Abstract
In cyanobacteria, the CO2-concentrating mechanism (CCM) is a vital biological process that provides effective photosynthetic CO2 fixation by elevating the CO2 level near the active site of Rubisco. This process enables the adaptation of cyanobacteria to various habitats, particularly in CO2-limited environments. Although CCM of freshwater and marine cyanobacteria are well studied, there is limited information on the CCM of cyanobacteria living under alkaline environments. Here, we aimed to explore the molecular components of CCM in 12 alkaliphilic cyanobacteria through genome-based analysis. These cyanobacteria included 6 moderate alkaliphiles; Pleurocapsa sp. PCC 7327, Synechococcus spp., Cyanobacterium spp., Spirulina subsalsa PCC 9445, and 6 strong alkaliphiles (i.e. Arthrospira spp.). The results showed that both groups belong to β-cyanobacteria based on β-carboxysome shell proteins with form 1B of Rubisco. They also contained standard genes, ccmKLMNO cluster, which is essential for β-carboxysome formation. Most strains did not have the high-affinity Na+/HCO3− symporter SbtA and the medium-affinity ATP-dependent HCO3− transporter BCT1. Specifically, all strong alkaliphiles appeared to lack BCT1. Beside the transport systems, carboxysomal β-CA, CcaA, was absent in all alkaliphiles, except for three moderate alkaliphiles: Pleurocapsa sp. PCC 7327, Cyanobacteriumstranieri PCC 7202, and Spirulina subsalsa PCC 9445. Furthermore, comparative analysis of the CCM components among freshwater, marine, and alkaliphilic β-cyanobacteria revealed that the basic molecular components of the CCM in the alkaliphilic cyanobacteria seemed to share more degrees of similarity with freshwater than marine cyanobacteria. These findings provide a relationship between the CCM components of cyanobacteria and their habitats.
Keywords: Inorganic carbon uptake, CO2-concentrating mechanism, Carbonic anhydrase, Carboxysomes, Alkaliphilic cyanobacteria, Genomic data
1. Introduction
CO2-concentrating mechanism (CCM) is an important process that maximizes the efficiency of inorganic carbon (Ci; CO2 and HCO3−) uptake and CO2 fixation in cyanobacteria and eukaryotic algae [1]. It elevates CO2 level near the active site of Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) enclosed in a polyhedral microcompartment called carboxysomes, thus enhancing photosynthetic performance [2]. In cyanobacteria, CCM is the key process that enables them to adapt to their diverse ranges of CO2-limited aquatic environments such as freshwater, marine, and alkaline lakes [3], [4], [5]. Insights into the basic molecular components of cyanobacterial CCM in relation to their habitats may provide us with an efficient strategy for improvement of photosynthetic CO2 fixation and biomass yield in these organisms [6], [7] and crop plants [8], [9].
In general, the cyanobacterial CCM consists of two primary components –Ci uptake systems and carboxysomes– as described below.
1.1. Ci Uptake Systems
The Ci uptake systems are comprised of two uptake systems of CO2 [10], [11] and three transport systems of HCO3− [12], [13]. The CO2 uptake systems, located at the thylakoid membrane, convert cytosolic CO2 into HCO3− [14]. These systems are based on NAD(P)H dehydrogenase type 1 (NDH-1) complexes comprising of NDH-13 and NDH-14 protein complexes. NDH-13 is the low-CO2 inducible high-affinity CO2 uptake system, encoded by ndhD3, ndhF3, and cupA(chpY). On the other hand, NDH-14 protein complex is the constitutive low-affinity CO2 uptake system encoded by ndhD4, ndhF4, and cupB(chpX) genes [15]. While protein subunits NdhD and NdhF are responsible for CO2 uptake [10], CupA and CupB catalyze the hydration reaction of CO2 into HCO3− [16]. For the transport of HCO3−, it is facilitated by three transporters, located at the plasma membrane, including BicA (a SulP-type sodium dependent HCO3− transporter), SbtA (a sodium-dependent HCO3− symporter), and BCT1 (an ATP-binding cassette (ABC)-type HCO3− transporter). These three transporters have different properties. BicA has low affinity for bicarbonate (Km = 70–350 μM), but high flux of HCO3− uptake, while SbtA has high affinity for bicarbonate (Km < 5 μM), but low flux of HCO3− uptake [12], [17]. BCT1 has medium substrate affinity for bicarbonate (Km = 10–15 μM) and low flux of HCO3− uptake [18]. The operation of the Ci uptake systems ends up with a cytosolic Ci pool in the form of HCO3−, which is subsequently diffused into carboxysomes.
1.2. Carboxysomes
Carboxysomes are specialized sub-cellular compartments composing of protein shells and two encapsulated enzymes, Rubisco and carbonic anhydrase (CA) [19], [20]. In carboxysomes, CA catalyzes HCO3− into CO2, which is a substrate for Rubisco [21]. There are two types of carboxysomes, α- and β-. The cso-type of shell proteins, encoded by cso operon, is termed α-carboxysomes, while the ccm-type of shell polypeptides, encoded by ccmKLMNO operon, is termed β-carboxysomes. Based on this criterion, the cyanobacterial species carrying form 1A of Rubisco within α-carboxysomes are classified as α-cyanobacteria while the species containing form 1B of Rubisco within β-carboxysomes are classified as β-cyanobacteria [22], [23]. Although the two carboxysome types are different in gene organization, formation, and species distribution, they have similar functions which are to limit CO2 leaking, reduce the risk of photorespiration, and enhance the carboxylase activity of Rubisco [20], [24]. Among the β-carboxysome proteins, which have been extensively studied, CcmK, CcmL, and CcmO were proposed to be in the outer shell layer [25], [26], while CcmM and CcmN were proposed to localize in the inner shell [27]. Concerning on CA, various carboxysomal CA have been reported. They are named β-CA (CcaA) and γ-CA (CcmM) in β-cyanobacteria [28] and named β-CA (CsoSCA) in α-cyanobacteria [29]. β-cyanobacterial species also contain two types of non-carboxysomal CAs, β-CA (EcaB) and α-CA (EcaA), localized in the cell membrane or in the periplasmic space [30]. However, the specific function of EcaA/B has not yet been confirmed. For Rubisco, it catalyzes CO2 fixation reaction to generate 3-phosphoglycerate as a precursor for the Calvin-Benson-Bassham cycle. This enzyme consists of eight small (RbcS; 12-18 kDa) and eight large (RbcL, 50–55 kDa) subunits [31]. Assembly of Rubisco requires chaperone proteins [32]. RbcX encoded by rbcX is a Rubisco assembly chaperone, which interacts with RbcL to facilitate the assembly of RbcL and RbcS to form Rubisco holoenzyme [33], [34]. It has been reported that RbcX is highly conserved in organisms having form 1B Rubisco [35].
Cyanobacteria tend to have different sets of CCM components depending on their habitats [1]. Studies have shown that α- and β-cyanobacteria occupy different environments [36]. Most of the α-cyanobacteria such as Prochlorococcus and Synechococcus strains inhabit marine while β-cyanobacteria such as Synechocystis sp. PCC 6803 [37], [38], [39], Anabaena variabilis [40], and Synechococcus elongatus PCC 7942 [41] live mainly in freshwater. The two distinct environments differ mainly in their conditions such as pH, Ci content, and salinity. The factor that affects CCM the most is pH because it is strongly linked to the equilibrium of Ci species (H2CO3, CO2, HCO3−, and CO32 −) in a system [42]. At high pH (> 9), Ci content is usually high with dominant CO32 − and HCO3− ions, while pH 6–8, HCO3− is mostly present. At low pH (< 6), Ci content is low with dominant CO2 and H2CO3 ions. It is reported that the Ci concentration in marine environment (pH ≈ 8.2) is fairly constant around 2 mM [3]. The Ci availability in freshwater (pH ≈ 7) is however lower and fluctuates [43]. Based on Ci content, freshwater cyanobacteria tend to have complete Ci uptake systems which allow them to cope with the Ci fluctuation, whereas marine strains appear to lack some Ci uptake systems because they mainly experience with stable environment [3]. In addition, some cyanobacteria can also survive in alkaline environments (pH = 8.5–11) [44]. An example of alkaline environments is soda lake which is characterized by the strong alkaline (pH ≥ 9.5) and high Ci concentration dominated with HCO3− and CO32 − ions [43]. Although CCM of freshwater and marine cyanobacteria are well studied, only a few observations of CCM in alkaliphilic cyanobacteria have been reported [43], [45]. Some researchers have hypothesized that CCM might not be necessary in the alkaliphilic cyanobacteria because of unlimited supply of inorganic carbon in the form of HCO3− and CO32 − in the alkaline environments. Nevertheless, CCM components have recently been identified in some alkaliphilic cyanobacteria. In 2007, Dudoladova et al. [46] discovered α- and β-classes of CA and their sub-cellular localization in Rhabdoderma lineare. Later, Mikhodyuk et al. [47] studied the transport systems for carbonate of the natronophilic cyanobacterium Euhalothece sp. Z-M001. Recently, with the availability of a complete genome sequence of Microcoleus sp. IPPAS B-353, Kupriyanova et al. [48] identified a whole set of putative CCM components in this alkaliphilic organism living in soda lakes. They found that composition of the CCM components of the Microcoleus strain is similar to that of Synechocystis sp. PCC 6803 and Synechococcus PCC 7002 which are freshwater and marine β-cyanobacteria, respectively.
