Abstract
Fear acquisition and extinction have been demonstrated as core mechanisms for the development and maintenance of mental disorders, with different contributions of processing cues vs contexts. The hypothalamic peptide oxytocin (OXT) may have a prominent role in this context, as it has been shown to affect fear learning. However, investigations have focused on cue conditioning, and fear extinction. Its differential role for cue and context fear acquisition is still not known. In a randomized, double-blind, placebo (PLC)-controlled design, we administered an intranasal dose of OXT or PLC before the acquisition of cue and context fear conditioning in healthy individuals (n = 52), and assessed brain responses, skin conductance responses and self-reports (valence/arousal/contingency). OXT compared with PLC significantly induced decreased responses in the nucleus accumbens during early cue and context acquisition, and decreased responses of the anterior cingulate cortex and insula during early as well as increased hippocampal response during late context, but not cue acquisition. The OXT group additionally showed significantly higher arousal in late cue and context acquisition. OXT modulates various aspects of cue and context conditioning, which is relevant from a mechanism-based perspective and might have implications for the treatment of fear and anxiety.
Keywords: oxytocin, fear conditioning, cue, context, magnetic resonance imaging
Introduction
Learning about environmental threats and the flexibility to adapt our behavior accordingly are important for our every-day life and associated with the development of fear and anxiety and related mental disorders (e.g. Morey et al., 2015; Sun et al., 2015).
To investigate fear learning processes, conditioning has been employed to assess the acquisition, consolidation, extinction and expression of fear in both human and rodent studies (Bustos et al., 2009; Nader and Einarsson, 2010). In pavlovian cue fear conditioning, an initially neutral or conditioned stimulus (CS) is repeatedly paired with an aversive unconditioned stimulus (US) until its presentation alone elicits a conditioned response (CR), often but not always similar to the unconditioned response (UR) (Pavlov, 1927). In context conditioning, an external situation (e.g. a certain spatial configuration) or internal state (e.g. certain mood) serves as CS that is less predictable than the CS in cue conditioning. Whereas cue conditioning rather induces fear, context conditioning has been related to anxiety.
Pharmacological interventions have been employed to unravel the neurobiological mechanisms of fear learning and as targets for interventions: to facilitate extinction of an aversive learned memory (Leslie et al., 2005; Do Monte et al., 2013) or to disrupt the reconsolidation of a fear memory (Nikitin et al., 2015; Otis et al., 2015).
One pharmacological target that has become more and more prominent in the context of anxiety disorders (Striepens et al., 2011; Knobloch et al., 2012; Eckstein et al., 2014) within the recent years is oxytocin (OXT). OXT is a small nonapeptide synthesized in the hypothalamus, which affects the hypothalamic-pituitary adrenal axis (Neumann et al., 2000), and acts centrally as a neurotransmitter and peripherally as a hormone (Churchland and Winkielman, 2012). It was demonstrated to be a key substrate with respect to social contexts, for example in social group living and pair bonds in humans (Scheele et al., 2012). Endogenous concentrations of OXT strongly act during situations of psychosocial or physiological challenge including changes in anxiety (Neumann and Slattery, 2016). OXT compared with placebo (PLC) resulted in enhanced social fear extinction (Acheson and Risbrough, 2015; Hou et al., 2015; Eckstein et al., 2016), and enhanced extinction recall, indicated by reduced startle responses to the CS (Acheson et al., 2013), or in attenuated negative evaluation of previously fear conditioned faces, when being administered after conditioning (Petrovic et al., 2008). Moreover, a single systemic injection of OXT after the reactivation of learned fear resulted in impaired reconsolidation of social fear memories (Hou et al., 2015). With respect to the acquisition of fear, a suppression of any recall effect for learned, social and non-social, stimuli was observed when OXT was administered 50 min before fear acquisition (Heinrichs et al., 2003; Rimmele et al., 2009; Weigand et al., 2013). Moreover, OXT resulted in heightened behavioral and skin conductance responses and increased responses during social fear acquisition (Eckstein et al., 2016). This indicates enhanced learning of fear-associated stimuli and suggests that OXT may enable a rapid adaptation to socially related fear signals.
Brain regions involved in fear conditioning comprise in particular the amygdala, insula, prefrontal cortical regions, the striatum and the hippocampus (Sehlmeyer et al., 2009). Although the amygdala is one of the key brain regions in fear conditioning (Bale et al., 2001; Kirsch et al., 2005; Domes et al., 2007; Eckstein and Hurlemann, 2013), in particular fear acquisition (Campbell-Smith et al., 2015), during extinction prefrontal cortical regions are central (Qi et al., 2009; Acheson et al., 2013). Significant effects of OXT in the brain including the prefrontal cortex (PFC) (also cingulate cortex), may be the result of a dampening of amygdala reactivity to threat through exogenous concentrations of OXT (Domes et al., 2007; Eckstein and Hurlemann, 2013). As highlighted earlier, fear can be acquired along different mechanistic routes, depending on relations to cues and/or contexts. In particular the hippocampus was shown being strongly involved in context compared with cue fear learning (e.g. Kim and Fanselow, 1992; Anagnostaras et al., 1999; Marschner et al., 2008; Seo et al., 2015). However, studies on OXT effects on fear acquisition in relation to cues vs contexts are lacking so far.
