Abstract
Psychometric research has identified stable traits that predict inter-individual differences in appetitive motivation and approach behavior. Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scales have been developed to quantitatively assess these traits. However, neural mechanisms corresponding to the proposed constructs reflected in BIS/BAS are still poorly defined. The ventral striatum (VS) and orbitofrontal cortex (OFC) are implicated in subserving reward-related functions that are also associated with the BAS. In this study, we examined whether functional connectivity between these regions predicts components of these scales. We employed resting-state functional connectivity and BIS/BAS scores assessed by a personality questionnaire. Participants completed a resting state run and the Behavioral Inhibition and Activation Systems (BIS/BAS) Questionnaire. Using resting-state BOLD, we assessed correlations between two basal ganglia ROIs (caudate and putamen) and bilateral OFC ROIs, establishing single subject connectivity summary scores. Summary scores were correlated with components of BIS/BAS scores. Results demonstrate a novel correlation between BAS-fun seeking and resting-state connectivity between middle OFC and putamen, implying that spontaneous synchrony between reward-processing regions may play a role in defining personality characteristics related to impulsivity.
Keywords: resting state, connectivity, fMRI, BIS/BAS, impulsivity
Introduction
Both normal behavior and pathological deviations, such as eating habits and addiction, are related to individual differences in reward-sensitivity and inhibitory control (Powell et al., 2002; Davis et al., 2007; Crews and Boettiger, 2009). Providing an integrative account of the biopsychological mechanisms subserving personality traits related to reward processing and impulsivity is therefore of interest to both basic and clinical researchers.
Studies examining variations in impulsive behavior using neuroimaging methods have largely focused on state-dependent activation in response to reward-driven behavior and rewarding stimuli. For example, individual differences in impulsivity and reward-seeking predict neural responses to rewarding stimuli including images of food (Beaver et al., 2006) and exposure to drugs (Daw et al., 2006; Franken et al., 2006). Relatively little, however, is known about the neural substrates of stable individual differences in trait impulsivity. Many studies have examined how task-dependent fMRI activation varies as a function of personality and behavior (Scheres and Sanfey, 2006, Hickey et al., 2010; Reineberg et al., 2015). However, few have examined how task-free, resting-state neural activity may reflect stable individual differences in personality.
Candidate biological substrates of a behavioral approach system
One major challenge in human cognitive neuroscience is to understand how specific differences in brain physiology relate to global psychological phenomena such as personality traits (see Depue & Collins 1999). This study is concerned with a subset of personality traits primarily relating to motivation and approach. To measure these traits, we employed the popular and validated Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) questionnaire (Carver and White, 1994, based upon Gray, 1987). The BIS/BAS questionnaire assesses three personality dimensions related to reward sensitivity (BAS) and one related to behavioral inhibition and anxiety (BIS). The three BAS scales include items probing the persistent pursuit of desired goals (drive scale), the desire for novel rewards and willingness to approach potentially rewarding events (fun-seeking scale), and positive responses to the occurrence or anticipation of reward (reward responsiveness scale). Of special interest to the current work is the BAS fun-seeking subscale, which correlates strongly with other measures of impulsivity, while the other subscales do not (Zelenski and Larsen, 1999).
BAS subscales have been shown to correlate with reward-related BOLD activation, implicating the mesocortical circuitry involved in reward sensitivity reflected by BAS (Scheres and Sanfey, 2006; Kahnt et al., 2012). Experimental paradigms that elicit behavior using reward and appetizing stimuli reliably activate regions in the subcortical striatum and orbitofrontal/ventromedial prefrontal cortex, both of which are the focus of numerous studies investigating reward coding and association (Elliot et al., 2000; Laricchiuta et al., 2014; Jarbo and Verstynen, 2015). Ventral striatum (VS), including caudate nucleus and putamen, as well as subdivisions of orbitofrontal cortex (OFC), are often implicated in fMRI studies as involved in processing reward and impulsive behavior (O’Doherty et al., 2001; McClure et al., 2004, Kringelbach, 2005; Delgado, 2007). Thus, to begin to understand whether and how personality traits reflected by measures of BIS/BAS constructs are related to functional connection of circuits, we look first to this system.
Recent work has made strides in providing a robust functional parcellation of the subdivisions of OFC and limbic circuitry in humans (e.g., Morris et al., 2016). As part of a larger circuit mediating goal-directed behavior, the striatum plays a central role as the input structure of the basal ganglia (Delgado, 2007; Pessoa and Engelmann, 2010), whose substructures have been linked to reward processing (e.g., Elliott et al., 2000). Evidence from DTI and functional studies examining coactivation during cognitive tasks suggest that VS projects to orbitofrontal cortices (Cohen et al., 2005; Jarbo and Verstynen, 2015). Additionally, putamen contains a white matter convergence zone consisting of voxels with unique projections from OFC (Jarbo and Verstynen, 2015). In turn, OFC has also been implicated in representing reward and affective value, directly influencing decision-making and impulsive behavior (Kringelbach and Radcliffe, 2005; Price, 2006; Pardey et al., 2013).