To further explore alkaliphilic cyanobacterial CCM, we aimed to probe unique features of molecular components of CCM in 12 alkaliphilic strains and relationship with their habitat. All the candidate genes/proteins involved in Ci uptake systems and carboxysomes of 12 alkaliphilic strains, including those inhabiting moderate (pH 8.5–9.4) and strong alkaliphilic (pH ≥ 9.5) environments, were identified. Computational identification of orthologous proteins was performed between the selected alkaliphiles and the ‘model’ β-cyanobacterium, Synechocystis sp. PCC 6803, whose CCM has been well studied. By sequence-based analysis, the variation of CCM components and potential orthologous sequences associated with such components was proposed. Comparative analyses within alkaliphilic, freshwater, and marine β-cyanobacteria were investigated, and the relationship between CCM components and ecological adaptation of alkaliphilic cyanobacteria were also emphasized. Since CCM is the crucial mechanism for CO2 fixation and photosynthesis in cyanobacteria, we believe that a better understanding of the CCM components could pave the way for future research towards cellular improvement of economically important cyanobacteria such as Arthrospira spp.
2. Materials and Methods
2.1. Protein Sequence Retrieval
Amino acid sequences of 27 proteins involved in CCM of β-cyanobacterium, Synechocystis sp. PCC 6803 (Assembly ID GCA_000009725.1), were retrieved from the CyanoBase (http://genome.kazusa.or.jp/cyanobase) as a reference target protein set. These proteins were NdhD4 (gene ID sll0027), NdhF4 (gene ID sll0026), CupB (gene ID slr1302), NdhD3 (gene ID sll1733), NdhF3 (gene ID sll1732), CupA (gene ID sll1734), BicA1 (gene ID sll0834), BicA2 (gene ID slr0096), SbtA (gene ID slr1512), SbtB (gene ID slr1513), CmpA (gene ID slr0040), CmpB (gene ID slr0041), CmpC (gene ID slr0043), CmpD (gene ID slr0044), CcmK1 (gene ID sll1029), CcmK2 (gene ID sll1028), CcmK3 (gene ID slr1838), CcmK4 (gene ID slr1839), CcmL (gene ID sll1030), CcmM (gene ID sll1031), CcmN (gene ID sll1032), CcmO (gene ID slr0436), RbcL (gene ID slr0009), RbcS (gene ID slr0012), RbcX (gene ID slr0011), CcaA (gene ID slr1347), and EcaB (gene ID slr0051).
The genome, protein sequences, and annotation data of the 12 selected alkaliphiles were retrieved from the database of the National Center for Biotechnology Information (NCBI) in October, 2016. The analyzed alkaliphilic cyanobacteria included Pleurocapsa sp. PCC 7327 (P7; GenBank CP003590) [49], Synechococcus sp. JA-2-3B′a(2–13) (S2; GenBank NC_007776) [50], Synechococcus sp. JA-3-3Ab (S3; GenBank NC_007775) [50], Cyanobacterium PCC 7702 (CP; GenBank NZ_KB235926) [49], Cyanobacterium stranieri PCC 7202 (CS; GenBank CP003940) [49], Spirulina subsalsa PCC 9445 (SS; GenBank NZ_JH980292) [49], Arthrospira platensis C1 (AC; GenBank NZ_CM001632) [51], Arthrospira platensis NIES-39 (AN; GenBank NC_016640) [52], Arthrospira platensis str. Paraca (AP; GenBank ACSK00000000) [53], Arthrospira maxima CS-328 (AM; GenBank ABYK00000000) [54], Arthrospira sp. PCC 8005 (A8; GenBank NZ_FO818640) [55], and Arthrospira sp. TJSD091 (AT; GenBank LAYT00000000) [56].
2.2. Identification of Orthologous Proteins
The bidirectional sequence alignment approach, namely reciprocal BLASTP [57], was employed to identify proteins of 12 studied species, which are homologous to the reference proteins of Synechocystis sp. PCC 6803. To avoid under- and over-estimation of sequence similarity of these related species, the candidate orthologous proteins were determined based on BLAST statistics with the E-value threshold (≤ 10− 6) [58], the identity (≥ 30) [58], and coverage percentage (≥ 60) [58]. Only protein sequences with the BLASTP scores above the set critical values were further analyzed for the conserved domain using the Pfam database 27.0, provided by the Sanger Centre, UK (http://pfam.xfam.org/search) [59]. The default E-value cut-off of 1.0 was applied for this study [60]. The GUIDANCE web-server tool (http://guidance.tau.ac.il/) [61] was used to evaluate a confidence score of multiple sequence alignments. Additionally, the genomic features were visualized by GView [62].
2.3. Phylogenetic Analysis
A phylogenetic tree of the 12 selected strains and reference cyanobacteria was constructed based on Rubisco large subunit (RbcL) amino acid sequences, which were used to infer the protein function and classification among the strains. Other phylogenetic trees based on protein sequences of CmpABCD of the HCO3− transporter BCT1 and sequences of NrtABCD of the nitrite/nitrate transporter were constructed to confirm the identity between the proteins. The reference species were selected according to types of carboxysomes (α- and β-classes), the existence of both CmpABCD and NrtABCD transporters in genomes, or their habitats. These strains included freshwater (Anabaena sp. PCC 7120, Anabaena variabilis ATCC 29413, Cyanothece sp. PCC 8801, Cyanothece sp. PCC 8802, Nostoc punctiforme ATCC 29133, Synechococcus sp. PCC 7942, and Synechocystis sp. PCC 6803) and marine (Lyngbya sp. PCC 8106, Trichodesmium erythraeum IMS101, Synechococcus sp. PCC 7002, Synechococcus sp. CC9605, Synechococcus sp. CC9902, Prochlorococcus marinus AS9601, NATL1A, and NATL2A, and Prochlorococcus marinus MIT 9211, 9215, 9301, 9303, 9312, 9313, and 9515) cyanobacteria. Their corresponding amino acid sequences were retrieved from the public databases, including the CyanoBase (http://genome.kazusa.or.jp/cyanobase) and the GenBank (http://www.ncbi.nlm.nih.gov/genbank) databases. A phylogenetic tree was created by performing multiple sequence alignment with MUSCLE [63], [64], and then constructed based on the Maximum Likelihood [65] through the MEGA 6.0 software [66]. The reliability of the trees/branches was estimated via the bootstrap method [67], with 3000 replications.
3. Results
3.1. Strains and Classification of Alkaliphilic Cyanobacterial CCM
In this study, 12 selected alkaliphilic cyanobacterial strains were defined based on their ability to grow in an alkaline environment (pH roughly 8.5–11). The chosen strains included both unicellular and filamentous blue-green algae, which have different original habitats. According to the habitat pH values, we classified the selected strains into two main groups: moderately alkaliphilic cyanobacteria (pH 8.5–9.4) and strongly alkaline cyanobacteria (pH ≥ 9.5) (Table 1). The first group, moderately alkaline cyanobacteria, was comprised of two subgroups: alkali-thermophile and alkali-mesophile. The subgroup alkali-thermophile consisted of four species isolated from alkaline hot spring environments (pH 8.5–8.8, 50–70 °C), P7, S2, S3, and CP. The alkali-mesophile group of cyanobacteria was composed of two euryhaline cyanobacteria, CS and SS, living under a saline and alkaline environment (pH 8.5–8.8, 30–45 °C). The strongly alkaline cyanobacteria group was comprised of six Arthrospira strains (AC, AN, AP, AM, A8, and AT), a dominant genus found in natural soda lakes, with growth optimum at pH range of 9.5–10.5 [68], [69]. More details about the ecological niches of all studied alkaliphilic strains are given in Table 1.
