Abstract
The Reward Hypersensitivity Model of bipolar disorder argues that hypersensitivity to reward-relevant cues characterizes risk for hypo/mania. This hypersensitivity leads to increased goal-directed motivation during reward-relevant life events that, in the extreme, is reflected in hypo/manic symptoms. In line with this perspective, individuals with bipolar disorder display elevated activation in a cortico-striatal reward circuit including the nucleus accumbens (NAcc) and medial orbitofrontal cortex (mOFC). To date, however, research on reward-related neural circuitry underlying bipolar symptoms focuses on syndromal bipolar disorder (bipolar I, bipolar II), and typically examines neural regions in isolation of each other. Accordingly, this study examines the relationship between subsyndromal hypo/mania proneness and structural connectivity between the NAcc and both the mOFC and amygdala in a medication-free sample. Fifty-four community participants completed diffusion-weighted imaging and a self-report measure of bipolar risk (hypo/mania proneness). As predicted, elevated structural connectivity between the NAcc and both the mOFC and amygdala were associated with elevated hypo/mania proneness. This relationship was specific to NAcc-centered reward connectivity, as there was no relationship between hypo/mania proneness and either whole-brain or cortico-amygdala connectivity. Results suggest that reward-relevant tractography from cortical (mOFC) and subcortical (amygdala) regions amplify NAcc-centered reward processing in bipolar risk.
Keywords: hypomania/mania, diffusion tensor imaging, reward processing, nucleus accumbens, amygdala, medial orbitofrontal cortex
Introduction
Bipolar disorder is associated with significant work impairment, high rates of divorce and substance abuse, a 10-year earlier mortality rate, and elevated suicidality (Angst et al., 2002; Kupfer et al., 2002). Within the bipolar disorder category, several disorders form a spectrum of severity from the milder variant of cyclothymia, to bipolar II disorder, to the most severe, bipolar I disorder (Birmaher et al., 2009; Alloy et al., 2012b). Importantly, this spectrum of severity extends to pre-clinical symptoms of bipolar disorder, as well as temperamental risk factors, as individuals with subsyndromal variants of the illness are at elevated risk for later developing bipolar disorder (Kwapil et al., 2000).
The Reward Hypersensitivity Model of bipolar disorder argues that abnormalities in reward processing are central to the pathophysiology of bipolar disorder (Johnson et al., 2012; Alloy et al., 2015). Reward processing relates to the value individuals place on potential rewards, the perceived probability of reward receipt, and the mechanisms by which an individual processes reward-relevant cues. In line with the Reward Hypersensitivity Model is growing evidence that risk for bipolar disorder is characterized by abnormalities in reward-related brain function (see Nusslock et al., 2014, for review). To date, however, research on reward-related neural circuitry underlying bipolar disorder largely focuses on syndromal (i.e. bipolar I and bipolar II disorder), as opposed to subsyndromal, bipolar symptoms. Examining subsyndromal dimensions helps dissociate whether neural signatures of bipolar symptoms are pre-existing risk factors, or consequences of the illness. Examining subsyndromal dimensions also negates the influence of psychotropic medications, many of which directly modulate reward-related neural circuitry (Abler et al., 2007). Additionally, research on reward-related neural circuitry in bipolar disorder typically focuses on neural regions in isolation of each other. This approach neglects important functional and structural connections between neural regions in modulating the relationship between reward sensitivity and risk for hypomania or mania (hypo/mania). To begin to address these issues, this study employed diffusion-weighted imaging (DWI) to examine the relationship between individual differences in risk for bipolar symptoms (i.e. subsyndromal hypo/mania proneness) and structural connectivity between prefrontal [i.e. medial orbitofrontal cortex (mOFC)] and subcortical [i.e. nucleus accumbens (NAcc), amygdala] regions involved in reward processing in a medication-free sample of participants who have not yet developed a bipolar spectrum disorder.