The aim of this study was therefore to compare cue and context fear acquisition following OXT vs PLC in a between-subjects design. We hypothesized that OXT compared with PLC lead to reduced response in the amygdala and insula, together with increased response in the PFC, including the anterior cingulate cortex (ACC), which should be more pronounced during context compared with cue conditioning. Moreover, the hippocampus may also be more strongly activated during context conditioning following OXT exposure. These findings would strengthen our knowledge on mechanistic models of fear learning.
Materials and methods
Participants
Fifty-two healthy volunteers of Caucasian ethnicity (mean age = 22.12, s.d. = 4.06 years, 28 female) participated in this study. Individuals with current or lifetime mental disorder as assessed by the German version of The Structured Clinical Interview for the Diagnostic and Statistical Manual-IV Axis I Disorders (Wittchen et al., 1997) were excluded. Further exclusion criteria were: hormonal problems, neurological conditions, claustrophobia, history of cardiac illness, regular medication except for oral contraceptives, pregnancy, tattoo, metal piercing, allergy to OXT. The participants received either OXT or PLC in a double-blind design (details see below). The two resulting treatment groups (OXT vs PLC) did not significantly differ in age, years of education, trait anxiety [assessed with the State-Trait Anxiety Inventory (STAI; Laux et al., 1981)], depression [assessed with the German version of the Center for Epidemiological studies Depression Scale (ADS; Hautzinger and Bailer, 1993) (see Table 1)], handedness and sex. Written consent was obtained before the experiment, which was approved by the Ethics Committee of the Medical Faculty Mannheim, Heidelberg University.
Table 1.
Overview on mean values (M) and standard deviation (SD) of anxiety and depression in the treatment groups (PLC and OXT)
| Questionnaires | PLC [M(SD)] n | OXT [M(SD)] n | P value for factor group |
|---|---|---|---|
| ADS | 12.44(5.38) 27 | 15.13(9.12) 25 | n.s. |
| STAI | 39.00(8.62) 27 | 39.52(8.72) 25 | n.s. |
Note: ADS, Center for Epidemiological studies Depression Scale; STAI, State-Trait Anxiety Inventory; n.s., non-significant.
Experimental design
We applied a randomized, PLC-controlled, double-blind, between-subject design. A parallel-group design avoids potentially confounding effects of repetitive fear conditioning that might occur in a crossover within-subject design. Participants were randomly assigned to either intranasal administration of OXT (Oxytocin Spray; InPhaSol, Heidelberg, Germany), 3 puffs per nostril, each with 4 intranasal units (IU) OXT for a total dose of 24 IU, or PLC, sodium chloride solution, in accordance with current guidelines (Guastella et al., 2013). The participants were informed that a pharmacological intervention is performed, but they did not know which kind of treatment they receive. Testing for OXT vs PLC effects resulted in no significant differences. The pharmacological treatment (OXT or PLC) was always administered 45 min before the acquisition phase of either cue or context conditioning. The order of cue and context conditioning was counterbalanced across subjects.
Experimental procedure
For both, cue and context conditioning, we used the design from our previous studies (Lang et al., 2009; Pohlack et al., 2012; Cacciaglia et al., 2013, 2015), as these produced reliable and valid responses. Such a reference is needed and important when performing a manipulation of effects as it is done with pharmacological interventions.
Cue conditioning. Two different geometric figures (a square and a diamond) were used as conditioned stimuli (CS + and CS−) and projected with a beamer on a mirror system, while the participants lay inside the scanner. The square or the diamond as CS + was counterbalanced across subjects. A loud but tolerable sound (scream) served as US. The US was presented through headphones for each participant. Throughout the experiment, both the CS + and the CS − lasted 6 s, and were presented with a random inter-stimulus-interval (ISI) varying between 7 and 12 s, while the US lasted 3 s and terminated with the CS (delay conditioning).
The conditioning procedure consisted of four phases: habituation, early and late acquisition and extinction. The habituation phase aimed at introducing the participants to the stimuli (and showed no significant differences between OXT vs PLC) and was conducted in the laboratory. The following acquisition and extinction phase were conducted in the scanner.
The habituation phase consisted of 10 CS + and 10 CS − as well as four US, which were presented together with a fixation cross and without any temporal contingency with the CS. During acquisition phase a total of 36 CSs were presented, 18 CS + and 18 CS−. The order of presentation was randomized, with the only constraint that a maximum of 3 CS of the same type (e.g. 3 CS−) could appear in consecutive order. Of the 18 CS+, 9 were coupled with the US (CS + paired), while the remaining 9 CS + were not reinforced (CS + unpaired). The CS − was never presented together with the US. Finally, during the extinction phase, each of the two CS (CS+, CS−) was presented 18 times, while the US was omitted. Participants were not informed about the CS–US contingency and were told to passively view the stimuli.
Context conditioning. The context conditioning protocol consisted of initial habituation (conducted in the laboratory—with no significant differences between OXT vs PLC), early and late acquisition and extinction. Two colors (orange and blue) were used to represent two different spatial contexts (CS±). The colors were slowly blended in and, after having reached their full spectrum for several seconds, passed into the next color to reinforce the feeling of context. The colors designated as CS + were counterbalanced across participants and the sequence of CS± was pseudo-randomized. The stimuli were projected via a mirror system, thus realizing a surround color, i.e. an actual context.
