Abstract
In order to evaluate the potential risk of nelfinavir (NFV) accumulation in human immunodeficiency virus (HIV)-hepatitis C virus (HCV)-coinfected patients with liver disease, we investigated the concentrations of NFV and M8, the active metabolite of NFV, in plasma HIV-positive (HIV+) patients coinfected with HCV. A total of 119 HIV+ subjects were included in our study: 67 HIV+ patients, 32 HIV+ and HCV-positive (HCV+) patients without cirrhosis, and 20 HIV+ and HCV+ patients with cirrhosis. Most of the enrolled patients (chronically treated) were taking NFV at the standard dosage of 1,250 mg twice a day. To assay plasma NFV and M8 concentrations, patients underwent serial plasma samplings during the dosing interval at steady state. Plasma NFV and M8 concentrations were measured simultaneously by a high-performance liquid chromatography method with UV detection. The HIV+ and HCV+ patients with and without cirrhosis had significantly lower NFV oral clearances than the HIV+ and HCV-negative individuals (28 and 58% lower, respectively; P < 0.05), which translated into higher areas under the concentration-time curves for cirrhotic and noncirrhotic patients. The NFV absorption rate was significantly lower in cirrhotic patients, resulting in a longer time to the maximum concentration in serum. The mean ratios of the M8 concentration/NFV concentration were significantly lower (P < 0.05) in HIV+ and HCV+ subjects with cirrhosis (0.06 ± 0.074) than in the subjects in the other two groups. The mean ratios for M8 and NFV were not statistically different between HIV+ and HCV-negative patients (0.16 ± 0.13) and HIV+ and HCV+ patients without cirrhosis (0.24 ± 0.17), but the interpatient variability was high. Our results indicate that the pharmacokinetics of NFV and M8 are altered in HIV+ and HCV+ patients, especially those with liver cirrhosis. Therefore, there may be a role for therapeutic drug monitoring in individualizing the NFV dosage in HIV-HCV-coinfected patients.
More than one-third of human immunodeficiency virus (HIV)-positive (HIV+) patients worldwide are coinfected with hepatitis C virus (HCV), as these viruses share some of the same modalities of transmission. Coinfection may reach a prevalence in excess of 50% in selected populations and in certain countries (9, 14, 22, 23). Patients coinfected with HIV and HCV are more susceptible to progression to cirrhosis and to end-stage liver disease than their monoinfected HCV-positive (HCV+) counterparts, as indicated by the increasing rates of hospitalization and death caused by liver conditions in observational cohorts (19). Moreover, liver toxicity associated with highly active antiretroviral therapy (HAART) is definitely more frequent in patients coinfected with HCV or hepatitis B virus (HBV) (7, 16, 21, 25).
Increased drug-associated liver toxicity in HIV-HCV-coinfected patients may be partially explained by the fact that antiretroviral compounds are generally metabolized by the liver and the changes induced by chronic viral infections hinge on the different metabolic pathways involved in drug metabolism. Unlike in renal failure (in which there is a linear correlation between creatinine clearance and the levels of drugs in plasma metabolized mainly by the kidneys), there is no standardized test to predict the effects of liver changes during chronic hepatitis on drug elimination, since elevated liver enzyme levels reflect cellular damage more than they reflect functional impairment (24).
As the liver damage progresses, the metabolizing capabilities of members of the cytochrome P450 enzyme family decrease (2) and increased concentrations of antiretrovirals are likely to be found in plasma (27).
For some antiretroviral drugs, increased levels in plasma have been shown to be associated with increased toxicity (6, 11, 12, 15).
Nelfinavir (NFV), like the other presently available protease inhibitors, is extensively metabolized by the hepatic cytochrome P450 system, mainly by the isoenzymes CYP3A4, CYP2C19, and CYP2D6, into its main oxidative metabolite, hydroxy-t-butylamide (M8). The generation of M8 appears to be exclusively catalyzed by the CYP2C19 isoenzyme.
As expected for a drug that undergoes hepatic metabolism, previous studies have suggested the potential risk of NFV accumulation in HIV-HCV-coinfected patients with liver disease, which may lead to higher toxicity rates (20, 26).
We investigated the levels of NFV and M8 in the plasma of HIV-HCV-coinfected patients and compared the findings with those for a control population.
MATERIALS AND METHODS
Study design.