Table 1.
Ecological niches of selected alkaliphilic cyanobacteria whose genome sequences are available (October 2016).
| Species | Isolation site | Characteristics and habitats | Classification | Genome status | Reference |
|---|---|---|---|---|---|
| Pleurocapsa sp. PCC 7327 (P7) | Hunters hot spring, Oregon, USA | A unicellular nitrogen-fixing cyanobacterium. It is a stenohaline strain that can only survive within a narrow range of salinities. It has been found in alkaline water, hot spring (53 °C pH 8.5). | Moderate alkali-thermophile | Finished | [49] |
|
Synechococcus sp. JA-2-3B′a(2–13) (S2) Synechococcus sp. JA-3-3Ab (S3) |
Octopus Spring, Yellowstone National Park | A group of small (2–5 μm) unicellular cyanobacteria. Non-nitrogen-fixing cyanobacteria. The strain is dominant in alkaline siliceous hot springs (50–70 °C pH 8.5). | Moderate alkali-thermophile | Finished | [50] |
| Cyanobacterium PCC 7702 (CP) | Alkaline hot spring, near Reykjavik, Iceland | A practically unicellular cyanobacterium. The strain is isolated from alkaline siliceous hot springs (50–70 °C pH 8.8). Nitrogen-fixing and non-motile. | Moderate alkali-thermophile | Permanent Draft | [49] |
| Cyanobacterium stranieri PCC 7202 (CS) | Alkaline pond, Chad | A unicellular non-nitrogen-fixing cyanobacterium capable of growth in both freshwater and seawater media. Thus, it is able to adapt to a wide range of salinities (euryhaline). Non-motile. | Moderate alkali-mesophile | Finished | [49] |
| Spirulina subsalsa PCC 9445 (SS) | Alkaline-saline volcanic lake, Pantelleria, Italy | A motile filamentous cyanobacterium. It is capable of growth under a saline and alkaline environment. | Moderate alkali-mesophile | Permanent draft | [49] |
|
Arthrospira platensis C1 (AC) Arthrospira platensis NIES-39 (AN) Arthrospira platensis str. Paraca (AP) Arthrospira maxima CS-328 (AM) Arthrospira sp. PCC 8005 (A8) Arthrospira sp. TJSD091 (AT) |
Unknown Lake Chad, Chad, East Africa Unknown Lake Chad, Chad, East Africa Seaside wetland, China, Bohai Unknown |
A group of filamentous cyanobacteria that have an important role in industrial applications. Non-heterocyst-forming and non-nitrogen-fixing cyanobacteria; hydrogen-producing strains. They grow naturally in a high-salt alkaline (carbonate/bicarbonate) open pond system. The optimum pH for growth of ordinary Arthrospira strains is in the range of 9.0–9.5. AC is a laboratory strain. | Strong alkaliphile | Permanent draft Permanent draft Permanent draft Permanent draft Permanent draft Permanent draft |
[51], [52], [53], [54], [55], [56] |
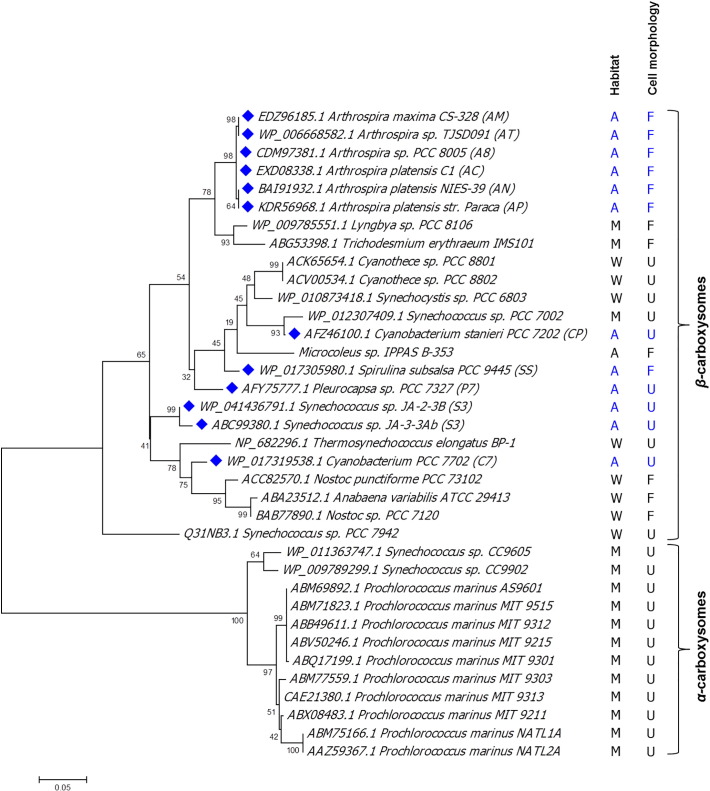
To examine the carboxysome type operating in the 12 investigated cyanobacteria, a phylogenetic tree was constructed based on RbcL amino acid sequences. RbcL was chosen because it is a well-conserved enzyme for CO2 fixation and has been used for the classification of cyanobacteria groups before [70]. A total of 36 protein sequences from 36 cyanobacteria were analyzed; 12 chosen alkaliphilic cyanobacteria and 24 reference species consisting of 8 freshwater, 15 marine, and a haloalkaliphilic species. The results showed that all 36 cyanobacterial strains were divided into two main groups, β- and α-cyanobacteria, according to their Rubisco forms (Fig. 1). All 12 studied alkaliphilic cyanobacteria were clustered together in the β-cyanobacteria branch, reflecting the presence of the Rubisco 1B form. However, they were not completely grouped in the same cluster, following neither to their habitat nor morphology. For instance, four moderately alkali-thermophilic cyanobacteria, P7, S2, S3, and CP, were located in different clusters. In addition, SS which is a filamentous cyanobacterium appeared to be in the same cluster with the unicellular species.
Fig. 1.
Phylogenetic tree based on Rubisco large subunit protein sequences. The 12 alkaliphilic cyanobacterial strains examined in this study are identified by the blue diamond, while other reference species are represented without diamond. Cyanobacterial habitat, cell arrangement, and carboxysome type (α- or β-) are displayed. Within the column for habitat, freshwater strains are denoted by W, marine by M, and alkaline niche by A. Unicellular and filamentous cell arrangement is represented by U and F, respectively.
3.2. Identification of Orthologous Proteins and Genes in Alkaliphilic Strains
Proteins corresponding to the CCM components of the 12 chosen alkaliphilic strains were identified as described in the Materials and Methods. All identified proteins had different degree of identity (40–80%, E-value threshold of ≤ 10− 20) with the reference sequences. They also contained conserved domain regions which were similar to the reference proteins. Annotation details, including gene and protein accession number, annotation scores, protein domain analysis, are available in Supplementary File. The presence and absence of genes encoding the CCM components of alkaliphilic strains are shown in Table 2. The comparison of molecular CCM components from the Synechocystis sp. PCC 6803 and from the analyzed species revealed that approximately 20 orthologous genes are present in the investigated alkaliphiles. Furthermore, the results showed that the moderately alkaline group possesses more CCM components than the strongly alkaline ones.
Table 2.