During habituation, the CSs were presented 10 times for 3–12 s in random order. The US was also a loud but tolerable sound (board scratch) played 10 times during the ISI (4–12 s) and lasted 3 s. During acquisition, 20 CS + (10 CS + paired, 10 CS + unpaired) and 20 CS − trials equally divided across early (10 CS+, 10 CS−) and late (10 CS+, 10 CS−) acquisition, were presented. The colors were blended in until they reached their full spectrum, which took 3–4 s. After additional 3–12 s the colors were blended off and passed into the next color. The CS + was paired with the US (aversive sound) in 50% of the trials, the CS − was never paired with the sound. The US onset was randomized over the time course of the CS + (variation between 3 and 10 s after the CS + reached the full spectrum) to maximize unpredictability, which constitutes an additional integral characteristic of context conditioning (Bouton, 1994; Grillon and Davis, 1997; Vansteenwegen et al., 2005; Grillon et al., 2006). In the extinction phase, the two colors (10 CS + unpaired, 10 CS−) were presented for 3–12 s each.
Participants were uninformed about the CS–US contingency and were told to passively view the stimuli.
Data acquisition and analysis
Skin conductance response and self-report data
Data acquisition. The skin conductance responses (SCRs) were recorded from two electrodes placed on the thenar and hypothenar eminence of the participants’ right hand using a sampling rate of 16 Hz and a VarioPort recording system (BECKER MEDITEC, Karlsruhe, Germany). Data analysis was performed using Ledalab software V3.4. (Benedek and Kaernbach, 2010) and SCR was defined as the maximum response amplitude initiated between 1 and 9 s after CS onset (Entire Interval Response, EIR). The SCR values were measured in microSiemens (µS) and were normalized using a logarithmic transformation [ln (1+ SCR)]. Subjects whose data were not usable due to movement artefacts were excluded (n = 8 in cue and n = 7 in context conditioning).
After each conditioning phase of both cue and context conditioning, the participants rated the emotional valence and arousal of the CSs (1 = very pleasant to 9 = very unpleasant, 1 = very calm to 9 = very arousing) and the CS-US contingency (1 = no CS–US association to 9 = perfect CS–US association).
Data analysis. Weighted beta values from the fMRI data and the SCRs and self-report data during acquisition were analyzed with the Predictive Analytics Software release 18.0.1 (PASW, SPSS Inc., Chicago, IL). Based on the aim of this study, we focused our analysis on the acquisition phase. None of the dependent variables violated the assumption of normality of distributions, which was assessed using the Kolmogorov–Smirnov test. To investigate effects of OXT vs PLC for cue and context acquisition, a repeated measures analysis of variance (rmANOVA) was used, including CS-type (two levels: CS+, CS−) and phase (two levels: early acquisition, late acquisition) as within-subjects factors, and treatment (two levels: OXT, PLC) as between-subjects factor. Moreover, to additionally examine differential effects of OXT vs PLC for the type of conditioning, we also performed an rmANOVA with type of conditioning (two levels: cue, context) as additional between-subject factor. To control for sex effects with relation to OXT, we used sex as a covariate in all our analyses. In case of sphericity violation, a Greenhouse–Geisser correction was applied. For significant interactions involving CS-type, Bonferroni corrected t-tests were conducted for each group. For each of the analyses the alpha level was set at 0.05.
Functional magnetic resonance imaging
Data acquisition. The fMRI data acquisition was performed using a 3T TRIO whole body scanner with a standard 12-channel head coil. Blood oxygenation level-dependent (BOLD) contrast whole-brain functional images were acquired using a T2*-weighted gradient-echo Echo Planar Imaging (EPI) sequence [echo time (TE) = 27 ms, repetition time (TR) = 2700 ms, flip angle = 90°, field of view = 220 × 220 mm, matrix size = 96 × 96]. Each image volume consisted of 40 contiguous, 2.3 mm-thick axial slices (interslice gap = 0.7 mm) recorded in descending order and positioned along the line passing through the anterior and posterior commissure (orientation). Before pre-processing, the first three volumes of each scanning session were discarded to allow for T1 equilibration effects.
