Abstract
Pediatric brain tumors including medulloblastoma and atypical teratoid/rhabdoid tumor are associated with significant mortality and treatment-associated morbidity. While medulloblastoma tumors within molecular subgroups 3 and 4 have a propensity to metastasize, atypical teratoid/rhabdoid tumors frequently afflict a very young patient population. Adjuvant treatment options for children suffering with these tumors are not only sub-optimal but also associated with many neurocognitive obstacles. A potentially novel treatment approach is oncolytic virotherapy, a developing therapeutic platform currently in early-phase clinical trials for pediatric brain tumors and recently US Food and Drug Administration (FDA)-approved to treat melanoma in adults. We evaluated the therapeutic potential of the clinically available oncolytic herpes simplex vector rRp450 in cell lines derived from medulloblastoma and atypical teratoid/rhabdoid tumor. Cells of both tumor types were supportive of virus replication and virus-mediated cytotoxicity. Orthotopic xenograft models of medulloblastoma and atypical teratoid/rhabdoid tumors displayed significantly prolonged survival following a single, stereotactic intratumoral injection of rRp450. Furthermore, addition of the chemotherapeutic prodrug cyclophosphamide (CPA) enhanced rRp450’s in vivo efficacy. In conclusion, oncolytic herpes viruses with the ability to bioactivate the prodrug CPA within the tumor microenvironment warrant further investigation as a potential therapy for pediatric brain tumors.
Keywords: oncolytic virus, brain tumors, medulloblastoma, pediatric oncology, herpes simplex virus, atypical teratoid rhabdoid tumor, cyclophosphamide
Introduction
Medulloblastoma is the most common malignant pediatric brain tumor subtype, accounting for approximately 20% of all childhood brain cancers.1, 2 These tumors are divided into four distinctive molecular subtypes: WNT (Wingless-type mammary tumor virus [MMTV] integration site family member), Sonic Hedgehog, group 3, and group 4. The group 3 and 4 subtypes are associated with the highest rate of metastases and the poorest prognosis, with 5-year overall survival rates ranging from 40% to 60% and 75%, respectively.1, 3, 4 Atypical teratoid/rhabdoid tumors (AT/RTs) are highly aggressive tumors of the CNS that predominantly afflict infants and younger children. AT/RTs, which are frequently associated with loss of function mutations in the hSNF5/INI-1 tumor suppressor gene, have a tendency to metastasize early and are known for their poor outcomes. The 5-year survival rates are in the range of 15% to 36%, with median survival times of less than 12 months.5, 6 Conventional treatments of medulloblastoma and AT/RT usually involve surgical resection, high-dose chemotherapy, and radiation (depending upon patient age). New therapies are desperately needed to treat AT/RT and medulloblastoma, because complete surgical resection is usually not possible and conventional radiotherapy and chemotherapy regimens have limited efficacy and debilitating side effects.1, 4
Oncolytic virotherapy, the use of live attenuated viruses to selectively infect and kill tumor cells, is a novel form of cancer therapy that is actively being pursued in pre-clinical and early-phase clinical trials; it was recently approved by the US Food and Drug Administration (FDA) for use in melanoma.7, 8 Oncolytic viruses (OVs) have pleomorphic effects, including direct lysis of infected malignant cells, disruption of the tumor blood supply, and stimulation of the immune system against tumor antigens. The safety and tolerability of these agents have been demonstrated in several recently completed clinical trials, where no evidence for a maximum tolerated dose has been reported.9, 10 Of the previous pre-clinical studies that have been performed using pediatric brain tumor models, measles virotherapy showed activity against localized and disseminated models of both medulloblastoma and AT/RTs.11, 12 In addition, multiple mutant variants of herpes simplex virus 1 (oncolytic herpes simplex virus [oHSV]) have demonstrated anti-tumor efficacy in localized tumor models of medulloblastoma.13, 14 Further determination of whether these observed responses are virus and/or tumor model specific has yet to be evaluated, and other clinically applicable oncolytic virus vectors need to be tested.
rRp450 is an attenuated herpes simplex 1 vector deficient in the viral-encoded ribonucleotide reductase (ICP6). In contrast with the oHSV mutant variants previously evaluated in medulloblastoma efficacy studies, rRp450 contains intact copies of the neurovirulence gene γ134.5 and expresses rat CYP2B1, an enzyme that activates the chemotherapeutic prodrug cyclophosphamide (CPA).15 Cells infected with rRp450 can bioactivate CPA, thus delivering active cytotoxic metabolites to the tumor microenvironment.16 We have previously demonstrated that in vivo intracranial (IC) rRp450 injection was well-tolerated and had no significant adverse effects associated with doses up to 1 × 108 plaque-forming units (PFUs).17 Therefore, we hypothesized that use of intratumoral administration of rRp450 would provide a targeted, less toxic approach for pediatric brain tumors. In this study we show that medulloblastoma and AT/RT cell lines supported viral replication and were susceptible to virus-induced cytotoxicity. Addition of CPA to rRp450-infected cells also resulted in cytotoxicity. Pre-clinical evaluation of rRp450 in multiple models of medulloblastoma and AT/RT demonstrated significant increases in survival and multiple complete responders. Lastly, in a murine xenograft model of AT/RT, CPA in combination with rRp450 increased the median survival and cured multiple animals compared with animals treated with rRp450 or CPA alone. These results indicate that further investigation involving rRp450 and CPA is warranted, and that pediatric brain tumors are strong candidates for clinical application involving this therapeutic platform.