This was a multicenter pharmacokinetic trial with HIV+ patients receiving HAART, including NFV plus two nucleoside reverse transcriptase inhibitors (NRTIs). Study data were collected from 15 infectious diseases centers in central and northern Italy (Ancona, Bolzano, Ferrara, Macerata, Mantua, Mestre, Modena, Parma, Ravenna, Rimini, Trento, Treviso, Trieste, Verona, and Vicenza) and were analyzed at the coordinating center, the Infectious Diseases Department of San Matteo Hospital in Pavia. Pharmacokinetic assays were centralized at the Pharmacology Department of the same institution.
Approval was first obtained from the institutional ethics committee of the coordinating center (Pavia) and then from those at the other participating centers. All patients enrolled in the study signed a written informed consent before they were started on any study procedure and were submitted to an accurate physical examination. Biochemical, immunological, and virological tests were performed at the baseline. Further liver function tests were carried out for the patients identified as HIV-HCV coinfected. Cirrhosis, diagnosed by liver biopsy or suggestive ultrasound imaging and biochemical plus clinical findings, was classified according to the Child-Pugh score (18).
The patients enrolled in the study were thus divided into three groups: HIV-HCV-coinfected patients without hepatic cirrhosis, HIV-HCV-coinfected patients with hepatic cirrhosis, and HIV+ patients without HCV infection (control subjects).
After the baseline procedures were performed, we assayed the patients' plasma for the concentrations of NFV and its metabolite, M8, as described below.
Study objectives.
The primary aim of the pharmacological study was to evaluate the pharmacokinetic parameters for NFV and M8 and their variabilities in HIV-HCV-coinfected patients and in HIV+ controls with negative serologic results for hepatitis.
Subjects.
All adult HIV+ patients (age range, 18 to 60 years) receiving NFV-based HAART (NFV plus two NRTIs) for at least 1 month were considered for enrollment in the study. This allowed the participation of both individuals receiving first-line antiretroviral therapy and experienced patients with a history of previous HAART or dual NRTI regimens. All study participants were required to sign a written informed consent, to attend the scheduled visits regularly, and to adhere to the HAART regimen.
The exclusion criteria were (i) pregnancy or breast-feeding, (ii) abuse of alcohol or other substances, (iii) therapy with drugs known to alter the pharmacokinetic parameters of NFV or to affect cytochrome P450 activity, (iv) therapy with nonnucleoside reverse transcriptase inhibitors, and (v) unstable clinical conditions (including HIV-related opportunistic infections and tumors).
Study drugs.
Most of the patients enrolled in the study were taking NFV at the standard dosage of 1,250 mg twice a day (b.i.d.).
NFV administration on the day that the samples were collected for pharmacokinetic analysis was not witnessed: the patients were asked to record the time that they had last taken an NFV dose on the day before the visit.
Protease inhibitors other than NFV were not allowed in the protocol.
Sample collection.
In order to assay plasma NFV and M8 concentrations, patients (chronically treated) underwent serial plasma samplings (seven or more times) during the dosing interval at steady state. Samples were collected at the following times after NFV ingestion: 0 h (to measure the trough concentration [Ctrough], i.e., the concentration just before NFV administration); 1, 2, 3, 4, 5, and 6 h; and 8 h for the regimen of NFV administration three times a day and 12 h for the b.i.d. regimen.
As part of the standard counseling for patients receiving NFV, the specific recommendation that the drug be taken with food was made before the start of treatment and at each visit.
Plasma samples were separated, inactivated in a bath at 56°C for 45 min, and then frozen at −20°C until analyses were performed. Plasma NFV and M8 concentrations were measured simultaneously by a modified high-performance liquid chromatography method with UV detection (28) validated over concentration ranges of 0.05 to 10.0 μg/ml for NFV and 0.03 to 5.0 μg/ml for M8 by using 250 μl of plasma. The rates of recovery of NFV and M8 from human plasma were 95.3 and 93.8%, respectively.
The intraday coefficients of variation of NFV and M8 quality control (QC) concentrations were 9.8 and 9.5%, respectively, for the low QC concentration and 7.2 and 8.4%, respectively, for the high QC concentration. The interday coefficients of variation for NFV and M8 were 8.9 and 9.3%, respectively, for the low QC concentration and 6.9 and 7.5%, respectively, for the high QC concentration. The corresponding accuracy rates ranged from 96.7 to 108.3%.
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic parameters were calculated by standard methods. The peak concentration (Cmax) was taken as the maximum observed concentration in serum, and the time to Cmax (Tmax) was taken as the sampling time when Cmax was observed. The areas under the plasma concentration-time curve (AUCs) from 0 to 8 h (AUC0-8) for the 8-h regimen and from 0 to 12 h (AUC0-12) for the 12-h regimen were calculated by using the linear trapezoidal rule. The apparent oral clearance, corrected for body weight [(CL/F) per kilogram of body weight, where F indicates oral bioavailability], was obtained by dividing the ratio of the dose and the AUC by body weight. The ratio of the M8 concentration in plasma to the NFV concentration in plasma was also evaluated.