Data analysis. The fMRI data analysis was performed applying preprocessing, single subject- and group(treatment)-analyses using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB R2010a (The MathWorks Inc., Natick, MA, USA). We conducted standard temporal and spatial pre-processing. Functional volumes were slice time corrected to reference slice 18 and realigned to the fourth volume using a rigid body transformation in order to correct for head movement. Images were normalized to the Montreal Neurological Institute International Consortium for Brain Mapping (MNI) space, using the EPI template provided by SPM8. Images were smoothed with an 8 × 8 × 10 mm full-width at half-maximum Gaussian kernel. To remove low-frequency noise, a high-pass filter (cutoff 1/128 Hz) was included and the time series were corrected for serial autocorrelations using first-order autoregressive functions AR(1). A fixed effects analysis was performed by setting up a general linear model including the following experimental conditions for the acquisition phase: CS + unpaired, CS − and CS + paired. These inputs were convolved with a canonical hemodynamic response function (first order expansion) to create the design matrix. The six parameters describing the rigid body transformation were implemented as confound variables in the statistical analysis to covary out signal that was correlated with head motion. At the group level, a random effects analysis was used, employing voxel-wise two-sample t-tests with the contrast of interest (CS + > CS−). In addition to whole brain analyses, we implemented a regions of interest (ROIs) approach, for which ROIs were bilaterally defined according to our a priori hypotheses using the MNI template Automated Anatomical Labeling (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003), implemented in the Wake Forest Pick Atlas (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003). We investigated treatment group differences in regions relevant for in fear conditioning, such as the amygdala, hippocampus insula and the PFC, including the ACC, and the striatum (e.g. Nielsen and Hansen, 2002; Cullen et al., 2015), with family wise error at < 0.05. Moreover, contrast estimates were obtained from these regions by extracting the weighted mean within the selected ROIs. Here, the BOLD values of each voxel within the ROI were taken as weights when computing the average across all the selected voxels. To this purpose, we used the REX toolbox for SPM8 (http://web.mit.edu/swg/software.htm).
Results
Skin conductance responses
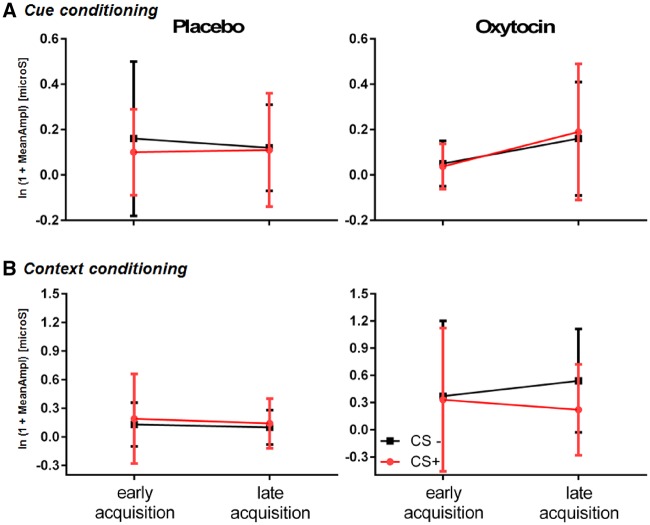
For the SCRs of both cue and context conditioning, we did not find any significant effects of treatment, i.e. OXT vs PLC (see Figure 1).
Fig. 1.
SCRs expressed by mean values (M) and s.d. in early and late acquisition for both treatment group (PLC and OXT). Note: CS+, conditioned stimulus paired; CS−, conditioned stimulus unpaired.
Self-report data
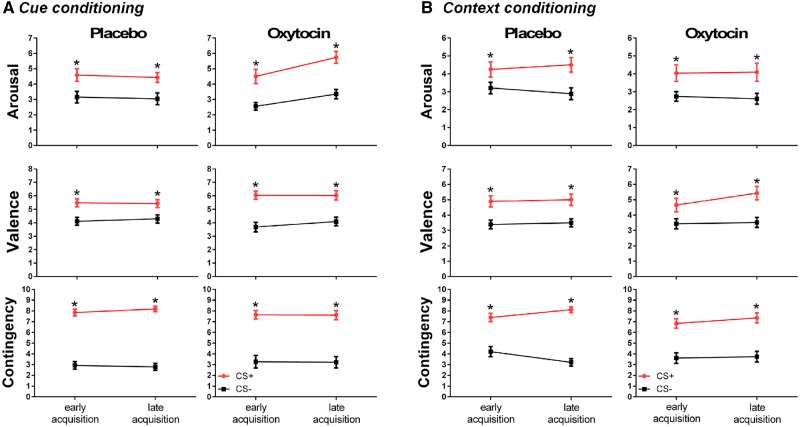
The ratings are displayed in Figure 2. For treatment (OXT vs PLC), we observed a significant difference in the arousal measure following late acquisition [F(1,49) = 6.220, P = 0.016], where the OXT group showed significantly higher arousal than the PLC group—for both cue and context conditioning.
Fig. 2.
Self-report data related to arousal, valence and contingency, acquired immediately after the early and late acquisition in (A) cue conditioning and (B) context conditioning. *P < 0.05 in comparison to the CS−. Note: For all measures (arousal, valence and contingency ratings) in both paradigms (cue and context conditioning) there was a significant difference between the CS+ and the CS− in the early [arousal: F(1,47) = 32.073, P < 0.001; valence: F(1,47) = 36.754, P < 0.001; contingency: F(1,47) = 83.888, P < 0.001] and late acquisition [arousal: F(1,49) = 58.095, P < 0.001; valence: F(1,49) = 30.407, P = 0.002; contingency: F(1,49) = 117.170, P < 0.001]. In these cases, higher arousal, valence and contingency ratings were observed for CS+ compared with CS−.
fMRI data
For the early acquisition phase (using the contrast CS + > CS− and treatment and conditioning procedure as factors), we found a significant main effect of treatment (OXT vs PLC) in the nucleus accumbens: the OXT group showed a significantly reduced response compared to the PLC group (see Figure 3). Moreover, we found a significant interaction of treatment and conditioning procedure: for context, but not cue conditioning, OXT compared with PLC resulted in a significantly reduced response in the ACC and the insula (see Figure 4). We did not observe any significant effects for the amygdala.
