Abstract
Auditory Hallucinations (AH) cause substantial suffering and dysfunction, yet remain poorly understood and modeled. Previous reports have linked AH to increases in negative emotions, suggesting a role for the autonomic nervous system (ANS) in underlying this link. Employing an Experience Sampling Method (ESM) approach, 40 individuals with schizophrenia completed a 36-hour ambulatory assessment of AH and cardiac autonomic regulation. Participants carried mobile electronic devices that prompted them to report 10 times/d the severity of their momentary AH, along with a Holter monitor that continuously recorded their cardiac autonomic regulation. The clocks of the devices and monitors were synchronized, allowing for high time-resolution temporal linking of the AH and concurrent autonomic data. Power spectral analysis was used to determine the relative vagal (parasympathetic) contribution to autonomic regulation during 5 minutes prior to each experience sample. The participants also completed interview-based measures of AH (SAPS; PSYRATS). The ESM-measured severity of AH was significantly correlated with the overall SAPS-indexed AH severity, along with the PSYRATS-indexed AH frequency, duration, loudness, degree of negative content, and associated distress. A mixed-effect regression model indicated that momentary increases in autonomic arousal, characterized by decreases in vagal input, significantly predicted increases in ESM-measured AH severity. Vagal input averaged over the 36-hour assessment displayed a small but significant inverse correlation with the SAPS-indexed AH. The results provide preliminary support for a link between ANS regulation and AH. The findings also underscore the highly dynamic nature of AH and the need to utilize high time-resolution methodologies to investigate AH.
Keywords: schizophrenia, psychosis, auditory hallucinations, autonomic regulation, arousal, cardiac, heart, stress, vagal, experience sampling method, negative emotions, heart rate variability, mobile devices
Introduction
Auditory Hallucinations (AH) are non-voluntary, internally generated auditory experiences, often perceived as originating from external sources. They are considered a core symptom of schizophrenia that is experienced by approximately 70% of affected individuals,1–4 often resulting in substantial suffering, comorbidity, and dysfunction.2,5,6 Over the past decade, research and clinical interest in AH has grown considerably, as evident by the advent of The International Consortium on Hallucination Research.7–9 However, despite this surge in research and clinical interests, AH remain poorly understood and modeled, and the neurobiological mechanism underlying them remain largely obscure.10
One notable characteristic of AH is their “ebb-and-flow” nature, with considerable variability both between and within individuals. The frequency of AH have been found to vary substantially among individuals with AH, with 48% reporting nearly constant AH, 26% frequent AH (11–20 times/wk), and the remainder reporting sporadic experiences (<once a day).1 Surveys also indicate considerable differences in AH durations—59% of individuals reported episodes lasted hours, 31% several minutes, and 12% a few seconds.1 Other studies found episodes of AH to last on average 190 and 299 minutes.11,12 Altogether, these findings underscore the dynamic nature of AH, but raise a number of fundamental questions—Why do AH occur at certain times but not others? What factors drive such patterns? While in recent years a number of models of AH have been put forward, the temporal nature of AH and the factors driving their “ebb-and-flow” characteristics have received little consideration.
One feature that seems to correspond with the “ebb-and-flow” of AH, and thus may potentially contribute to such patterns, is emotional experience. Consistent with this view, a number of models of psychosis highlight the roles negative emotions and stress play in the contributing to the development and exacerbation of psychotic symptoms, including AH.13–17 This view is also supported by the world of clinical experiences in which individuals with schizophrenia often experience the onset of psychosis or symptom exacerbations in response to stressful events.18–20 Such associations have also been documented among individuals at clinical high-risk for psychosis.21 Consistent with this view, studies of AH found temporal links between momentary elevations in negative emotions,11,12 delusional intensity,22 and hallucinatory episodes. Notably, Delespaul and colleagues found that negative emotions elevate well before the onset of the AH episodes and decline toward the latter part of the episode, suggesting that negative emotions are a cause, rather than a consequence of AH.12
The concomitant experience of negative emotions suggests increased physiological arousal and a putative role for the autonomic nervous system (ANS) in the onset and/or exacerbation of AH.23,24 Specifically, these reports suggest a model in which autonomic arousal precedes and may trigger the onset and exacerbation of AH. An alternative view would suggest that autonomic arousal may amplify or modulate already present AH. This distinction has important theoretical and therapeutic implications; however, previous reports provide little information to clarify this issue. While laboratory studies have reported associations between psychotic symptom exacerbations and increased cardiac autonomic arousal characterized by low vagal (parasympathetic) inputs,25–28 these studies have been limited by the use of “stationary” assessments of arousal, with participants typically assessed during rest and over brief periods (<1 h). Additionally, the assessment of psychotic symptoms was retrospective and thus vulnerable to the influence of memory difficulties and cognitive biases and reframing.29,30 These issues are noteworthy given the substantial episodic memory deficits experienced by many individuals with schizophrenia,31 making the use of retrospective assessments problematic in this population. Thus, despite the ostensible clinical plausibility of the arousal-psychosis link, there is a dearth of evidence pointing to temporal associations between momentary increases in autonomic arousal and psychotic experiences, including AH. Consequently, the underlying neurobiology of AH remains understudied and unclear, hindering theoretical and therapeutic progress. Our group and others have argued that the paucity of evidence on this question stem from the limitations of the methodologies employed of previous studies,29,32–34 making it difficult to characterize AH’s dynamic nature.