Results
In Vitro Viral Replication
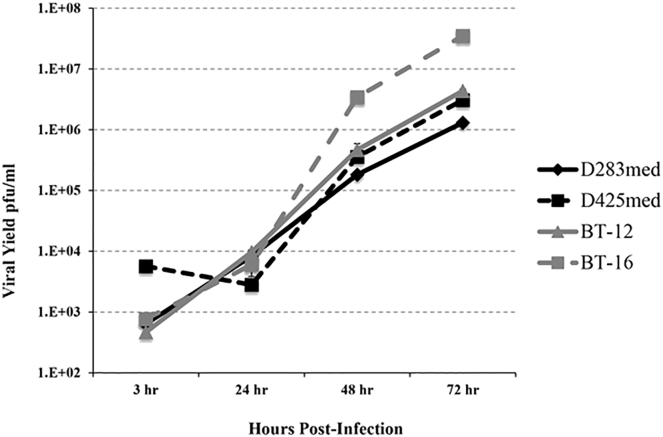
We examined pediatric brain tumor cell lines for permissivity to oHSV replication in vitro (Figure 1). The AT/RT cell lines displayed higher permissiveness to oHSV rRp450 viral replication than did the group 3/4 medulloblastoma cell lines tested. The AT/RT cell line BT-16 showed the highest amount of replication (>4 logs), whereas BT-12 displayed approximately 4 logs of viral replication. The group 3 medulloblastoma cell line D425med and the group 4 medulloblastoma cell line D283med both exhibited >3 logs of replication. All cell lines evaluated displayed increased viral replication over time except D425med, which had slightly lower replication at 24 hr compared with 3 hr.
Figure 1.
Replication of rRp450 in Medulloblastoma and AT/RT Cell Lines
Replication of rRp450 in medulloblastoma (D283med, D425med) and AT/RT (BT-12, BT-16) cells is demonstrated by the titers obtained following infection at an MOI of 0.01. Cells and supernatants were collected at the times designated, and virus titers were determined by standard plaque assays on Vero cells. Means ± SEM are shown (n = 3).
In Vitro Cytotoxicity
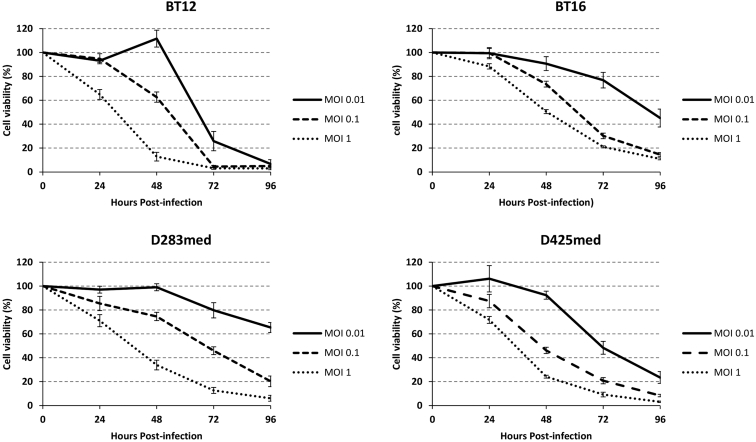
We tested the viability of the two AT/RT and two medulloblastoma cell lines following infection by rRp450 in vitro (Figure 2). All four cell lines displayed sensitivity to the virus in a dose- and time-dependent manner. The AT/RT cell line BT-12 displayed greater sensitivity to viral killing than did the AT/RT cell line BT-16, whereas the medulloblastoma cell line D425med displayed slightly greater sensitivity to rRp450 than the medulloblastoma cell line D283med. BT-12 showed the highest amount of cytotoxicity at 48 hr postinfection at the highest MOI (MOI = 1 infectious virion per cell), with 12.8% of cells viable, compared with 30.5% for BT-16. The medulloblastoma cell lines displayed similar sensitivities to rRp450 at 48 hr postinfection at an MOI of 1, with 33.8% and 22.4% viable cells for D283med and D425med, respectively. After 48 hr, greater than 90% of BT-12 cells and 80% of BT-16, D283med, and D425med cells were no longer viable. Likewise, by 96 hr postinfection, greater than 80% of all cells were killed with an MOI of 0.1.
Figure 2.
In Vitro rRp450-Mediated Cytotoxicity
The viability of AT/RT (BT-12, BT-16) and medulloblastoma (D283med, D425med) cells following administration of rRp450 at an MOI of 0.01, 0.1, and 1. The number of viable cells was determined daily for 4 days and normalized against the number of mock-infected cells. Means ± SEM are shown (n = 8).
In Vivo Efficacy
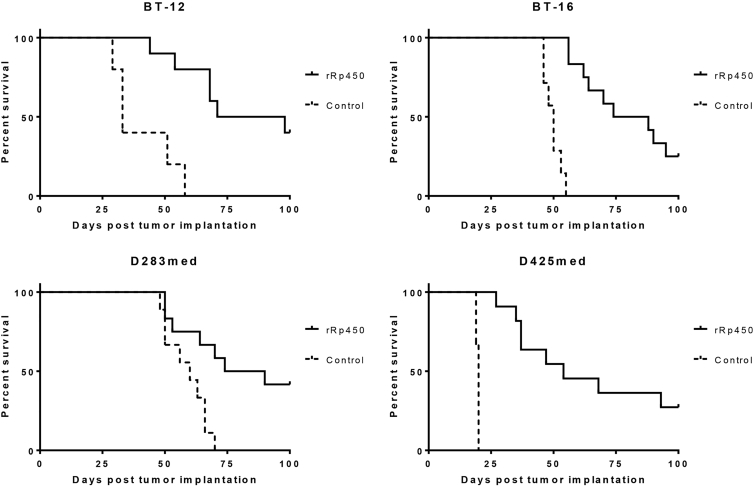
All orthotopic xenograft pediatric brain tumor models tested showed statistically significant improvement in length of survival with intracranial oHSV rRp450 treatment as compared with treatment with vehicle (Figure 3). The group 4 medulloblastoma D283med showed the most significant response, with 5/12 (41.6%) mice exhibiting complete response (CR) upon the conclusion of the study at day 100 post-tumor implantation. The group 3 medulloblastoma D425med-bearing mice displayed 3/11 (27.3%) mice cured after treatment, whereas the AT/RT-bearing mice showed 4/10 CR (40%) and 3/12 CR (25%) for BT-12 and BT-16, respectively.