An integral part of the limbic system, the cingulate is influential in emotion formation and linking behavioral outcomes to motivation (Cardinal et al., 2002). Aspects of the anterior cingulate cortex (ACC) converge with amygdala, nucleus accumbens, hypothalamus, and insula to assess the salience of motivational information (Allman et al., 2001). It is clear that the description of anatomical connectivity is improving; however, an account of individual differences in connectivity, and how they are realized functionally, is lacking.
Resting-state functional correlation
Correlations among spontaneous, low-frequency (< 0.1 Hz) fluctuations in BOLD signal among disparate regions may offer insight into how and when certain brain areas work in tandem. Various properties of both the intra- and inter-regional activity of the resting brain, including stable state-independent networks, are well documented and promote our understanding of the brain as a complex network (Cohen et al., 2009; Cauda et al., 2011; Choi et al., 2012, Zuo et al, 2013). Li and colleagues (2013) have shown that resting-state functional correlations (RSFC) in regions such as striatum and prefrontal cortex are predictive of impulsivity in a delay-discounting task. While their results suggest that RSFC predicts differences in impulsive choices, this study does not address individual differences in underlying trait impulsivity. Studies of the relationship between low-frequency BOLD oscillations and psychopathologies associated with personality (Wolf et al., 2011; Tang et al., 2013) have shown that clinical deviations in personality traits are correlated with variations in resting-state functional correlation. Thus, RSFC may allow us to clarify the role functional connectivity plays in the variation of personality traits in healthy populations (Fulwiler et al., 2012).
Of particular interest to capturing the nature of reward-driven and impulsive behavior is Gray’s model of personality and addiction (Gray, 1970; Franken et al., 2006). Gray’s Behavioral Activation system has been proposed as a biologically motivated system underlying stable appetitive motivation and approach traits, while a related Behavioral Inhibition System is proposed to underlie inhibition of behavior in response to cues that signal negative outcomes (Sutton and Davidson, 1997; Johnson et al., 2003). Approach behavior and disinhibition have been proposed to reflect reactivity in a ventral striatal–orbitofrontal circuit (Pickering and Gray, 1999), but it remains unclear how variability in trait impulsivity is reflected in brain structure and connectivity. In an attempt to connect the Behavioral Activation System with neurobiological structures, we examined in this study how trait personality characteristics relate to, and are perhaps influenced by, underlying RSFC.
Materials and methods
General procedure
Forty-seven healthy subjects participated in the study (ages 18–38, mean ± SD of 22 ± 3.8 years; 44.6% male). This was a convenience sample of participants in several different task-related fMRI studies. All subjects completed the same structural and resting-state functional scans, as well as a BIS/BAS questionnaire. All subjects were right-handed, with no history of neurological or psychiatric disease. Each subject provided signed, informed consent for this research as approved by the Institutional Review Board of the University of Maryland and the University of Delaware. All subjects completed the same 3D MPRAGE structural scan. Task-based functional MRI scans were acquired prior to resting-state scans, and each subject completed one of two behavioral tasks. Twenty-four subjects completed a simple game task; 23 completed a reward-association learning task. Behavioral runs were followed by a functional resting-state scan and, in most cases, a diffusion-weighted scan.
Following each scanning session, subjects completed the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) questionnaire (Carver and White, 1994). The BIS subscale relates to anxiety and avoidance of cues to negative outcomes (e.g. ‘Even is something bad is about to happen to me, I rarely experience fear or nervousness’). The three BAS subscales include items probing the persistent pursuit of desired goals (drive scale; e.g., ‘When I want something, I go all-out to get it’), the desire for novel rewards and willingness to approach potentially rewarding events, related to impulsivity (fun-seeking scale, e.g., ‘I crave excitement and new sensations’), and positive responses to the occurrence or anticipation of reward (reward responsiveness scale; e.g., ‘When I get something I want, I feel excited and energized’). Responses were made on a 4-point scale, with 1 indicating strong agreement, 2 indicating slight agreement, 3 indicating slight disagreement, and 4 indicating strong disagreement. The questionnaire was administered electronically, with questions appearing in a randomized order for each participant.