Fig. 3.
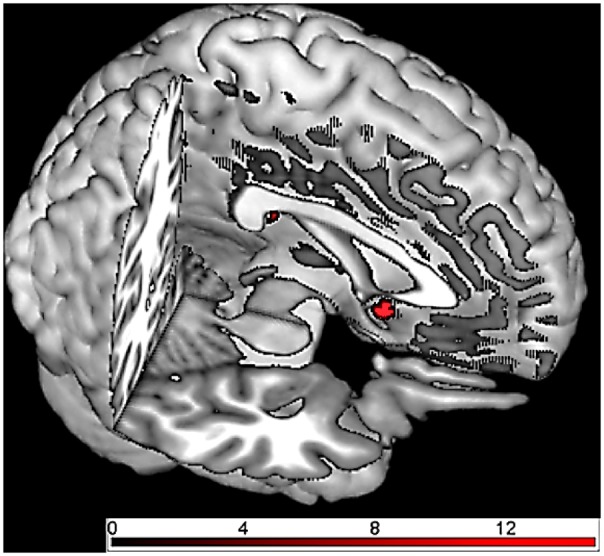
Brain responses in early acquisition phase (group comparison OXT vs PLC using the contrast CS + > CS− and cue vs context conditioning as factor): the OXT group showed reduced responses in nucleus accumbens compared with the PLC group during both cue and context conditioning.
Fig. 4.
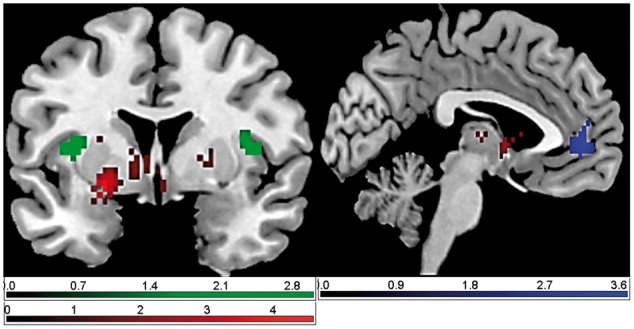
Brain responses in early acquisition phase (group comparison OXT vs PLC using the contrast CS + > CS− and cue vs context conditioning as factor): the OXT group showed reduced response in the ACC and the insula compared with the PLC group during context conditioning.
For the late acquisition phase, we found a significant interaction of conditioning procedure and treatment in the hippocampus: the OXT group showed a significantly increased response in the hippocampus during context, but not cue conditioning (see Figure 5). This hippocampal response was significantly predicted by the response in the ACC and the insula during early acquisition.
Fig. 5.
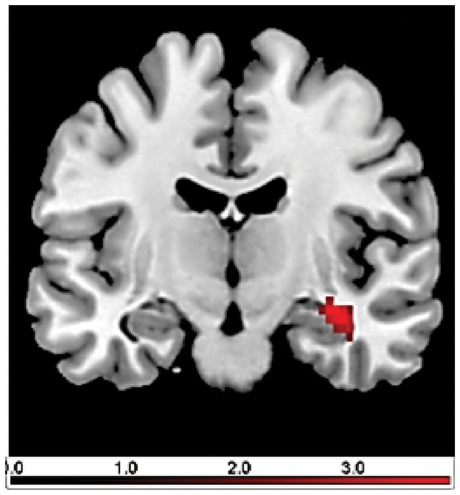
Brain responses in late acquisition phase (group comparison OXT vs PLC using the contrast CS + > CS− and cue vs context conditioning as factor): the OXT group showed an increased response in the hippocampus compared with the PLC group during context conditioning.
Discussion
Up to date, OXT effects on fear conditioning have been investigated in particular using social stimuli and with respect to fear extinction. These findings have already provided important insights into (social) fear learning processes. However, to further understand OXT effects on different the mechanisms of fear learning, we examined the role OXT in the acquisition of non-social fear responses and not only for cue, but also context-related processes in healthy individuals. We found a reduction in early fear acquisition following OXT vs PLC in the nucleus accumbens for both cue and context conditioning. This was accompanied by increased ratings of arousal following OXT vs PLC. For the late acquisition phase, OXT compared with PLC resulted in an increased response in the hippocampus during contextual, but not cue fear learning.
In a recent study on the effects of OXT on fear learning, Eckstein et al. (2016) used a cue fear conditioning procedure with both social and non-social conditioned stimuli in healthy males and observed faster reaction times, and higher skin conductance responses as well as increased response in the subgenual ACC, independent of social aspects. Interestingly, the authors did not find any significant differences between OXT and PLC for the amygdala responsivity. In line with this study, we also identified the ACC as a central region for OXT effects on fear acquisition and did not observe significant OXT effects on amygdala activation. The ACC response in our study was significantly reduced in context vs cue conditioning following OXT. This highlights a role for OXT also in contextual learning mechanisms, which might even been stronger than for cue conditioning. OXT might thus be a target for building aversive context associations, which is crucial in everyday life and fits with interpretations that OXT enables extremely rapid and flexible adaptation to fear signals in social contexts. This may confer an evolutionary advantage, but could also elevate vulnerability for the pathological sequelae of interpersonal trauma such as rapid avoidance behavior (Eckstein et al., 2016).