Figure 3.
rRp450 Increases Survival in Orthotopic Mouse Models of Medulloblastoma and AT/RT
Medulloblastoma (D283med, D425med) and AT/RT (BT-12, BT-16) tumors were established in the right frontal lobe of athymic nude mice. Seven days after tumor implantation, the mice received 1 × 106 PFUs of rRp450. Mice treated with rRp450 had significantly longer survival times than mice receiving Opti-MEM vehicle only. Kaplan-Meier curves depict differences in survival and statistical differences determined using the log rank test. D283med, p = 0.0059; D425med, p < 0.0001; BT-12, p = 0.0007; BT-16, p < 0.0001.
The median survival was significantly prolonged for all oHSV-treated mice compared with vehicle-treated animals. The median survival of mice bearing D283med tumors was 82 days (range 50–100 days, n = 12) compared with 60 days (range 48–70 days, n = 9) in vehicle-treated mice. Median survival for oHSV-treated D425med tumor-bearing mice was 54 days (range 27–100 days, n = 11) versus 20 days (range 19–20 days, n = 9) for vehicle-treated mice. rRp450-treated BT-12 tumor-bearing mice had a median survival of 84.5 days (range 44–100 days, n = 10) compared with 33 days in vehicle-treated mice (range 29–58 days, n = 5). The median survival for oHSV-treated BT-16 tumor-bearing mice was 81 days (range 56–100 days, n = 12) versus 50 days in vehicle-treated mice (range 46–55 days, n = 7).
In Vivo Detection of oHSV Infection by Immunohistochemistry
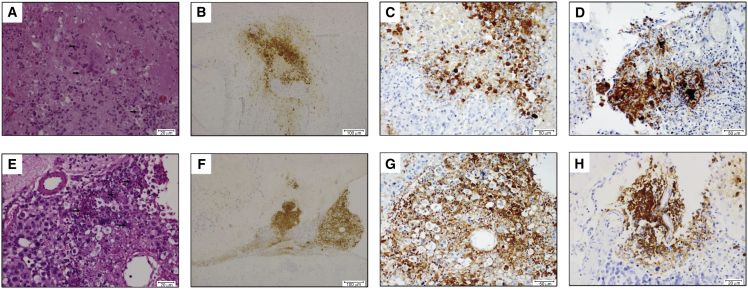
We found evidence by immunohistochemical staining of oHSV infection in the brains of all tumor-bearing animals at time of death. Staining for HSV-1 showed association of oHSV infection with tumor cell necrosis (Figure 4). Viral inclusion bodies were noted within the nuclei of some tumor tissues (Figure 4). Cured animals showed no evidence of tumor upon brain dissection and no significant oHSV staining (data not shown).
Figure 4.
Histologic Examination of Mice Treated with rRp450
(A and E) H&E staining of BT-12 (A) and D425med (E) tumors demonstrates HSV inclusion bodies (arrows). (B–D and F–H) Immunohistochemical staining of brains from tumor-bearing mice shows strong focal expression of oHSV-1 in BT-12 (B and C), BT-16 (D), D425med (F and G), and D283med (H) cells. Staining is cytoplasmic and nuclear in location and is associated with tumor cell necrosis.
rRp450 Bioactivates the Prodrug CPA In Vitro
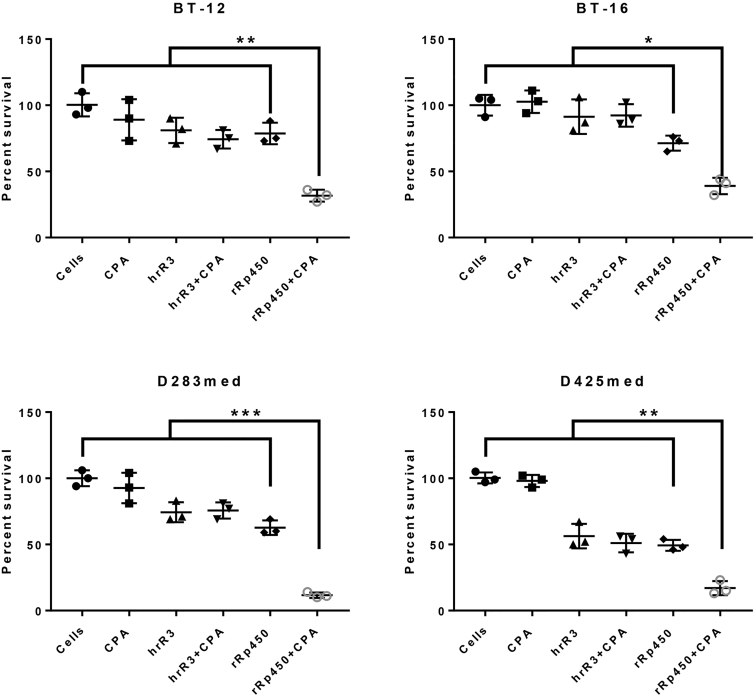
Because rRp450 contains the gene for rat Cyp2b1, which bioactivates the cancer chemotherapeutic prodrug CPA,15 we wanted to investigate whether we could build upon our initial in vivo results demonstrating significant increases in survival following rRp450 treatment alone. We thus evaluated rRp450-mediated cytotoxicity in the presence or absence of CPA. Because rRp450 is very cytotoxic to our cell populations (Figure 2), we performed the assays at 39.8°C, a temperature that is non-conducive to ICP6 mutant viral replication. Thus, we could isolate cytotoxicity associated with CPA bioactivation in the absence of oncolysis.15, 18 Figure 5 demonstrates that addition of CPA to rRp450-infected cells significantly increases cytotoxicity in all four cell lines examined. However, CPA failed to increase cytotoxicity of the ICP6-deficient, γ134.5 wild-type HSV-1 mutant hrR3.19 Unlike rRp450, hrR3 does not contain CYP2B1, the gene product allowing bioactivation of CPA in viral-infected cells. Addition of CPA alone also had no cytotoxic effect on the cells.