Imaging procedures
Resting-state fMRI (rs-fMRI) data were collected on a Siemens 3T TIM Trio system (Siemens, Erlangen, Germany). Foam pads were used for positioning and immobilization of the subject’s head within a 32-channel coil. T2*-weighted images were collected using a multi-band (MB) echo planar sequence (TR = 1000 msec, TE = 39.4 msec, flip angle = 90°, MB acceleration factor = 6) with 66 contiguous transverse slices of 2.2 mm thickness covering the whole brain. Despite a comparatively longer TE, which in standard EPI protocols may lead to signal dropout in areas such as OFC, MB EPI provides significant signal gain per unit volume of tissue and unit length of time, along with improved characteristics in typically problematic regions such as OFC, and this gain more than makes up for the effect of lengthened TE (Setsompop et al., 2012; Xu et al., 2013). Matrix size was 96 × 96 pixels with a 210 mm × 210 mm field of view (FOV), corresponding to an in-plane resolution of 2.2 mm × 2.2 mm. High-resolution T1-weighted anatomical images were acquired using three-dimensional magnetization prepared rapid acquisition gradient recalled echo (3D MP-RAGE) using the following parameters: in-plane resolution 0.9 mm × 0.9 mm 192 slices with 0.9 mm thickness, FOV of 230 mm, TR = 1900 msec, TE = 2.32 msec, flip angle = 9°. During rs-fMRI scans, all participants were asked to remain still and relaxed, without falling asleep, while fixating on an unchanging cross-shaped stimulus presented on the screen.
Data analysis
fMRI processing pipeline
We adapted the Duke Brain Imaging and Analysis Center’s (BIAC) resting-state pipeline employing FSL (FMRIB Software Library, University of Oxford, UK) functions to pre-process and analyze rs-fMRI data as follows (Chen et al., 2009). Motion correction was performed using FSL’s linear image registration tool (MCFLIRT). The functional run was meaned across time and submitted to FSL’s Brain Extraction Tool (BET) to create a brain mask and perform skull-stripping. Data were normalized using FLIRT to the MNI-152 2mm standardized brain, after registration of functional to structural and structural to MNI-152 template images. White matter (WM) and cerebrospinal fluid (CSF) masks were generated, and the fMRI signal was averaged within those masks for each timepoint. Band-pass filtering was used to remove high- and low-frequency noise, using a pass-through range of 0.001–0.08 Hz. Time-series were submitted to a regression that included regressors of no interest, specifically six-parameter rigid body head motion (obtained from motion correction), the signal averaged over WM, and the signal averaged over CSF regions to reduce non-neuronal contributions to BOLD correlations. The MNI automated anatomical labeling atlas (AAL) was used to extract the average time-series for each of the regions of interest based on the residuals of this regression. Finally, the time-series were employed to produce a functional BOLD correlation matrix, consisting of bivariate correlation coefficients between each pair of regions. Fisher’s z-transformation was then applied to each subjects’ coefficients. Selection parameters of the main regions of interest are delineated below.
ROI selection
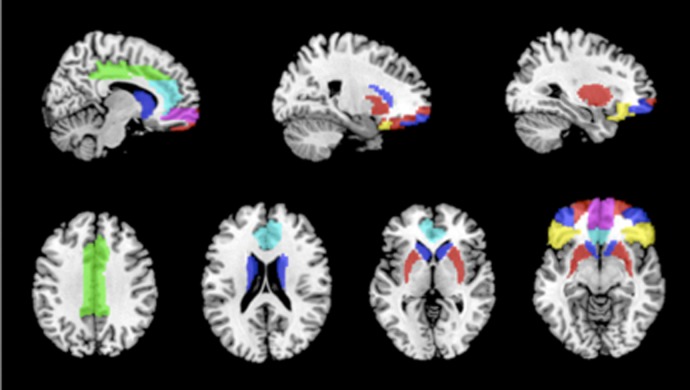
Subcortical and frontal regions of interest (ROIs) were picked a priori from areas classically involved in reward processing, particularly regions implicated in a dopaminergic circuit originating in basal ganglia and terminating in OFC (Middleton and Strick, 2000; Delgado, 2007; Verstynen et al., 2012). We additionally examined connectivity to cingulate cortex. Focusing our examination on these critical reward-processing regions, we selected subcortical ROIs to include bilateral caudate nucleus and putamen. Bilateral inferior, middle, medial, superior orbitofrontal cortices plus anterior cingulate and middle cingulate make up our prefrontal ROIs. To focus on mesocortical pathways between frontal and subcortical regions, and to limit the number of statistical comparisons, we focused only on connectivity between basal ganglia ROIs, on the one hand, and frontal ROIs, on the other, in our final analysis. For instance, we examined putamen’s correlation with middle OFC, but not middle-OFC’s connectivity to middle cingulate. In total, 14 connectivity scores per subject were entered into our final analysis (Figure 1). We computed temporal signal-to-noise ratios (tSNR) to ensure optimal functional signal from each region of interest (basal ganglia, 89.60–96.03; cingulate, 101.67–106.01; OFC 79.98–82.28; these values compare favorably to previous work, see e.g. Welvaert and Rosseel, 2013).
Fig. 1.
Regions of interest. Putamen (red) and caudate (blue) connectivity with inferior OFC (yellow), middle OFC (blue), medial OFC (fuchsia), superior OFC (red), anterior cingulate (cyan), and middle cingulate (green).