The response in the ACC as well as the insula during early acquisition furthermore predicted an increased OXT-related hippocampal response during late acquisition in context conditioning. Cullen et al. (2015) have demonstrated that activation in the ACC and the ventral hippocampus are related to increases in contextual fear generalization. Moreover, a selective role for the ACC in generalized contextual fear memory expression was reported by Einarsson et al. (2015), and also the insula was shown to be involved in contextual fear learning (e.g. Alvarez et al., 2008). Our results on reduced responses in those two brain regions may induce a preference for or enables building spatial, and thus contextual, associations, which appear then at later stages of the learning process, indicated by the increased response in the hippocampus. This may be seen in line with an animal study which indicated differences in context-related behavioral learned responses in mice depending on the time point of learning disruption (Alescio-Lautier and Soumireu-Mourat, 1998). Vasopressin, whose effects are related to OXT receptors, was administered at the beginning or the middle of the learning processes and thus at different stages of memorizing context information and resulted in altered memory processes related to the ventral hippocampus (Alescio-Lautier and Soumireu-Mourat, 1998). Our findings indicate a differential role of OXT with respect to core mechanistic routes of fear acquisition, with an involvement of emotionally driven cognitive control processes related to brain regions including the ACC and the insula, but without targeting the amygdala.
Moreover, our results showed a reduced response in the nucleus accumbens during early cue and context fear acquisition following OXT vs PLC. This decreased response in the nucleus accumbens was accompanied by increased ratings of arousal. In mice, it was demonstrated that the rewarding properties of social interaction require a coordinated activity of OXT and 5-HT in the nucleus accumbens (Dölen et al., 2013). The nucleus accumbens is a ventral extent of the striatum, the main input nucleus of the basal ganglia (Nicola, 2007), which receives input from important limbic nuclei such as the amygdala, the hippocampus, and the PFC and sends output to the basal ganglia (Jones and Mogenson, 1980). Due to this neural network integration and to dopamine dynamics, the nucleus accumbens has also been associated with learning processes involving prediction error and stimulus salience (Saddoris et al., 2013). OXT can decrease fear learning at early stages due to its action on the nucleus accumbens, considering its strategic localization and specific input/output to key structures involved in fear learning (e.g. amygdala, hippocampus and the PFC). Our observed effects of OXT on brain responses were not accompanied by changes in SCRs. This indicates that the central OXT effects during fear acquisition did not converge into the psychophysiological level and thus did not induce a learning-based system comprehensive adaptation. This was also often shown in general for associations between responses in the brain and peripheral physiological measures in pavlovian fear conditioning (e.g. Birbaumer et al., 2005; Wessa and Flor, 2007).
OXT has also been demonstrated as a promising substance for the treatment of several mental disorders including anxiety disorders, posttraumatic stress disorder, schizophrenia and autism. Experimentally, contextual fear, as opposed to cued fear, may ideally reflect the feeling of aversive expectations about potential danger that characterizes anxiety (Grillon, 2002; Steiger et al., 2015). Studies have reported that OXT administration dampened amygdala reactivity towards emotional faces in PTSD patients (Ragen et al., 2015), or improved social interaction deficits in children with autism (Yatawara et al., 2015), and controlled social cognition in patients with schizophrenia (Woolley et al., 2014). Together with these studies, our findings also suggest that intranasal administration of OXT could serve as a feasible method for clinical use of neuropeptides which do not cross the blood–brain-barrier and would require a direct pathway to the human brain (Born et al., 2002).
In this study, we used the cue and context conditioning procedures from our own previous studies (Lang et al., 2009; Pohlack et al., 2012; Cacciaglia et al., 2013, 2015), which differ in their number of stimuli. One could argue that this different number of stimuli could have impacted the present findings. However, the used conditioning procedures reflect the natural intrinsic differences between cue and context processing, with slower learning rates often observed for context compared with cue CRs. When performing a manipulation of effects as it is done with pharmacological interventions, it is important to refer to stable procedures. Nevertheless, it might be interesting to address the aspect of stimulus and trial number in detail in future studies.
In conclusion, this work has indicated a differential OXT-driven modulation of cue and context fear acquisition on both a neural and verbal-subjective level, with specific effects on contextual learning processes. This profile of OXT action provides interesting insight into the processes of fear learning as adaptive mechanism for our behavior in everyday life and may be introduced in learning-based treatment approaches as additional therapeutic target.
Funding
This study was supported by grant SFB636/C1 to H.F. and SFB636/Z3 and by SFB1158/B03 to F.N. and H.F. from the Deutsche Forschungsgemeinschaft, and by grant from CAPES/Alexander von Humboldt (AvH) provided to J.C.
Conflict of interest. None declared.
