Abstract
Schizophrenia is characterized by neuropsychological deficits across many cognitive domains. Cognitive phenotypes with high heritability and genetic overlap with schizophrenia liability can help elucidate the mechanisms leading from genes to psychopathology. We performed a meta-analysis of 170 published twin and family heritability studies of >800 000 nonpsychiatric and schizophrenia subjects to accurately estimate heritability across many neuropsychological tests and cognitive domains. The proportion of total variance of each phenotype due to additive genetic effects (A), shared environment (C), and unshared environment and error (E), was calculated by averaging A, C, and E estimates across studies and weighting by sample size. Heritability ranged across phenotypes, likely due to differences in genetic and environmental effects, with the highest heritability for General Cognitive Ability (32%–67%), Verbal Ability (43%–72%), Visuospatial Ability (20%–80%), and Attention/Processing Speed (28%–74%), while the lowest heritability was observed for Executive Function (20%–40%). These results confirm that many cognitive phenotypes are under strong genetic influences. Heritability estimates were comparable in nonpsychiatric and schizophrenia samples, suggesting that environmental factors and illness-related moderators (eg, medication) do not substantially decrease heritability in schizophrenia samples, and that genetic studies in schizophrenia samples are informative for elucidating the genetic basis of cognitive deficits. Substantial genetic overlap between cognitive phenotypes and schizophrenia liability (average rg = −.58) in twin studies supports partially shared genetic etiology. It will be important to conduct comparative studies in well-powered samples to determine whether the same or different genes and genetic variants influence cognition in schizophrenia patients and the general population.
Keywords: neuropsychology, heritability, meta-analysis, endophenotypes, twin study, cognition
Introduction
There is extensive evidence that cognition is significantly impaired in schizophrenia. Relative to healthy individuals, schizophrenia patients as a whole have a generalized impairment across many cognitive functions, demonstrated by meta-analyses of over 200 schizophrenia neuropsychological studies.1,2 At the individual patient level, 75%–100% of patients exhibit cognitive impairments, depending on the method of classifying deficits.3 A meta-analysis by Mesholam-Gately and colleagues4 of neuropsychological functioning in first episode schizophrenia patients showed that, in the early stages of disease when the brain is less affected by antipsychotic medications, performance across several cognitive domains is impaired on average 0.91 SDs below the healthy control mean. Individuals at clinical high-risk for psychosis are less impaired than first episode patients, although there are greater deficits in high-risk individuals who later develop a full psychotic illness than those who do not.5,6 In addition, unaffected family members at genetic risk for developing the disorder have poorer cognition compared to unaffected individuals without a family history of schizophrenia.7–10 Cognitive deficits are relatively unimproved by antipsychotic medications and consequently have a substantial impact on the functional outcome of patients.11–17 Elucidating the molecular mechanisms underlying cognition is essential for identifying novel targets for the development of new treatments that improve cognitive functioning in patients with schizophrenia.
Recent advances in the field of psychiatric genetics have led to the identification of a large number of common genetic variants that confer risk for schizophrenia.18–27 Determining how these risk variants contribute to brain-based phenotypes that are abnormal in schizophrenia, such as cognitive deficits, can greatly contribute to our understanding of the biological pathways leading from risk genes to disease. A prerequisite for such studies is evidence that the phenotypes are heritable and have a genetic basis that overlaps with that of schizophrenia liability.28–30 Measures of General Cognitive Ability are highly heritable (h2 > 65%),31,32 although there is less consistent evidence for heritability of measures indexing specific cognitive domains. Toulopoulou, Owens and colleagues estimated from a series of twin studies that genetic correlations between cognitive measures and liability of schizophrenia range between −.09 and −1.00, averaging −.58,33–38 indicating that a substantial proportion of the variance in cognition and schizophrenia liability is due to common genetic factors.
We performed a systematic review of twin- and family-based heritability studies of cognitive phenotypes in schizophrenia and nonpsychiatric populations with several objectives in mind. Given the lack of available quantitative studies, we performed meta-analyses of data available for many neuropsychological tests and cognitive domains to determine the best estimates of heritability. We determined whether studies of nonpsychiatric individuals and schizophrenia patients differ in heritability to examine whether, as claimed, heritability in patients is lower due to environmental factors and illness-related moderators, such as higher rates of smoking and substance use, fluctuations in medication, or clinical state during testing.39–41 Further, as family studies cannot disentangle genetic and shared environmental sources of phenotypic variance, we compared variance component estimates from family and monozygotic/dizygotic (MZ/DZ) twin study designs.
Methods
Detailed methods are provided in the supplementary material.
Data Collection
A literature search performed in PubMed and PsycINFO resulted in >2000 papers published prior to January 2016. Following abstract review, identification of additional articles from the reference lists, and exclusion of studies according to pre-established criteria (supplementary methods, supplementary tables 1–4), 170 empirical articles describing twin or family studies were included in the analyses (supplementary figure 1). These studies investigated the heritability of >600 neuropsychological test variables in 64 independent cohorts (supplementary tables 1, 5–8). See supplementary material for a complete list of references included in the meta-analyses.