Reward hypersensitivity model of bipolar disorder
The Reward Hypersensitivity Model proposes that a core mechanism underlying risk for hypo/mania is a hypersensitivity to cues of possible reward (Johnson et al., 2012; Nusslock et al., 2014; Alloy et al., 2015). Individuals with bipolar disorder are at risk for experiencing an excessive increase in approach motivation during life events involving the pursuit or attainment of reward. In the extreme, this increase in motivation is reflected in hypo/manic symptoms such as elevated mood, decreased need for sleep, increased goal-directed activity, grandiosity, and distractibility. Thus, according to the Reward Hypersensitivity Model, symptoms of hypo/mania involve extreme expressions along core brain-behavior dimensions of positive valence, reward-processing and incentive motivation. In line with this perspective, individuals with bipolar I disorder (Meyer et al., 2001; Salavert et al., 2007), and either bipolar II disorder or cyclothymia (Alloy et al., 2008) self-report higher levels of reward sensitivity than healthy controls, and elevated reward sensitivity is associated with a greater likelihood of progressing to a more severe bipolar diagnosis (Alloy et al., 2012a). Even in remission, individuals with bipolar spectrum disorders exhibit higher self-reported reward sensitivity (Salavert et al., 2007; Alloy et al., 2008), suggesting that elevated reward sensitivity is independent of mood-related biases. Growing evidence suggests that elevated self-reported reward sensitivity is a pre-existing risk factor for bipolar disorder, as opposed to a consequence of the illness. Individuals prone to hypo/manic symptoms, but who have not yet developed the disorder, self-report higher levels of reward sensitivity than healthy controls (Meyer et al., 1999), and elevated self-reported reward sensitivity is associated with a greater likelihood of prospectively developing a first onset of a bipolar spectrum episode (Alloy et al., 2012b).
At the neural level, reward processing has been linked to an integrated cortico-striatal neural circuit involving the NAcc (a subnuclei of the ventral striatum) and the mOFC, among other regions (Haber and Knutson, 2010). The NAcc is involved in processing both primary (e.g. food) and secondary (e.g. monetary) rewards, and plays an important role in incentive motivation and facilitating reward-directed behavior (Floresco, 2015). The mOFC is important for encoding reward value, assessing the probability of reward receipt, and maintaining goal oriented behavior (Zald et al., 2014; Samanez-Larkin and Knutson, 2015). The mOFC is also a critical node in a top-down regulatory feedback loop, involving the ventromedial prefrontal cortex and the dorsal anterior cingulate cortex among other regions, which modulates both approach and avoidant motivational states (Phillips et al., 2008; Haber and Behrens, 2014). Subcortical regions, including the amygdala, have also been implicated in reward processing (Wassum and Izquierdo, 2015). Importantly, excitatory input from the amygdala to the NAcc has been shown to directly modulate reward processing during both reward anticipation and outcome (Janak and Tye, 2015), highlighting the importance of the amygdala in reward-related emotions.
In line with the Reward Hypersensitivity Model, growing evidence indicates that bipolar disorder and, in particular, risk for hypo/manic symptoms are associated with structural and functional abnormalities in the cortico-striatal circuit (see Nusslock et al., 2014, for review). Structural imaging studies report abnormalities in prefrontal volume (Lopez-Larson et al., 2002) and increased striatal volume (Strakowski et al., 2002) in individuals with bipolar disorder. Functional imaging studies document abnormally elevated striatal, OFC, and amygdala activation to reward-relevant cues in bipolar disorder. Elevated reward-related neural activation has been observed across bipolar mood states (i.e. mania and remission; Abler et al., 2008; Bermpohl et al., 2009, 2010; Nusslock et al., 2012; Caseras et al., 2013), and across the bipolar spectrum (i.e. bipolar I and bipolar II disorder; Abler et al., 2008; Bermpohl et al., 2010; Nusslock et al., 2012; Caseras et al., 2013; Chase et al., 2013).