Variation of the genes involved in CO2-concentrating mechanism among alkaliphilic cyanobacterial strains. Pleurocapsa sp. PCC 7327 (P7), Synechococcus sp. JA-2-3B′a(2–13) (S2), Synechococcus sp. JA-3-3Ab (S3), Cyanobacterium PCC 7702 (CP), Cyanobacterium stranieri PCC 7202 (CS), Spirulina subsalsa PCC 9445 (SS), A. platensis C1 (AC), A. platensis NIES-39 (AN), A. platensis Paraca (AP), A. maxima CS-328 (AM), Arthrospira sp. PCC 8005 (A8), and Arthrospira sp. TJSD091 (AT) Numbers represent copy number of genes; nf is referred to not found; genes coding for putative NrtABCD are denoted by the symbol “?”.
| Component | Gene | Alkaliphilic strains |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate alkaliphilic cyanobacteria |
Strong alkaliphilic cyanobacteria |
||||||||||||||
| Thermophiles |
Mesophiles |
||||||||||||||
| P7 | S2 | S3 | CP | CS | SS | AC | AN | AP | AM | A8 | AT | ||||
| Ci uptake systems | CO2 uptake | NDH-14 complex | ndhF4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ndhD4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| cupB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| NDH-13 complex | ndhF3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| ndhD3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| cupA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| HCO3− transport | BicA | bicA1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| bicA2 | nf | nf | nf | nf | nf | 1 | 1 | 1 | 1 | 1 | 1 | nf | |||
| SbtA SbtA regulator | sbtA | nf | nf | nf | nf | nf | 1 | nf | 1 | 1 | nf | nf | nf | ||
| sbtB | nf | nf | nf | nf | nf | 1 | nf | 1 | 1 | nf | nf | nf | |||
| BCT1 | cmpA | 1 | 1 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ||
| cmpB | 1 | 1 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | |||
| cmpC | 1 | 1 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | |||
| cmpD | 1 | 1 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | |||
| Carboxysomes | Shell proteins | β-Carboxysomal shell proteins | ccmK1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ccmK2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| ccmK3 | 1 | nf | nf | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| ccmK4 | 1 | nf | nf | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| ccmL | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| ccmM (containing γ-CA domain) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| ccmN | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| ccmO | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Encapsulated enzymes | Rubisco | rbcL | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| rbcS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| β-CA | ccaA | 1 | nf | nf | nf | 1 | 1 | nf | nf | nf | nf | nf | nf | ||
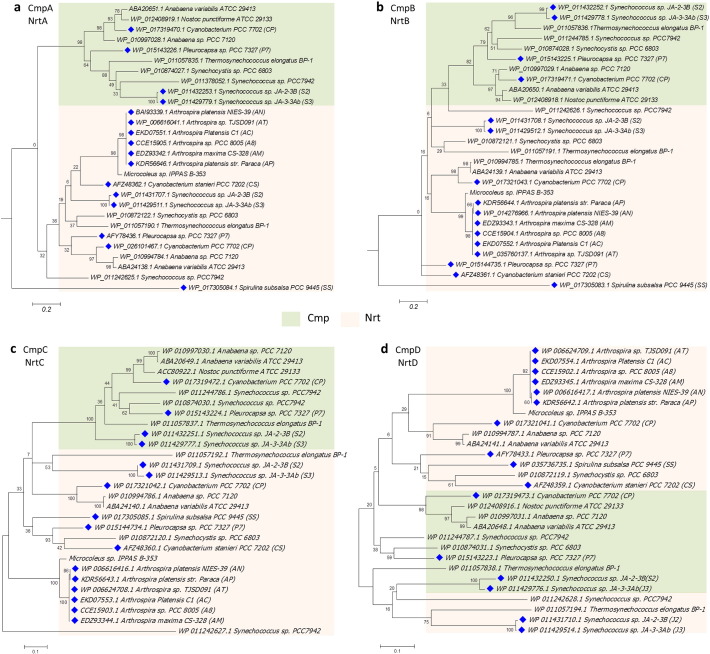
Focusing on the Ci transport systems, there were up to five systems identified in the 12 alkaliphilic cyanobacteria: i) a low-affinity NDH-14 complex (NdhD4/NdhF4/CupB); ii) a high-affinity NDH-13 complex (NdhD3/NdhF3/CupA); iii) a SulP-type low-affinity Na+-dependent HCO3− BicA transporter; iv) a high-affinity Na+/HCO3− symporter SbtA; and v) a high-affinity ATP-binding cassette BCT1(CmpABCD). All protein sequences of NDH-14 and NDH-13 showed a high sequence similarity with the reference sequences. Genes encoding each NDH-1 complex were localized together (Fig. 2). All studied strains showed high degree of homology with the BicA of Synechocystis sp. PCC 6803 (≥ 60% of amino acid identity). However, the orthologs of the SbtA transporter were found only in SS, AN, and AP. SbtB gene encoding SbtB protein that possibly functions as SbtA regulator [71] was also found nearby sbtA in the opposite direction in these three strains (Fig. 2). For the third HCO3− transporter, BCT1, the orthologs of CmpABCD and NrtABCD (nitrate/nitrite transport system) cluster were observed in all studied alkaliphiles. They exhibited a moderate sequence similarity (55–71%) with the reference proteins. Both CmpABCD and NrtABCD protein sequences contained a similar protein domain, PBP2_NrtA_CpmA. In addition, a confidence score of multiple sequence alignments from the GUIDANCE web-server tool (http://guidance.tau.ac.il/) [61] showed highly conserved regions among these two protein clusters. Since the CmpABCD and NrtABCD protein sequences have been previously reported to share high similarity in sequences belonging to the same ABC transporter family [72], the experimentally confirmed proteins CmpABCD of Synechococcus sp. PCC 7942, were included in the subsequent analysis to verify the previous annotation. The BLAST's results showed a high homology between CmpABCD of Synechococcus sp. PCC 7942 and the sequences retrieved from four species, P7, S2, S3, and CP, with ~ 65–75% identity and an E-value of ~ 10− 100. Moreover, the sequences were further identified using the phylogenetic analysis (Fig. 3). The trees showed that the candidate sequences of CmpABCD of four species, P7, S2, S3, and CP, were clustered into the CmpABCD of Synechococcus sp. PCC 7942 and the other reference cyanobacteria, while the putative NrtABCD sequences of eight cyanobacteria, CS, SS, and six Arthrospira spp., were clustered into the NrtABCD of the reference cyanobacterium. As such, it is likely that BCT1 is present only in the 4 out of the 12 studied alkaliphilic cyanobacteria. Nevertheless, an experimental study of the specificity of BCT1 to a certain substrate should be further performed to clarify the function of putative protein subunits (CmpABCD) in these four alkaliphilic cyanobacteria.
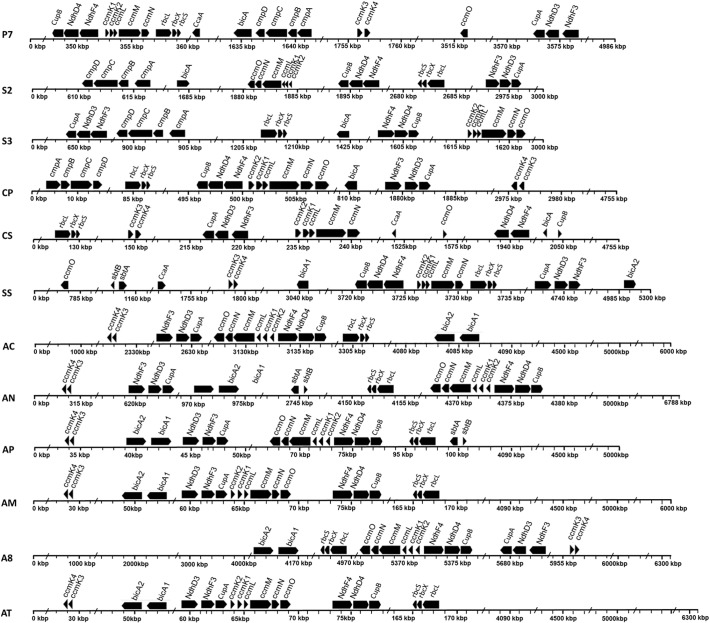
Fig. 2.
Comparative genomic structure and gene organization of the CCM in all 12 alkaliphilic cyanobacteria. Solid arrow boxes indicate genes and the direction of transcription. The completed genome sequence was available for P7, S2, S3, and CS, while for other strains there is only permanent draft genome information.
Fig. 3.
Phylogenetic trees of the CmpABCD proteins and NrtABCD proteins of the selected alkaliphilic cyanobacteria. The outgroup cyanobacteria includes Synechococcus sp. PCC 7942, which has an experimental study of HCO3− transporter BCT1 and nitrite/nitrate transport system, NRT [12]. (a) Phylogenetic tree based on the CmpA and NrtA protein sequences. (b) Phylogenetic tree based on the CmpB and NrtB protein sequences. (c) Phylogenetic tree based on the CmpC and NrtC protein sequences. (d) Phylogenetic tree based on the CmpD and NrtD protein sequences. The alkaliphilic cyanobacteria are identified by the blue diamond, respectively. Cmp and Nrt families are highlighted in orange and green, respectively. Bootstrap values with 3000 replicates are shown at the nodes of the tree. The scale bars indicate the number of nucleotide substitutions per site.