Figure 5.
Cyclophosphamide Potentiates In Vitro rRp450-Mediated Cytotoxicity
Medulloblastoma (D283med, D425med) and AT/RT (BT-12, BT-16) cells were infected with rRp450 or hrR3 in the presence or absence of cyclophosphamide (CPA) followed by a temperature shift to 39.8°C. Surviving cells were enumerated 5 days later and normalized against the number of cells observed in the absence of virus and CPA. Statistical analysis was performed using two-way ANOVA. Statistical values represent the difference between rRp450 plus CPA co-treatment and all other treatment groups. *p < 0.05; **p < 0.01; ***p < 0.001.
CPA Enhances rRp450 Efficacy In Vivo
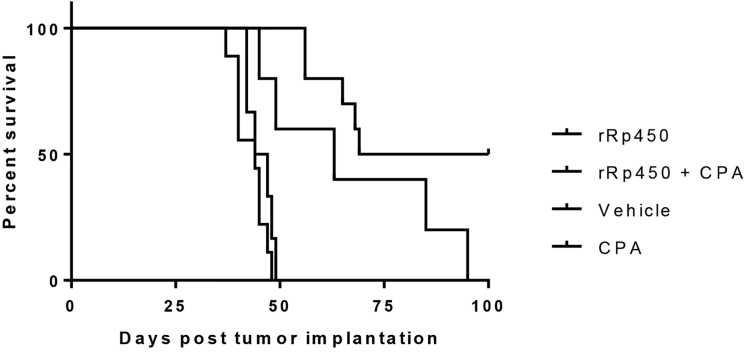
To confirm CPA potentiation of rRp450 cytotoxicity, we conducted a proof-of-principle study evaluating the efficacy of rRp450 and CPA co-treatment in an orthotopic model of BT-12. The addition of CPA to rRp450-treated animals increased the median survival to 84.5 days (range 56–100 days, n = 10), compared with a median survival of 63 days (range 45–95 days, n = 5) for animals treated with rRp450 alone (Figure 6). While co-treatment failed to reach strictly defined statistical significance, it did produce a strong trend toward increased survival (p = 0.0611), and 50% (5/10) of the animals displayed CR compared with no animals (0/5) in the rRp450-alone-treated group. Administration of CPA alone failed to increase animal survival over vehicle treatment. The median survival of animals treated with only CPA was 45.5 days (range 37–48 days, n = 9) compared with 44 days (range 42–49 days, n = 6) for vehicle-treated animals. Animals treated with either rRp450 or rRp450 plus CPA had a statistically significant increase in survival compared with vehicle- or CPA-treated animals (rRp450 versus vehicle, p = 0.0220; rRp450 versus CPA, p = 0.0035; rRp450 + CPA versus vehicle, p < 0.0001; rRp450 + CPA versus CPA, p < 0.0001).
Figure 6.
Cyclophosphamide Enhances rRp450-Mediated Survival in a Mouse Model of AT/RT
Kaplan-Meier survival analysis of mice implanted with BT-12 AT/RT cells treated 7 days later with 5 × 105 PFUs of rRp450. Cyclophosphamide (CPA), 2 mg dose, was administered 24 and 48 hr following virus treatment.
Discussion
With the recent FDA approval of the oHSV Imlygic (talimogene laherparepvec) for the treatment of melanoma, oncolytic viruses are poised for greater acceptance in treating other forms of cancer. Imlygic and similar oncolytic viruses have displayed remarkable specificity for infecting and destroying neoplastic cells while leaving the normal surrounding tissue undamaged.20, 21, 22 No dose-limiting toxicities have been reported for oHSVs that have advanced to clinical trials, and adverse events, if present, have typically been limited to mild to moderate flu-like symptoms.20, 22, 23 As such, these therapies may have considerable potential for pediatric brain tumor patients, because many tumors are refractory to conventional treatment modalities (i.e., surgical resection, chemotherapy, and radiation) that are associated with devastating and often permanent neurocognitive defects.
In the current study, we investigated the efficacy of the oHSV rRp450 against medulloblastoma and AT/RT cell lines and orthotopic xenograft models. The rRp450 virus was originally derived from the KOS HSV-1 strain and attenuated by replacement of the UL39 gene (encoding ICP6, the large subunit of ribonucleotide reductase) with the rat Cyp2b1 gene (encoding a cytochrome P450 enzyme, which functions as a convertase for oxazaphosphorine prodrugs). Compared with its wild-type counterpart, rRp450 replication in normal human cells is attenuated by three to four orders of magnitude. Also, whereas intracerebral inoculation of FVB/N mice with 1 × 105 PFUs of wild-type virus is uniformly fatal, doses as high as 1 × 108 PFUs of rRp450 are well tolerated.17 rRp450 is currently being studied in a phase I clinical trial for adult patients with primary liver cancer (NCT01071941).