One methodology that overcomes many of these limitations is experience sampling method (ESM), an ecologically-valid, time sampling of self-reports developed to study the dynamic process of person–environment interactions.11,30,35 ESM offers a number of advantages over retrospective assessments, including allowing assessment of real-world, real-time experiences (ie, in situ and in vivo) with limited need of episodic memory input and minimal impact of cognitive biases. Technological advances over the past decade have made it feasible to employ ESM using electronic mobile devices,29,30,35–39 including among hospitalized individuals.30,40 Such devices provide precise time-stamps of the participants’ responses, allowing for ascertainment of temporal links to concurrent autonomic indicators.29,30
To address these gaps in the literature, our goal was to investigate the link between autonomic arousal and AH during “real-world” daily functioning among individuals with psychosis using ESM and measures of autonomic arousal. Our aims were to: (1) examine the links of daily “real-world” experience of AH with multi-domain interview-based measures of AH; and (2) determine the impact of momentary changes in autonomic arousal on changes in AH severity.
Methods and Materials
Participants
The study was conducted at the New York State Psychiatric Institute (NYSPI) at the Columbia University Medical Center and was approved by the NYSPI’s Institutional Review Board. All participants provided written informed consent. The inclusion criteria were ages 18–55; English speaking; a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of schizophrenia or related disorders; moderate or more severe psychotic symptoms (≥3 on SAPS hallucinations/delusions); and capacity to provide informed consent. The exclusion criteria were a diagnosis of mental retardation (IQ < 80); abnormalities on ECG; history of serious hypertension/cardiac conditions; use of anti-cholinergic, beta-blockers, anti-histamine, or anti-hypertensive medications; history of severe neurological/medical conditions; and a recent use of street drugs (confirmed by a urine toxicology test).
Measures
AH were assessed using ambulatory and interview-based measures. Ambulatory momentary ratings of AH during “real-world” daily functioning were collected using ESM with mobile devices (iESP software ver. 3.3; Intel Research Center). Interview-based AH were indexed by the AH subscales of the Scale for Assessment of Positive Symptoms (SAPS) and the Psychotic Symptom Rating Scale (PSYRATS) which were administered on the day immediately following the completion of the ambulatory assessments, thus including the ambulatory assessment period.
Autonomic regulation during “real-world” daily functioning was indexed by cardiac vagal contribution to heart rate variability (HRV). Cardiac functioning is mediated by the ANS, with both the sympathetic and parasympathetic ANS branches innervating the myocardium. The continuous interplay between these branches reflects the ANS’ ability to respond to stressors and return to homeostasis,41 thus contributing to an individuals’ ability to function effectively within changing environments. The balance between the ANS’ branches can be indexed by the heart’s beat-to-beat variability. Power spectral analysis of HRV data can determine the relative contribution of the parasympathetic branch to cardiac autonomic regulation. Specifically, the high frequency band (HF; 0.15–0.40 Hz) has been found to reflect almost exclusive efferent vagal (parasympathetic) contributions to cardiac regulation. HRV is advantageous over salivary cortisol (SC) in allowing high time-resolution examination of changes in autonomic arousal (eg, seconds), as peak elevations in SC are typically reached 20–40 minutes after the presentation of stressors,42 making its use impractical for assessment of dynamic phenomena such as AH.
Concurrent momentary autonomic regulation data were recorded using a LifeShirt System (VivoMetrics), a vest-like undergarment embedded with sensors designed to record ambulatory cardiopulmonary data. The electro-encephalogram (ECG) electrodes were placed on the left and right upper chest and on the left anterior axillary line at the 10th intercostal space. Using this methodology, our group has previously demonstrated that momentary increases in stress were correlated with lower vagal input.29 Diagnoses were determined using the Diagnostic Interview for Genetic Studies. Affective symptoms were evaluated using the Beck depression and anxiety inventories.
Study Procedure
After satisfying the inclusion/exclusion criteria, data on momentary AH and autonomic regulation were collected continually over a 36-hour period (day 1, 10 AM to day 2, 10 PM), including during sleep. A 36-hour assessment period was selected in consideration of the participants’ comfort level, as participants could not take showers during this period due to the need to disconnect and reattach the ECG electrodes by themselves. On the morning of the first day, participants were fitted with the LifeShirt vest and were given a brief introductory session to the basic operations of the mobile device. The digital clocks of the mobile device and LifeShirt were synchronized, allowing for a high time-resolution, second-by-second temporal linking of the participants’ reports of AH with their concurrent cardiac autonomic regulation data. Participants were then provided with the mobile devices, which they were to carry daily (10 AM–10 PM) throughout the 36-hour ambulatory assessment period.29,30
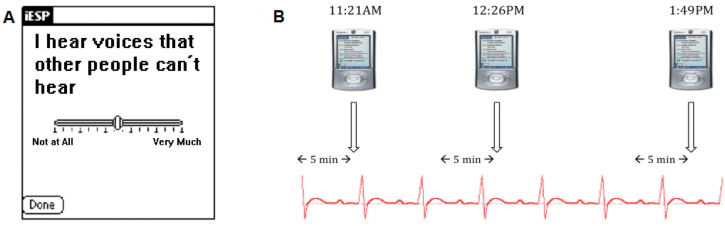
The device was programmed to beep 10 times/d at random times between 10 AM and 10 PM to elicit reports about AH. There were no scheduled beeps at nighttime, as to not interfere with the participants’ sleep schedule. Upon hearing the beep, participants were instructed to respond to a brief questionnaire presented on the screen of the device, which asked a single question about the severity of their AH just prior to the beep (ie, “I hear voices that other people can’t hear”; see figure 1A).11,29,30,37 The employment of a single global AH dimensional item to measure severity has ecological validity, with reported variation linked to symptom severity.11 There is a long tradition in ESM studies of assessing AH using Likert-like scales.11,12,22,29 This approach is not dissimilar from popular instrument used to measure AH (eg, Positive and Negative Syndrome Scale [PANSS]), which also employ a dimensional approach rather than a “Yes/No” categorical view. For example, AH on the PANSS (Item P3) employs a 3 rating for AH that do not result in distortions of thinking/behavior; rating of 4 indicates minor impact on thinking/behavior; rating of 5 indicates tendency to distort thinking/disrupt behavior, etc.). Consistent with previous ESM studies, the questionnaire also included questions about mood, other symptoms, activities and social context (not analyzed in present article).11,29,30,37,43 Responses were represented in the output as values between 1 (“not at all”) and 100 (“very much”). To ensure timely responses, the participants had to respond within 5 minutes of the beep, after which the device was programmed to turn off until the next experience sample.