According to the observed Ci uptake systems (Table 2), the 12 analyzed alkaliphilic strains could be divided into three genotypes: I) strains containing NDH-13, NDH-14, BicA and BCT1, II) strains containing NDH-13, NDH-14, and BicA, and III) strains containing NDH-13, NDH-14, BicA, and SbtA. While the moderate alkali-thermophiles possessed genotype I, the moderate alkali-mesophiles (euryhaline) and strong alkaliphiles seemed to possess either genotype II or III. These results revealed that all alkaliphilic strains shared the same CO2 uptake systems. However, their distinctions were observed by the presence of BCT1, a HCO3− transporter. This transporter appeared to exist only in the moderate alkali-thermophiles, but absent in all moderate alkali-mesophiles and strong alkaliphiles.
From the organization of the CCM genes, genes encoding carboxysome shell proteins in all alkaliphiles, except P7 and SS, were found to arrange in a cluster, ccmKLMNO, consisting of ccmK1, ccmK2, ccmL, ccmM, ccmN and ccmO (Fig. 2). In addition, ccmK3 and ccmK4 were also found to be present in the 10 strains, but S2 and S3. The protein sequences shared significant moderate similarity with the reference sequences retrieved from the model organism. The observed maximal homology was around ~ 60–70% identity, with an E-value of ~ 10− 50 (see Supplementary File). Of these, weak homologs (~ 40% similarity) were found only for CcmN sequences. Protein domain analysis showed that CcmK1-K4 and CcmO contained bacterial microcompartment (BMC) domain (Pfam00936), whereas CcmL (BMC-P) contained ethanolamine utilization (EutN) domain (Pfam03319). Regarding to CcmN, although low similarity was observed, multiple protein sequence alignment among all examined cyanobacteria with Synechococcus sp. PCC 7942 revealed two functionally conserved distinct regions at N- and C-terminals, which were separated by a poorly conserved linker. These results supported the functions of CcmKLMNO as carboxysome shell proteins in alkaliphilic cyanobacteria. Beside the shell proteins, the amino acid sequences of Rubisco subunits, RbcL and RbcS, were moderately conserved (60% identity with the reference sequences) in their sequences; this was compared with the assembly chaperone RbcX protein (~ 45% identity with the reference sequences). The rbcLSX gene clusters were found to appear in all investigated genomes, located up- and down-streams of the ccmKLMNO cluster as shown in Fig. 2.
Focusing on β-CAs, which are enclosed in the carboxysome (CcaA) or localized in periplasmic space of β-cyanobacteria (EcaB), moderate similarity of amino acid sequence (~ 60% identity with reference protein) was found for CcaA proteins in only three studied cyanobacteria, P7, CS, and SS (Table 2). EcaB orthologs were not detected in any of the studied organisms. However, CcmM proteins of all alkaliphilic strains were found to have the γ-CA-like domain at N-terminal region. We further searched for other recognized CA classes in all 12 alkaliphilic cyanobacteria by using the protein sequences of α-CA, EcaA (all2929) of Anabaena sp. PCC 7120. The results showed no homologs sequences of EcaA in all 12 studied cyanobacteria. As a result, we concluded that all alkaliphilic species possessed γ-CA (CcmM), of which three moderate alkaliphiles contained additional β-CA (CcaA). To further evaluate a potential function of CcmM as an active CA, the comparative analysis of the γ-CA-like domain in N-terminal protein sequence was performed. The CcmM sequences from Thermosynechococcus elongatus BP-1 and Nostoc sp. PCC 7120 were included as functional γ-CA [73], [74]. The CcmM from S. elongatus PCC 7942 and Synechocystis sp. PCC 6803 were also included as a non-functional γ-CA [28], [75]. Fig. 4 shows the important amino acid residues in γ-CA-like domain of the 11 alkaliphilic strains, except SS, which were structurally similar to those of active CcmM in T. elongatus BP-1 [73] and Nostoc sp. PCC 7120 [74]. This result implied that the CcmM proteins of such 11 species might potentially have CA activity when the carboxysomal β-CA, CcaA, was missing.
Fig. 4.
Partial alignment of CcmM amino acid sequences of 12 studied alkaliphiles with those of Synechococcus elongatus PCC 7942 (Syn7942), Synechocystis sp. PCC 6803 (Syn6803), Thermosynechococcus elongatus BP-1 (BP-1), and Nostoc sp. PCC 7120 (Noc7120) (GenBank accession no. BAA16773.1, Q03513.1, NP_681734, and BAB72822.1, respectively). The sequence order is based on the alignment. Boxes represent conserved regions of the N-terminal domain of CcmM that are assumed to be necessary for CA activity, according to Pena et al. [73]. Shaded cysteine amino acids showed essential residues participating in the disulfide bond in the C-termini of active CcmM protein. Asterisks indicate conserved amino acids inside such regions.
3.3. Comparative Analysis of CCM Components Among β-Cyanobacteria
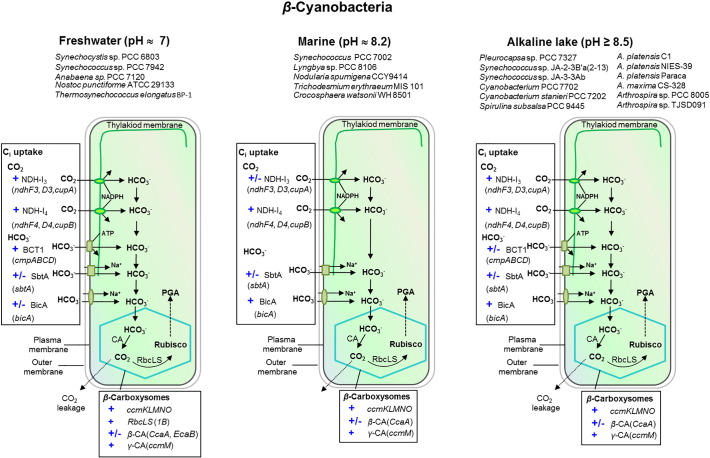
Comparative analysis of CCM components among β-cyanobacteria, living in freshwater (pH ~ 7), marine (pH ~ 8.2), and alkaliphilic (pH 8.5–11) strains were performed. Fig. 5 shows different CCM components among the three groups. The overall compositions of CCM components in alkaliphilic cyanobacteria were more similar to the freshwater than the marine groups. The cyanobacteria inhabiting freshwater and alkaline ecological niches possessed both CO2 uptake systems, NDH-13 and NDH-14, while most strains inhabiting marine habitats seemed to lack the NDH-13. Focusing on the HCO3− transport system, the results showed that marine and some alkaliphilic cyanobacteria consistently lacked the BCT1 type of the HCO3− transporter. In addition, the freshwater β-cyanobacteria possessed the highest abundance of CAs, β-CA (CcaA and EcaB), α-CA (EcaA), and γ-CA (CcmM), while the alkaliphilic cyanobacteria were likely to possess only two conventional CAs, carboxysomal β-CA (CcaA) and γ-CA (CcmM). However, it should be noted that nine out of the twelve investigated alkaliphiles appeared to have only γ-CA (CcmM).
Fig. 5.
Diversity in characteristic components of the cyanobacterial CCM living in three different pH environments; freshwater (pH ~ 7), marine (pH ~ 8.2), and alkaline (pH > 8.5). The scheme is based on the literature data and is depicted for β-cyanobacteria. The species that were used to derive the groups are shown on the figure. CCM components of freshwater and marine cyanobacteria are adapted from [3]. CCM components of high alkaliphilic cyanobacterial type were identified in this study. + and ± indicate that the particular component is ‘always present’ and ‘sometimes present’, respectively. Designation: NDH-14, low-affinity CO2 uptake system NDH-14 complex; NDH-13, low CO2-inducible high-affinity CO2 uptake system NDH-13 complex; BCT1, ATP-binding cassette (ABC)-type high-affinity HCO3− transporter; SbtA, high-affinity sodium-dependent HCO3− symporter; BicA, SulP-type low-affinity sodium dependent HCO3− transporter; CA, carbonic anhydrase; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase.