We initially examined the efficacy of rRp450 against medulloblastoma and AT/RT cell lines in vitro and found that all cell lines were susceptible and permissive to virus infection. This efficacy was also observed in vivo, where we inoculated mice bearing established orthotopic medulloblastoma or AT/RT tumors with a single intratumoral dose of 1 × 106 PFUs of rRp450. Mice in these virus-treated groups displayed significantly prolonged survival times compared with their respective vehicle-treated controls, with several mice within each tumor group surviving to the experimental endpoint at day 100 (Figure 3). Importantly, none of the rRp450-treated mice displayed any adverse symptoms that could be attributed to the virus during the course of the study. The mice that did succumb in these treatment groups had prominent brain tumors at the time of necropsy, presumably because of pockets of viable tumor cells that escaped rRp450 infection or its associated mechanisms of tumor cell killing. In contrast, the mice that survived to the experiment endpoint were tumor-free as determined by H&E staining and pathologist review.
Our findings are closely aligned with previous results demonstrating increased survival of medulloblastoma xenografts models following treatment with mutant variant oHSVs.13, 14 In the study completed by Lasner and colleagues,13 D283med tumors were shown to be susceptible to the oHSV variant 1716. They observed persistent viral replication within tumors, as well as acute viral replication within murine cells of the brain. Recently, the oHSVs G207 and M002 were used to treat a panel of group 3 medulloblastoma xenograft models.14 Virus treatment led to a significant prolongation of survival in each xenograft model, with no reported instances of neurotoxicity. Moreover, the authors demonstrated that each oHSV could infect the CD133+ or CD15+ subpopulation of tumor-initiating cells, which are difficult for conventional therapies to effectively target and kill. Unlike the rRp450 virus used in our studies, the G207, M002, and 1716 viruses used in these previous studies were attenuated by deletions in both copies of their ICP34.5 genes. The ICP34.5 product, the so-called herpes virus neurovirulence factor, counteracts the inhibitory activities of interferon-induced protein kinase R (PKR) on virus replication.24 While the pathways leading to PKR activation are typically impaired in most cancer cells regardless, the loss of ICP34.5 can have a negative impact on the overall potency of an oHSV.25 Our in vitro cytotoxicity data suggest that rRp450 is more cytolytically potent than the ICP34.5-deleted vectors, but a direct head-to-head comparison would be required to confirm this claim.
Another potential benefit of the rRp450 virus is its inclusion of the aforementioned rat Cyp2b1 gene and the ability of its encoded cytochrome P450 to activate chemotherapeutic drugs such as CPA. Multiple studies have demonstrated that CPA enhances the cytotoxicity of rRp450-infected cells both in vitro and in vivo.17, 26, 27 Furthermore, it has been shown that infected cells can bioactivate CPA, and the active chemotherapeutic metabolites can diffuse and kill non-infected bystander tumor cells.26, 28, 29 Because CPA is commonly used in chemotherapy regimens to treat both medulloblastoma and AT/RT, we postulated that studies investigating its inclusion with rRp450 in the treatment regimen were rational and may yield similar improvements.30, 31, 32, 33, 34 To evaluate the sensitivity to CPA in the context of rRp450 infection, we infected medulloblastoma and AT/RT cells with rRp450 in the presence or absence of CPA. We demonstrated that addition of CPA to rRp450-infected cells significantly enhanced cell death (Figure 5). In contrast, CPA failed to enhance the cytotoxicity of the oHSV hrR3. HrR3, the parental virus to rRp450, is also ICP6 deficient, but does not encode the Cyp2b1 gene. Thus, this virus cannot activate the CPA prodrug. While we did not address the role of CPA in rRp450 replication, it has previously been demonstrated in multiple cell types that CPA has minimal to no impact on viral replication.15, 26, 27
The AT/RT cell line BT-12 was used in a proof-of-principle study to evaluate whether CPA could enhance rRp450 cytotoxicity in vivo. Because all cell lines are equally sensitive to rRp450 therapy in vivo (Figure 3), and equally sensitive to CPA addition to rRp450 in vitro (Figure 5), we chose BT-12 because therapeutic modalities for AT/RT patients are severely inadequate. The addition of CPA 24 and 48 hr following rRp450 administration increased the animals’ median survival compared with animals treated solely with rRp450 or CPA. More importantly, 50% of the animals treated with CPA and rRp450 were tumor-free at the end of the study. All of the animals in the CPA and rRp450 treatment groups succumbed to tumor disease.
In conclusion, this study provides evidence of oHSV rRp450 efficacy against human medulloblastoma and AT/RT cell lines and orthotopic xenograft models. To our knowledge, this is the first study to evaluate efficacy of oHSV in pre-clinical models of AT/RTs. While in vitro permissivity to virus infection and replication could greatly vary among cell lines, mouse survival for each tumor model benefited significantly from rRp450 treatment, with multiple animals determined to be tumor-free at the experiment endpoint. No virus-associated toxicity was observed in the treated animals despite direct intracerebral inoculation with an ICP34.5-intact oHSV. Taking advantage of the Cyp2b1 gene encoded in rRp450, we demonstrated that the addition of CPA enhanced the killing of rRp450 in all four cell lines examined. We also found CPA-mediated enhancement of rRp450 cytotoxicity in the BT-12 animal model of AT/RT. We observed an increased median survival and percentage of complete responders when CPA was added to the rRp450 treatment regimen. The capacity of rRp450-infected cells to convert CPA to its active metabolites provides an attractive approach to augment lytic death associated with the virus. Furthermore, this strategy allows increased local concentrations of active chemotherapy in the tumor microenvironment causing bystander killing to non-infected tumor cells.28, 29 This work provides further support that pediatric brain tumors may benefit from oncolytic virotherapy and suggests future studies involving CPA potentiation of rRp450 oncolysis are warranted.