Fig. 1.
Sample screenshot of the mobile device and a schematic diagram of sampling of momentary auditory hallucinations and concurrent autonomic regulation. (A) A screenshot of an ESM-based question presented on the mobile device assessing severity of momentary auditory hallucinations; (B) Schematic diagram of ambulatory sampling of momentary auditory hallucinations and epochs of cardiac autonomic regulation during daily functioning; ESM—Experience Sampling Method.
The mobile device’s software was set up to divide each day’s assessment period by the number of questionnaires to create 10 equal time windows of 72 minutes (12 h × 60 min/ 10 questionnaires) and then scheduled 1 questionnaire randomly within each time window. Thus, the period of time between experience samples could range from 1 minute (from last min of one window to the first min of the next one) to 143 minutes (from first min of one window to last min of the next one). Our group has established the validity of this methodology in individuals with schizophrenia.29,30
Following the completion of the 36-hour period, the data from the mobile device and LifeShirt vest were uploaded to a PC computer. The precise time of each questionnaire was identified, and using VivoLogic software (VivoMetrics Inc.), the corresponding time point on the continuous stream of cardiac autonomic data was marked. We then demarcated a 5-minute epoch before each questionnaire’s time and used power spectral analysis to quantify autonomic regulation during each epoch (figure 1B). For each 5-minute epoch, we calculated spectral power in high-frequency band (0.15–0.40 Hz), which reflects parasympathetic contribution to cardiac autonomic regulation.44 The ECG signals were digitized at 200 Hz using the VivoLogic software. Prior to computing fast Fourier transforms, the software subtracted the linear trend and used multiplication of a Hanning window for each segment. The software then averaged and scaled the magnitude power spectrum for each sub-segment to form a 1-sided periodogram and calculated the area under the curve for each frequency range.
Statistical Analyses
Analyses were performed in SAS version 9.2. Descriptive statistics about demographic and clinical data were computed, along with person-averaged ESM measures of AH and vagal input over the 36-hour period. The high-frequency data derived from the power spectral analysis of each 5-minute epoch was log transformed to correct for violation of the normality assumption. To examine associations between interview- and ESM-measured AH, partial Spearman’s correlations were used to accommodate non-normal distributions adjusting for antipsychotic dosage. Associations were also explored between interview-based AH measures and mean ambulatory-measured vagal input. Average variability in ESM-measured AH was computed as the mean person-level SD of all observations within each person. A mixed effects regression model with a random person level intercept and an autoregressive (AR1) covariance structure within individual was used to model ESM-measured AH predicted by ambulatory vagal input, while adjusting for participants’ group (inpatient vs outpatient), age, gender, education, family history of mental illness, antipsychotic dosage, along with paternal age.45 The primary test of interest is the one-degree of freedom t test for the coefficient for vagal input, which represents the momentary change in AH given a 1-unit change in vagal input. Further exploratory analyses were conducted to examine moderators on the vagal input and AH severity relationship.
Results
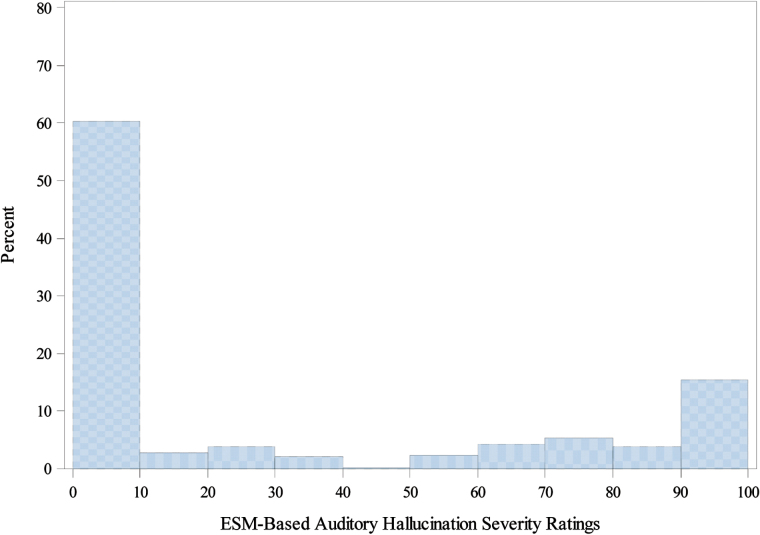
Data was collected on 40 individuals with DSM-IV schizophrenia and non-affective psychoses (27 schizophrenia; 7 schizoaffective disorder, 3 schizophreniform disorder, and 3 psychosis not otherwise specific [NOS]). On average, participants were 30.5 year old (SD = 8.2) and completed 15 years of education (SD = 4.0). The majority of participants were males (n = 25, 62%). The average antipsychotic medication dosage as indexed by chlorpromazine equivalence was 316.1 mg/d (SD = 350.9). The average Interview-based (SAPS) AH severity was 3.08 (SD = 2.13). The participants responded to 89.8% of the ESM prompts over the 36-hours assessment period, completing on average 17.8 (SD = 2.8) responses. The average ESM-measured AH severity was 30.68 (SD = 34.80). Participants reported AH during 40% of the experience samples, of which 61% were severe (rating >70; see figure 2).