Preliminary data indicate that abnormalities in cortico-striatal neural circuitry reflect a pre-existing risk factor for bipolar spectrum disorders as opposed to a consequence of the illness. Gray matter deficits in the ventral striatum are present in individuals at genetic risk for bipolar disorder but who have not yet developed the illness (McDonald et al., 2004). Research employing fMRI reward paradigms indicates that both individuals with elevated hypo/mania proneness (O’Sullivan et al., 2011) and individuals with a hypo/manic temperament who have not yet developed a bipolar spectrum disorder (Harada et al., 2013) display elevated OFC and striatal activation during reward processing compared with healthy controls. Comparable results have been reported in neurophysiological studies, in which individuals with elevated hypo/mania proneness, but not a bipolar spectrum diagnosis, displayed greater reward- or approach-related electroencephalogram activity than healthy controls (Harmon-Jones et al., 2002; Peterson and Harmon-Jones, 2008; Mason et al., 2012).
A connectivity perspective of reward-related neural circuitry and risk for bipolar disorder
Research on reward-related brain function in bipolar disorder typically focuses on neural regions in isolation of each other (although see Redlich et al., 2015; Satterthwaite et al., 2015, for exceptions), and no studies examine the relationship between subsyndromal risk for bipolar disorder and either functional or structural connectivity within the cortico-striatal reward circuit. This approach is limited by the fact that risk for psychiatric disorders may be characterized as much by abnormalities in the connections between neural regions as they are by abnormalities in any one specific area of the brain. To begin to address this issue, as well as to extend research on reward-related neural circuitry in subsyndromal risk for bipolar disorder, this study used DWI to examine the relationship between cortico-striatal structural connectivity and proneness to bipolar symptoms. DWI assesses the mobility of water molecules in brain tissue, which is affected by microstructural qualities such as fiber density, axonal diameter, and myelination in specific white matter tracts. DWI can also be used to calculate tractography, which estimates white matter tracts that connect gray matter areas and the strength of these connections (Jones et al., 2013). Proneness to bipolar symptoms was measured in this study using the Hypomanic Personality Scale (HPS; Eckblad and Chapman, 1986). In developing the HPS, Eckblad and Chapman (1986) referred to ‘hypomanic personality’ as an ‘overactive, gregarious style in which episodes of hypomanic euphoria are likely to occur’. They report that ∼77% of high HPS scorers met lifetime criteria for a hypo/manic episode, whereas no control participants received a diagnosis. In a 10-year longitudinal study, elevated HPS score prospectively predicted first onset of hypo/manic episodes (Kwapil et al., 2000), demonstrating that the HPS is a good index of risk for bipolar disorder. Furthermore, both neurophysiological studies (Harmon-Jones et al., 2002; Peterson and Harmon-Jones, 2008; Mason et al., 2012) and functional neuroimaging research using an established fMRI reward paradigm (O’Sullivan et al., 2011) report that elevated HPS scores are associated with elevated reward-related neural activation. This study extends this work by examining for the first time the relationship between HPS scores and structural connectivity within the cortico-striatal reward circuit.
Analyses centered on both cortical and subcortical connectivity with the NAcc. The NAcc is a hub of reward processing that integrates emotional and cognitive input to modulate goal-directed behavior (Haber and Knutson, 2010; Floresco, 2015). Although it is only a single node in a larger neural network, the NAcc is a unique node with respect to the diversity of cortical and subcortical input it receives to modulate incentive processing (Haber and Knutson, 2010). Thus, the NAcc is an optimal seed region for analyses in this study.