Statistical analysis
The output of the above-described resting-state pipeline is a set of functional correlation scores (temporal correlations) for each subject and each pair of AAL regions. From this matrix, correlations between prefrontal and subcortical ROIs were extracted. In order to test the hypothesis that individual differences in BAS scores would vary according to the strength of functional corticostriatal connectivity, bivariate correlations were computed between the BAS scales and these RSFC summary scores for each ROI combination. We employed a Bonferroni correction to our statistical threshold to account for multiple comparisons, which were 48 in total (12 ROI pairs and 4 BIS/BAS subscales).
Results
BIS/BAS scoring
Group-level correlation coefficients were computed between pairs of all four subscores of the BIS/BAS inventory. While the BIS/BAS yields separate subscale scores for these components of behavioral activation, they were intercorrelated in our sample. These correlations are presented in Table 1.
Table 1.
BIS/BAS subscale correlations
| BIS | BASd | BASf | BASr | |
|---|---|---|---|---|
| BIS | — | r = −0.35 | r = −0.36 | r = −0.056 |
| P = 0.016 | P = 0.013 | P = 0.71 | ||
| BASd | r = −0.35 | — | r = 0.67 | r = 0.54 |
| P = 0.016 | P < 0.0001 | P < 0.0001 | ||
| BASf | r = −0.36 | r = 0.67 | — | r = 0.67 |
| P = 0.013 | P < 0.0001 | P < 0.0001 | ||
| BASr | r = −0.056 | r = 0.54 | r = 0.67 | — |
| P = 0.71 | P < 0.0001 | P < .0001 |
In our sample, mean total scale scores for the BAS scales were as follows: BAS-fun seeking, range of 10–15, mean ± SD of 12.51 ± 2.76; BAS drive, range of 7–13 mean ± SD of 12.05 ± 2.64; BAS-reward responsiveness, range of 15-20 mean ± SD of 18.32 ± 1.86; and BIS, range of 19-25 mean ± SD of 21.15 ± 4.5. Male and female scores did not differ significantly in reward responsiveness scales (m = 18.37, f = 18.27)*.1
Primary analysis
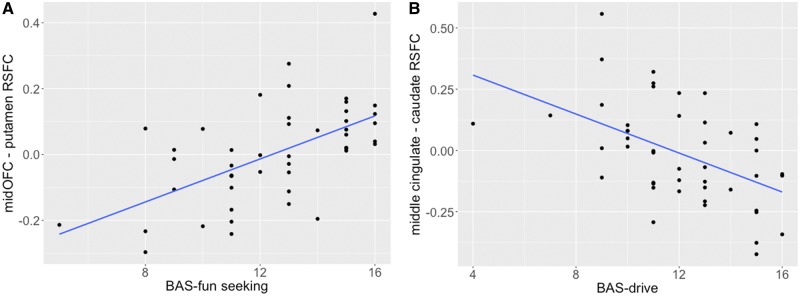
Table 2 below shows the bivariate correlation coefficients computed between putamen/caudate and our frontal ROIs’ connectivity summary scores, and the BIS/BAS subscale scores, along with uncorrected P-values. We found two significant correlations after correction (Bonferroni. BAS-fun seeking (BASf) was positively correlated with resting-state connectivity between middle OFC and putamen (r = 0.59, P < 0.0001). Additionally, middle cingulate and caudate RSFC connectivity was negatively correlated with BAS drive (BASd, r = −0.50, P = 0.0004). These results are shown in Figure 2 below.
Table 2.
ROI RSFC correlations with BIS/BAS scores
| BIS | BASf | BASd | BASr | |
|---|---|---|---|---|
| midOFC-putamen | r = −0.21 | r = 0.59 | r = 0.28 | r = 0.31 |
| P = .15 | P < 0.0001* | P = 0.058 | P = 0.033 | |
| midOFC-caudate | r = −.23 | r = −0.038 | r = −0.10 | r = −0.13 |
| P = .05 | P = 0.80 | P = 0.49 | P = 0.38 | |
| medOFC-putamen | r = −0.24 | r = −0.057 | r = −0.19 | r = 0.038 |
| P = 0.11 | P = .70 | P = 0.20 | P = 0.80 | |
| medOFC-caudaute | r = −0.075 | r = -.10 | r = −0.20 | r = −0.08 |
| P = 0.61 | P = .50 | P = 0.18 | P = 0.59 | |
| supOFC-putamen | r = −0.35 | r = .19 | r = −0.027 | r = −0.074 |
| P = 0.015 | P = .21 | P = 0.86 | P = 0.62 | |
| supOFC-caudate | r = −0.14 | r = .014 | r = −0.14 | r = 0.044 |
| P = 0.34 | P = 0.93 | P = 0.37 | P = 0.77 | |
| infOFC-putamen | r = −0.08 | r = 0.39 | r = 0.16 | r = 0.32 |
| P = 0.57 | P = 0.007 | P = 0.30 | P = 0.028 | |
| infOFC-caudate | r =-0.23 | r = 0.38 | r = 0.39 | r = −0.13 |
| P = 0.12 | P = 0.80 | P = 0.49 | P = 0.38 | |
| ACC putamen | r = −0.11 | r = −0.052 | r = −0.054 | r = −0.011 |
| P = 0.46 | P = 0.73 | P = 0.72 | P = 0.94 | |
| ACC caudate | r = −0.090 | r = .034 | r = −0.31 | r = −0.054 |
| P = 0.55 | P = 0.82 | P = 0.034 | P = 0.72 | |
| MC putamen | r = −0.041 | r = −0.071 | r = −0.12 | r = −0.22 |
| P = 0.78 | P = 0.63 | P = 0.44 | P = 0.14 | |
| MC caudate | r = 0.14 | r = −0.18 | r = −0.50 | r = −0.19 |
| P = 0.34 | P = 0.24 | P = 0.0004* | P = 0.19 |
Fig. 2.