Fig. 2.
Distribution of ESM-based auditory hallucinations severity ratings. Distribution of frequency ESM-based ratings of auditory hallucinations severity during the course of daily functioning—range from 0 (“not at all”) to 100 (“very much”); ESM—Experience Sampling Method.
We first examined the links between momentary- and interview-based measures of AH. Table 1 presents the means, SDs and partial Spearman’s correlations of the mean ESM-measured AH with domains of interview-based AH measures, adjusting for antipsychotic medication. A significant positive association was found between the mean severities of ESM- and interview-based SAPS-indexed measures of AH, as well as PSYRATS-indexed AH-related domains of frequency, duration, loudness, degree of negative content, and intensity of distress, with trend-associations found for amount of negative content, amount of distress, and controllability.
Table 1.
Mean, SD, and Associations of ESM- and Interview-Based Measures of Auditory Hallucinations
| Auditory Hallucinations | Mean (SD) | Correlations With Mean ESM-Measured Auditory Hallucinationsa | |
|---|---|---|---|
| ρ | P value | ||
| SAPS | |||
| Severity | 3.08 (2.13) | 0.47 | .004 |
| PSYRATS | |||
| Frequency | 1.85 (1.52) | 0.48 | .017 |
| Duration | 1.88 (1.41) | 0.60 | .002 |
| Location | 1.19 (1.53) | 0.31 | .139 |
| Loudness | 1.96 (0.90) | 0.46 | .025 |
| Origin of voices | 2.15 (1.48) | 0.32 | .126 |
| Amount of negative content | 1.92 (1.62) | 0.36 | .087 |
| Degree of negative content | 2.08 (1.62) | 0.47 | .020 |
| Amount of distress | 1.81 (1.44) | 0.38 | .070 |
| Intensity of distress | 1.31 (1.20) | 0.50 | .013 |
| Disruption to life caused by voices | 2.50 (0.93) | 0.17 | .428 |
| Controllability of voices | 1.96 (1.61) | 0.35 | .095 |
| ESM | |||
| Auditory hallucinations | 30.68 (34.80) | — | — |
Note: Bold type indicates significant correlations; SAPS—Scale for Assessment of Positive Symptoms (SAPS item #1; range 0–5); PSYRATS—Psychotic Symptom Rating Scales (range 0–5); ESM—Experience Sampling Method (range from 1–100); Higher scores indicated increased severity.
aPartial Spearman correlations controlling for antipsychotic medications as indexed by chlor-promazine equivalence.
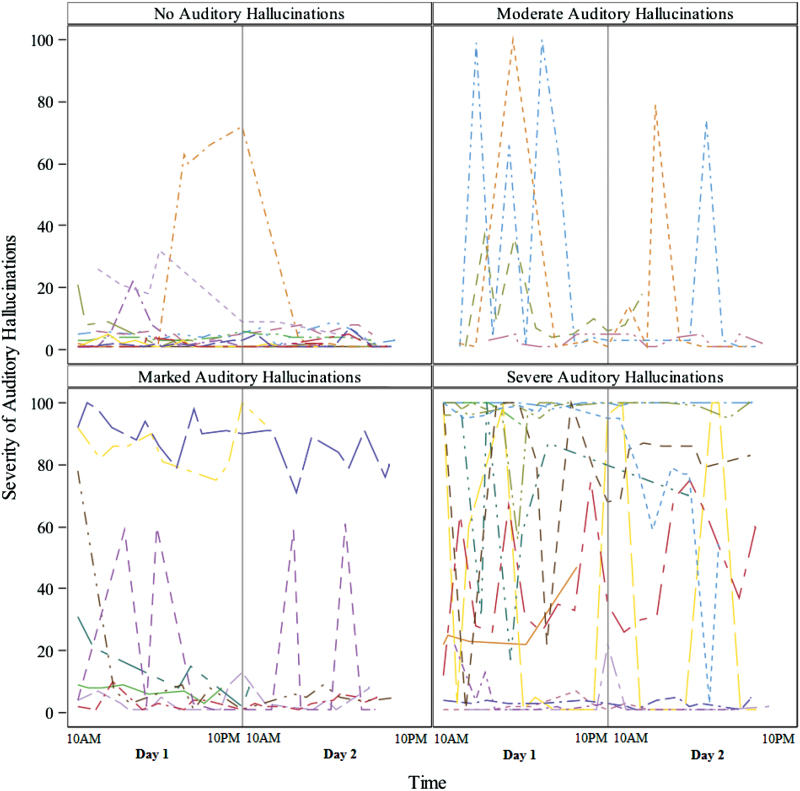
Examination of the link between ESM-measured AH severity over the 36-hour assessment grouped by SAPS AH ratings indicated some individuals with SAPS-indexed severe AH (SAPS = 5) reported few or substantial variability in ESM-measured AH (range 0–100; see figure 3). Similarly, some individuals who reported no AH in clinical interviews (SAPS = 0), endorsed AH in their ESM-measured assessments. The mean within-individual SD of ESM-measured AH was 5.5, 21.1, 8.4, and 13.5 for SAPS ratings of no AH, moderate, marked, and severe AH, respectively (SAPS item #1 values of 0, 3, 4, and 5).
Fig. 3.
Individuals’ ESM-based auditory hallucinations grouped by interview-based auditory hallucination severity ratings. Each line represent one individual’s ESM-based severity of auditory hallucinations across time. Ratings of no Hallucinations as well as moderate, marked, and severe hallucinations correspond with SAPS item #1 rating of 0, 3, 4, and 5 (respectively). No individuals had questionable or mild hallucinations ratings (SAPS item #1 ratings of 1 or 2, respectively); ESM—Experience Sampling Method; SAPS—Scale for Assessment of Positive Symptoms.