Materials and Methods
Cell Culture
The Vero African green monkey kidney cell line was obtained from the American Type Culture Collection (ATCC). The human medulloblastoma cell lines D283med and D425med were acquired from the ATCC and Dr. Darrell Bigner (Duke University), respectively. The human AT/RT cell lines BT-12 and BT-16 were supplied by Dr. Peter Houghton (Greehey Children’s Cancer Research Institute, UT Health Science Center) and have been previously characterized.35 All cell lines were maintained in DMEM supplemented with 1% penicillin/streptomycin and 10%–20% fetal bovine serum (FBS; 10% serum concentration for Vero cells, 20% serum concentration for all other cells). Firefly luciferase-expressing variants of the medulloblastoma and AT/RT cell lines were generated as described previously.36 All cell lines were verified to be free of mycoplasma contamination by the MycoAlert Mycoplasma Detection Kit (Lonza) prior to use.
Oncolytic Herpes Virus rRp450
The ICP6-deficient oHSV vector hrR3 was a kind gift from Dr. Sandra Weller (University of Connecticut School of Medicine). The ICP6-deficient oHSV vector rRp450 (KOS strain) was a kind gift from Dr. Antonio Chiocca (Brigham and Women’s Hospital). rRp450 was derived from hrR3 by replacing the lacZ gene inserted into the ICP6 gene locus with the rat Cyp2b1 gene.15 Viral stocks were prepared with the 7b cell line in 10-layer Nunc Cell Factories (Thermo Fisher Scientific) at an MOI of 0.005 and purified under good laboratory practice-like conditions as described elsewhere.37, 38 Virus titers were determined in triplicate on 7b cells according to standard protocols.38
In Vitro Assessment of Cytotoxicity
Cells were plated in 96-well plates at a density of 1.0 × 104 cells per well for BT-12 and BT-16 or 2.5 × 104 cells per well for D283med and D425med. Twenty-four hours after seeding, the cells were infected for 2 hr with various MOIs of rRp450 in 0.1 mL of Opti-MEM I reduced serum medium (Thermo Fisher Scientific) at 37°C. Each MOI was assessed in eight replicates. At the end of the incubation period, the virus was removed, and the cells were maintained in DMEM containing 10% FBS. Uninfected cells were used as controls. Cell viability was quantified by the CellTiter 96 Aqueous One Solution Cell Proliferation MTS assay (Promega). The percentage of surviving cells was calculated by dividing the absorbance at 490 nm recorded from the infected wells by the absorbance from the uninfected wells corresponding to the same time point.
In Vitro CPA Activation and Cytotoxicity
For in vitro CPA bioactivation and enhancement of rRp450 cytotoxicity experiments, cells were plated in six-well plates at a density of 2.5 × 105 cells per well for BT-12 and BT-16 or 1.0 × 106 cells per well for D283med and D425med. Twenty-four hours later, media were removed, cells were washed with PBS, and cells were infected with either rRp450 or hrR3 at an MOI of 0.1 in 700 μL of DMEM containing 10% FBS. Following 1-hr incubation at 37°C with gentle shaking every 20 min, CPA (SANDOZ), reconstituted in DMEM containing 10% FBS, was added at a final concentration of 250 μM. The final volume per well was 2 mL. Cells were incubated an additional 3 hr at 37°C before viral replication was attenuated by transferring the plates to a separate 39.8°C incubator. Surviving cells were enumerated 5 days later. All experimental groups were performed in triplicate. Results represent the means of three biological replicates evaluated in triplicate. Statistical analysis was performed using two-way ANOVA (GraphPad Prism 7.00).
In Vitro Virus Replication Assays
Cells were plated in 12-well plates at a density of 1.5 × 105 cells per well for BT-12 and BT-16 or 5.0 × 105 cells per well for D283med and D425med. Cells were infected 24 hr later with rRp450 at an MOI of 0.01 in 100 μL of Opti-MEM. Following a 2-hr incubation at 37°C with gentle shaking every 20 min, both cells and supernatants were collected at 3, 24, 48, and 72 hr after initial infection. These samples were then freeze-thawed three times, centrifuged to pellet debris, and serially diluted onto previously plated Vero cells. Virus titers were determined by standard plaque assays. Results represent three biological replicates evaluated in triplicate.
Animal Studies
Orthotopic xenograft models of medulloblastoma and AT/RT were used as described previously.12, 36 In brief, 5 × 105 D283med, D425med, BT-12, or BT-16 cells suspended in 2 μL of Opti-MEM were implanted into the caudate nucleus of 5-week-old Hsd:Athymic Nude-Foxn1nu mice (Envigo). Bioluminescent imaging was performed 1 week later with the Xenogen IVIS Spectrum (Caliper Life Sciences) to confirm that tumor burdens were roughly equivalent and that no dissemination of tumor into the spinal canal had occurred (data not shown). Animals that failed to meet these criteria were excluded from further analysis. Treatment with rRp450 (1 × 106 PFUs/dose) delivered in 5 μl total volume or an equivalent volume of Opti-MEM was administered at this time using the same injection coordinates as tumor cell implantation. The animals were then monitored for survival and euthanized if they developed neurological deficits such as hemiparesis or lethargy. At the time of necropsy, brains were collected, fixed overnight with 10% formalin, paraffin embedded, cut into 5-μM tissue sections, and stained with H&E.
For animal studies evaluating rRp450 and CPA co-treatment, 5 × 105 BT-12 cells suspended in 2 μL of Opti-MEM were stereotaxically implanted as described above. Bioluminescent imaging was performed 1 week later to confirm equal tumor burdens and exclude from further studies animals that exhibited disseminated disease. Treatment with rRp450 (5 × 105 PFUs/dose) delivered in 5 μl total volume or an equivalent volume of Opti-MEM was administered at this time using the same injection coordinates as tumor cell implantation. 24 and 48 hr following virus injection, 2 mg of CPA, reconstituted daily in 0.9% sodium chloride (Hospira) or 0.9% sodium chloride, was administered to animals via intraperitoneal (i.p.) injection. Animals were monitored and brains processed as described above. All animal experiments were approved by the Nationwide Children’s Hospital Institutional Animal Care and Use Committee.