(A, B) bivariate correlations of significant associations between RSFC and BIS/BAS subscale scores.
Principal components analysis
Given the observed intercorrelation of the four BIS/BAS measures, we performed a principal components analysis (PCA) to determine four new dimensions with different loadings on each dimension, thereby summarizing the correlated components of the BAS measures (Beckmann et al., 2005). This was done by z-scoring each component across participants, and then submitting z-scored BIS/BAS scale measures to a PCA. Consistent with the above analysis, RSFC of mid-OFC and putamen was strongly correlated with the first principal component (r = 0.4804, P = 0.0003). This first component had a similar positive loading on the three BAS subscales (with strongest loading on BAS-fun seeking), and a weaker negative loading on the BIS subscale (BAS-reward: 0.56, BAS-fun seeking: 0.590, BAS drive: 0.50, and BIS: −0.28).
General linear model
To additionally confirm the primary results, a multiple regression model was computed in order to account for the intercorrelation among the BIS/BAS subscales and simultaneously estimate the association between subscales and RSFC summary scores. We composed two regression models, one for each of the middle OFC-putamen and middle cingulate-caudate connectivity summary scores, and included as regressors the following variables: all four individual subject BIS/BAS subscale scores, both relevant ROI tSNRs estimates, age, gender and binary variables coding for experiment participation. Shared variation amongst IVs in this model, including shared variation across BIS/BAS scores, would thus not be modeled and not attributed to any IV. Confirming our prior results, the only BIS/BAS subscale score parameter estimate significantly associated with variation in middle OFC and putamen functional correlation is BAS-fun seeking (Table 3).
Table 3.
GLM results. Estimates and corresponding significance results of GLM coefficients (coefficients of control variables not shown). (A) Middle orbitofrontal cortex-putamen RSFC model results. As expected, the only parameter that significantly contributes to variation remains BAS-fun seeking. (B) Middle cingulate caudate RSFC model results. BAS drive remains the only significantly contributing parameter estimate.
| A | Estimate | Std. Error | t-value | Pr(>|t|) |
|---|---|---|---|---|
| BASd | −0.013 | 0.0096 | −1.36 | 0.18 |
| BASf | 0.032 | 0.011 | 2.93 | 0.006* |
| BASr | −0.0015 | 0.014 | −0.11 | 0.91 |
| BIS | 0.0017 | 0.0047 | 0.37 | 0.71 |
| B | Estimate | Std. Error | t value | Pr(>|t|) |
| BASd | −0.049 | 0.017 | −2.95 | 0.0056* |
| BASf | 0.018 | 0.019 | 0.94 | 0.35 |
| BASr | −0.0082 | 0.024 | −0.35 | 0.73 |
| BIS | −0.00055 | 0.0087 | −0.063 | 0.95 |
Discussion
The present study investigated how the strength of spontaneous fluctuations in BOLD signal that are synchronized between regions implicated in reward processing may relate to individual differences in personality measures linked to trait reward responsiveness, impulsivity, drive and inhibition. Aiming to mechanistically account for such variable features, a wealth of research has associated the BIS/BAS scales with behavioral and state-induced neural changes believed to be concomitant with individual variation in reward sensitivity. Previous neuroimaging and neurophysiology studies have shown the crucial roles of the striatum and OFC in high-level processing of reward, learning, and decision-making (O’Doherty et al., 2001; Cohen et al., 2009; Kahnt et al., 2010). Additionally, intrinsic, task-free activity found in the resting brain has been shown to predict task-based patterns of BOLD activity (Mennes et al., 2010; Li et al., 2013). The findings from this study complement correlations between impulsivity, neurobiology, and task-dependent activation in previous work. The results of our analyses may provide further insight into the strength of resting-state functional correlations in an orbitostriatal dopamine (DA) circuit and its relationship with patterns of impulsive behavior.