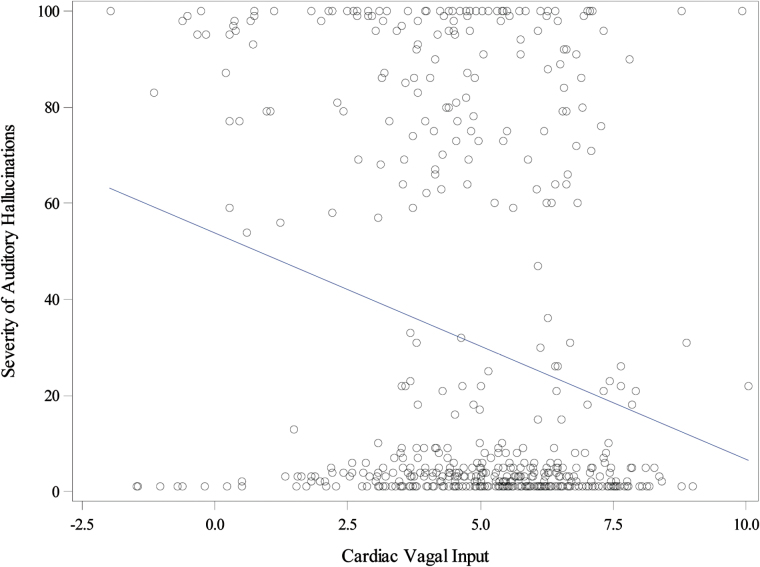
Our second aim was to determine the association of momentary changes in autonomic arousal on AH. A mixed-effect regression model showed a significant negative association between ambulatory-measured vagal input and ESM-measured AH (b = −1.44, SE = 0.73, P = .049; effect size = −0.08, see figure 4), after adjusting for covariates. For each 1-unit increase in ambulatory-measured HF on the log scale (ie, an exp(1) = 2.7-fold increase in HF), the ESM-measured AH decreases by 1.44 points (the 5th–95th percentile of HF is 2.4–2368.5). All covariates (group [inpatient vs outpatient: b = 6.10, SE = 18.6, P = .743]; age [b = 1.07, SE = 1.18, P = .365]; gender [b = 3.44, SE = 13.8, P = .803]; education [b = −0.37, SE = 1.77, P = .834]; family history of mental illness [b = −2.77, SE = 13.5, P = .838]; paternal age [under 30 vs missing: b = −0.839, SE = 16.1, P = .958; 30 and over vs missing: b = 6.29, SE = 21.3, P = .768], and antipsychotic dosage [b = −0.004, SE = 0.023, P = .875; dosage missing group: b = −2.09, SE = 26.4, P = .937]) included were not significant, and removing them from the model still produced a significant negative relationship (b = −1.58, SE = 0.72, P = .028; effect size = −0.08). Similarly, there was a small but significant inverse correlation between mean vagal input and SAPS-measured AH severity (r = −.36, P = .03).
Fig. 4.
The association of momentary auditory hallucinations and concurrent cardiac vagal input. Severity of auditory hallucinations—ambulatory ESM-based assessment of momentary auditory hallucinations; ESM—Experience Sampling Method; Cardiac Vagal Input—parasympathetic contribution to cardiac functioning during 5-min epoch prior to each experience sample (as indexed by log transformed High-Frequency data analyzed using power spectral analysis).
Discussion
The most important finding of the present study is the identification of a temporal link between autonomic regulation and AH. Specifically, momentary increases in cardiac autonomic arousal characterized by lower vagal input predicted transitory increases in AH. These findings extend results from previous reports linking negative emotions in individuals with schizophrenia to both autonomic arousal29 and AH exacerbations.11,12 Our results provide preliminary support for a model in which increases in autonomic arousal contribute to onset and/or exacerbation of AH.23 The findings, based on the within person (time-varying) effect between autonomic regulation and AH, suggest that this effect is in the small range. This may be attributed, in part, to standardization of both autonomic regulation and AH using the whole sample population. Given the substantial person-to-person variability on these measures, the resulting effect is rather small.
Our results suggest autonomic arousal precedes and may trigger the onset and exacerbation of AH. Establishment of a cause and effect relationship requires demonstration of covariation of the cause and effect, the presence of temporal precedence, and elimination of plausible alternative explanations.46 Our results demonstrate significant relationship between changes in autonomic arousal and AH, satisfying the covariation requirement. The measurement of autonomic arousal preceded the assessment of momentary AH, suggesting temporal precedence. Yet, while unlikely, it is possible the elevated autonomic arousal reflects association with “already in progress” AH episodes (ie, in the event that all/most experience samples occurred at the end of the AH episodes). Finally, we adjusted our model for a number of variables including clinical status, age, gender, education, family history of mental-illness, paternal age, and antipsychotic medication dosage in an attempt to address alternative explanations.
Additional indirect evidence supporting the arousal-AH link is available from treatments targeting AH. Breathing and relaxation exercises designed to reduce physiological arousal have long been a staple of treatments techniques aiming on stress reduction, with preliminary evidence demonstrating benefits in schizophrenia.47 Similarly, cognitive-behavioral therapies, which frequently include emotion regulation and stress-reducing components have also been found to benefit AH.48–50 Finally, studies of patients’ self-initiated strategies for coping with AH have indicated that strategies aimed to reduce stress-related arousal are among the most commonly used.51,52 Related to this, it has been suggested that negative symptoms and social withdrawal may reflect attempts to down regulate stress and related arousal.53 Future studies should aim to confirm our findings, by examining the arousal-AH link among first-episode patients, as well as individuals at clinical high-risk for psychosis who report anomalous auditory experiences.