Statistical analysis.
Demographic and pharmacokinetic data were summarized as group means by using the standard deviation. Pharmacokinetic and statistical calculations were performed with KINETICA (version 4.0) software (INNAPHASE Corporation, Philadelphia, Pa.). One-way analysis of variance was used to examine any differences in the values of the pharmacokinetic parameters between the three groups. A P value of ≤0.05 was considered statistically significant for all tests.
Data abstraction.
Biochemical, virological, and clinical data were collected from each patient's clinical chart at every visit. Adherence was assessed with a self-reported semiquantitative scale and covered the previous 2 weeks.
Cirrhosis diagnosis was based on either the liver biopsy report (grade 4 fibrosis or cirrhosis) or clinical and ultrasonographic diagnostic criteria (liver surface nodularity, caudate lobe hypertrophy, and changes in hepatic venous flow) (1, 5, 8).
RESULTS
One-hundred nineteen patients were enrolled in the study and were subsequently divided into three groups according to their biochemical and clinical characteristics: 67 were HIV+ without HCV coinfection, 32 were HIV-HCV coinfected without cirrhosis, and 20 were HIV-HCV coinfected with cirrhosis. Two patients were HCV-HBV coinfected (HBsAg and HBV DNA positive). The results of liver biochemistry tests for the three groups of subjects are summarized in Table 1.
TABLE 1.
Baseline patient characteristics
| Parameter | HCV− (A) | HCV+ (B) | HCV+ with cirrhosis (C) | Significance (P < 0.05) |
|---|---|---|---|---|
| No. of patients | 67 | 32 | 20 | |
| Sex (no. of males/no. of females) | 46/21 | 24/8 | 12/8 | |
| Age (yr)a | 41 ± 8 | 42 ± 6 | 41 ± 6 | |
| Body wt (kg)a | 68.1 ± 12.0 | 69.9 ± 13.0 | 63.1 ± 10.0 | |
| ASTb concn (U/liter)a | 24.0 ± 14.6 | 47.8 ± 30.8 | 71.3 ± 38.1 | A vs B, C vs A and B |
| ALT concn (U/liter)a | 25.6 ± 20.8 | 62.8 ± 44.3 | 82.2 ± 35.5 | A vs B and C |
| No. of CD4+ cells/μla | 376.8 ± 244.6 | 462.14 ± 230.7 | 421.7 ± 288.8 |
Values are reported as means ± standard deviations.
AST, aspartate aminotransferase.
All patients were at steady state for NFV treatment, which was part of their antiretroviral regimen, at 1,250 mg b.i.d. (74% of patients) or 750 mg three times a day (17% of patients). About seven plasma samples were obtained from each patient and were analyzed for NFV and M8 pharmacokinetics by validated assays. The pharmacokinetics of NFV were measured in all 119 subjects, while the pharmacokinetics of M8 were monitored only in the last 71 consecutive patients enrolled after our laboratory received the pure substance (courtesy of Pfizer Inc.).
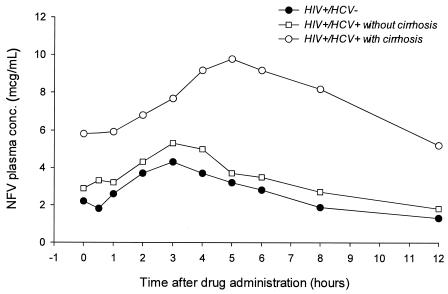
The mean plasma concentration-time profiles for NFV in HIV+ and HCV-negative (HCV−) patients and in HIV+ and HCV+ patients with and without cirrhosis are shown in Fig. 1; the values of the main pharmacokinetic parameters are given in Tables 2 and 3.
FIG. 1.
Plasma concentration-time curve for NFV in HIV+ and HCV− patients and in HIV+ and HCV+ patients with and without cirrhosis. Data are only for patients taking NFV every 12 h and were normalized to a regimen of 1,250 mg b.i.d.
TABLE 2.
