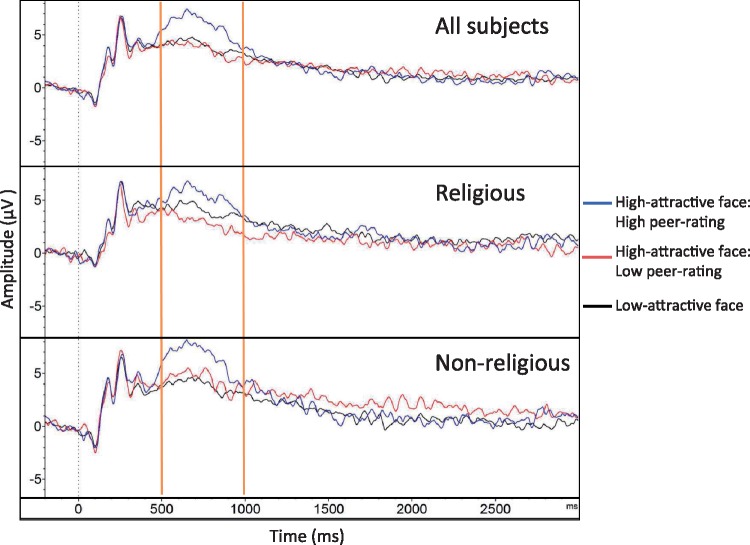
A visual examination of waveforms during the peer-rating slide across all subjects clearly showed that High-attractive face: High peer-ratings produced a larger LPP than High-attractive face: Low peer-ratings in the 500-1000ms time range (Figure 4). Thus, we submitted LPP amplitudes during the 500-1000ms window to a repeated measures ANOVA, with Trial Type (High-attractive face: High peer-rating, High-attractive face: Low peer-rating, Low-attractive face) as a within-subjects factor, and Group (Non-religious, Religious) as a between-subjects factor. That revealed only a main effect of Trial Type [F(2,86) = 16.07, P < 0.001, p2= 0.27].
Fig. 4.
LPP waveforms (at site Pz) to peer-rating presentation by trial type in the religious and non-religious group. The 500–1000 ms time range used for analyses is highlighted for clarity.
Confirming our visual inspection, a paired-samples t-test revealed that High-attractive face: High peer-ratings (M = 5.94, s.d. = 4.51) robustly elicited a stronger LPP than High-attractive face: Low peer-ratings (M = 3.63, s.d. = 3.71) in the 500–1000 ms time range, [t(44) = 4.79, P < 0.001]. Crucially, both the Religious and the Non-religious group showed this effect (both ps < 0.01), suggesting that the peer-rating manipulation effectively altered anticipatory arousal in both groups. Furthermore, the strength of this effect (defined as the difference score in LPP between High-attractive face: High peer-ratings minus High-attractive face: Low peer-ratings) did not differ between the Religious (M = 2.56, s.d. = 3.14) and Non-religious (M = 2.07, s.d. = 3.36) groups [t < 1]. Collectively, these findings suggest that the lower social conformity at the LPP level found among non-religious participants is unlikely to reflect greater skepticism towards the peer-ratings, as both groups appear to have been equally responsive to our peer-rating manipulation in anticipatory arousal6.
Discussion
In this study, we examined whether non-religious individuals are less sensitive to social conformity at various units of analysis (i.e. neural and self-report). In order to assess conformity in a domain unrelated to religion, we used a paradigm that involved judgments of facial attractiveness. Findings showed that, at the self-report level, peer-influences affected ratings of attractiveness in both groups. Crucially, however, neural responses to facial beauty among non-religious individuals were less sensitive to peer-influences (compared to religious subjects). Specifically, although peer-influences powerfully impacted LPP responses to facial beauty in religious individuals (successfully replicating prior findings in unselected subjects; Thiruchselvam et al., 2016), they did not exert any impact on non-religious individuals. These findings demonstrate for the first time a link between religiosity and social conformity.
Social psychologists have long held that conformity pressures can modify both outward behavior and private judgments, and that these two types of changes are distinct (Deutsch and Gerard, 1955; Petty and Cacioppo, 1986; Zaki et al., 2011). Our findings attest to this: among non-religious participants, peer-influences altered self-reported ratings but did not affect LPP responses. A plausible interpretation of this finding is that, among non-religious individuals, conformity pressures modified outward responses but not private appraisals of facial attractiveness. In contrast, religious individuals yielded to conformity pressures in both their outward responses and private appraisals. As several authors have posited (Cialdini and Goldstein, 2004; Zaki et al., 2011), these two types of conformity may reflect different motivations: individuals may wish to avoid social exclusion and thus alter their outward responses to simply appear consistent with the group (normative conformity); alternatively, they may want to use social input to inform their judgments of the world, and modify their actual private appraisals accordingly (informational conformity). A plausible interpretation of our findings then is that both groups displayed sensitivity to normative conformity, but that non-religious individuals were unaffected by informational conformity.