Our results invite speculation about the putative brain circuitry that may link cardiac autonomic regulation to AH. Extensive data from neuroimaging studies have linked AH to both structural and functional abnormalities in a distributed network of brain regions that includes Broca’s area,54–56 the insula,54,57–59 the amygdala-hippocampal complex,54,57,60 and subcortical regions.55,61 Specifically, the temporoparietal junction of the left posterior superior temporal cortex has been implicated in both the anatomical and functional pathology of AH.62 Relevant to our findings, the left temporoparietal junction is architecturally adjacent to the left insula and is connected to it by short association fibers.63–65 Previous reports have linked cardiac autonomic regulation to insula activation66,67 and the left anterior insula has been identified as one of the brain regions with the highest likelihood of activating during AH.58,68 Furthermore, studies of time course regional brain activation indicate insula activation may serve as a precursor event leading to AH, as the insula and adjacent regions (left inferior frontal gyrus) have been found to activate 4.5 seconds57 and 9 seconds55 prior to AH onset, respectively. Future studies should examine and experimentally confirm these putative associations.
Our findings have implications for the assessment of AH. Given the dynamic nature AH, interview-based measures that ask participants to retrospectively report on their symptoms may be limited in their ability to characterize hallucinatory experiences. Such assessments are vulnerable to the impact of episodic memory deficits and cognitive biases and reframing,29,69,70 which are issues critical in schizophrenia.71–73 The use of high time-resolution methodologies that capture “in vivo, in situ” experiences during daily-functioning offers distinct advantages in characterizing the clinical richness of AH, along with their physiological, cognitive, behavioral, affective, and environmental correlates.35 Notably, the ESM AH measure significantly correlated with some, but not all PSYRATS domains, suggesting that a number of AH dimensions (ie, location, origin of voices, amount of negative content, disruption in life) may be less closely related to “in vivo” AH measures. Thus, ESM-based measurements may complement clinical interviews by providing unique data.
The findings of an arousal-AH link also have clinical implications, suggesting a potential role for emotion regulation strategies in down-regulating arousal and negative emotions. Emotional difficulties are ubiquitous in schizophrenia, and are considered a core component of the disorder.74 Individuals with schizophrenia75,76 and those at clinical high-risk for psychosis77 report using substantially more suppression and less reappraisal compared to healthy controls and their use of reappraisal is ineffective, as indicated by event-related potentials studies.78,79 Such difficulties may be associated with reduced activation in the bilateral striatum, ventromedial prefrontal, and right orbitofrontal cortices during regulation of negative emotions.80 These findings are consistent with reports linking suppression use with more severe AH.81 Yet, Grezellschak et al82 reported successful application of instructed-reappraisal in down-regulating anxiety, suggesting a potential for intervention development. Future studies should further clarify the link between emotional regulation, negative emotions, arousal, and psychosis.
The present study has a number of potential limitations. One is the 36-hour assessment period, which was shorter than typical ESM schizophrenia studies. Another limitation is the use of a single ESM question to inquire about the presence of AH. While such strategy has been used successfully in previous ESM AH studies,11,12,22,83,84 having additional questions would have allowed a more granular analysis. Future ESM AH studies should investigate temporal changes in other AH dimensions including duration, location (inside vs outside head), loudness, content and associated distress, as well as controllability. Additionally, we could not exclude the possibility that other concurrent symptoms (eg, paranoia) would impact the outcome. Finally, recent reports have suggested the presence of multiple subtypes of AH.1 The limited number ESM AH questions and the relatively modest sample size prevented us from examining this issue, which may have important implications for treatment development.85
Funding
National Institute of Mental Health (K23MH077653 to D.K.).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. McCarthy-Jones S, Trauer T, Mackinnon A, Sims E, Thomas N, Copolov DL. A new phenomenological survey of auditory hallucinations: evidence for subtypes and implications for theory and practice. Schizophr Bull. 2014;40:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larøi F, Sommer IE, Blom JD, et al. The characteristic features of auditory verbal hallucinations in clinical and nonclinical groups: state-of-the-art overview and future directions. Schizophr Bull. 2012;38:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waters F, Allen P, Aleman A, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford JM, Morris SE, Hoffman RE, et al. Studying hallucinations within the NIMH RDoC framework. Schizophr Bull. 2014;40(suppl 4):S295–S304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimhy D, Goetz R, Yale S, Corcoran C, Malaspina D. Delusions in individuals with schizophrenia: factor structure, clinical correlates, and putative neurobiology. Psychopathology. 2005;38:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harkavy-Friedman JM, Kimhy D, Nelson EA, Venarde DF, Malaspina D, Mann JJ. Suicide attempts in schizophrenia: the role of command auditory hallucinations for suicide. J Clin Psychiatry. 2003;64:871–874. [PubMed] [Google Scholar]