With respect to cortical connectivity, we focused on white matter tracts between the mOFC and NAcc for a number of reasons. First, the mOFC is important for encoding reward value and assessing the probability of reward receipt (Haber and Knutson, 2010). Second, both non-human animal and human research document the role of excitatory white matter tracts from the mOFC to the NAcc in modulating or upregulating reward valuation (Haber and Knutson, 2010; Sesack and Grace, 2010). Third, individual differences in functional connectivity between the mOFC and NAcc relate to reward sensitivity (Costumero et al., 2013), reward dependence (Cohen et al., 2009), and reward-based learning (Haber and Behrens, 2014), all of which relate to risk for bipolar spectrum disorders (Alloy et al., 2015). Finally, individuals with bipolar I disorder display abnormally elevated mOFC activation during reward-anticipation in fMRI studies (Nusslock et al., 2014). Thus, an abnormal coupling between the mOFC and NAcc during reward processing may be central to the pathophysiology of risk for bipolar disorder. Specifically, we predict that proneness to hypo/mania will be associated with elevated structural connectivity between the mOFC and the NAcc, suggesting that individuals at risk for bipolar disorder may engage the mOFC in a manner that up-regulates or amplifies NAcc-based reward processing.
With respect to subcortical connectivity, analyses focused on tracts between the amygdala and the NAcc. In addition to playing a central role in threat-processing and aversive learning (Janak and Tye, 2015), the amygdala has also been implicated in reward learning, appetitive motivation and goal-directed behavior (Wassum and Izquierdo, 2015). Post-mortem micro-circuitry studies document direct excitatory input from the basolateral amygdala to the NAcc (Haber and Knutson, 2010; Sesack and Grace, 2010). The basolateral amygdala provides input to the NAcc regarding reward prediction in learning paradigms, and the central nucleus of the amygdala marks reward outcome to guide the modulation of behaviors through the NAcc (Janak and Tye, 2015). Integrating expected and actual reward values is particularly aberrant in the NAcc of bipolar individuals, as demonstrated by time series analyses of reward responsivity in bipolar mania (Abler et al., 2008). In non-clinical samples, functional connectivity between the amygdala and NAcc relates to novelty seeking (Cohen et al., 2009) and reward sensitivity (Costumero et al., 2013), both of which are implicated in risk for bipolar disorder (Alloy et al., 2015). Finally, preliminary data indicate that hypo/manic individuals display abnormally elevated amygdala activation to positive stimuli (Bermpohl et al., 2009). Accordingly, we predict that proneness to hypo/mania will be associated with elevated structural connectivity between the amygdala and the NAcc. We further propose that enhanced connectivity between the amygdala and NAcc may reflect a subcortical amplification circuit through which the amygdala enhances reward processing in the NAcc; in the extreme, may elevate risk for hypo/manic symptoms.
In addition to examining the single order relationships between hypo/mania proneness and both NAcc-amygdala and NAcc-mOFC connectivity, we also conducted a multiple regression model with both NAcc-amygdala and NAcc-mOFC connectivity scores entered simultaneously as predictors of HPS scores to assess whether each tract provides unique vs shared contributions to hypo/mania proneness.
Finally, we implemented two strategies to assess whether hypo/mania proneness was specifically related to connectivity within the NAcc reward circuit or structural connectivity more generally. First, we examined the relationship between hypo/mania proneness and whole-brain white matter integrity. Second, we examined the relationship between hypo/mania proneness and cortico-amygdala structural connectivity within the uncinate fasciculus. The uncinate fasciculus is a white matter tract between the OFC and amygdala that is central to regulating threat-processing, and appears to be unrelated to reward-based learning (see Wassum and Izquierdo, 2015 for review). Reduced structural connectivity within the uncinate fasciculus is associated with poor emotion regulation capacity of negative affect, and elevated symptoms of general distress in both unipolar depression (Tayloret al., 2007; Aghajani et al., 2014) and anxiety (Kim and Whalen, 2009; Phan et al., 2009). We predict that hypo/mania proneness will be uniquely related to connectivity within the NAcc reward circuit and unrelated to both whole-brain white matter integrity and connectivity related to more negative emotionality (i.e. uncinate fasciculus). Results in line with this prediction will have important implications for establishing the specificity of NAcc-centered connectivity, and abnormalities in reward processing more generally, in risk for bipolar disorder.