Statistical Analyses
We performed separate meta-analyses for studies of nonpsychiatric twins (129 studies), nonpsychiatric families (13), and schizophrenia families (22). We meta-analyzed neuropsychological test variables that were reported in at least 2 independent studies. Additionally, neuropsychological test variables were assigned to one of the following 11 domains based on prior meta-analyses2,4 of cognition in schizophrenia: General Cognitive Ability, Attention/Processing Speed, Attention/Vigilance, Working Memory, Verbal Learning and Memory, Nonverbal Learning and Memory, Executive Function, Verbal Ability, Visuospatial Ability, Motor Skills, and Social Cognition. Meta-analyses of cognitive domains utilized all tests within a domain regardless of how many studies analyzed a particular test variable. If multiple tests within a cognitive domain were available for a given cohort, we included the average estimates for additive genetic effects (A), common (shared) environment (C), as well as unique (unshared) environment and error (E) across those tests in the domain meta-analysis. Supplementary table 9 describes the tests included in this meta-analysis, the cognitive functions they assess, and the cognitive domains they index.
For each neuropsychological test or cognitive domain, the proportion of total variance accounted for by A, C, and E was calculated by averaging each component across studies from independent cohorts while weighting by sample size. Family studies without twins cannot distinguish the influences of A and C, therefore the estimate of familiality (A+C) was used as A. Heritability was calculated as the proportion of total variance due to additive genetic effects (A/ A+C+E). All analyses were carried out in R (http://www.r-project.org) using custom scripts.
Wald tests42 were applied to identify significant differences in the heritability of cognitive phenotypes estimated from meta-analyses of schizophrenia and nonpsychiatric family studies, and nonpsychiatric twin and family studies. P-values were Bonferroni-corrected for 15 phenotypes tested for each of the 2 comparisons (.05/15 = .0033).
Results
Twin Correlations as Indicators of Heritability
Supplementary figure 2 illustrates that nonpsychiatric MZ twin correlations for cognitive phenotypes averaged .58 (range 0–.94), while DZ twin correlations averaged .32 (range 0–.88), suggesting sizeable influences of A and E, and minor influences of C. General Cognitive Ability had the highest MZ/DZ correlation ratios (mean rMZ/ rDZ = .75/ .44), indicating that a large proportion of the variation can be attributed to A, consistent with reports of high heritability.43 Although more variable, ratios for Verbal Ability and Visuospatial Ability were also high (.66/ .40 and .55/ .30).
Meta-analyses of Heritability of Cognitive Phenotypes
Nonpsychiatric Twins.
The studies of nonpsychiatric twins aged >16 (supplementary table 5) reported 375 test variables from all 11 cognitive domains. Of these, 48 variables from 8 domains were studied in 2–11 independent cohorts and meta-analyzed. Heritability estimates ranged between 20% and 80% (figure 1). High heritability (h2) was observed for measures of General Cognitive Ability (h2 = 53%) and the Index Scores obtained from the Wechsler Adult Intelligence Scale (WAIS) (h2 = 53%–80%), as well as WAIS Information (h2 = 63%), Block Design (h2 = 60%), Digit Symbol Coding (h2 = 59%) and Arithmetic (h2 = 58%), Thurstone Picture Memory (h2 = 63%), and the Educational Testing Service (ETS) tests (h2 = 67%–74%). Overall, the cognitive domain showing the highest heritability was Visuospatial Ability (h2 = 51%), followed by Verbal Ability (h2 = 41%) and Attention/Processing Speed (h2 = 45%), primarily driven by the high heritability of the WAIS subtests within each of these 3 domains.
Fig. 1.
Variance component estimates for cognitive phenotypes based on meta-analysis of nonpsychiatric twin studies. Percentage variance explained by A (Additive genetic influences), C (Common environment), and E (Unique environment and Error) for individual test variables (plain font) and cognitive domains (bold font). Error bars and numbers in parentheses indicate 95% CIs. The sample size for a given cognitive domain differs from the summed sample size for test variables within that domain because inclusion criteria differ (supplementary methods).
Nonpsychiatric Families.
The family studies of nonpsychiatric individuals (supplementary table 6) reported 64 test variables from 10 of the 11 cognitive domains (except Motor Skills), of which 9 variables were studied in 2–3 independent family cohorts and meta-analyzed. Heritability estimates ranged from 30% to 55% (figure 2). Heritability was high for measures of General Cognitive Ability (h2 = 53%), as well as the IQ subtest scores of WAIS Letter–Number Sequencing (h2 = 55%) and WAIS Matrix Reasoning (h2 = 53%). Overall, the individual cognitive domains showing the highest heritability were Visuospatial Ability (h2 = 52%), which was primarily driven by the high heritability of Matrix Reasoning; and Verbal Ability (h2 = 52%).
Fig. 2.
Variance component estimates for cognitive phenotypes based on meta-analysis of nonpsychiatric family studies. Percentage variance explained by A + C (Additive genetic + Common environment influences) and E (Unique environment and Error) for individual test variables (plain font) and cognitive domains (bold font). A and C are combined because they cannot be disentangled in the family design. Error bars and numbers in parentheses indicate 95% CIs. The sample size for a given cognitive domain differs from the summed sample size for test variables within that domain because inclusion criteria differ (supplementary methods).
Schizophrenia Families.
The family studies of schizophrenia (supplementary table 7) reported 155 test variables from all 11 cognitive domains, of which 41 variables were studied in 2–7 independent cohorts and meta-analyzed. Heritability estimates ranged from 15 to 74% (figure 3). Heritability was high for measures of General Cognitive Ability (h2 = 63%) and WAIS Vocabulary (h2 = 74%), as well as WRAT Reading (h2 = 74%), often used as a proxy measure for premorbid IQ. Furthermore, high heritability was apparent for the Wechsler Memory Scale (WMS) subtest Logical Memory Immediate Recall, CVLT Long Delay Free Recall, Penn Word Memory, WAIS Block Design and Similarities, and Judgment of Line Orientation (h2 = 52%–62%). Overall, the cognitive domain showing the highest heritability was Verbal Ability (h2 = 55%), followed by Visuospatial Ability (h2 = 51%), again driven mostly by the high heritability of the WAIS subtests within each domain.