Exploratory analyses also intriguingly revealed that both religious and non-religious individuals were sensitive to the peer-rating manipulation itself: both groups exhibited larger LPPs in response to seeing high peer-ratings (vslow peer-ratings) for upcoming attractive faces during the 500-1000ms window of peer-rating presentation. Crucially, there was no group difference in the size of this effect, suggesting that our peer-rating manipulation effectively altered anticipatory arousal in both religious and non-religious participants to a similar extent. This finding may illuminate potential mechanisms underlying the lower informational conformity observed in the non-religious group. Specifically, it suggests that non-religious individuals’ lower conformity at the LPP level is not likely attributable to a lack of attention or greater skepticism about the authenticity of the peer-ratings. Rather, non-religious individuals appear to have effectively encoded the peer-ratings, but did not subsequently use these ratings to inform personal judgments of facial attractiveness. More broadly, the lower conformity at the LPP level observed in non-religious individuals thus appears to have arisen from reduced assimilation – rather than reduced encoding – of peer-information into one’s personal evaluations.
Prior studies have shown interesting cognitive differences between non-religious and religious individuals. In particular, those high on religious conviction show a dampened neural response to errors (i.e. the error-related negativity; ERN) arising from the anterior cingulate cortex (Inzlicht et al., 2009). Furthermore, priming religious concepts (Inzlicht and Tullett, 2010) as well as thoughts of God’s benevolent nature (Good et al., 2015) among the faithful elicits this effect. Inzlicht et al. (2011) argue that such effects may account for the anxiety-reducing impact of religion. Using a ‘motivated meaning-making’ model of religion, they propose that religious belief systems offer individuals a way to create and sustain meaning (i.e. perceived coherence between mental representations), allowing them to view the world as an orderly place.
Interestingly, like response conflict (Braver et al., 2001), a failure to conform to group norms also elicits error-related signals in the brain (Klucharev et al., 2009). It is possible that an aversion towards social deviance and an aversion towards response conflict are both supported by similar mechanisms that favor consistency and coherence in the mental representations underlying one’s cognitive machinery (i.e., Festinger, 1957). To the extent that individuals value peer-information as input to inform their mental representations of the world, deviating from group norms could threaten such internal consistency.
Our findings also bear on the theoretical proposals of Graham and Haidt (2010), who argue that a core function of religion is to promote group cohesion. According to their social-functionalist perspective, the central appeal of religion lies not in the benefits conferred by specific belief systems, but rather in the group cooperation and communality that emerges as individuals organize their behaviors around these beliefs. We believe that a plausible hypothesis is that individuals with a reduced tendency towards social conformity are less amenable to the social-binding functions of religion, and are therefore less inclined to become religious; however, claims to causality depend on manipulating either religiosity or social conformity. Our findings add to Graham and Haidt’s theorizing by raising the possibility that social cohesion and the ensuing pressures to conform to group norms may offer a crucial mechanism by which religious beliefs themselves become entrenched: that is, greater social cohesion might not only be an end (i.e., functional consequence) of religion, but it could also offer via conformity pressures a central means through which religious belief systems enter and remain in the minds of the faithful. Crucially then, our finding of a link between informational conformity and religiosity highlights the possibility that the social-cohesive (Graham and Haidt, 2010) and motivated meaning-making (Inzlicht et al., 2011) functions of religion could interact with each other.
Although our findings are consistent with a priori theoretical predictions about the causal impact of social conformity on the rejection of religious faith, strong claims of a causal nature cannot be made from the present data as we did not manipulate conformity. Future studies can strive to experimentally alter social conformity in religious individuals (e.g., by manipulating perceived group size), and examine whether this impacts their momentary endorsement of faith-based doctrines.
We have argued that, since people adopt religious doctrines on faith (i.e., without available personal evidence), social conformity may be a critical mechanism for the transmission of religious belief. In predominantly religious societies, a diminished tendency towards conformity may therefore disincline individuals towards religion. An intriguing question for future research is whether this relationship also holds in predominantly secular societies. One possibility is that a lower tendency towards conformity simply leads individuals to reject the status quo or the prevailing set of social norms; thus, in strongly secular societies, those with decreased conformity may be more—rather than less—likely to adopt religion. Another scenario—one that we believe is more plausible—is that diminished conformity renders individuals less likely to adopt others’ beliefs on faith; thus, insofar as religion depends on faith, lower conformity would be associated with less religiosity even in secular societies.