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded tissues in an automated fashion using the BondMAX IHC system (Leica Microsystems) with an antibody targeted against HSV-1 (rabbit polyclonal antibody [PP108AA] from Biocare Medical). The prediluted antibody is ready to use, and no epitope retrieval was needed. Signal was detected using the Refine Polymer, 3,3'-diaminobenzidine (DAB; Leica Microsystems). Negative controls were obtained by substituting the primary antibody with a Universal Negative Control Serum (Biocare Medical). The sections were reviewed by the neuropathologist author (C.R.P.). Tumors were harvested post-virus treatment; BT-12 (14 days), BT-16 (21 days), D283med (50 days), and D425med (28 days) staining was performed.
Statistical Analysis
Kaplan-Meier survival curves were generated with the GraphPad Prism 7.00 software. Statistical significance (p < 0.05) between the PBS- and rRp450-treated groups was determined using the log rank test.
Author Contributions
A.W.S., B.J.H., T.P.C., E.M.J., and J.R.L. were involved in the scientific design of study. A.W.S., B.J.H., C.R.P., K.B.H., and T.P.C. developed the methodology utilized. A.W.S., B.J.H., and C.R.P. performed the studies. C.R.P., T.P.C., E.M.J., and J.R.L. provided materials and funding for the experiments performed. A.W.S., C.R.P., K.B.H., and T.P.C. analyzed the data. A.W.S., B.J.H., C.R.P., and K.B.H. prepared the manuscript. E.M.J and J.R.L. contributed equally as senior authors. All authors have reviewed the manuscript.
Conflicts of Interest
The authors report no conflict of interest.
Acknowledgments
The authors would like to acknowledge Dr. Darrell Bigner (Duke University, Durham, NC, USA) for the D425med cell line and Dr. Peter Houghton (Greehey Children’s Cancer Research Institute, UT Health Science Center, San Antonio, TX) for the human AT/RT cell lines BT-12 and BT-16. The authors would also like to thank Dr. E. Antonio Chiocca (Brigham and Women’s Hospital, Boston, MA) for the oHSV rRp450 and Dr. Sandra Weller (University of Connecticut School of Medicine, Farmington, CT) for the oHSV hrR3. Funding sources for this work include start-up funding from Nationwide Children’s Hospital (to E.M.J. and J.R.L.).
References
- 1.Skowron P., Ramaswamy V., Taylor M.D. Genetic and molecular alterations across medulloblastoma subgroups. J. Mol. Med. (Berl.) 2015;93:1075–1084. doi: 10.1007/s00109-015-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui C.H., Gajjar A.J., Kane J.R., Qaddoumi I.A., Pappo A.S. Challenging issues in pediatric oncology. Nat. Rev. Clin. Oncol. 2011;8:540–549. doi: 10.1038/nrclinonc.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSouza R.M., Jones B.R., Lowis S.P., Kurian K.M. Pediatric medulloblastoma - update on molecular classification driving targeted therapies. Front. Oncol. 2014;4:176. doi: 10.3389/fonc.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajjar A.J., Robinson G.W. Medulloblastoma-translating discoveries from the bench to the bedside. Nat. Rev. Clin. Oncol. 2014;11:714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 5.Versteege I., Sévenet N., Lange J., Rousseau-Merck M.F., Ambros P., Handgretinger R., Aurias A., Delattre O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 6.Morgenstern D.A., Gibson S., Brown T., Sebire N.J., Anderson J. Clinical and pathological features of paediatric malignant rhabdoid tumours. Pediatr. Blood Cancer. 2010;54:29–34. doi: 10.1002/pbc.22231. [DOI] [PubMed] [Google Scholar]
- 7.Pol J., Bloy N., Obrist F., Eggermont A., Galon J., Cremer I., Erbs P., Limacher J.M., Preville X., Zitvogel L. Trial watch: oncolytic viruses for cancer therapy. OncoImmunology. 2014;3:e28694. doi: 10.4161/onci.28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolgin E. Oncolytic viruses get a boost with first FDA-approval recommendation. Nat. Rev. Drug Discov. 2015;14:369–371. doi: 10.1038/nrd4643. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann J.K., Chiocca E.A. Oncolytic virotherapy for gliomas: steps toward the future. CNS Oncol. 2013;2:389–392. doi: 10.2217/cns.13.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly O.G., Errington-Mais F., Prestwich R., Harrington K., Pandha H., Vile R., Melcher A.A. Recent clinical experience with oncolytic viruses. Curr. Pharm. Biotechnol. 2012;13:1834–1841. doi: 10.2174/138920112800958904. [DOI] [PubMed] [Google Scholar]
- 11.Studebaker A.W., Hutzen B., Pierson C.R., Russell S.J., Galanis E., Raffel C. Oncolytic measles virus prolongs survival in a murine model of cerebral spinal fluid-disseminated medulloblastoma. Neuro-oncol. 2012;14:459–470. doi: 10.1093/neuonc/nor231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studebaker A.W., Hutzen B., Pierson C.R., Shaffer T.A., Raffel C., Jackson E.M. Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors. Neuro-oncol. 2015;17:1568–1577. doi: 10.1093/neuonc/nov058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasner T.M., Kesari S., Brown S.M., Lee V.M., Fraser N.W., Trojanowski J.Q. Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J. Neuropathol. Exp. Neurol. 1996;55:1259–1269. doi: 10.1097/00005072-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Friedman G.K., Moore B.P., Nan L., Kelly V.M., Etminan T., Langford C.P., Xu H., Han X., Markert J.M., Beierle E.A., Gillespie G.Y. Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro-oncol. 2016;18:227–235. doi: 10.1093/neuonc/nov123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chase M., Chung R.Y., Chiocca E.A. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 16.Clarke L., Waxman D.J. Oxidative metabolism of cyclophosphamide: identification of the hepatic monooxygenase catalysts of drug activation. Cancer Res. 1989;49:2344–2350. [PubMed] [Google Scholar]