Fig. 3.
Variance component estimates for cognitive phenotypes based on meta-analysis of schizophrenia family studies. Percentage variance explained by A + C (Additive genetic + Common environment influences) and E (Unique environment and Error) for individual test variables (plain font) and cognitive domains (bold font). A and C are combined because they cannot be disentangled in the family design. Error bars and numbers in parentheses indicate 95% CIs. The sample size for a given cognitive domain differs from the summed sample size for test variables within that domain because inclusion criteria differ (supplementary methods).
Supplementary figures 3–13 show heritability data from individual studies included in the meta-heritability estimates. The forest plots show the heritability of individual neuropsychological test variables and the overarching cognitive domains. The resulting meta-estimates of individual tests (bold) and domains (bold italic) correspond to the data in figures 1–3.
In summary, twin and family studies show highest heritability for the following tests: WAIS Digit Symbol Coding, Letter–Number Sequencing, Digit Span, Vocabulary, Information, and Block Design, as well as ETS Hidden Patterns and Identical Pictures, and Thurstone Picture Memory.
Heritability Comparisons Between Schizophrenia and Nonpsychiatric Samples
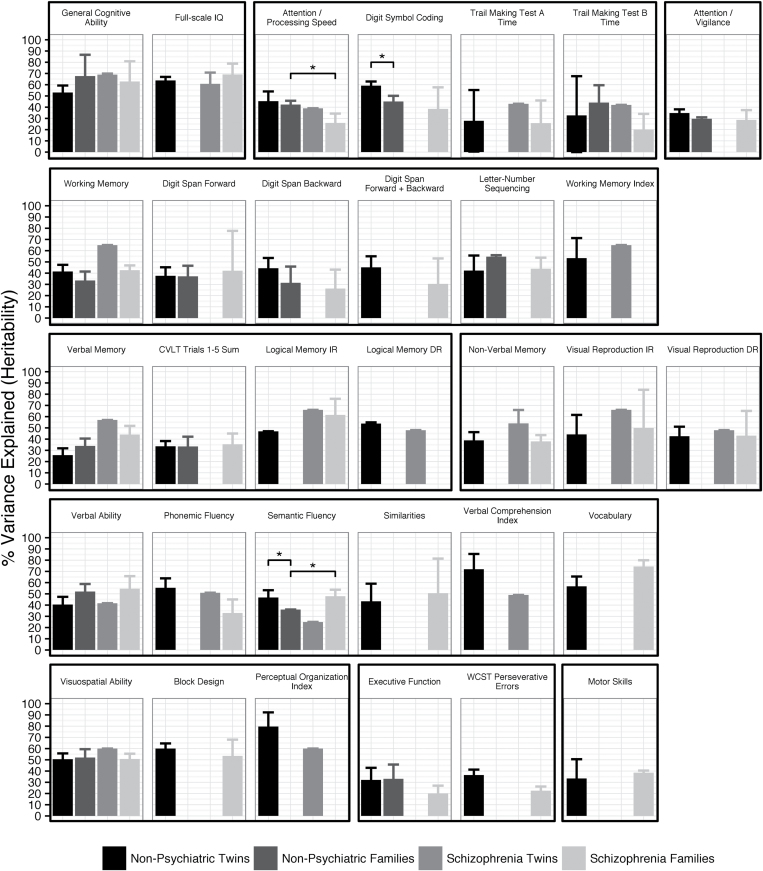
Comparison of heritability estimates from schizophrenia and nonpsychiatric samples indicated that cognitive phenotypes do not have lower heritability in patients, in contrast to what has been thought due to environmental factors and illness-related moderators. Figure 4 shows the results from each study design for phenotypes that were meta-analyzed in the nonpsychiatric twin design and at least one other study design. Of the 15 phenotypes available in both schizophrenia and nonpsychiatric family studies, 13 phenotypes did not significantly differ in heritability. Attention/Processing Speed had significantly lower heritability and Semantic Fluency had significantly higher heritability in schizophrenia families compared to nonpsychiatric families (Wald test P = 3.5 × 10−4 and P = 3.9 × 10−5). However, the difference in Semantic Fluency may not be accurate, as it was driven by low heritability in nonpsychiatric families based on only 2 studies. Heritability estimates also did not markedly differ between 15 phenotypes available in nonpsychiatric twin and family designs, with only WAIS Digit Symbol Coding and Semantic Fluency having significantly lower heritability in families than twins (Wald test P = 7.3 × 10−6 and P = 1.0 × 10−3). The CIs of the heritability estimates were typically narrower for phenotypes with larger numbers of studies and larger total sample sizes, as expected. Taking the sample sizes into account, heritability estimates appeared equally precise for schizophrenia and nonpsychiatric studies.
Fig. 4.
Heritability of cognitive phenotypes across study designs. Phenotypic variance explained was determined by meta-analysis for nonpsychiatric twin, nonpsychiatric family, and schizophrenia family studies, or from the original study estimates for schizophrenia twin studies. Cognitive domains (bold) and test variables (plain) meta-analyzed in the nonpsychiatric twin and at least one other study design are shown. Error bars indicate 95% CIs. *P < .05 (Bonferroni-corrected). WCST = Wisconsin Card Sorting Test; CVLT = California Verbal Learning Test; IR, DR = Immediate, Delayed Recall.