- 7. Thomas N, Rossell SL, Waters F. The changing face of hallucination research: the International Consortium on Hallucination Research (ICHR) 2015 Meeting Report. Schizophr Bull. 2016;42:891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waters F, Woods A, Fernyhough C. Report on the 2nd International Consortium on Hallucination Research: evolving directions and top-10 “hot spots” in hallucination research. Schizophr Bull. 2014;40:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waters F, Aleman A, Fernyhough C, Allen P. Report on the inaugural meeting of the International Consortium on Hallucination Research: a clinical and research update and 16 consensus-set goals for future research. Schizophr Bull. 2012;38:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull. 2010;36:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delespaul P. Assessing Schizophrenia in Daily Life. Maastricht, The Netherlands: IPSER; 1995. [Google Scholar]
- 12. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37:97–104. [DOI] [PubMed] [Google Scholar]
- 13. Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195. [DOI] [PubMed] [Google Scholar]
- 14. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. [DOI] [PubMed] [Google Scholar]
- 15. Holtzman CW, Trotman HD, Goulding SM, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 18. Thompson JL, Kelly M, Kimhy D, et al. Childhood trauma and prodromal symptoms among individuals at clinical high risk for psychosis. Schizophr Res. 2009;108:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heins M, Simons C, Lataster T, et al. Childhood trauma and psychosis: a case-control and case-sibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am J Psychiatry. 2011;168:1286–1294. [DOI] [PubMed] [Google Scholar]
- 20. Freeman D, Emsley R, Dunn G, et al. The stress of the street for patients with persecutory delusions: a test of the symptomatic and psychological effects of going outside into a busy urban area. Schizophr Bull. 2015;41:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM. Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2013;43:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oorschot M, Lataster T, Thewissen V, Bentall R, Delespaul P, Myin-Germeys I. Temporal dynamics of visual and auditory hallucinations in psychosis. Schizophr Res. 2012;140:77–82. [DOI] [PubMed] [Google Scholar]
- 23. Bentall RP. The illusion of reality: a review and integration of psychological research on hallucinations. Psychol Bull. 1990;107:82–95. [DOI] [PubMed] [Google Scholar]
- 24. Dawson ME, Nuechterlein KH, Schell AM, Gitlin M, Ventura J. Autonomic abnormalities in schizophrenia. State or trait indicators? Arch Gen Psychiatry. 1994;51: 813–824. [DOI] [PubMed] [Google Scholar]
- 25. Valkonen-Korhonen M, Tarvainen MP, Ranta-Aho P, et al. Heart rate variability in acute psychosis. Psychophysiology. 2003;40:716–726. [DOI] [PubMed] [Google Scholar]
- 26. Toichi M, Kubota Y, Murai T, et al. The influence of psychotic states on the autonomic nervous system in schizophrenia. Int J Psychophysiol. 1999;31:147–154. [DOI] [PubMed] [Google Scholar]
- 27. Bär KJ, Letzsch A, Jochum T, Wagner G, Greiner W, Sauer H. Loss of efferent vagal activity in acute schizophrenia. J Psychiatr Res. 2005;39:519–527. [DOI] [PubMed] [Google Scholar]
- 28. Bär KJ, Wernich K, Boettger S, et al. Relationship between cardiovagal modulation and psychotic state in patients with paranoid schizophrenia. Psychiatry Res. 2008;157:255–257. [DOI] [PubMed] [Google Scholar]
- 29. Kimhy D, Delespaul P, Ahn H, et al. Concurrent measurement of “real-world” stress and arousal in individuals with psychosis: assessing the feasibility and validity of a novel methodology. Schizophr Bull. 2010;36:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kimhy D, Delespaul P, Corcoran C, Ahn H, Yale S, Malaspina D. Computerized experience sampling method (ESMc): assessing feasibility and validity among individuals with schizophrenia. J Psychiatr Res. 2006;40:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. [DOI] [PubMed] [Google Scholar]
- 32. Mathalon DH, Ford JM. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Front Hum Neurosci. 2012;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ford JM, Dierks T, Fisher DJ, et al. Neurophysiological studies of auditory verbal hallucinations. Schizophr Bull. 2012;38:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27:307–317. [DOI] [PubMed] [Google Scholar]
- 35. Kimhy D, Myin-Germeys I, Palmier-Claus J, Swendsen J. Mobile assessment guide for research in schizophrenia and severe mental disorders. Schizophr Bull. 2012;38:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ben-Zeev D. Mobile technologies in the study, assessment, and treatment of schizophrenia. Schizophr Bull. 2012;38:384–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Granholm E, Loh C, Swendsen J. Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophr Bull. 2008;34:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Demeulemeester M, Kochman F, Fligans B, Tabet AJ, Thomas P, Jardri R. Assessing early-onset hallucinations in the touch-screen generation. Br J Psychiatry. 2015;206:181–183. [DOI] [PubMed] [Google Scholar]
- 39. Palmier-Claus JE, Ainsworth J, Machin M, et al. The feasibility and validity of ambulatory self-report of psychotic symptoms using a smartphone software application. BMC Psychiatry. 2012;12:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimhy D, Vakhrusheva J, Liu Y, Wang Y. Use of mobile assessment technologies in inpatient psychiatric settings. Asian J Psychiatr. 2014;10:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. [DOI] [PubMed] [Google Scholar]
- 42. Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. [DOI] [PubMed] [Google Scholar]
- 43. Myin-Germeys I, Nicolson NA, Delespaul PA. The context of delusional experiences in the daily life of patients with schizophrenia. Psychol Med. 2001;31:489–498. [DOI] [PubMed] [Google Scholar]
- 44. Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381. [PubMed] [Google Scholar]
- 45. Antonius D, Kimhy D, Harkavy-Friedman J, Crystal S, Goetz R, Malaspina D. Paternal age related schizophrenia and cardiac autonomic regulation profiles. Schizophr Res. 2011;127:273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shadish W, Cook T, Campbell D. Experimental and Quasi-Experimental Designs for Generilized Causal Inference. Boston, MA: Houghton Mifflin; 2002. [Google Scholar]
- 47. Helgason C, Sarris J. Mind-body medicine for schizophrenia and psychotic disorders: a review of the evidence. Clin Schizophr Relat Psychoses. 2013;7:138–148. [PubMed] [Google Scholar]