Materials and methods
Participants
Data were collected on 54 (26 female) community participants from the Chicago area. Participants were on average 21.2-years old (range: 18–26; s.d.: 1.75 years) and ethnically diverse (Caucasian = 42%; Asian = 25%, Multiracial = 22%, African American = 11%). At screening, participants had no self-reported history of a psychiatric or neurological disorder,1 were not taking psychotropic medication, and were right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). This study was approved by the Institutional Review Board at Northwestern University.
Procedure
After completing the HPS (Eckblad and Chapman, 1986), participants completed DWI to assess structural connectivity. Participants were provided with financial compensation for their participation at the end of the scanning session.
Proneness to hypo/mania assessment
Individual differences in hypo/mania proneness were measured using the HPS (Eckblad and Chapman, 1986), which identifies individuals at risk for bipolar disorder. The HPS contains 48 true–false items that include positively coded items, such as ‘I frequently find that my thoughts are racing’, and negatively coded items, such as ‘I consider myself to be pretty much an average kind of person’. In line with convention (Eckblad and Chapman, 1986), we weighted each item equally into a single total summary score. The HPS has a published coefficient alpha reliability of 0.87 in an undergraduate sample (n = 1519), and a test-retest reliability of 0.81 after an interval of 15 weeks (n = 89; Eckblad and Chapman, 1986). Elevated HPS score have been shown to prospectively predict bipolar disorder onset over a 10-year follow-up period (Kwapil et al., 2000), demonstrating that the HPS is a good index of risk for bipolar disorder. Both neurophysiological studies (Harmon-Jones et al., 2002; Peterson and Harmon-Jones, 2008; Mason et al., 2012) and functional neuroimaging research using an established fMRI reward paradigm (O’Sullivan et al., 2011) report that elevated HPS scores are associated with elevated reward-related neural activation.
HPS scores ranged from 8 to 37 in the present sample, with a mean score of 18.27 (s.d. = 6.55). This represents a good distribution of scores for analyses of individual differences that is consistent with prior studies with the HPS in non-clinical samples (Eckblad and Chapman, 1986; Kwapil et al., 2000; Harmon-Jones et al., 2002, 2008). Furthermore, the range and variation of HPS scores in the present sample overlaps considerably with previous research using the HPS with bipolar spectrum participants [Walsh et al., 2015; range: 10–42; mean = 20.1 (s.d. = 10.1)]. One participant in this study was classified as ultra-high risk according to prior cross-sectional and prospective studies with the HPS (HPS score > 36; Eckblad and Chapman, 1986; Kwapil et al., 2000). Cronbach’s alpha in this study was 0.90.
Data acquisition and processing
Structural i mages . A high-resolution MPRAGE, diffusion-weighted data, and echo planar images were acquired on a Siemens 3T Trio scanner with a 32-channel head coil. The MPRAGE was acquired using echo planar imaging (TR = 2300, TE = 2.91, 176 sagittal slices, resolution 1.0 × 1.0 × 1.0 mm). Structural images were then reconstructed using FreeSurfer’s reconstruction and auto-segmentation program, which strips the skull from the image, normalizes the field intensity, uses the remaining intensity values to create white matter and gray matter edges, and moves the Desikan-Killany Atlas into the subjects’ native space (Fischl and Dale, 2000). These labels and white matter edges were visually inspected to ensure they were aligned with the participant’s anatomy.
Diffusion -w eighted i maging. Diffusion-weighted data were acquired using echo planar imaging (TR = 9.6 s, TE = 87 ms, 65 axial slices, resolution 2.0 × 2.0 × 2.0 mm). Diffusion weighting was isotropically distributed across 64 directions (b-value = 1000 s/mm2). By using this high angular distribution of diffusion-weighting directions the signal to noise ratio was increased and the directional bias was decreased resulting in a robust probabilistic density estimate. A single volume was collected at the beginning of the diffusion series without weighting (b-value = 1000 s/mm2) for use as an anatomical reference for image alignment. These images were processed using the Tracts Constrained by Underlying Anatomy pipeline (Yendiki et al., 2011), which combines processing steps from FDT (FSL software for diffusion tensor analysis; Behrens et al., 2003) with the surface, volume, and automatic segmentation derived from the FreeSurfer software package (Fischl et al., 2002, Fischl and Dale 2000).