Genetic Overlap Between Schizophrenia Liability and Cognition
To assess the genetic overlap between schizophrenia liability and cognitive functions, we summarized data reported in the Maudsley Twin and Family Studies and by the overarching Schizophrenia Twins and Relatives Consortium (STAR Consortium),44 a series of studies of twins and other family members concordant or discordant for schizophrenia (supplementary table 8).33–38Figure 5 shows the cross-twin-cross-trait correlations, ie, the correlations between phenotype 1 in co-twin 1 and phenotype 2 in co-twin 2, and vice versa.36,37 Significantly larger cross-twin-cross-trait correlations for MZ twin pairs (rMZCT–CT) than for DZ twins (rDZCT–CT) resulted in greater genetic overlap (rg) with schizophrenia liability, as noted for verbal and nonverbal memory measures (Logical Memory [−.34 to −.58], Verbal Paired Associates [−.26 to −.50], and Visual Reproduction [−.34 to −.99]), Full-scale IQ (−.46 to −.69), and WAIS Index Scores (−.34 to −.79), which are major components of IQ. Some cognitive phenotypes had very high genetic correlations, (rg > .8, Trail Making Test A and B, Semantic Fluency) which may be overestimations since the CIs for those correlations were relatively wide (supplementary table 8). The genetic overlap between cognitive phenotypes and schizophrenia liability ranged widely, possibly due to small samples causing imprecise estimates, but overall was relatively high (average rg = −.58; SD = 0.22).
Fig. 5.
Genetic overlap between cognitive phenotypes and schizophrenia liability based on twin data. Cross-twin cross-trait (CT–CT) correlations for cognition in co-twin 1 and schizophrenia liability in co-twin 2 for MZ pairs (rMZCT–CT) and DZ pairs (rDZCT–CT). rph is the total phenotypic correlation between the cognitive phenotype and schizophrenia liability, of which rph-a is the amount due to additive genetic influences. rg is the genetic correlation between the cognitive phenotype and schizophrenia liability. All correlations are negative (ie, poor cognition associated with high liability) but are shown as positive values for plotting consistency. Data are maximum likelihood estimates reported in references.34–37 Study reference numbers are shown in parentheses. CT–CT correlations were only reported for studies Toulopoulou et al36 and Toulopoulou et al.37 FIQ and WMS subtests were analyzed in multiple studies; only data from studies reporting CT–CT correlations are shown. See supplementary table 8 for all reported data. FIQ = Full-Scale Intelligence Quotient; IR, DR = Immediate, Delayed Recall; WAIS = Wechsler Adult Intelligence Scale; WMS = Wechsler Memory Scale.
Discussion
This study reports a comprehensive meta-analysis of heritability data across cognitive domains in nonpsychiatric individuals and those with schizophrenia. We confirm the high heritability of multiple cognitive domains and demonstrate that this applies to both schizophrenia and nonpsychiatric samples. To our knowledge, this is the first systematic, meta-analytic demonstration that the heritabilities of cognitive phenotypes in schizophrenia are equivalent to those in healthy populations.
The most heritable phenotypes (h2 > 64%) were IQ, Spearman’s “g,” and some WAIS index scores that comprise IQ (Verbal Comprehension, Perceptual Organization), as well as tests that comprise or are highly correlated with IQ, such as WAIS Vocabulary and Block Design (h2 = 57%–60%). Interestingly, phenotypes within the Attention/Processing Speed domain differed substantially in heritability, with measures indexing information processing speed (eg, Digit Symbol Coding, ETS Hidden Patterns) having relatively high heritability (h2 = 59%–74%), and timed measures dependent on motor response having lower heritability (h2 = 28%–50%). For the most part, tests that we considered indexes of executive functioning (eg, Wisconsin Card Sorting Test, Tower of London) had relatively low heritability (h2 = 20%–40%). Given the multifactorial nature of neuropsychological tests, our categorization of tests into cognitive domains is not the only possible arrangement. For example, Stroop Interference and Trail Making Test B could be grouped into the Executive Function domain instead of the Attention/Processing Speed domain; however, doing so would not increase the Executive Function heritability estimate since the heritability of these tests was also low. Thus, we provided individual test data in the tables to enable evaluation of test-specific heritability estimates, in addition to domain estimates.
The fact that we report substantial heritability across cognitive phenotypes has important implications. While high heritability does not indicate that any particular gene has a large effect on the phenotype, it does suggest that the phenotype has a sizeable genetic component that improves precision for defining gene function in cognition. The range in heritability across phenotypes could result from differences in genetic architecture, where different genes and/or the same genes with different effects (possibly due to different genetic variants) mediate the phenotypes. Alternatively, differences in environmental effects or measurement error between phenotypes could affect heritability, since heritability is a proportion of the total phenotypic variance, which also comprises variance due to common environment (C), and unique environment and measurement error (E). Measurement error is likely not a major factor, however, since test–retest reliability is generally high (intra-class correlations > .7) for most neuropsychological tests.45–48 An exception is Executive Function tasks that tend to have low reliability49,50 (eg, intra-class correlations < .7 for WCST indices51). This might be due to their sensitivity to practice effects,50,52 which may explain their relatively low heritability in our meta-analyses. In contrast, environmental factors have substantial influences on some cognitive phenotypes; eg, verbal tests are particularly sensitive to education and socioeconomic status.53,54 In reality, both genetic and environmental factors likely underlie the range in heritability observed across the cognitive phenotypes. An important implication is that cognitive phenotypes with similar degrees of genetic effects could differ in heritability due to different environmental effects, a notion that is typically not appreciated by heritability studies, which tend to invoke genetic explanations for heritability differences.