Values of main NFV pharmacokinetic parameters for the three groups of HIV+ patientsa
| PKb parameter | HIV+ and HCV− (A)
|
HIV+ and HCV+ (B)
|
HIV+ and HCV+ with cirrhosis (C)
|
Significance (P < 0.05) | |||
|---|---|---|---|---|---|---|---|
| No. of patients | Value of PK parameter | No. of patients | Value of PK parameter | No. of patients | PK parameters | ||
| CL/F (liters/h/kg) | 67 | 0.68 ± 0.38 | 32 | 0.49 ± 0.19 | 20 | 0.29 ± 0.13 | A vs B and C, B vs C |
| Ctrough (μg/ml)c | 57 | 2.1 ± 1.5 | 23 | 2.8 ± 1.8 | 17 | 6.6 ± 3.6 | C vs B and A |
| Cmax (μg/ml)c | 38 | 4.7 ± 2.00 | 18 | 5.6 ± 2.5 | 12 | 11.3 ± 5.3 | C vs B and A |
| Tmax (h)c | 38 | 2.9 ± 1.0 | 18 | 3.0 ± 1.1 | 12 | 4.1 ± 1.4 | C vs B and A |
| AUC0-12 (μg · h/ml)c | 38 | 32.21 ± 10.81 | 18 | 40.25 ± 17.64 | 12 | 85.95 ± 44.70 | A vs B and C, B vs C |
Values are reported as means ± standard deviations.
PK, pharmacokinetic.
Data are only for patients taking NFV every 12 h; the values are normalized to 1,250 mg/12 h.
TABLE 3.
Values of main M8 pharmacokinetic parameters for the three groups of HIV-positive patientsa
| PKb parameter | HIV+ and HCV−
|
HIV+ and HCV+ without cirrhosis
|
HIV+ and HCV+ with cirrhosis
|
|||
|---|---|---|---|---|---|---|
| No. of patients | Value of PK parameter | No. of patients | Value of PK parameter | No. of patients | Value of PK parameter | |
| Ctrough (μg/ml)c | 36 | 0.4 ± 0.4 | 18 | 0.6 ± 0.7 | 17 | 0.2 ± 0.2 |
| Cmax (μg/ml)c | 18 | 1.4 ± 0.9 | 15 | 1.4 ± 1.1 | 13 | 0.8 ± 0.6 |
| Tmax (h)c | 18 | 3.8 ± 1.2 | 15 | 3.6 ± 0.8 | 10 | 4.1 ± 1.8 |
| AUC0-12 (μg · h/ml)c | 18 | 8.26 ± 5.50 | 15 | 6.21 ± 4.55 | 13 | 4.32 ± 4.3 |
| NFV + M8 AUC0-12 (μg · h/mL)c | 18 | 39.02 ± 14.03 | 15 | 46.71 ± 21.34 | 13 | 90.74 ± 51.34 |
| M8 AUC/NFV AUC ratio | 36 | 0.24 ± 0.17 | 19 | 0.16 ± 0.13 | 16 | 0.06 ± 0.07 |
Values are reported as means ± standard deviations.
PK, pharmacokinetic.
Data are only for patients taking NFV every 12 h; the values are normalized to 1,250 mg/12 h.
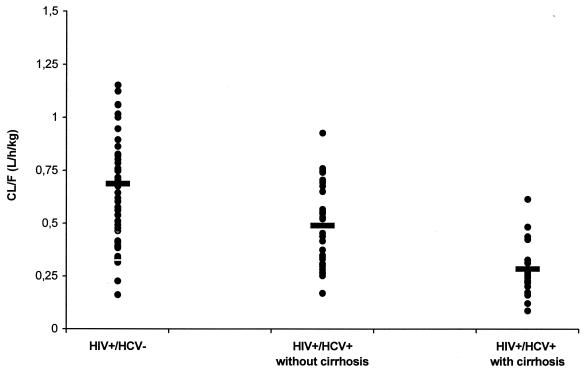
The HIV+ and HCV+ patients with and without cirrhosis had significantly lower NFV CL/F than the HIV+ and HCV− individuals (28 and 58% lower, respectively; P < 0.05), which translated into higher levels of systemic drug exposure in cirrhotic and noncirrhotic patients (Fig. 2). The AUCs for NFV in HIV+ and HCV+ patients with and without cirrhosis were about 2.5-fold and about 1.3-fold higher, respectively, than the corresponding value observed in HIV+ and HCV− patients.
FIG. 2.
Distribution of NFV CL/F in HIV+ and HCV− patients and in HIV+ and HCV+ patients with and without cirrhosis. Bars indicate mean values.
In contrast, the AUCs for M8 were reduced by about 50% and about 30% in HIV+ and HCV+ patients with and without cirrhosis, respectively, but the changes were not statistically significant.