4. Discussion
Based on Rubisco phylogeny, all studied alkaliphilic cyanobacteria fall into β-cyanobacteria group (Fig. 1). It is obviously that the phylogeny of the studied alkaliphiles based on RbcL sequences can classify the cyanobacterial types; however, the tree is insufficient to elucidate the evolutionary relationship within the group based on cell morphology and habitats. Komarek et al. [76] previously performed a phylogenetic tree of 146 cyanobacterial OTUs using 31 conserved protein sequences and reported that the tree could not be clustered based on their morphology. Thus, phylogenetic analysis may not be an appropriate technique to unveil evolutionary relationship of cyanobacterial morphology and environments.
The presence of the ccmKLMNO cluster (Fig. 2) in all the 12 studied strains indicates the genes conserved in the ccm cluster of β-carboxysomes. Our finding suggests that all the investigated alkaliphilic cyanobacteria possess complete standard genes, which are essential for carboxysome formation. In addition, since CcmK3 and CcmK4 were considered as an accessory protein improving the functionality of the shell [26], the 10 studied strains found to possess CcmK1–4 would have a better shell protein function than the others. However, there is no obvious correlation between numbers of ccmK genes and environment niche (moderately to strongly alkaline habitat) of the examined strains.
Two systems of CO2 uptake, NDH-13 and NDH-14 complexes, were identified in all 12 analyzed strains (Fig. 5). Recently, Kupriyanova et al. [48] confirmed the presence of NDH-13 and NDH-14 in an alkaliphilic cyanobacterium Microcoleus sp. IPPAS B-353 and showed that genes corresponding to the NDH-13 were transcribed and probably constitutively expressed. Both CO2 uptake systems were also observed in freshwater β-cyanobacteria and 20 strains of M. aeruginosa living in brackish waters and eutrophic lakes [77]. In contrast, the absence of NDH-13 and/or NDH-14 was reported in the oceanic α-cyanobacteria, Prochlorococcus species [36], and the marine β-cyanobacteria, Trichodesmium erythraeum species [3]. Thus, existence of NDH-13 and NDH-14 is apparently related to environments with varying CO2 availability. This is due to the distinct property of each complex in that NDH-I3 has a higher substrate affinity for CO2 than NDH-I4 [16]. The presence of both complexes in all 12 strains of alkaliphilic cyanobacterial seems to have an essential role in the survival and maintenance under CO2 fluctuation, particularly in alkaline environments (i.e. hot spring and soda lake).
Our finding for HCO3− transport systems, BicA and SbtA, revealed that several alkaliphilic cyanobacteria strains have only one of the two transporters, preferably BicA. This may be due to the difference in their affinity for bicarbonate. BicA is a low-affinity and high flux rate bicarbonate transport system (Km = 70–350 μM), while SbtA is high-affinity (Km < 5 μM) HCO3− transporter [17]. Possibly, the high-affinity SbtA is not necessary in most alkaliphilic strains typically inhabiting HCO3− rich environment. If so, the organisms would most likely possess BicA rather than SbtA. Meanwhile, the presence of high-affinity transporter SbtA in SS, AN, and AP may, though indirectly, indicates that these organisms are able to face low concentrations of exogenous Ci. Therefore, the presence of both BicA and SbtA in such strains might give a selective advantage in HCO3− uptake and allow cell growth, enabling them to adapt in response to different Ci concentrations. These cyanobacteria might have a greater ability to maintain their higher growth rate than the other alkaliphiles, particularly when they face a wide dynamic range in HCO3− availability, i.e. during cyanobacterial bloom, by utilizing BicA at high HCO3− and SbtA at low HCO3− conditions.
In regards to BCT1, it has been reported as an inducible transporter under Ci limitation [12], [78] and high-light stress [79]. This transporter is the medium substrate affinity class (Km = 10–15 μM) [18]. Comparative genome analysis showed that this transporter is found only in freshwater β-cyanobacteria and four strains of the moderate alkali-thermophiles, but not in other moderate alkali-mesophiles, strong alkaliphiles, and the marine cyanobacteria (Table 2 and Fig. 5). Since the ATP binding cassette transporter BCT1 helps facilitating bicarbonate transportation, its presence is crucial for cyanobacteria living in freshwater where the contents of inorganic carbon and ions are extremely low. However, the reason why BCT1 is required in all the moderate alkali-thermophiles is not obvious. It is speculated that high temperature might limit the solubility of inorganic carbon and ions in such environment. This is supported by Kamennaya et al. [80] who reported that some inorganic carbons can form insoluble carbonate, of which its solubility is decreased with increasing temperature. The lack of BCT1 in all of the alkali-mesophiles and highly-alkaliphiles indicates the unnecessity of this transporter and the adaptation of such cyanobacterial groups residing in the saline alkaline environments with enriched carbonate ion.
Finally, diversity of CAs was observed among freshwater, marine, and alkaliphilic β-cyanobacteria. While carboxysomal γ-CA, CcmM, was observed in all cyanobacteria, β-CA, CcaA, was only found in some cyanobacteria (Fig. 5). Nine out of the twelve investigated alkaliphilic strains were found with the absence of CcaA. The reason why the strains living in the high pH conditions tend to lose CcaA is still unclear. On the contrary, the existence of CcmM in all studied strains is not surprising given the role it plays in β-carboxysomes. The evolution of CcaA and CcmM within the carboxysomes of these alkaliphiles remains to be evaluated. Thus far, it has been believed that CcmM not only functions as a shell protein for carboxysome but also as CA activity for the strains lacking CcaA. Peña et al. in 2010 [73] reported the CA activity in Thermosynechococcus elongatus BP-1 possessing only CcmM, and later de Araujo et al. in 2014 [74] suggested that activity of γ-CA might be regulated by RbcS-like domains in CcmM. Recently, Kupriyanova et al. 2016 [48] has attempted to reveal the function of CA in the haloalkaliphilic cyanobacterium Microcoleus sp. IPPAS B-353 possessing both CcaA and CcmM by using Western blotting and CA activity assay. Results showed that CcaA functions as an active non-carboxysomal CA, whereas CcmM did not have CA activity in this alkaliphilic cyanobacterium.
5. Conclusion
The molecular components of CCM in 12 alkaliphilic cyanobacteria were identified in this study. The diversity and adaptability in the Ci uptake systems and CAs of such cyanobacterial species were observed. Remarkably, the existence of HCO3− transporters greatly differs among the alkaliphiles. It seems likely that alkaliphilic cyanobacteria tend to modify their CCM components in response to the environmental influence (moderately to strongly alkaline habitat). These reflect the capability of the strains to survive and establish competitive growth by using different Ci uptake strategies at changes of CO2 and HCO3− levels. This insight into the CCM components of the alkaliphiles provides fundamental knowledge for further research towards improvement of photosynthetic CO2 fixation in some economically important cyanobacterial strains and crops.
The following is the supplementary data related to this article.
Detailed annotation of CCM genes and protein of 12 studied alkaliphilic cyanobacteria.
Conflicts of Interest
The authors declare no conflicts of interest.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Tayvich Vorapreeda for his suggestion in sequence analysis and all members of the Systems Biology and Bioinformatics (SBI) research group at King Mongkut's University of Technology Thonburi for their assistance. AK was supported by grant P-11-01089, which was provided by the National Center for Genetic Engineering and Biotechnology (BIOTEC), NSTDA, Thailand.
Contributor Information
Peerada Prommeenate, Email: peerada.pro@biotec.or.th.
Asawin Meechai, Email: asawin.mee@kmutt.ac.th.