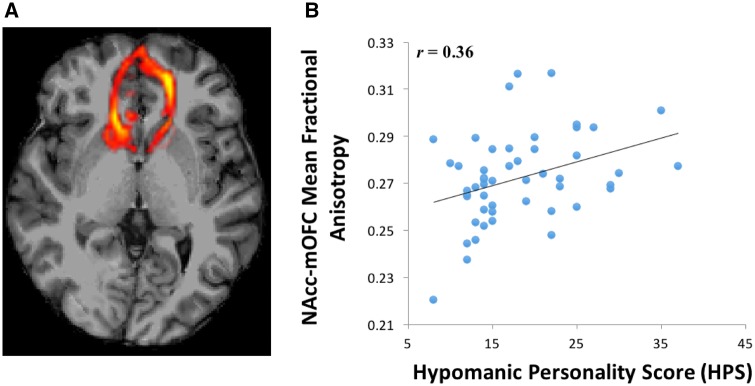
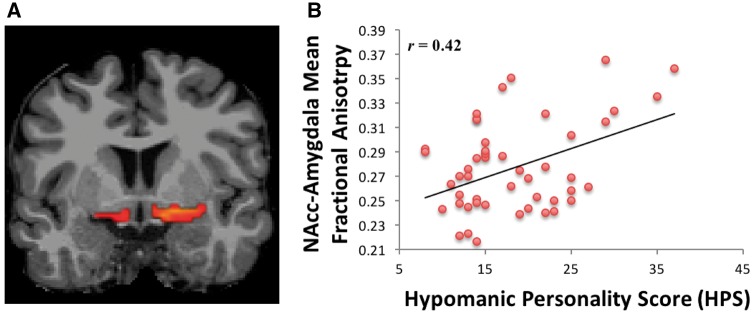
Diffusion pre - processing. Diffusion pre-processing involved correcting for field heterogeneities detected in the initial unweighted image, eddy current correction, masking the brain, and extracting the skull. FreeSurfer’s border based registration (bbregister) created a transformation matrix by registering the high resolution MPRAGE with the extracted unweighted brain image. This transformation matrix was then applied to the cortical and subcortical automatic segmentation labels, which moved the labels from MPRAGE to the diffusion matrix dimensions and created the seed regions for the tractography. The labels were then visually inspected for each subject. Individually defined masks were calculated unilaterally for the NAcc, amygdala, mOFC and OFC (used to calculate the uncinate fasciculus tract). These hypotheses-driven seed regions were used for diffusion weighted probabilistic tractography. Probabilistic tracts were created from 5000 bootstrapped, Euler streamlined, and fractional anisotropy constrained paths, and thresholded to leave tracts above the 97th percentile. These probabilistic tracts were then combined into bilateral masks because we had no a priori predictions regarding laterality, and also to minimize Type I error. Mean fractional anisotropy was extracted from the NAcc-mOFC (Figure 1A), NAcc-amygdala (Figure 2A), and amygdala-OFC (i.e. uncinate fasciculus) probabilistic tracts (Cohen et al., 2009; Samanez-Larkin et al., 2010). Mean fractional anisotropy was calculated for a whole-brain mask to assess the relationship between whole-brain white matter integrity and hypo/mania proneness. Mean fractional anisotropy values across all voxels within each tract and within the whole-brain mask were extracted from FreeSurfer and imported into R (version 3.1.2) for analyses (http://cran.r-project.org/). The logic of Fisher’s protected t-tests (Cohen et al., 2003) was employed to minimize familywise error rate, which requires a significant omnibus test in order to proceed to or interpret pairwise follow-up tests.
Fig. 1.
(A) Probabilistic tractography between the NAcc and mOFC. (B) Relationship between HPS scores and NAcc–mOFC fractional anisotropy.
Fig. 2.