The current study also compared heritability between schizophrenia and nonpsychiatric study designs and did not find consistent differences. This is perhaps the most important and novel finding of this study, and has several important implications. First, similar heritability suggests that genetic factors contributing to schizophrenia do not disrupt normal genetic influences on cognition in the general population. Second, it suggests that schizophrenia and nonpsychiatric samples should be equally informative for genetic studies of cognition. Third, similar heritability indicates that illness-related moderators (eg, medication, higher rates of smoking and substance use in patients) and environmental factors, which are often assumed to have larger effects in schizophrenia samples, have negligible effects on heritability. However, the lack of marked differences between schizophrenia and nonpsychiatric samples in the measurement accuracy of neuropsychological tests46–48 suggests that fluctuations in illness-related moderators have negligible effects on cognitive performance. While there is some evidence that illness-related moderators affect some cognitive domains,55–59 2 large cohort studies did not find consistently higher variances in several cognitive phenotypes in schizophrenia patients compared to healthy subjects (eg, BACS composite score, Continuous Performance Test, CVLT, Letter–Number Sequencing).60–63 Also, we found similar CI widths for heritability estimates of cognitive phenotypes across schizophrenia and nonpsychiatric samples, indicating that heritability in schizophrenia samples is fairly consistent despite differences in environmental factors and illness-related moderators (due to differences in ascertainment, inclusion/exclusion criteria, etc.). Therefore, the degree to which genetic studies of cognition in schizophrenia will be informative for elucidating the biology and informing novel treatment approaches for cognitive impairment will depend on the variance explained by genetic factors (ie, the heritability), rather than illness-related moderators and environmental factors. This is an important issue because it is commonly thought that environmental factors and illness-related moderators hinder the detection of genetic effects in schizophrenia samples. For this reason, some studies have focused on unaffected relatives of patients, who carry genetic risk for schizophrenia and have impaired cognition (although less severe than patients),9,64–67 but lack or have milder illness-related moderators, as a means to examine the genetics of cognition in schizophrenia. Instead, similar heritability suggests that studying schizophrenia samples is valuable and, some might argue, more informative for understanding the relationship between cognition and schizophrenia.
Some cognitive domains that are impaired in schizophrenia1,4 are relatively understudied genetically, particularly Social Cognition, Attention/Vigilance, Nonverbal Memory, and Executive Function. Fewer studies and smaller sample sizes can lead to inaccurate heritability estimates that may explain, in part, the low heritability observed for some of these domains. Heritability could be inaccurate in family studies since they do not distinguish influences of genetic effects (A) and common environment (C); therefore, these influences are combined into “familiality.” However, the lack of consistent differences in heritability using a family design compared to the MZ/DZ twin design suggests that C has negligible influence on most cognition phenotypes. Thus, for family studies, A+C is almost entirely A, and “familiality” is a good proxy for heritability.
An emerging method to estimate heritability utilizes population-based SNP data to determine the collective variance in a phenotype that is explained by common genetic variation.68,69 SNP-based heritability of cognitive phenotypes in adults is estimated to be 29% for General Cognitive Ability (Spearman’s g)70 and 19%–56% for other cognitive phenotypes.71–76 These estimates are lower than those from our meta-analyses, but in line with a twin study that reported considerably lower heritability estimated from SNP data than from variance components.73 This “missing heritability” suggests that other inherited factors not indexed by SNPs, such as rare variants and structural variation, as well as heritable epigenetic modifications and other factors, contribute to individual variation in cognitive phenotypes.77 It will be important to develop analytical methods that incorporate other potential sources of genetic variation to estimate heritability using population-based approaches.
We also examined the genetic overlap between cognitive phenotypes and schizophrenia liability from a summary of schizophrenia twin studies.33–38 We found moderate to high genetic correlations (average rg = −.58), consistent with a previous report of significant correlations between several cognitive domains and negative symptoms and disorganization.78 However, the twin study genetic correlations ranged widely across phenotypes, possibly due to small samples causing imprecise estimates, or because some cognitive constructs have stronger genetic relationships to schizophrenia than others. The genetic overlap suggests that shared genes regulate neurodevelopmental processes mediating both cognition and psychosis.79 Alternatively, the genes may have pleiotropic effects reflecting multiple roles in neural processes that govern cognition and other mechanisms underlying schizophrenia. In an attempt to assess causality between cognition and schizophrenia liability, Toulopoulou et al38 performed multivariate structural equation modeling in schizophrenia twin-family samples and found evidence that cognitive deficits lie upstream of liability, with a genetic correlation of −.51. Population-based studies also support genetic overlap of cognition with schizophrenia liability, although possibly lower (eg, genetic correlation of −.26)80 than that estimated by schizophrenia studies. Molecular genetic overlap between cognition and schizophrenia is supported by recent GWAS mega-analyses.19,81–83 Specifically, polygenic variation influencing cognitive functioning in healthy cohorts was significantly associated with schizophrenia case status in independent datasets.19 In parallel, polygenic risk for schizophrenia was significantly associated with poorer cognition in healthy cohorts.81–83 These studies suggest that polygenic variants influencing schizophrenia risk modulate neural processes involved in cognition in the general population, although the contribution is small (explaining <1% variance in cognition).81–83 It is conceivable that schizophrenia genetic factors have a stronger impact on cognition in patients, possibly because dysfunctional neural circuits are more sensitive to the genetic effects than normally functioning circuits. Unfortunately, the few patient studies examining schizophrenia genetic variants in cognition produced inconsistent results, possibly due to small sample sizes.84–86 In addition, there have been no studies specifically examining the genetic basis of the degree of cognitive impairment in patients separate from the genetic basis of cognitive ability. While schizophrenia risk genes may influence the degree of impairment, as suggested by the presence of cognitive deficits in unaffected relatives genetically predisposed to schizophrenia,9,64–67 other genes that are not causal in schizophrenia may be involved. Indeed, the partial genetic overlap between cognition and schizophrenia liability indicates that genes not involved in schizophrenia also have a substantial influence on cognition.