References
- Acheson D.T., Risbrough V.B. (2015). Oxytocin enhancement of fear extinction: a new target for facilitating exposure-based treatments?. Biological Psychiatry, 78(3), 154–5. [DOI] [PubMed] [Google Scholar]
- Acheson D., Feifel D., de Wilde S., McKinney R., Lohr J., Risbrough V. (2013). The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology (Berlin), 229(1), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alescio-Lautier B., Soumireu-Mourat B. (1998). Role of vasopressin in learning and memory in the hippocampus. Progress in Brain Research, 119, 501–21. [DOI] [PubMed] [Google Scholar]
- Alvarez R.P., Biggs A., Chen G., Pine D.S., Grillon C. (2008). Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. The Journal of Neuroscience, 28(24), 6211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras S.G., Maren S., Fanselow M.S. (1999). Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. The Journal of Neuroscience, 19, 1106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Davis A.M., Auger A.P., Dorsa D.M., McCarthy M.M. (2001). CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. The Journal of Neuroscience, 21(7), 2546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Kaernbach C. (2010). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N., Veit R., Lotze M., et al. (2005). Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry, 62, 799–805. [DOI] [PubMed] [Google Scholar]
- Born J., Lange T., Kern W., McGregor G.P., Bickel U., Fehm H.L. (2002). Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience, 5, 514–6. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. (1994). Context, ambiguity, and classical conditioning. Current Directions in Psychological Science, 3(2),49–53. [Google Scholar]
- Bustos S.G., Maldonado H., Molina V.A. (2009). Disruptive effect of midazolam on fear memory reconsolidation: decisive influence of reactivation time span and memory age. Neuropsychopharmacology, 34, 446–57. [DOI] [PubMed] [Google Scholar]
- Cacciaglia R., Nees F., Pohlack S.T., et al. (2013). A risk variant for alcoholism in the NMDA receptor affects amygdala activity during fear conditioning in humans. Biological Psychology, 94, 74–81. [DOI] [PubMed] [Google Scholar]
- Cacciaglia R., Pohlack S.T., Flor H., Nees F. (2015). Dissociable roles for hippocampal and amygdalar volume in human fear conditioning. Brain Structure and Function, 220, 2575–86. [DOI] [PubMed] [Google Scholar]
- Campbell-Smith E.J., Holmes N.M., Lingawi N.W., Panayi M.C., Westbrook R.F. (2015). Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats. Learning and Memory, 22(5), 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland P.S., Winkielman P. (2012). Modulating social behavior with oxytocin: how does it work? What does it mean?. Hormones and Behavior, 61(3), 392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P.K., Gilman T.L., Winiecki P., Riccio D.C., Jasnow A.M. (2015). Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiology of Learning and Memory, 124, 19–27. [DOI] [PubMed] [Google Scholar]
- Do Monte F.H., Souza R.R., Bitencourt R.M., Kroon J.A., Takahashi R.N. (2013). Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behavioural Brain Research, 250, 23–7. [DOI] [PubMed] [Google Scholar]
- Dölen G., Darvishzadeh A., Huang K.W., Malenka R.C. (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Gläscher J., Büchel C., Braus D.F., Herpertz S.C. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–90. [DOI] [PubMed] [Google Scholar]
- Eckstein M., Hurlemann R. (2013). Oxytocin: evidence for a therapeutic potential of the social neuromodulator. Nervenarzt, 11, 1321–8. [DOI] [PubMed] [Google Scholar]
- Eckstein M., Scheele D., Patin A., et al. (2016). Oxytocin Facilitates Pavlovian Fear Learning in Males. Neuropsychopharmacology, 41(4), 932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M., Scheele D., Weber K., Stoffel-Wagner B., Maier W., Hurlemann R. (2014). Oxytocin facilitates the sensation of social stress. Human Brain Mapping, 35, 4741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson E., Pors J., Nader K. (2015). Systems reconsolidation reveals a selective role for the anterior cingulate cortex in generalized contextual fear memory expression. Neuropsychopharmacology, 40(2), 480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. (2002). Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry, 52(10), 958–75. [DOI] [PubMed] [Google Scholar]
- Grillon C., Baas J.M., Cornwell B., Johnson L. (2006). Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biological Psychiatry, 60(7), 752–9. [DOI] [PubMed] [Google Scholar]
- Grillon C., Davis M. (1997). Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology, 34(4), 451–8. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Hickie I.B., McGuinness M.M., et al. (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38(5), 612–25. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M. (1993) Die Allgemeine Depressionsskala [General Depression Scale]. Göttingen: Hogrefe.