One limitation is that we did not manipulate peer-influences in the low-attractive faces. We decided against doing so primarily because generating peer-ratings to decrease expectations in the low-attractive faces (i.e. by subtracting 1.8 points from the mean attractiveness level for each face obtained from our independent sample) would attach a peer-rating value of 1 (the lowest value on our scale) to a substantial number of faces. We believed this would likely produce suspicion about the authenticity of the peer-ratings and thus did not include such a manipulation. Future research can potentially address this limitation by using faces of moderate (rather than low) attractiveness to allow investigators to generate sufficiently low peer-ratings for each face.
Conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
In order to mask the study’s focus on religiosity, this question was embedded within a larger set of irrelevant questions (e.g., about college major, Facebook usage, etc.).
Since all of our religious and non-religious participants were blind to the study’s true goals when they first agreed to complete the screening survey, self-selection is unlikely to explain any of the differences between the two groups reported in this study.
In a few cases, adding 1.8 points to a face with an initially high mean attractiveness score produced a value greater than 9 (the upper limit on our rating scale); we assigned those faces a maximum peer-rating value of 9.
The number (standard deviation) of trials remaining in the High-attractive: High peer-rating, High-attractive: Low peer-rating, and Low-attractive trial types within each Group are as follows: 24.67 (3.64), 24.41 (3.79), 48.16 (7.31) in the Non-religious group; 23.77 (4.91), 23.95 (5.16), 47.18 (9.68) in the Religious group.
For these exploratory analyses, to extract the LPP during the peer-rating window, we used a processing path nearly identical to that employed for the face-elicited LPP reported earlier. However, as there were a substantial amount of eye-blinks during the peer-rating window, we corrected for eye-blinks using the widely employed Gratton et al. (1983) ocular artifact procedure rather than discard trials containing eye-blinks. The LPP was again coded at site Pz. One additional participant in the Non-Religious group was excluded from these exploratory analyses due to a large number (greater than 60%) of unusable trials.
Although peer-ratings powerfully altered anticipatory arousal in the 500–1000 ms range in both groups, this effect disappeared by the end of the peer-rating presentation window: during the final 2500–3000 ms range of the peer-rating slide, there was no LPP difference between High-attractive face: High peer-ratings and High-attractive face: Low peer-ratings in either group (both ps > 0.42). Thus, it is unlikely that the anticipatory arousal generated upon first seeing the peer-rating carried forward and influenced the LPP that was subsequently elicited by the face in each trial.
References
- Asch S.E. (1951). Effects of group pressure upon the modification and distortion of judgments. Groups, Leadership, and Men, 222–36. [Google Scholar]
- Bradley, M. M., Hamby, S., Löw, A., Lang, P. J. (2007). Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology, 44(3), 364–73. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex, 11(9), 825–36. [DOI] [PubMed] [Google Scholar]
- Brooks A.C. (2008). Gross National Happiness: Why Happiness Matters for America—and How We Can Get More of It. New York: Basic Books. [Google Scholar]
- Cialdini R.B., Goldstein N.J. (2004). Social influence: Compliance and conformity. Annual Review of Psychology, 55, 591–621. [DOI] [PubMed] [Google Scholar]
- Chartrand T.L., Bargh J.A. (1999). The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology, 76(6), 893.. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Danchin É., Giraldeau L.A., Valone T.J., Wagner R.H. (2004). Public information: from nosy neighbors to cultural evolution. Science, 305(5683), 487–91. [DOI] [PubMed] [Google Scholar]
- Dawkins R. (2016). The god delusion. Random House. [Google Scholar]
- De Cesarei, A., Codispoti, M. (2006). When does size not matter? Effects of stimulus size on affective modulation. Psychophysiology, 43(2), 207–15. [DOI] [PubMed] [Google Scholar]
- de Rover M., Brown S.B., Boot N., et al. (2012). Beta receptor-mediated modulation of the late positive potential in humans. Psychopharmacology, 219(4), 971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch M., Gerard H.B. (1955). A study of normative and informational social influences upon individual judgment. The Journal of Abnormal and Social Psychology, 51(3), 629.. [DOI] [PubMed] [Google Scholar]
- Festinger L. (1957). Cognitive Dissonance Theory. 1989) Primary Prevention of HIV/AIDS: Psychological Approaches. Newbury Park, California, Sage Publications. [Google Scholar]
- Foti D., Hajcak G. (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20(6), 977–88. [DOI] [PubMed] [Google Scholar]