It has been suggested that projections from OFC to striatum provide routes by which the OFC can influence behavior (Winstanley et al., 2004; Rolls, 2005; Jarbo and Verstynen, 2015). Our results, consistent with these findings, support a network of corticostriatal connections integrating reward, attention and executive processes. Previous psychopharmacological studies have implicated OFC in the integration of value-encoding and resulting impulsive action and choices. For example, Pardey et al. (2013) conclude that reduced DA D2 receptor functioning may contribute to an increase in impulsive behavior. Additionally, it has been argued that impulsive and sensation-seeking behaviors are phenotypic of substance addiction (Ersche et al., 2010), which is directly associated with striatal dopamine D2 receptor availability (Martinez et al., 2004). Taken together with the present study, these findings may suggest that variation in DA D2 receptor functioning in OFC and striatum contribute to variation in an underlying approach system that is in turn instantiated in individual differences in trait impulsivity.
Multiple cognitive functions rely on the striatum and its connections between various cortical and subcortical networks (McClure et al., 2004; Cohen et al., 2009). Interestingly, the strongest association in this study between BAS measures and orbitostriatal connectivity occurs in bilateral putamen and bilateral middle OFC, suggesting that this functional connection is driven by putamen in VS and not caudate nucleus. This finding is consistent with recent studies of converging functional and structural connectivity involving putamen and orbitofrontal projections (Jarbo and Verstynen, 2015). Results showing the strength of putamen/mid-OFC RSFC, and its relationship to BAS functioning, offer further specificity to a neurobiology of approach behavior and reward-sensitivity. It is possible that these relationships could also predict a predisposition to the atypical inhibitory control symptomatic of dopamine-related psychopathologies.
According to Gray’s model of personality and addiction (Franken et al., 2006), individuals displaying high levels of BAS sensitivity are predisposed to pathological approach behaviors. Our findings may have relevance for the pathophysiology of disorders such as addiction, ADHD, and bipolar disorder, whose etiologies are broadly characterized by aberrant dopaminergic activity. For example, symptoms of OCD are associated with orbitostriatal hyperactivation (Evans et al., 2004). Substance abuse disorders, and the motivating relationship between impulsivity and executive function, are shown to be related to changes in orbitofrontal cortical structure and dopamine receptor availability (Martinez et al., 2004; Dalley et al., 2008; Crews and Boettiger, 2009). The findings presently discussed may support future investigations needed to characterize the RSFC of regions in this orbitostriatal circuit underlying psychiatric and neurological populations.
The observed correlation between mid-OFC/putamen connectivity and trait impulsivity suggests potential psychological mechanisms at play in reward-seeking behavior. The OFC is implicated in guiding choice behavior according to estimated reward value of those choices (e.g. Elliot et al., 2000; Tanji and Hoshi, 2001), as well as playing a role in inhibition of impulsive behavior, suggesting that OFC may integrate representations of positive and negative consequences. Increased resting-state correlations between regions of basal ganglia associated with ‘wanting’ (Brown et al., 1999; Schmidt et al., 2008) and the mid-OFC implies that, perhaps, this region’s role in suppressing impulsive behavior might be moderated by increased communication with regions responsible for assessing desirable outcomes. That is, if OFC integrates wanting reward with avoiding consequence, connectivity with regions more associated with wanting than assessing negative consequences may tip the balance of behavior towards wanting.
We have primarily cast impulsivity itself as a construct. However, a different emerging view (e.g., Carver et al., 2009) places impulsivity in the context of a dual-mode supervisory system (MacDonald, 2008). According to such a view, impulsive actions may arise out of responses from the more reactive, fast-processing mode, and impulsivity may derive from lack of constraint by a supervisory system. This framework is grounded by work suggesting that pathological impulsivity is related to disordered serotonergic functioning (Raine 2008). Impulsive behavior may be due in part to variation in serotonin receptor density, which shares common projections with a corticostriatal dopamine loop. Under this view, the connection we observed in the present study between mid-OFC – putamen connectivity and impulsivity may reflect variations in control by the supervisory system: when such a supervisory system generally exerts less control, impulsivity may result, perhaps in part from stable differences in the inhibition of communication between putamen and mid-OFC. Further research incorporating serotonergic receptor functioning, resting-state correlations, and trait impulsivity could be illuminating.
While we found only two pairs of regions whose connectivity was significantly correlated with BIS/BAS subscales, it is important to note some limitations in our study that may have prevented us from finding a broader set of robust correlations. Several regional interactions were correlated with BIS/BAS subscales at levels that failed to reach stringent statistical correction, but were significant at an uncorrected alpha of .05. First, our sample size is limited, and with a larger sample there would be more power to detect such correlations. Secondly, as noted previously, the BIS/BAS subscales exhibited surprisingly high degrees of intercorrelation. We hope that the present study will encourage follow-up studies including larger samples, and seek either to employ alternative subscales with weaker intercorrelations or focus on correlations of rs-fMRI with components derived from PCA or similar dimensionality reduction techniques.
