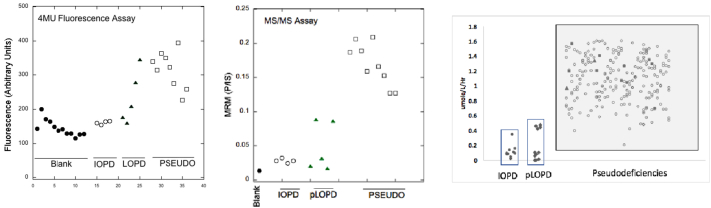
Sir - while we have no issues with the data presented in the Letter to the Editor [1], we are compelled to give an alternative conclusion. The majority of newborn labs set their own screen cutoffs, and Illinois uses much higher cutoffs than Missouri. It is only meaningful to compare the number of below-cutoff enzyme activity samples with the use of equivalent cutoffs [2], [3], [4]. For example, for mucopolysaccharidosis-I, Missouri steadily lowered their cutoff from 20% to 7% of mean iduronidase activity over the past few years [5], whereas Illinois currently uses a much higher cutoff of 14% [6]. With a 7% cutoff the number of screen positives per 100,000 newborns by digital microfluidics fluorescence in Missouri was 43 [5], whereas tandem mass spectrometry (MS/MS) gave 9 in Washington [4], [6], 12 in Illinois [6], and 16 in New York [6]. For Pompe disease with equivalent cutoffs [4], [6], Missouri reports 52 screen positives per 100,000 [5], Washington 23 [4], [6], Illinois 14 [6], and New York 21 [6]. We hypothesize that the lower number of screen positives with MS/MS is due to the high analytical range (not synonymous with dynamic range) of this method compared to fluorometry [7]. Perhaps the clearest evidence that MS/MS performs better than fluorometry comes from a study in Taiwan using an identical set of DBS showing that MS/MS but not fluorometry separates the pseudodeficiency from the Pompe-affected DBS samples (Fig. 1) [8]. This shows that variation due to DBS sampling is not a dominant effect and disproves the statement that the “analytical/dynamic range is irrelevant” [1]. Additionally, there is no correlation seen in one enzymatic activity compared to another for 111,000 DBS tested for iduronidase, -glucosidase, and -galactosidase [9], showing that variation in white cell count or other DBS quality factors are not dominant effects.
Fig. 1.
(left) Fluorometer plate reader response for assay of GAA in DBS using the fluorometric assay with 4MU substrate for blank (filled circles), clinically confirmed infantile-onset Pompe patients (IOPD, open circles), potential late-onset Pompe patients (pLOPD, triangles), and pseudodeficiencies (squares). (middle) MS/MS assay of GAA on the same set of DBS (product-to-internal standard ion count ratio). (right) Expanded MS/MS study showing that 96% of 230 pseudodeficiency DBS separate from the IOPD/pLOPD cohort (GAA activity in mole/h/L). From [8].
References
- 1.Millington D.S., Bali D. Misinformation regarding tandem mass spectrometric vs fluorometric assays to screen newborns for LSDs. Mol. Genet. Metab. Rep. 2017 doi: 10.1016/j.ymgmr.2017.04.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelb M.H., Turecek F., Scott C.R. Newborn screening for lysosomal storage diseases. Clin. Chem. 2015;16:335–346. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schielen P.C.J.I., Kemper E.A., Gelb M.H. Newborn screening for lysosomal storage diseases: a concise review of the literature on screening methods, therapeutic possibilities and regional programs. Int. J. Neonatal Screen. 2017;3:6. doi: 10.3390/ijns3020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott S. Pilot study of newborn screening for six lysosomal storage diseases using tandem mass spectrometry. Mol. Genet. Metab. 2016;118:304–309. doi: 10.1016/j.ymgme.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NBSTRN . 2017 April 6. Joint Pompe and MPS1 Pilot Monthly Webinar. [Google Scholar]
- 6.NBSTRN . 2017 May 4. Joint Pompe and MPS1 Pilot Monthly Webinar. [Google Scholar]
- 7.Kumar A.B. Tandem mass spectrometry has a larger analytical range that fluorescence assays of lysosomal enzymes: applications to newborn screening and diagnosis of Mucopolysaccharidoses types II, IVA, and VI. Clin. Chem. 2015;61:1363–1371. doi: 10.1373/clinchem.2015.242560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao H.-C. Mass spectrometry but not fluorometry distinguishes affected and pseudodeficiency patients in newborn screening for Pompe disease. Clin. Chem. 2017;63:842–851. doi: 10.1373/clinchem.2016.269027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turecek F., Gelb M.H., Scott C.R. Identification of infants at risk for developing Fabry, Pompe, or Mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. (unpublished data from S. Elliot et al.) [DOI] [PMC free article] [PubMed] [Google Scholar]