The mean ratios of the M8 concentration/NFV concentration were significantly lower (P < 0.05) in HIV+ and HCV+ subjects with cirrhosis (0.06 ± 0.074) than in the other two groups. The mean ratios were not statistically different between the HIV+ and HCV+ patients without cirrhosis (0.16 ± 0.13) and the HIV+ and HCV− patients (0.24 ± 0.17), but the interpatient variability was high. Among the patients in the HIV+ and HCV− group, four patients had no measurable M8 concentrations.
For patients taking the b.i.d. NFV regimen, the mean peak concentrations of NFV (after normalization to 1,250 mg/12 h) were 4.7 ± 2.0 μg/ml in HIV+ and HCV− patients and were higher (7.8 ± 4.7 μg/ml; P < 0.05) in HIV-HCV-coinfected subjects. The same trend was observed for Ctrough. When the HIV+ and HCV+ group is considered, Cmaxs and Ctroughs were markedly higher in the patients with cirrhosis (Cmax, 11.3 ± 5.3 μg/ml; Ctrough, 6.6 ± 3.6 μg/ml) than in those without cirrhosis (Cmax, 5.6 ± 2.5 μg/ml; Ctrough, 2.8 ± 1.8 μg/ml). After oral administration the maximum concentration of NFV in plasma occurred significantly (P < 0.05) later (Tmax, 4.1 h) in HIV+ and HCV+ patients with cirrhosis than in HIV+ and HCV+ patients without cirrhosis (Tmax, 2.9 h). The Cmaxs and Ctroughs of M8 were markedly lower in HIV+ and HCV+ patients with cirrhosis than in HIV+ and HCV+ patients without cirrhosis and HIV+ and HCV− patients. The median Tmax was about 3.6 to 4.1 h after oral administration in all patient groups.
DISCUSSION
We evaluated plasma NFV levels in 67 HIV+ patients, 32 HIV+ and HCV+ patients without cirrhosis, and 20 HIV+ and HCV+ patients with cirrhosis.
The disposition of NFV in HIV-HCV-coinfected patients was altered compared to that in the population infected only with HIV. The plasma NFV concentrations were higher and the CL/Fs were lower in the HIV-HCV-coinfected patients than in the HIV+ and HCV− patients. This result is consistent with the data of Landman et al. (13). This difference appeared to be even greater when cirrhotic patients were compared to HCV− controls.
NFV is extensively metabolized by cytochrome P450 isozymes and produces two main metabolites: its main active hydroxy-t-butylamide metabolite (M8) and a minor metabolite (M1), which has not been shown to be active against HIV. CYP3A4 and CYP2C19 are the main contributors to NFV metabolism. CYP2C19 is a polymorphic enzyme that exclusively catalyzes the conversion of NFV to M8, which is in turn metabolized by CYP3A4.
NFV and M8 demonstrate similar antiretroviral activities on the basis of the 50% effective concentration in vitro, and the sum of the NFV concentration plus the M8 concentration in plasma may represent the cumulative antiviral activity after parent drug administration.
Disease states influence the metabolic capacity of the individual and alter cytochrome P450-dependent drug elimination. Not surprisingly, diseases with hepatic involvement are most commonly associated with impaired drug elimination (3).
Several viral and bacterial infections have been associated with impaired cytochrome P450-mediated drug metabolism. During infectious disease episodes the mixed-function oxidase system is depressed and the capability of the liver to metabolize drugs can be compromised in both animals and humans. This has been considered, at least partly, the result of cytokine release, since interleukin-2, interleukin-4, and gamma interferon have been shown to down-regulate the expression and catalytic activities of cytochrome P450 enzymes and genes. Some isoforms may be more susceptible than others to down-regulation (i.e., a reduction in protein content, most likely due to altered synthesis of the corresponding mRNA species), and previous observations indicated that CYP2C19 is exquisitely sensitive to the presence of liver disease (2). Therefore, HCV-infected patients are likely to have an impaired P450-dependent NFV metabolism (23).
The mechanisms of liver damage in HIV+ individuals coinfected with HCV are not well understood. HCV infection is not always associated with liver injury, since some HCV RNA-positive subjects may have a normal liver histology and normal liver biochemistry test results or may exhibit some degree of liver damage. Twenty to 30% of people infected with HCV have persistently normal alanine aminotransferase (ALT) levels (17). Most HCV+ individuals with persistently normal ALT levels exhibit less serious disease progression and milder disease. However, some of these patients with normal ALT levels do not fit so neatly into this category, and a small percentage of these patients may have moderate to severe liver damage.