Forthcoming research illustrating orbitostriatal structural connectivity using diffusion-weighted MRI could offer insight into whether and how variations in white matter microstructure between these regions also correlate with personality traits. Additionally, we are left questioning the causal direction between personality and RSFC. It is possible that this a bottom-up relationship, by which deviations in RSFC functioning in these subregions lead to differences in the expression of personality traits. Conversely, decreased functional correlations between regions may lead to a decrease in dopamine receptor availability in these regions. Psychopharmacological work examining the association between RSFC, personality, and variations in catecholamine levels may allow us to further specify the functional relationship of regions involved in reward-sensitivity, approach behavior, and inhibitory control.
Funding
This study was funded by a University of Delaware Research Foundation Grant in conjunction with NSF BCS 1558535 and NSF OIA 1632849 to Timothy Vickery.
Conflict of interest. None declared.
Footnotes
Female participants did score significantly higher on average on BIS than did male participants (f = 23.1, m = 18.9).
References
- Allman J., Hakeem A., Erwin J., Nimchinsky E., Hof P. (2001). The anterior cingulate cortex: the evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences 935, 107–17. [PubMed] [Google Scholar]
- Beaver J.D., Lawrence A.D., van Ditzhuijzen J., Davis M.H., Woods A., Calder A.J. (2006). Individual differences in reward drive predict neural responses to images of food. The Journal of Neuroscience, 26, 5160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 360, 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Bullock D., Grossberg S. (1999). How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. The Journal of Neuroscience, 19, 10502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews, 26, 321–52. [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319. [Google Scholar]
- Carver C.S., Johnson S.L., Joormann J. (2009). Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Current Directions in Psychological Science, 18, 195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N.K., Chou Y.H., Song A.W., Madden D.J. (2009). Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Structure and Function, 213, 571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X., Young J., Baek J.M., Kessler C., Ranganath C. (2005). Individual differences in extraversion and dopamine genetics predict neural reward responses. Cognitive Brain Research, 25, 851–61. [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Schoene-Bake J.C., Elger C.E., Weber B. (2009). Connectivity-based segregation of the human striatum predicts personality characteristics. Nature Neuroscience, 12, 32–4. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Boettiger C.A. (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology Biochemistry and Behavior, 93, 237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Patte K., Levitan R., Reid C., Tweed S., Curtis C. (2007). From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite, 48, 12–9. [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Mar A.C., Economidou D., Robbins T.W. (2008). Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacology Biochemistry and Behavior, 90, 250–60. [DOI] [PubMed] [Google Scholar]
- Daw N.D., O'doherty J.P., Dayan P., Seymour B., Dolan R.J. (2006). Cortical substrates for exploratory decisions in humans. Nature, 441, 876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward‐related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Depue R.A., Collins P.F. (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–517. [DOI] [PubMed] [Google Scholar]
- Elliott R., Friston K.J., Dolan R.J. (2000). Dissociable neural responses in human reward systems. The Journal of Neuroscience, 20, 6159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.W., Lewis M.D., Iobst E. (2004). The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive–compulsive disorder. Brain and Cognition, 55, 220–34. [DOI] [PubMed] [Google Scholar]
- Ersche K.D., Turton A.J., Pradhan S., Bullmore E.T., Robbins T.W. (2010). Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biological Psychiatry, 68, 770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken I.H., Muris P., Georgieva I. (2006). Gray's model of personality and addiction. Addictive Behaviors, 31, 399–403. [DOI] [PubMed] [Google Scholar]
- Fulwiler C.E., King J.A., Zhang N. (2012). Amygdala-orbitofrontal resting-state functional connectivity is associated with trait anger. Neuroreport, 23, 606.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.A. (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8, 249–66. [DOI] [PubMed] [Google Scholar]
- Gray J.A. (1987). The psychology of fear and stress (Vol. 5). CUP Archive.