It is unclear whether cognition genes have the same effects in schizophrenia and nonpsychiatric populations, or whether the effects vary despite heritability being similar. It may be that different genes support cognition in schizophrenia and healthy individuals, possibly due to pleiotropy or due to genes being inactive under certain conditions. While there has been considerable effort to elucidate the genetic architecture of cognition in nonpsychiatric populations,43,70,81,87 there is a dearth of high-powered genetic studies of cognition in schizophrenia samples. Analyses of large, well-phenotyped samples consisting of both patients and healthy individuals, which we are actively undertaking, will be important to clarify this issue. If the same genes influence cognition in schizophrenia patients and the general population, the neural mechanisms regulated by these genes may nonetheless operate differently in patients, perhaps due to genetic variation associated with cognitive impairment. A similar genetic basis in schizophrenia and nonpsychiatric populations would not invalidate cognitive markers as endophenotypes of schizophrenia, since cognitive deficits in patients meet the criteria for endophenotypes notwithstanding.28 Cognition is a particularly important endophenotype because delineating its underlying genetic mechanisms may identify promising targets for improving cognitive functioning in patients.
At this time, it is difficult to recommend specific neuropsychological tests or cognitive domains that are the most appropriate for examining the genetic basis of cognition in schizophrenia. This judgment depends on both the heritability of the phenotype and its genetic overlap with schizophrenia. Our meta-analyses identified several cognitive phenotypes having high heritability, such as IQ, Spearman’s g, and WAIS index scores mentioned above, which could be prioritized for genetic studies. However, the genetic correlation data from twin studies are too limited to conclusively identify phenotypes having the largest genetic overlap with schizophrenia. Similarly, analyses of the effects of schizophrenia risk genes and polygenic factors across multiple cognitive phenotypes are limited, although 2 recent studies reported that polygenic risk explains more variance in Attention/Language than Verbal Memory,83 and Performance IQ than Verbal IQ and Full-Scale IQ.82 Additional studies are required to determine which cognitive phenotypes have the strongest genetic relationships to schizophrenia and are most appropriate as markers for studying the genetics of cognition in schizophrenia.
Recent studies have also identified genetic overlap of cognitive phenotypes with brain structural phenotypes.38 Further, the heritability of specific cognitive abilities is comparable to the heritability of volume and cortical thickness of brain structures subserving those abilities,88,89 many of which involve the prefrontal cortex. Schizophrenia onset during adolescence and early adulthood coincides with the maturation of brain regions that are abnormal in schizophrenia, such as the temporal and frontal lobes.90–92 Indeed a putative mechanism underlying schizophrenia might be mistiming of brain maturation processes that are important for higher order cognitive functions.93 Brain maturational stages would therefore be important to consider when interpreting genetic relationships between schizophrenia and cognition or other phenotypes. It would also be important to consider sex differences in cognitive deficits given known sexual dimorphism in normal neurodevelopmental processes and their timing, as well as those associated with schizophrenia.94
Conclusions
Taken together, our results show that most cognitive phenotypes have moderate to high heritability, although estimates range widely, likely due to differences in both genetic and environmental influences. Our results also indicate that heritability of cognitive phenotypes does not markedly differ between schizophrenia and nonpsychiatric populations, suggesting that schizophrenia samples are valuable for studying the genetic basis of cognitive impairment in patients. Genetic overlap between schizophrenia and cognitive phenotypes supports a shared genetic etiology; however, more studies are required to verify whether the same genes influence cognitive variation in schizophrenia patients and the general population.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) grant number R01MH092380 to T.L.P. supporting the Genetics of Endophenotypes of Neurofunction to Understand Schizophrenia (GENUS) Consortium. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplementary Material
Acknowledgments
The authors would like to thank Melissa DeLeon, Emma Parrish, and Gautami Shashidhar for their contributions to the preparation of the supplementary tables. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 3. O’Carroll R. Cognitive impairment in schizophrenia. Adv Psychiatr Treat. 2000;6:161–168. [Google Scholar]
- 4. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 5. Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–571. [DOI] [PubMed] [Google Scholar]
- 6. Giuliano AJ, Li H, Mesholam-Gately RI, et al. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415. [DOI] [PubMed] [Google Scholar]
- 7. Bhojraj TS, Francis AN, Montrose DM, Keshavan MS. Grey matter and cognitive deficits in young relatives of schizophrenia patients. Neuroimage. 2011;54(suppl 1):S287–S292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gur RE, Calkins ME, Gur RC, et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keefe RS, Sweeney JA, Gu H, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. [DOI] [PubMed] [Google Scholar]
- 12. Allott K, Liu P, Proffitt TM, Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr Res. 2011;125:221–235. [DOI] [PubMed] [Google Scholar]
- 13. Allott KA, Cotton SM, Chinnery GL, et al. The relative contribution of neurocognition and social cognition to 6-month vocational outcomes following Individual Placement and Support in first-episode psychosis. Schizophr Res. 2013;150:136–143. [DOI] [PubMed] [Google Scholar]