Cirrhosis, a disease state most commonly associated with impaired P450-dependent drug elimination, can markedly affect the metabolic capacity to biotransform NFV. In our study, the HIV+ and HCV+ patients with cirrhosis had a significantly lower (weight-adjusted) NFV CL/F (58% lower; P < 0.001) than HIV+ and HCV− individuals. Significant but less marked reductions (28% lower; P < 0.05) were also seen in the HIV+ and HCV+ patients without cirrhosis. Moreover, in HIV-HCV-coinfected patients with cirrhosis, the NFV absorption rate was significantly lower, resulting in a longer Tmax.
M8 concentrations were markedly variable. CYP2C19 is a polymorphic enzyme whose activity is related to a specific gene sequence. This enzyme is completely deficient due to genetic constitution in about 3% of Caucasian individuals (29), making them pharmacokinetically like patients with liver disease with respect to drugs such as NFV, which has a clinically established CYP2C19-catalyzed metabolism. Therefore, changes in M8 concentrations may be due to an inherited CYP2C19 deficiency, disease-related hepatic dysfunction, or enzyme inhibition. While enzyme inhibition may be assessed from medical data, the differentiation between genetic and acquired CYP2C19 deficiency would require genotyping or coadministration of a nonpolymorphically metabolized test substrate. None of our patients was receiving drugs known to inhibit the CYP2C19 enzyme.
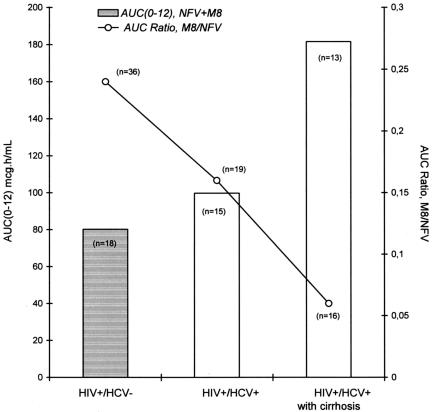
The ratio of the M8 concentration/NFV concentration was altered in HIV-HCV-coinfected patients with and without cirrhosis. The sum of the AUCs for NFV and M8 was found to be lowest in the HIV+ and HCV− group: the sum was 1.2-fold higher in the HIV+ and HCV+ patients without cirrhosis (P > 0.05) and 2.3-fold higher in the HIV+ and HCV+ patients with cirrhosis (P < 0.001) (Fig. 3). The increase in the NFV concentrations in HIV+ and HCV+ patients with and without cirrhosis appeared to be higher than the decrease in the M8 concentrations, thus leaving the sum of the NFV concentration plus that of its active metabolite slightly and markedly higher, respectively, than that in the control group. Most of the intergroup difference in the sum of the NFV concentration and that of its active metabolite was due more to changes in NFV concentrations than to changes in M8 concentrations.
FIG. 3.
Changes in AUC0-12s for NFV and M8 and in the M8 concentration/NFV concentration ratio in HIV+ and HCV− patients and in HIV+ and HCV+ patients with and without cirrhosis.
Our results indicate that liver cirrhosis considerably changes the pharmacokinetics of NFV and its active metabolite, M8, in the HIV+ and HCV+ patients, who had higher plasma NFV concentrations as a result of a lower CL/F than the HIV+ and HCV− patients. Such a change should not affect the interpretation of the plasma drug concentrations, expressed as the concentration of NFV plus that of its metabolite, in terms of efficacy, because in vitro data indicate that NFV and M8 have similar antiretroviral activities. On the other hand, more caution is needed when toxicity is considered, as M8 is less cytotoxic than NFV in vitro (10).
Our study was limited in that cirrhosis was defined by using uneven data from diagnostic imaging, clinical records, or, when they were available, histological findings. Moreover, we grouped together individuals with different Child-Pugh scores, and this could have masked the real impact of different disease stages on the pharmacokinetics of NFV. However, only 2 of 14 patients belonged to Child-Pugh class B, and 1 patient belonged to Child-Pugh class C and had had many episodes of liver decompensation before enrollment in the study. The fact that two other patients were also infected with HBV may add to the population heterogeneity, but their liver function tests showed no significant differences from those for the rest of the group.
Even with these limitations, our findings can help define the NFV disposition characteristics in different populations of HIV-HCV-coinfected subjects.