- Hickey C., Chelazzi L., Theeuwes J. (2010). Reward guides vision when it's your thing: Trait reward-seeking in reward-mediated visual priming. PLoS One, 5, e14087.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K., Verstynen T.D. (2015). Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. The Journal of Neuroscience, 35, 3865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.L., Turner R.J., Iwata N. (2003). BIS/BAS levels and psychiatric disorder: An epidemiological study. Journal of Psychopathology and Behavioral Assessment, 25, 25–36. [Google Scholar]
- Kahnt T., Chang L.J., Park S.Q., Heinzle J., Haynes J.D. (2012). Connectivity-based parcellation of the human orbitofrontal cortex. The Journal of Neuroscience, 32, 6240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T., Heinzle J., Park S.Q., Haynes J.D. (2010). The neural code of reward anticipation in human orbitofrontal cortex. Proceedings of the National Academy of Sciences, 107, 6010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience, 6, 691–702. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D., Petrosini L., Piras F., et al. (2014). Linking novelty seeking and harm avoidance personality traits to basal ganglia: volumetry and mean diffusivity. Brain Structure and Function, 219, 793–803. [DOI] [PubMed] [Google Scholar]
- Li N., Ma N., Liu Y., et al. (2013). Resting-state functional connectivity predicts impulsivity in economic decision-making. The Journal of Neuroscience, 33, 4886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K.B. (2008). Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychological Review, 115, 1012.. [DOI] [PubMed] [Google Scholar]
- Martinez D., Broft A., Foltin R.W., et al. (2004). Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology, 29, 1190–202. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306, 503–7. [DOI] [PubMed] [Google Scholar]
- McClure S.M., York M.K., Montague P.R. (2004). The neural substrates of reward processing in humans: the modern role of FMRI. The. Neuroscientist, 10, 260–8. [DOI] [PubMed] [Google Scholar]
- Mennes M., Kelly C., Zuo X.N., et al. (2010). Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage, 50, 1690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. (2000). Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain and Cognition, 42, 183–200. [DOI] [PubMed] [Google Scholar]
- Morris L.S., Kundu P., Dowell N., et al. (2016). Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex, 74, 118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J., Kringelbach M.L., Rolls E.T., Hornak J., Andrews C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4, 95–102. [DOI] [PubMed] [Google Scholar]
- Pardey M.C., Kumar N.N., Goodchild A.K., Cornish J.L. (2013). Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. Journal of Psychopharmacology, 27, 203–12. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Engelmann J.B. (2010). Embedding reward signals into perception and cognition. Frontiers in Neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A.D., Gray J.A. (1999) The neuroscience of personality In: Pervin L. A., John O. P. (eds). Handbook of Personality: Theory and Research. New York: Guilford; Ed 2: 277–299. [Google Scholar]
- Powell J., Dawkins L., Davis R.E. (2002). Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biological Psychiatry, 51, 151–63. [DOI] [PubMed] [Google Scholar]
- Price J.L. (2006) Connections of orbital cortex In: Zald D.H., Rauch S.L. (Eds.), The Orbitofrontal Cortex. Oxford: University Press, Oxford, 39–55. [Google Scholar]
- Reineberg A.E., Andrews-Hanna J.R., Depue B.E., Friedman N.P., Banich M.T. (2015). Resting-state networks predict individual differences in common and specific aspects of executive function. Neuroimage, 104, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (2005) Emotion Explained. Oxford University Press, Oxford. [Google Scholar]
- Scheres A., Sanfey A.G. (2006). Individual differences in decision making: Drive and reward responsiveness affect strategic bargaining in economic games. Behavioral and Brain Functions, 2, 35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L., d’Arc B.F., Lafargue G., et al. (2008). Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain, 131, 1303–10. [DOI] [PubMed] [Google Scholar]
- Setsompop K., Gagoski B.A., Polimeni J.R., Witzel T., Wedeen V.J., Wald L.L. (2012). Blipped‐controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g‐factor penalty. Magnetic Resonance in Medicine, 67, 1210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S.K., Davidson R.J. (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8, 204–10. [Google Scholar]
- Tang Y., Jiang W., Liao J., Wang W., Luo A. (2013). Identifying individuals with antisocial personality disorder using resting-state FMRI. PloS One, 8, e60652.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J., Hoshi E. (2001). Behavioral planning in the prefrontal cortex. Current Opinion in Neurobiology, 11, 164–70. [DOI] [PubMed] [Google Scholar]
- Verstynen T.D., Badre D., Jarbo K., Schneider W. (2012). Microstructural organizational patterns in the human corticostriatal system. Journal of Neurophysiology, 107, 2984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A., Theobald D.E., Cardinal R.N., Robbins T.W. (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of Neuroscience, 24, 4718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R.C., Sambataro F., Vasic N., et al. (2011). Aberrant connectivity of resting-state networks in borderline personality disorder. Journal of Psychiatry & Neuroscience, 36, 402.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welvaert M., Rosseel Y. (2013). On the definition of signal-to-noise ratio and contrast-to-noise ratio for fMRI data. PLoS ONE, 8, e77089.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Moeller S., Auerbach E.J., et al. (2013). Evaluation of slice accelerations using multiband echo planar imaging at 3T. Neuroimage, 83, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenski J.M., Larsen R.J. (1999). Susceptibility to affect: A comparison of three personality taxonomies. Journal of Personality, 67, 761–91. [DOI] [PubMed] [Google Scholar]
- Zuo X.N., Xu T., Jiang L., et al. (2013). Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage, 65, 374–86. [DOI] [PMC free article] [PubMed] [Google Scholar]