- 17.Currier M.A., Gillespie R.A., Sawtell N.M., Mahller Y.Y., Stroup G., Collins M.H., Kambara H., Chiocca E.A., Cripe T.P. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol. Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein D.J., Weller S.K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein D.J., Weller S.K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J.C., Coffin R.S., Davis C.J., Graham N.J., Groves N., Guest P.J., Harrington K.J., James N.D., Love C.A., McNeish I. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman H.L., Kim D.W., DeRaffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 22.Nomura N., Kasuya H., Watanabe I., Shikano T., Shirota T., Misawa M., Sugimoto H., Kanazumi N., Nomoto S., Takeda S., Nakao A. Considerations for intravascular administration of oncolytic herpes virus for the treatment of multiple liver metastases. Cancer Chemother. Pharmacol. 2009;63:321–330. doi: 10.1007/s00280-008-0742-6. [DOI] [PubMed] [Google Scholar]
- 23.Kaur B., Chiocca E.A., Cripe T.P. Oncolytic HSV-1 virotherapy: clinical experience and opportunities for progress. Curr. Pharm. Biotechnol. 2012;13:1842–1851. doi: 10.2174/138920112800958814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou J., Chen J.J., Gross M., Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanai R., Zaupa C., Sgubin D., Antoszczyk S.J., Martuza R.L., Wakimoto H., Rabkin S.D. Effect of γ34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J. Virol. 2012;86:4420–4431. doi: 10.1128/JVI.00017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlik T.M., Nakamura H., Mullen J.T., Kasuya H., Yoon S.S., Chandrasekhar S., Chiocca E.A., Tanabe K.K. Prodrug bioactivation and oncolysis of diffuse liver metastases by a herpes simplex virus 1 mutant that expresses the CYP2B1 transgene. Cancer. 2002;95:1171–1181. doi: 10.1002/cncr.10776. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik T.M., Nakamura H., Yoon S.S., Mullen J.T., Chandrasekhar S., Chiocca E.A., Tanabe K.K. Oncolysis of diffuse hepatocellular carcinoma by intravascular administration of a replication-competent, genetically engineered herpesvirus. Cancer Res. 2000;60:2790–2795. [PubMed] [Google Scholar]
- 28.Freeman S.M., Abboud C.N., Whartenby K.A., Packman C.H., Koeplin D.S., Moolten F.L., Abraham G.N. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 29.Bi W.L., Parysek L.M., Warnick R., Stambrook P.J. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum. Gene Ther. 1993;4:725–731. doi: 10.1089/hum.1993.4.6-725. [DOI] [PubMed] [Google Scholar]
- 30.Hagler S., Currimbhoy Z.E., Tinsley M. Cerebellar medulloblastoma chemotherapeutic remission with vincristine cyclophosphamide and methotrexate. Cancer. 1968;21:912–919. doi: 10.1002/1097-0142(196805)21:5<912::aid-cncr2820210514>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Friedman H.S., Mahaley M.S., Jr., Schold S.C., Jr., Vick N.A., Falletta J.M., Bullard D.E., D’Souza B.J., Khandekar J.D., Lew S., Oakes W.J. Efficacy of vincristine and cyclophosphamide in the therapy of recurrent medulloblastoma. Neurosurgery. 1986;18:335–340. doi: 10.1227/00006123-198603000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Friedman H.S., Bigner S.H., Bigner D.D. Cyclophosphamide therapy of medulloblastoma: from the laboratory to the clinic and back again (and again and again) J. Neurooncol. 1995;24:103–108. doi: 10.1007/BF01052667. [DOI] [PubMed] [Google Scholar]
- 33.Kim H., Kang H.J., Lee J.W., Park J.D., Park K.D., Shin H.Y., Ahn H.S. Irinotecan, vincristine, cisplatin, cyclophosphamide, and etoposide for refractory or relapsed medulloblastoma/PNET in pediatric patients. Childs Nerv. Syst. 2013;29:1851–1858. doi: 10.1007/s00381-013-2163-z. [DOI] [PubMed] [Google Scholar]
- 34.Gotti G., Biassoni V., Schiavello E., Spreafico F., Antonelli M., Calareso G., Pecori E., Gandola L., Massimino M. A case of relapsing spinal atypical teratoid/rhabdoid tumor (AT/RT) responding to vinorelbine, cyclophosphamide, and celecoxib. Childs Nerv. Syst. 2015;31:1621–1623. doi: 10.1007/s00381-015-2755-x. [DOI] [PubMed] [Google Scholar]
- 35.D’cunja J., Shalaby T., Rivera P., von Büren A., Patti R., Heppner F.L., Arcaro A., Rorke-Adams L.B., Phillips P.C., Grotzer M.A. Antisense treatment of IGF-IR induces apoptosis and enhances chemosensitivity in central nervous system atypical teratoid/rhabdoid tumours cells. Eur. J. Cancer. 2007;43:1581–1589. doi: 10.1016/j.ejca.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Studebaker A.W., Kreofsky C.R., Pierson C.R., Russell S.J., Galanis E., Raffel C. Treatment of medulloblastoma with a modified measles virus. Neuro-oncol. 2010;12:1034–1042. doi: 10.1093/neuonc/noq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krisky D.M., Wolfe D., Goins W.F., Marconi P.C., Ramakrishnan R., Mata M., Rouse R.J., Fink D.J., Glorioso J.C. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 38.Goins W.F., Huang S., Cohen J.B., Glorioso J.C. Engineering HSV-1 vectors for gene therapy. Methods Mol. Biol. 2014;1144:63–79. doi: 10.1007/978-1-4939-0428-0_5. [DOI] [PubMed] [Google Scholar]