- Good M., Inzlicht M., Larson M.J. (2015). God will forgive: reflecting on God’s love decreases neurophysiological responses to errors. Social Cognitive and Affective Neuroscience, 10(3), 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J., Haidt J. (2010). Beyond beliefs: Religions bind individuals into moral communities. Personality and Social Psychology Review, 14(1), 140–50. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–84. [DOI] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35(2), 129–55. [DOI] [PubMed] [Google Scholar]
- Henrich J., Boyd R. (1998). The evolution of conformist transmission and the emergence of between-group differences. Evolution and Human Behavior, 19(4), 215–41. [Google Scholar]
- Henrich J., McElreath R. (2003). The evolution of cultural evolution. Evolutionary Anthropology: Issues, News, and Reviews, 12(3), 123–35. [Google Scholar]
- Inzlicht M., McGregor I., Hirsh J.B., Nash K. (2009). Neural markers of religious conviction. Psychological Science, 20(3), 385–92. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam, R., Hajcak, G., Gross, J. J. (2012). Looking inward shifting attention within working memory representations alters emotional responses. Psychological Science, 23(12), 1461–6. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Tullett A.M. (2010). Reflecting on god religious primes can reduce neurophysiological response to errors. Psychological Science, 21(8), 1184–90. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Tullett A.M., Good M. (2011). The need to believe: A neuroscience account of religion as a motivated process. Religion, Brain & Behavior, 1(3), 192–212. [Google Scholar]
- Johnston V.S., Oliver-Rodriguez J.C. (1997). Facial beauty and the late positive component of event-related potentials. Journal of Sex Research, 34, 188–98. [Google Scholar]
- Klucharev V., Hytönen K., Rijpkema M., Smidts A., Fernández G. (2009). Reinforcement learning signal predicts social conformity. Neuron, 61(1), 140–51. [DOI] [PubMed] [Google Scholar]
- Kundu P., Cummins D.D. (2013). Morality and conformity: The Asch paradigm applied to moral decisions. Social Influence, 8(4), 268–79. [Google Scholar]
- Liu Y., Huang H., McGinnis-Deweese M., Keil A., Ding M. (2012). Neural substrate of the late positive potential in emotional processing. The Journal of Neuroscience, 32(42), 14563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner J.K., Rouby D.A., Gonzaga G.C. (2008). Automatic inattention to attractive alternatives: The evolved psychology of relationship maintenance. Evolution and Human Behavior, 29(5), 343–9. [Google Scholar]
- Mason M.F., Dyer R., Norton M.I. (2009). Neural mechanisms of social influence. Organizational Behavior and Human Decision Processes, 110(2), 152–9. [Google Scholar]
- Morgan L.K., Kisley M.A. (2014). The effects of facial attractiveness and perceiver's mate value on adaptive allocation of central processing resources. Evolution and Human Behavior, 35(2), 96–102. [Google Scholar]
- Moscovici S., Lage E., Naffrechoux M. (1969). Influence of a consistent minority on the responses of a majority in a color perception task. Sociometry, 365–80. [PubMed] [Google Scholar]
- Petty R., Cacioppo J.T. (1986). The elaboration likelihood model in persuasion In: Berkowitz L., editors. Advances in Experimental Social Psychology, Vol. 19, pp. 123–192. New York, NY: Academic Press. [Google Scholar]
- Sabatinelli D., Lang P.J., Keil A., Bradley M.M. (2007). Emotional perception: correlation of functional MRI and event-related potentials. Cerebral Cortex 17(5),1085–91. [DOI] [PubMed] [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A.. 2002. E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools, Inc. [Google Scholar]
- Sherif M. (1936). The psychology of social norms.
- Stallen M., Smidts A., Sanfey A. (2013). Peer influence: neural mechanisms underlying in-group conformity. Frontiers in Human Neuroscience, 7, 50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R., Blechert J., Sheppes G., Rydstrom A., Gross J.J. (2011). The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology, 87(1), 84–92. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R., Hajcak G., Gross J.J. (2012). Looking inward shifting attention within working memory representations alters emotional responses. Psychological Science, 23(12), 1461–6. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R., Harper J., Homer A.L. (2016). Beauty is in the belief of the beholder: cognitive influences on the neural response to facial attractiveness. Social Cognitive and Affective Neuroscience, 11(12), 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooff J.C., Crawford H., van Vugt M. (2010). The wandering mind of men: ERP evidence for gender differences in attention bias towards attractive opposite sex faces. Social, Cognitive, and Affective Neuroscience, 6, 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Schirmer J., Mitchell J.P. (2011). Social influence modulates the neural computation of value. Psychological Science, 22(7), 894–900. [DOI] [PubMed] [Google Scholar]
- Zuckerman P. (2005). Atheism: Contemporary rates and patterns In Martin M. (Ed.), The Cambridge Companion to Atheism (pp. 47–67). Cambridge, England: Cambridge University Press. [Google Scholar]