- 14. Nuechterlein KH, Subotnik KL, Green MF, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37(suppl 2):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaragoza Domingo S, Bobes J, García-Portilla M-P, Morralla C. EPICOG-SCH Study Group. Cognitive performance associated to functional outcomes in stable outpatients with schizophrenia. Schizophr Res Cogn. 2015;2:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 17. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 18. Schizophrenia Psychiatric Genome-Wide Association Study Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Schizophrenia Consortium , Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi J, Levinson DF, Duan J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi Y, Li Z, Xu Q, et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet. 2011;43:1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Irish Schizophrenia Genomics Consortium, the Wellcome Trust Case Control Consortium. Genome-wide association study implicates HLA-C*01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol Psychiatry. 2012;72:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikeda M, Aleksic B, Kinoshita Y, et al. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry. 2011;69:472–478. [DOI] [PubMed] [Google Scholar]
- 27. Yue WH, Wang HF, Sun LD, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet. 2011;43:1228–1231. [DOI] [PubMed] [Google Scholar]
- 28. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 29. Glahn DC, Knowles EE, McKay DR, et al. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rieder RO, Gershon ES. Genetic strategies in biological psychiatry. Arch Gen Psychiatry. 1978;35:866–873. [DOI] [PubMed] [Google Scholar]
- 31. Briley DA, Tucker-Drob EM. Explaining the increasing heritability of cognitive ability across development: a meta-analysis of longitudinal twin and adoption studies. Psychol Sci. 2013;24:1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haworth CM, Wright MJ, Luciano M, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2010;15:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Owens SF, Picchioni MM, Ettinger U, et al. Prefrontal deviations in function but not volume are putative endophenotypes for schizophrenia. Brain. 2012;135:2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owens SF, Picchioni MM, Rijsdijk FV, et al. Genetic overlap between episodic memory deficits and schizophrenia: results from the Maudsley Twin Study. Psychol Med. 2011;41:521–532. [DOI] [PubMed] [Google Scholar]
- 35. Owens SF, Rijsdijk F, Picchioni MM, et al. Genetic overlap between schizophrenia and selective components of executive function. Schizophr Res. 2011;127:181–187. [DOI] [PubMed] [Google Scholar]
- 36. Toulopoulou T, Goldberg TE, Mesa IR, et al. Impaired intellect and memory: a missing link between genetic risk and schizophrenia? Arch Gen Psychiatry. 2010;67:905–913. [DOI] [PubMed] [Google Scholar]
- 37. Toulopoulou T, Picchioni M, Rijsdijk F, et al. Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch Gen Psychiatry. 2007;64:1348–1355. [DOI] [PubMed] [Google Scholar]
- 38. Toulopoulou T, van Haren N, Zhang X, et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol Psychiatry. 2015;20:1386–1396. [DOI] [PubMed] [Google Scholar]
- 39. Roth M, Hong LE, McMahon RP, Fuller RL. Comparison of the effectiveness of Conners’ CPT and the CPT-identical pairs at distinguishing between smokers and nonsmokers with schizophrenia. Schizophr Res. 2013;148:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hahn E, Vollath A, Ta TT, et al. Assessing long-term test-retest reliability of the CPT-IP in schizophrenia. PLoS One. 2014;9:e84780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cole VT, Weinberger DR, Dickinson D. Intra-individual variability across neuropsychological tasks in schizophrenia: a comparison of patients, their siblings, and healthy controls. Schizophr Res. 2011;129:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Engle RF. Wald, Likelihood Ratio, and Lagrange Multiplier Tests in Econometrics. In: Intriligator MD, Griliches Z, eds. Handbook of Econometrics II. Amsterdam, The Netherlands: Elsevier Science Publishers BV; 1983:796–801. [Google Scholar]
- 43. Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry. 2015;20:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Haren NE, Rijsdijk F, Schnack HG, et al. The genetic and environmental determinants of the association between brain abnormalities and schizophrenia: the schizophrenia twins and relatives consortium. Biol Psychiatry. 2012;71:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 46. Light GA, Swerdlow NR, Rissling AJ, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7:e39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilk CM, Gold JM, Bartko JJ, et al. Test-retest stability of the Repeatable Battery for the Assessment of Neuropsychological Status in schizophrenia. Am J Psychiatry. 2002;159:838–844. [DOI] [PubMed] [Google Scholar]
- 48. Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. [DOI] [PubMed] [Google Scholar]
- 49. Rabbitt P. Methodology of Frontal and Executive Function. Hove, UK: Psychology Press Ltd; 1997. [Google Scholar]
- 50. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press, Inc; 2006. [Google Scholar]
- 51. Paolo AM, Axelrod BN, Tröster AI. Test-retest stability of the Wisconsin Card Sorting Test. Assessment. 1996;3:137–143. [Google Scholar]
- 52. Lowe C, Rabbitt P. Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Cambridge Neuropsychological Test Automated Battery. International Study of Post-Operative Cognitive Dysfunction. Neuropsychologia. 1998;36:915–923. [DOI] [PubMed] [Google Scholar]
- 53. Jefferson AL, Gibbons LE, Rentz DM, et al. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J Am Geriatr Soc. 2011;59:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Contador I, Bermejo-Pareja F, Del Ser T, Benito-León J. Effects of education and word reading on cognitive scores in a community-based sample of Spanish elders with diverse socioeconomic status. J Clin Exp Neuropsychol. 2015;37:92–101. [DOI] [PubMed] [Google Scholar]