- 48. Thomas N, Hayward M, Peters E, et al. Psychological therapies for auditory hallucinations (voices): current status and key directions for future research. Schizophr Bull. 2014;40(suppl 4):S202–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sommer IE, Slotema CW, Daskalakis ZJ, Derks EM, Blom JD, van der Gaag M. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr Bull. 2012;38:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baker R, Owens M, Thomas S, et al. Does CBT facilitate emotional processing? Behav Cogn Psychother. 2012;40:19–37. [DOI] [PubMed] [Google Scholar]
- 51. Frederick J, Cotanch P. Self-help techniques for auditory hallucinations in schizophrenia. Issues Ment Health Nurs. 1995;16:213–224. [DOI] [PubMed] [Google Scholar]
- 52. Wahass S, Kent G. Coping with auditory hallucinations: a cross-cultural comparison between western (British) and non-western (Saudi Arabian) patients. J Nerv Ment Dis. 1997;185:664–668. [DOI] [PubMed] [Google Scholar]
- 53. David G., Kingdon DT. Cognitive Therapy of Schizophrenia. New York, NY: Guilford Press; 2005. [Google Scholar]
- 54. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. [DOI] [PubMed] [Google Scholar]
- 55. Shergill SS, Brammer MJ, Amaro E, Williams SC, Murray RM, McGuire PK. Temporal course of auditory hallucinations. Br J Psychiatry. 2004;185:516–517. [DOI] [PubMed] [Google Scholar]
- 56. McGuire PK, Shah GM, Murray RM. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. [DOI] [PubMed] [Google Scholar]
- 57. Hoffman RE, Anderson AW, Varanko M, Gore JC, Hampson M. Time course of regional brain activation associated with onset of auditory/verbal hallucinations. Br J Psychiatry. 2008;193:424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thoma RJ, Chaze C, Lewine JD, et al. Functional MRI evaluation of multiple neural networks underlying auditory verbal hallucinations in schizophrenia spectrum disorders. Front Psychiatry. 2016;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yun JY, Kim SN, Lee TY, Chon MW, Kwon JS. Individualized covariance profile of cortical morphology for auditory hallucinations in first-episode psychosis. Hum Brain Mapp. 2016;37:1051–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dierks T, Linden DE, Jandl M, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. [DOI] [PubMed] [Google Scholar]
- 61. Silbersweig DA, Stern E, Frith C, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. [DOI] [PubMed] [Google Scholar]
- 62. Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010;67:912–918. [DOI] [PubMed] [Google Scholar]
- 63. Hatton SN, Lagopoulos J, Hermens DF, Hickie IB, Scott E, Bennett MR. Short association fibres of the insula-temporoparietal junction in early psychosis: a diffusion tensor imaging study. PLoS One. 2014;9:e112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nazeri A, Chakravarty MM, Felsky D, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oishi K, Zilles K, Amunts K, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. [DOI] [PubMed] [Google Scholar]
- 67. Christensen H, Boysen G, Christensen AF, Johannesen HH. Insular lesions, ECG abnormalities, and outcome in acute stroke. J Neurol Neurosurg Psychiatry. 2005;76:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 69. Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (Berl). 2000;149:319–325. [DOI] [PubMed] [Google Scholar]
- 70. Phillips LK, Voglmaier MM, Deldin PJ. A preliminary study of emotion processing interference in schizophrenia and schizoaffective disorder. Schizophr Res. 2007;94:207–214. [DOI] [PubMed] [Google Scholar]
- 71. Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychol Rev. 2009;19:312–323. [DOI] [PubMed] [Google Scholar]
- 73. Blum LH, Vakhrusheva J, Saperstein A, et al. Depressed mood in individuals with schizophrenia: a comparison of retrospective and real-time measures. Psychiatry Res. 2015;227:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. [DOI] [PubMed] [Google Scholar]
- 75. Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, Gross JJ. Emotion awareness and regulation in individuals with schizophrenia: implications for social functioning. Psychiatry Res. 2012;200:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kimhy D, Vakhrusheva J, Khan S, et al. Emotional granularity and social functioning in individuals with schizophrenia: an experience sampling study. J Psychiatr Res. 2014;53:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kimhy DG, Gill KE, Brucato G, et al. The impact of emotion awareness and regulation on social functioning in individuals at clinical high-risk for psychosis. Psychol Med. 2016;46:2907–2918. [DOI] [PubMed] [Google Scholar]
- 78. Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Lee BG, Gold JM. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophr Bull. 2013;39:872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Horan WP, Hajcak G, Wynn JK, Green MF. Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychol Med. 2013;43:2377–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Eack SM, Wojtalik JA, Barb SM, Newhill CE, Keshavan MS, Phillips ML. Fronto-limbic brain dysfunction during the regulation of emotion in schizophrenia. PLoS One. 2016;11:e0149297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Badcock JC, Paulik G, Maybery MT. The role of emotion regulation in auditory hallucinations. Psychiatry Res. 2011;185:303–308. [DOI] [PubMed] [Google Scholar]
- 82. Grezellschak S, Lincoln TM, Westermann S. Cognitive emotion regulation in patients with schizophrenia: evidence for effective reappraisal and distraction. Psychiatry Res. 2015;229:434–439. [DOI] [PubMed] [Google Scholar]
- 83. Hartley S, Haddock G, Vasconcelos e Sa D, Emsley R, Barrowclough C. The influence of thought control on the experience of persecutory delusions and auditory hallucinations in daily life. Behav Res Ther. 2015;65:1–4. [DOI] [PubMed] [Google Scholar]
- 84. Mulligan LD, Haddock G, Emsley R, Neil ST, Kyle SD. High resolution examination of the role of sleep disturbance in predicting functioning and psychotic symptoms in schizophrenia: a novel experience sampling study. J Abnorm Psychol. 2016;125:788–797. [DOI] [PubMed] [Google Scholar]
- 85. Smailes D, Alderson-Day B, Fernyhough C, McCarthy-Jones S, Dodgson G. Tailoring cognitive behavioral therapy to subtypes of voice-hearing. Front Psychol. 2015;6:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]