References
- 1.Badger M.R., Price G.D., Long B.M., Woodger F.J. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J Exp Bot. 2006;57:249–265. doi: 10.1093/jxb/eri286. [DOI] [PubMed] [Google Scholar]
- 2.Badger M.R., Andrews T.J., Whitney S.M., Ludwig M., Yellowlees D.C., Leggat W. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot. 1998;76:1052–1071. [Google Scholar]
- 3.Price G.D., Badger M.R., Woodger F.J., Long B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot. 2008;59:1441–1461. doi: 10.1093/jxb/erm112. [DOI] [PubMed] [Google Scholar]
- 4.Burnap R.L., Hagemann M., Kaplan A. Regulation of CO2 concentrating mechanism in cyanobacteria. Life (Basel) 2015;5:348–371. doi: 10.3390/life5010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagemann M., Kern R., Maurino V.G., Hanson D.T., Weber A.P., Sage R.F. Evolution of photorespiration from cyanobacteria to land plants, considering protein phylogenies and acquisition of carbon concentrating mechanisms. J Exp Bot. 2016;67:2963–2976. doi: 10.1093/jxb/erw063. [DOI] [PubMed] [Google Scholar]
- 6.Mangan N., Brenner M. Systems analysis of the CO2 concentrating mechanism in cyanobacteria. Elife. 2014;10(7554) doi: 10.7554/eLife.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudana S.B., Zarzycki J., Moparthi V.K., Kerfeld C.A. Bioinformatic analysis of the distribution of inorganic carbon transporters and prospective targets for bioengineering to increase Ci uptake by cyanobacteria. Photosynth Res. 2015;126:99–109. doi: 10.1007/s11120-014-0059-8. [DOI] [PubMed] [Google Scholar]
- 8.Hanson M.R., Lin M.T., Carmo-Silva A.E., Parry M.A. Towards engineering carboxysomes into C3 plants. Plant J. 2016;87:38–50. doi: 10.1111/tpj.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long B.M., Rae B.D., Rolland V., Forster B., Price G.D. Cyanobacterial CO2-concentrating mechanism components: function and prospects for plant metabolic engineering. Curr Opin Plant Biol. 2016;31:1–8. doi: 10.1016/j.pbi.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Shibata M., Ohkawa H., Kaneko T., Fukuzawa H., Tabata S., Kaplan A. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci. 2001;98:11789–11794. doi: 10.1073/pnas.191258298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W., Ogawa T. Oxygenic photosynthesis-specific subunits of cyanobacterial NADPH dehydrogenases. IUBMB Life. 2015;67:3–8. doi: 10.1002/iub.1341. [DOI] [PubMed] [Google Scholar]
- 12.Omata T., Price G.D., Badger M.R., Okamura M., Gohta S., Ogawa T. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci U S A. 1999;96:13571–13576. doi: 10.1073/pnas.96.23.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata M., Katoh H., Sonoda M., Ohkawa H., Shimoyama M., Fukuzawa H. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. J Biol Chem. 2002;277:18658–18664. doi: 10.1074/jbc.M112468200. [DOI] [PubMed] [Google Scholar]
- 14.Price G.D. Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism. Photosynth Res. 2011;109:47–57. doi: 10.1007/s11120-010-9608-y. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa T., Mi H. Cyanobacterial NADPH dehydrogenase complexes. Photosynth Res. 2007;93:69–77. doi: 10.1007/s11120-006-9128-y. [DOI] [PubMed] [Google Scholar]
- 16.Maeda S., Badger M.R., Price G.D. Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol Microbiol. 2002;43:425–435. doi: 10.1046/j.1365-2958.2002.02753.x. [DOI] [PubMed] [Google Scholar]
- 17.Price G.D., Woodger F.J., Badger M.R., Howitt S.M., Tucker L. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci U S A. 2004;101:18228–18233. doi: 10.1073/pnas.0405211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omata T., Takahashi Y., Yamaguchi O., Nishimura T. Structure, function and regulation of the cyanobacterial high-affinity bicarbonate transporter, BCT1. Funct Plant Biol. 2002;29 doi: 10.1071/PP01215. [DOI] [PubMed] [Google Scholar]
- 19.Rae B.D., Long B.M., Whitehead L.F., Forster B., Badger M.R., Price G.D. Cyanobacterial carboxysomes: microcompartments that facilitate CO2 fixation. J Mol Microbiol Biotechnol. 2013;23:300–307. doi: 10.1159/000351342. [DOI] [PubMed] [Google Scholar]
- 20.Kerfeld C.A., Melnicki M.R. Assembly, function and evolution of cyanobacterial carboxysomes. Curr Opin Plant Biol. 2016;31:66–75. doi: 10.1016/j.pbi.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Espie G.S., Kimber M.S. Carboxysomes: cyanobacterial RubisCO comes in small packages. Photosynth Res. 2011;109:7–20. doi: 10.1007/s11120-011-9656-y. [DOI] [PubMed] [Google Scholar]
- 22.Tabita F.R. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a different perspective. Photosynth Res. 1999;60:1–28. [Google Scholar]
- 23.Badger M.R., Hanson D., Price G.D. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol. 2002;29:161–173. doi: 10.1071/PP01213. [DOI] [PubMed] [Google Scholar]
- 24.Kinney J.N., Axen S.D., Kerfeld C.A. Comparative analysis of carboxysome shell proteins. Photosynth Res. 2011;109:21–32. doi: 10.1007/s11120-011-9624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samborska B., Kimber M.S. A dodecameric CcmK2 structure suggests beta-carboxysomal shell facets have a double-layered organization. Structure. 2012;20:1353–1362. doi: 10.1016/j.str.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Rae B.D., Long B.M., Badger M.R., Price G.D. Structural determinants of the outer shell of beta-carboxysomes in Synechococcus elongatus PCC 7942: roles for CcmK2, K3–K4, CcmO, and CcmL. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long B.M., Tucker L., Badger M.R., Price G.D. Functional cyanobacterial beta-carboxysomes have an absolute requirement for both long and short forms of the CcmM protein. Plant Physiol. 2010;153:285–293. doi: 10.1104/pp.110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cot S.S., So A.K., Espie G.S. A multiprotein bicarbonate dehydration complex essential to carboxysome function in cyanobacteria. J Bacteriol. 2008;190:936–945. doi: 10.1128/JB.01283-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon G.C., Heinhorst S., Kerfeld C.A. Carboxysomal carbonic anhydrases: structure and role in microbial CO2 fixation. Biochim Biophys Acta. 1804;2010:382–392. doi: 10.1016/j.bbapap.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 30.So A.K., Espie G.S. Cloning, characterization and expression of carbonic anhydrase from the cyanobacterium Synechocystis PCC6803. Plant Mol Biol. 1998;37:205–215. doi: 10.1023/a:1005959200390. [DOI] [PubMed] [Google Scholar]
- 31.Andersson I., Taylor T.C. Structural framework for catalysis and regulation in ribulose-1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys. 2003;414:130–140. doi: 10.1016/s0003-9861(03)00164-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu C., Young A.L., Starling-Windhof A., Bracher A., Saschenbrecker S., Rao B.V. Coupled chaperone action in folding and assembly of hexadecameric Rubisco. Nature. 2010;463:197–202. doi: 10.1038/nature08651. [DOI] [PubMed] [Google Scholar]
- 33.Saschenbrecker S., Bracher A., Rao K.V., Rao B.V., Hartl F.U., Hayer-Hartl M. Structure and function of RbcX, an assembly chaperone for hexadecameric Rubisco. Cell. 2007;129:1189–1200. doi: 10.1016/j.cell.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Bracher A., Starling-Windhof A., Hartl F.U., Hayer-Hartl M. Crystal structure of a chaperone-bound assembly intermediate of form I Rubisco. Nat Struct Mol Biol. 2011;18:875–880. doi: 10.1038/nsmb.2090. [DOI] [PubMed] [Google Scholar]
- 35.Tabita F.R. Rubisco: the enzyme that keeps on giving. Cell. 2007;129:1039–1040. doi: 10.1016/j.cell.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Badger M.R., Price G.D. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot. 2003;54:609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- 37.Xu M., Bernát G., Singh A., Mi H., Rögner M., Pakrasi H.B. Properties of mutants of Synechocystis sp. strain PCC 6803 lacking inorganic carbon sequestration systems. Plant Cell Physiol. 2008;49:1672–1677. doi: 10.1093/pcp/pcn139. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S., Spann K.W., Frankel L.K., Moroney J.V., Bricke T.M. Identification of two genes, sll0804 and slr1306, as putative components of the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 2008;190:8234–8237. doi: 10.1128/JB.01126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnap R.L., Nambudiri R., Holland S. Regulation of the carbon-concentrating mechanism in the cyanobacterium Synechocystis sp. PCC6803 in response to changing light intensity and inorganic carbon availability. Photosynth Res. 2013;118:115–124. doi: 10.1007/s11120-013-9912-4. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan A., Badger M.R., Berry J.A. Photosynthesis and the intracellular inorganic carbon pool in the bluegreen alga Anabaena variabilis: response to external CO2 concentration. Planta. 1980;149:219–226. doi: 10.1007/BF00384557. [DOI] [PubMed] [Google Scholar]