- 55. Lee J, Green MF, Calkins ME, et al. Verbal working memory in schizophrenia from the Consortium on the Genetics of Schizophrenia (COGS) study: the moderating role of smoking status and antipsychotic medications. Schizophr Res. 2015;163:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wing VC, Bacher I, Sacco KA, George TP. Neuropsychological performance in patients with schizophrenia and controls as a function of cigarette smoking status. Psychiatry Res. 2011;188:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang XY, Chen DC, Xiu MH, et al. Cigarette smoking and cognitive function in Chinese male schizophrenia: a case-control study. PLoS One. 2012;7:e36563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahlers E, Hahn E, Ta TM, Goudarzi E, Dettling M, Neuhaus AH. Smoking improves divided attention in schizophrenia. Psychopharmacology (Berl). 2014;231:3871–3877. [DOI] [PubMed] [Google Scholar]
- 59. Stone WS, Mesholam-Gately RI, Braff DL, et al. California Verbal Learning Test-II performance in schizophrenia as a function of ascertainment strategy: comparing the first and second phases of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hill SK, Reilly JL, Keefe RS, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Greenwood TA, Lazzeroni LC, Calkins ME, et al. Genetic assessment of additional endophenotypes from the Consortium on the Genetics of Schizophrenia Family Study. Schizophr Res. 2016;170:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Millard SP, Shofer J, Braff D, et al. Prioritizing schizophrenia endophenotypes for future genetic studies: an example using data from the COGS-1 family study. Schizophr Res. 2016;174:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nuechterlein KH, Green MF, Calkins ME, et al. Attention/vigilance in schizophrenia: performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barrantes-Vidal N, Aguilera M, Campanera S, et al. Working memory in siblings of schizophrenia patients. Schizophr Res. 2007;95:70–75. [DOI] [PubMed] [Google Scholar]
- 65. Calkins ME, Ray A, Gur RC, et al. Sex differences in familiality effects on neurocognitive performance in schizophrenia. Biol Psychiatry. 2013;73:976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horan WP, Braff DL, Nuechterlein KH, et al. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2008;103:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roalf DR, Gur RC, Almasy L, et al. Neurocognitive performance stability in a multiplex multigenerational study of schizophrenia. Schizophr Bull. 2013;39:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee SH, DeCandia TR, Ripke S, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bulik-Sullivan BK, Loh PR, Finucane HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davies G, Armstrong N, Bis JC, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N=53949). Mol Psychiatry. 2015;20:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hatzimanolis A, Bhatnagar P, Moes A, et al. Common genetic variation and schizophrenia polygenic risk influence neurocognitive performance in young adulthood. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kirkpatrick RM, McGue M, Iacono WG, Miller MB, Basu S. Results of a “GWAS plus:” general cognitive ability is substantially heritable and massively polygenic. PLoS One. 2014;9:e112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Plomin R, Haworth CM, Meaburn EL, Price TS, Wellcome Trust Case Control C , Davis OS. Common DNA markers can account for more than half of the genetic influence on cognitive abilities. Psychol Sci. 2013;24:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rowe SJ, Rowlatt A, Davies G, et al. Complex variation in measures of general intelligence and cognitive change. PLoS One. 2013;8:e81189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Toro R, Poline JB, Huguet G, et al. Genomic architecture of human neuroanatomical diversity. Mol Psychiatry. 2015;20:1011–1016. [DOI] [PubMed] [Google Scholar]
- 76. Vogler C, Gschwind L, Coynel D, et al. Substantial SNP-based heritability estimates for working memory performance. Transl Psychiatry. 2014;4:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen LS, Rice TK, Thompson PA, Barch DM, Csernansky JG. Familial aggregation of clinical and neurocognitive features in sibling pairs with and without schizophrenia. Schizophr Res. 2009;111:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40:744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fowler T, Zammit S, Owen MJ, Rasmussen F. A population-based study of shared genetic variation between premorbid IQ and psychosis among male twin pairs and sibling pairs from Sweden. Arch Gen Psychiatry. 2012;69:460–466. [DOI] [PubMed] [Google Scholar]
- 81. Lencz T, Knowles E, Davies G, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry. 2014;19:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hubbard L, Tansey KE, Rai D, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2016;42:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liebers DT, Pirooznia M, Seiffudin F, Musliner KL, Zandi PP, Goes FS. Polygenic risk of schizophrenia and cognition in a population-based survey of older adults. Schizophr Bull. 2016;42:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martin AK, Robinson G, Reutens D, Mowry B. Common genetic risk variants are associated with positive symptoms and decision-making ability in patients with schizophrenia. Psychiatry Res. 2015;229:606–608. [DOI] [PubMed] [Google Scholar]
- 85. van Scheltinga AF, Bakker SC, van Haren NE, et al. Schizophrenia genetic variants are not associated with intelligence. Psychol Med. 2013;43:2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yeo RA, Gangestad SW, Walton E, et al. Genetic influences on cognitive endophenotypes in schizophrenia. Schizophr Res. 2014;156:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Verhaaren BF, Vernooij MW, Koudstaal PJ, et al. Alzheimer’s disease genes and cognition in the nondemented general population. Biol Psychiatry. 2013;73:429–434. [DOI] [PubMed] [Google Scholar]
- 88. Blokland GA, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 2012;15:351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stone WS, Seidman LJ. Neuropsychological and structural neuroimaging endophenotypes in schizophrenia. In: Cicchetti D, ed. Developmental Psychopathology. Vol 2, Developmental Neuroscience. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2016:931–965. [Google Scholar]
- 90. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. [DOI] [PubMed] [Google Scholar]
- 92. DeLisi LE, Stritzke P, Riordan H, et al. The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry. 1992;31: 241–254. [DOI] [PubMed] [Google Scholar]
- 93. Catts VS, Fung SJ, Long LE, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.