The latest available guidelines maintain that hepatopathic patients are among those most likely to benefit from therapeutic drug monitoring (http://aidsinfo.nih.gov). Our results demonstrated large interpatient variations in the relationship between the NFV dose and plasma NFV concentrations in subjects with different degrees of liver impairment. Therefore, the application of pharmacokinetic analysis to hepatopathic patients might greatly decrease the changes in the plasma NFV concentration. The assumption of a clear relationship between the NFV concentration and the pharmacological effect has been demonstrated only in terms of antiretroviral efficacy in HIV+ drug treatment-naïve patients and not in terms of toxicity (4). The development of diarrhea, the most common side effect of NFV, has not been shown to correlate with plasma NFV levels (20). This does not mean that other adverse events may not be related to increased NFV exposure in the medium or long term. In fact, at least one report (26) has linked high plasma NFV levels and lipodystrophy.
In conclusion, there may be a role for therapeutic drug monitoring in individualizing the NFV dosage in HIV-HCV-coinfected patients who, as demonstrated, often have high plasma drug levels. This may lead to the long-needed individualization of antiretroviral drug dosing, with a significant reduction in costs, pill burden, and, possibly, long-term treatment-related toxicities.
REFERENCES
- 1.Aube, C., F. Oberti, N. Korali, M. A. Namour, D. Loisel, J. Y. Tanguy, E. Valsesia, C. Pilette, M. C. Rousselet, P. Bedossa, H. Rifflet, M. Y. Maiga, D. Penneau-Fontbonne, C. Caron, and P. Cales. 1999. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J. Hepatol. 30:472-478. [DOI] [PubMed] [Google Scholar]
- 2.Branch, R. A. 1998. Drugs in liver disease. Clin. Pharmacol. Ther. 64:462-464. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer, K. L. R., G. E. Dukes, and J. R. Powell. 1996. Influence of liver function on drug disposition. .In W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics, principles of therapeutic drug monitoring. Applied Therapeutics, Vancouver, Wash.
- 4.Burger, D., P. Hugen, P. Reiss, I. Gyssens, M. Schneider, F. Kroon, G. Schreij, K. Brinkman, C. Richter, J. Prins, R. Aarnoutse, J. Lange, and the ATHENA Cohort Study Group. 2003. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naïve HIV-1-infected individuals. AIDS 17:1157-1165. [DOI] [PubMed] [Google Scholar]
- 5.Colli, A., M. Fraquelli, B. Marino, E. Zuccoli, and D. Conte. 2003. Severe liver fibrosis or cirrhosis: accuracy of US for detection—analysis of 300 cases. Radiology 227:89-94. [DOI] [PubMed] [Google Scholar]
- 6.Dielemann, J. P., I. C. Gyssens, M. E. van der Ende, S. de Marie, and D. M. Burger. 1999. Urological complaints in relation to indinavir plasma concentrations in HIV infected patients. AIDS 13:473-478. [DOI] [PubMed] [Google Scholar]
- 7.Dieterich, D. T., M. Fischl, and G. Sepulveda. 2002. The safety/efficacy of protease inhibitors (PIs) in hepatitis C co-infected patients, board 1729. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 8.Di Lelio, A., C. Cestari, A. Lomazzi, and L. Beretta. 1989. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 172:389-392. [DOI] [PubMed] [Google Scholar]
- 9.Dodig, M., and A. S. Tavill. 2001. Hepatitis C and human immunodeficiency virus coinfections. J. Clin. Gastroenterol. 33:367-374. [DOI] [PubMed] [Google Scholar]
- 10.Donahue, J. P., D. Dowdy, K. K. Ratnam, T. Hulgan, J. Price, D. Unutmaz, J. Nicotera, S. Raffanti, M. Becker, and D. W. Haas. 2003. Effects of nelfinavir and its M8 metabolite on lymphocyte P-glycoprotein activity during antiretroviral therapy. Clin. Pharmacol. Ther. 73:78-86. [DOI] [PubMed] [Google Scholar]
- 11.Gatti, G., A. D. Biagio, R. Casazza, C. De Pascalis, M. Bassetti, M. Cruciani, S. Vella, and D. Bassetti. 1999. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS 13:2083-2089. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez, F., S. Padilla, A. Navarro, M. Masia, I. Hernandez, J. A. Ramos Esteban, and A. Martin-Hidalgo. 2003. Lopinavir plasma concentration and changes in lipid levels during salvage therapy with lopinavir/ritonavir-containing regimens. J. Acquir. Immune Defic. Syndr. 15:3592-3600. [DOI] [PubMed] [Google Scholar]
- 13.Landman, R., G. Peytavin, C. Lamotte, H. Aumaitre, A. Trylesinsky, S. Legag, F. Mentre, F. Brun-Vezinet, R. Farinotti, P. Yeni, and the LIVIR IMEA 014 Study Group. 2001. 2nd International Workshop on Clinical Pharmacology, abstr. 6.4.