- 41.Long B.M., Badger M.R., Whitney S.M., Price G.D. Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA. J Biol Chem. 2007;282:29323–29335. doi: 10.1074/jbc.M703896200. [DOI] [PubMed] [Google Scholar]
- 42.Kalff J. 2002. Limnology: inland water ecosystems. San Francisco, United States: Pearson education (US) pp. 218–222. [Google Scholar]
- 43.Kupriyanova E.V., Samylina O.S. CO2-concentrating mechanism and its traits in haloalkaliphilic cyanobacteria. Microbiol Mol Biol Rev. 2015;84:112–124. [PubMed] [Google Scholar]
- 44.Horikoshi K. Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev. 1999;63:735–750. doi: 10.1128/mmbr.63.4.735-750.1999. [table of contents] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diaz M.M., MaBerly S.C. Carbon-concentrating mechanisms in acidophilic algae. Phycologia. 2009;48:77–85. [Google Scholar]
- 46.Dudoladova M.V., Kupriyanova E.V., Markelova A.G., Sinetova M.P., Allakhverdiev S.I., Pronina N.A. The thylakoid carbonic anhydrase associated with photosystem II is the component of inorganic carbon accumulating system in cells of halo- and alkaliphilic cyanobacterium Rhabdoderma lineare. Biochim Biophys Acta. 1767;2007:616–623. doi: 10.1016/j.bbabio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Mikhodyuk O.S., Zavarzin G.A., Ivanovsky R.N. Transport systems for carbonate in the extremely natronophilic cyanobacterium Euhalothece sp. Microbiology. 2008;77:412–418. [PubMed] [Google Scholar]
- 48.Kupriyanova E.V., Cho S.M., Park Y.I., Pronina N.A., Los D.A. The complete genome of a cyanobacterium from a soda lake reveals the presence of the components of CO2-concentrating mechanism. Photosynth Res. 2016 doi: 10.1007/s11120-016-0235-0. [DOI] [PubMed] [Google Scholar]
- 49.Shih P.M., Wu D., Latifi A., Axen S.D., Fewer D.P., Talla E. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci U S A. 2013;110:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhaya D., Grossman A.R., Steunou A.S., Khuri N., Cohan F.M., Hamamura N. Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J. 2007;1:703–713. doi: 10.1038/ismej.2007.46. [DOI] [PubMed] [Google Scholar]
- 51.Cheevadhanarak S., Paithoonrangsarid K., Prommeenate P., Kaewngam W., Musigkain A., Tragoonrung S. Draft genome sequence of Arthrospira platensis C1 (PCC9438) Stand Genomic Sci. 2012;6:43–53. doi: 10.4056/sigs.2525955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujisawa T., Narikawa R., Okamoto S., Ehira S., Yoshimura H., Suzuki I. Genomic structure of an economically important cyanobacterium, Arthrospira (Spirulina) platensis NIES-39. DNA Res. 2010;17:85–103. doi: 10.1093/dnares/dsq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lefort F., Calmin G., Crovadore J., Falquet J., Hurni J.P., Osteras M. Whole-genome shotgun sequence of Arthrospira platensis strain Paraca, a cultivated and edible cyanobacterium. Genome Announc. 2014;2 doi: 10.1128/genomeA.00751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrieri D., Ananyev G., Lenz O., Bryant D.A., Dismukes G.C. Contribution of a sodium ion gradient to energy conservation during fermentation in the cyanobacterium Arthrospira (Spirulina) maxima CS-328. Appl Environ Microbiol. 2011;77:7185–7194. doi: 10.1128/AEM.00612-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen P.J., Morin N., Mergeay M., Leroy B., Wattiez R., Vallaeys T. Genome sequence of the edible cyanobacterium Arthrospira sp. PCC 8005. J Bacteriol. 2010;192:2465–2466. doi: 10.1128/JB.00116-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong S., Chen J., Wang S., Wu Y., Hou H., Li M. Draft genome sequence of cyanobacteria Arthrospira sp. TJSD091 isolated from seaside wetland. Mar. Genomics. 2015;24:197–198. doi: 10.1016/j.margen.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 58.Pearson W.R. 2013. An introduction to sequence similarity (“homology”) searching. Current protocols in bioinformatics/editoral board, Andreas D. Baxevanis… [et al.] [Chapter 3:Unit 3 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.UniProt C. UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penn O., Privman E., Ashkenazy H., Landan G., Graur D., Pupko T. GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Res. 2010;38:W23–W28. doi: 10.1093/nar/gkq443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petkau A., Stuart-Edwards M., Stothard P., Van Domselaar G. Interactive microbial genome visualization with GView. Bioinformatics. 2010;26:3125–3126. doi: 10.1093/bioinformatics/btq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edgar R.C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinf. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 66.Hall B.G. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30:1229–1235. doi: 10.1093/molbev/mst012. [DOI] [PubMed] [Google Scholar]
- 67.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 68.Vonshak A. Taylor & Francis; London: 1997. Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. [Google Scholar]
- 69.Dadheech P.K., Glockner G., Casper P., Kotut K., Mazzoni C.J., Mbedi S. Cyanobacterial diversity in the hot spring, pelagic and benthic habitats of a tropical soda lake. FEMS Microbiol Ecol. 2013;85:389–401. doi: 10.1111/1574-6941.12128. [DOI] [PubMed] [Google Scholar]
- 70.Tomitani A., Knoll A.H., Cavanaugh C.M., Ohno T. The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proc Natl Acad Sci U S A. 2006;103:5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du J., Forster B., Rourke L., Howitt S.M., Price G.D. Characterisation of cyanobacterial bicarbonate transporters in E. coli shows that SbtA homologs are functional in this heterologous expression system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omata T. Structure, function and regulation of the nitrate transport system of the cyanobacterium Synechococcus sp. PCC7942. Plant Cell Physiol. 1995;36:207–213. doi: 10.1093/oxfordjournals.pcp.a078751. [DOI] [PubMed] [Google Scholar]
- 73.Pena K.L., Castel S.E., de Araujo C., Espie G.S., Kimber M.S. Structural basis of the oxidative activation of the carboxysomal gamma-carbonic anhydrase, CcmM. Proc Natl Acad Sci U S A. 2010;107:2455–2460. doi: 10.1073/pnas.0910866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Araujo C., Arefeen D., Tadesse Y., Long B.M., Price G.D., Rowlett R.S. Identification and characterization of a carboxysomal gamma-carbonic anhydrase from the cyanobacterium Nostoc sp. PCC 7120. Photosynth Res. 2014;121:135–150. doi: 10.1007/s11120-014-0018-4. [DOI] [PubMed] [Google Scholar]
- 75.So A.K.C., Espie G.S. Cyanobacterial carbonic anhydrases. Can J Bot. 2005;83:721–734. [Google Scholar]
- 76.Komarek J., Kastovsky J., Mares J., Johansen J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia. 2014;86:295–335. [Google Scholar]
- 77.Sandrini G., Matthijs H.C.P., Verspagen J.M.H., Muyzer G., Huisman J. Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. ISME J. 2014;8:589–600. doi: 10.1038/ismej.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Omata T., Ogawa T. Biosynthesis of a 42 kDa polypeptide in the cytoplasmic membrane of the cyanobacterium Anacystis nidulans strain-R2 during adaptation to low CO2 concentration. Plant Physiol. 1986;80:525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reddy K.J., Masamoto K., Sherman D.M., Sherman L.A. DNA sequence and regulation of the gene (cbpA) encoding the 42 kilodalton cytoplasmic membrane carotenoprotein of the cyanobacterium Synechococcus sp. strain PCC7942. J Bacteriol. 1989;171:3486–3493. doi: 10.1128/jb.171.6.3486-3493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamennaya N.A., Ajo-Franklin Caroline M., Northen T., Jansson C. Cyanobacteria as biocatalysts for carbonate mineralization. Minerals. 2012;2:338–364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed annotation of CCM genes and protein of 12 studied alkaliphilic cyanobacteria.