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54, 1389–98. [DOI] [PubMed] [Google Scholar]
- Hou Y., Zhao L., Zhang G., Ding L. (2015). Effects of oxytocin on the fear memory reconsolidation. Neuroscience Letters, 6(594), 1–5. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Mogenson G.J. (1980). Nucleus accumbens to globus pallidus GABA projection subserving ambulatory activity. American Journal of Physiology, 238, R65–9. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. (1992). Modality-specific retrograde amnesia of fear. Science, 256, 675–7. [DOI] [PubMed] [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch H.S., Charlet A., Hoffmann L.C., et al. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73(3), 553–66. [DOI] [PubMed] [Google Scholar]
- Lang S., Kroll A., Lipinski S.J., et al. (2009). Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. European Journal of Neuroscience, 29(4), 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux L., Glanzmann P., Schaffner P., Spielberger C.D. (1981) Das State-Trait-Angstinventar [State-Trait Anxiety Inventory]. Weinheim: Beltz. [Google Scholar]
- Leslie J.C., Shaw D., Gregg G., McCormick N., Reynolds D.S., Dawson G.R. (2005). Effects of reinforcement schedule on facilitation of operant extinction by chlordiazepoxide. Journal of the Experimental Analysis of Behavior, 84(3), 327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Marschner A., Kalisch R., Vervliet B., Vansteenwegen D., Büchel C. (2008). Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience, 28(36), 9030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Haswell C.C., Li W., et al. (2015). Assessment of Myelin Compromise in mild traumatic brain injury with quantitative susceptibility mapping. Biological Psychiatry, 77(9), 40S. [Google Scholar]
- Nader K., Einarsson E.O. (2010). Memory reconsolidation: an update. Annals of the New York Academy of Sciences, 1191, 27–41. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Krömer S.A., Toschi N., Ebner K. (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory Peptides, 96(1–2), 31–8. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Slattery D.A. (2016). Oxytocin in general anxiety and social fear: a translational approach. Biological Psychiatry, 79(3), 213–21. [DOI] [PubMed] [Google Scholar]
- Nicola S.M. (2007). The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berlin), 191(3), 521–50. [DOI] [PubMed] [Google Scholar]
- Nielsen F.A., Hansen L.K. (2002). Modeling of activation data in the BrainMap database: detection of outliers. Human Brain Mapping, 15(3), 146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin V.P., Kozyrev S.A., Solntseva S.V. (2015). Reconsolidation of reminder-induced amnesia: role of NMDA and AMPA glutamate receptors. Bulletin of Experimental Biology and Medicine, 160(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Otis J.M., Werner C.T., Mueller D. (2015). Noradrenergic regulation of fear and drug-associated memory reconsolidation. Neuropsychopharmacology, 40(4), 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I. (1927) Conditioned Reflexes. England: Oxford University Press. [Google Scholar]
- Petrovic P., Kalisch R., Singer T., Dolan R.J. (2008). Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of Neuroscience, 28(26), 6607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack S.T., Nees F., Ruttorf M., Schad L.R., Flor H. (2012). Activation of the ventral striatum during aversive contextual conditioning in humans. Biological Psychiatry, 91(1), 74–80. [DOI] [PubMed] [Google Scholar]
- Qi J., Yang J.Y., Wang F., Zhao Y.N., Song M., Wu C.F. (2009). Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology, 56(5), 856–65. [DOI] [PubMed] [Google Scholar]
- Ragen B.J., Seidel J., Chollak C., Pietrzak R.H., Neumeister A. (2015). Investigational drugs under development for the treatment of PTSD. Expert Opinion on Investigational Drugs, 24(5), 659–72. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Hediger K., Heinrichs M., Klaver P. (2009). Oxytocin makes a face in memory familiar. The Journal of Neuroscience, 29(1), 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris M.P., Sugam J.A., Cacciapaglia F., Carelli R.M. (2013). Rapid dopamine dynamics in the accumbens core and shell: learning and action. Frontiers in Bioscience (Elite Ed), 1(5), 273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Striepens N., Güntürkün O., et al. (2012). Oxytocin modulates social distance between males and females. The Journal of Neuroscience, 32, 16074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C., Schöning S., Zwitserlood P., et al. (2009). Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One, 4(6), e5865.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D.O., Carillo M.A., Chih-Hsiung Lim S., Tanaka K.F., Drew M.R. (2015). Adult hippocampal neurogenesis modulates fear learning through associative and nonassociative mechanisms. The Journal of Neuroscience, 35(32), 11330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger F., Nees F., Wicking M., Lang S., Flor H. (2015). Behavioral and central correlates of contextual fear learning and contextual modulation of cued fear in posttraumatic stress disorder. International Journal of Psychophysiology, 98, 584–93. [DOI] [PubMed] [Google Scholar]
- Striepens N., Kendrick K.M., Maier W., Hurlemann R. (2011). Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Frontiers in Neuroendocrinology, 32(4), 426–50. [DOI] [PubMed] [Google Scholar]
- Sun J., Zhu G., Liu Y., et al. (2015). UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis. Cell Reports, 12(3), 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D., Hermans D., Vervliet B., et al. (2005). Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behaviour Research and Therapy, 43(3), 323–36. [DOI] [PubMed] [Google Scholar]
- Weigand A., Feeser M., Gärtner M., et al. (2013). Effects of intranasal oxytocin prior to encoding and retrieval on recognition memory. Psychopharmacology (Berlin), 227(2), 321–9. [DOI] [PubMed] [Google Scholar]
- Wessa M., Flor H. (2007). Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. The American Journal of Psychiatry, 164, 1684–92. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Zaudig M., Frydrich T. (1997) Strukturiertes Klinisches Interview Für DSM-IV [Structured Clinical Interview for DSM-IV]. Goettingen, Germany: Hogrefe. [Google Scholar]
- Woolley J.D., Chuang B., Lam O., et al. (2014). Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology, 47, 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara C.J., Einfeld S.L., Hickie I.B., Davenport T.A., Guastella A.J. (2015). The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Molecular Psychiatry, 27, 1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]