- 14.Lauer, G., and B. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 15.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1 infected individuals. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 16.Nunez, M., R. Lana, J. Mendoza, L. Martin-Carbonero, and V. Soriano. 2001. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 27:426-431. [DOI] [PubMed] [Google Scholar]
- 17.Pradat, P., A. Alberti, T. Poynard, J. I. Esteban, O. Weiland, P. Marcellin, S. Badalamenti, and C. Trepo. 2003. Predictive value of ALT levels for histologic findings in chronic hepatitis C: a European collaborative study. Hepatology 37:950-951. [DOI] [PubMed] [Google Scholar]
- 18.Pugh, R. N. H., I. M. Murray-Lyon, J. L. Dawson, M. C. Pietroni, and R. Williams. 1973. Transection of the esophagus in bleeding oesophageal varices. Br. J. Surg. 60:648-652. [DOI] [PubMed] [Google Scholar]
- 19.Qurishi, N., C. Kreutberg, and G. Luchters. 2003. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitits C coinfection. Lancet 362:1708-1713. [DOI] [PubMed] [Google Scholar]
- 20.Reijers, M. H. E., H. M. Weigel, A. A. M. Hart, R. W. Ten Kate, J. W. Mulder, P. Reiss, H. Schuitemaker, Hoetelmans, R. M., G. J. Weverling, and J. M. Lange. 2000. Toxicity and drug exposure in a quadruple drug regimen in HIV-1 infected patients participating in the ADAM study. AIDS 14:59-68. [DOI] [PubMed] [Google Scholar]
- 21.Saves, M., S. Vandentorren, V. Daucourt, C. Marimoutou, M. Dupon, P. Couzigou, N. Bernard, P. Mercie, and F. Dabis. 1999. Severe hepatic cytolysis: incidence and risk factors in patients treated with antiretroviral combinations. AIDS 17:115-121. [DOI] [PubMed] [Google Scholar]
- 22.Sherman, K., S. Roustrer, R. Chung, and N. Rajicic. 2000. Hepatitis C: prevalence in HIV-infected patients: a cross-sectional analysis of the US ACTG. Antivir. Ther. 5(Suppl. 1):64-65. [Google Scholar]
- 23.Soriano, V., J. Garcìa-Samaniego, R. Rodriguez-Rosado, J. Gonzalez, and J. Pedreira. 1999. Hepatitis C and HIV infection: biological, clinical and therapeutic implications. J. Hepatol. 31(Suppl. 1):119-123. [DOI] [PubMed] [Google Scholar]
- 24.Soriano, V., O. Kirk, F. Antunes, M. Johnson, A. d'Arminio Monforte, L. S. Teglbjorg, F. D. Goebel, and J. D. Lundgren. 2000. The influence of hepatitis C on the prognosis of HIV: the EuroSIDA Study, abstr.ThOrB655. XIIIth International AIDS Conference.
- 25.Soriano, V., M. Sulkowski, C. Bergin, A. Hatzakis, P. Cacoub, C. Katlama, A. Cargnel, S. Mauss, D. Dieterich, S. Moreno, C. Ferrari, T. Poynard, and J. Rockstroh. 2002. Editorial review—care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. AIDS 16:813-828. [DOI] [PubMed] [Google Scholar]
- 26.Treluyer, J. M., J. P. Morini, J. Dimet, R. E. Gorin, J. Deleuze, P. F. Ceccaldi, J. P. Escande, G. Pons, and N. Dupin. 2002. High concentration of nelfinavir as an independent risk factor for lipodystrophy in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 46:4009-4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veronese, L., J. Rautareau, B. M. Sadler, and C. Gillotin. 2000. Single-dose pharmacokinetics of amprenavir, a human immunodeficency virus type 1 protease inhibitor, in subjects with normal and impaired hepatic function. Antimicrob. Agents Chemother. 44:821-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, E. Y., J. M. Wilkinson, D. G. Naret, V. L. Daniels, L. J. Williams, and D. A. Khalil. 1997. High-performance liquid chromatographic method for the determination of nelfinavir, a novel HIV-1 protease inhibitor, in human plasma. J. Chromatogr. B Biochem. Sci. Appl. 695:373-380. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, K. E., E. Wu, A. K. Patick, B. Kerr, M. Zorbas, A. Lankford, T. Kobayashi, Y. Maeda, B. Shetty, and S. Webber. 2001. Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: structural identification, levels in plasma and antiviral activity. Antimicrob. Agents Chemother. 45:1086-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]