(A) Probabilistic tractography between the NAcc and amygdala. (B) Relationship between HPS scores and NAcc–amygdala fractional anisotropy.
Results
Demographic analyses
Age did not correlate with either HPS scores or mean fractional anisotropy in any of the specified tracts, all rs < 0.08, Ps > 0.55. There were gender differences, as males displayed a non-significant trend towards elevated HPS scores, t(52) = 1.76, P = 0.08 [Mean HPS scores for Males = 19.75 (s.d. = 7.26); Mean HPS scores for Females = 16.69 (s.d. = 5.39)], and males displayed elevated fractional anisotropy in both the NAcc-amygdala, t(52) = 2.62, P = 0.01 and NAcc-mOFC, t(52) = 5.03, P < 0.001, tracts. Importantly, however, all of the subsequent findings remained significant after controlling for gender, and a comparison of model fit indicated that there were no significant differences in the relationship between HPS scores and either the NAcc-mOFC tract or the NAcc-amygdala tract based on whether or not gender was included as a factor (Fs < 0.08, Ps > 0.41).
Single order relationships between hypo/mania proneness and structural connectivity with the NAcc
To examine the single order relationships between hypo/mania proneness and cortico-striatal connectivity, we first separately regressed HPS scores onto mean fractional anisotropy in the NAcc-mOFC and NAcc-amygdala tracts. In line with predictions, elevated proneness to hypo/mania was associated with increased fractional anisotropy (i.e. elevated structural connectivity) in both the NAcc-mOFC tract, F(1, 52) = 5.32, r = 0.36, P = 0.03 (Figure 1B), and the NAcc-amygdala tract, F(1, 52) = 9.68, r = 0.42, P < 0.01 (Figure 2B). Thus, proneness to hypo/mania was characterized by elevated cortical (NAcc-mOFC) and subcortical (NAcc-amygdala) structural connectivity with the NAcc.2
Unique vs shared contributions to relationship between hypo/mania proneness and structural connectivity with the NAcc
We next conducted a multiple regression with both NAcc-mOFC and NAcc-amygdala fractional anisotropy scores entered simultaneously as predictors of HPS scores to assess whether each tract provided unique vs shared contributions to hypo/mania proneness. The combined model was significant, F(3, 51) = 6.50, R2 = 0.20, P < 0.01. Furthermore, both elevated NAcc-mOFC fractional anisotropy, t(1,51) = 1.72, β = 0.22, P = 0.08 and elevated NAcc-amygdala fractional anisotropy, t(1, 51) = 2.65, β = 0.34, P = 0.01 were uniquely associated with elevated HPS scores (with the NAcc-mOFC relationship reflecting a non-significant trend, P = 0.08). This suggests that structural connectivity within both the NAcc-mOFC tract and the NAcc-amygdala tract contribute unique variance to hypo/mania proneness.
Specificity of the relationship between hypo/mania proneness and structural connectivity with the NAcc
To assess the specificity of the relationship between hypo/mania proneness and structural connectivity with the NAcc, we separately regressed HPS scores onto mean fractional anisotropy in the OFC-amygdala tract (i.e. uncinate fasciculus) and the whole-brain mask. There was no relationship between HPS scores and whole-brain white matter integrity (i.e. mean fractional anisotropy), F(1, 53) = 0.2, r = −0.06, P = 0.65, indicating that the relationship between hypo/mania proneness and NAcc-centered connectivity was not an artifact of overall structural integrity within the brain. There was also no relationship between HPS scores and mean fractional anisotropy in the OFC-amygdala tract (i.e. uncinate fasciculus), F(1, 53) = 0.62, r = 11, P = 0.43. This suggests that proneness to hypo/mania relates to NAcc-centered reward connectivity and not to emotional processing tractography more generally.
Discussion
To date, research on reward-related neural circuitry underlying bipolar symptoms has largely focused on syndromal bipolar disorder (bipolar I and bipolar II), and typically examined neural regions in isolation of each other. This study is the first investigation of the relationship between proneness to hypo/mania (Kwapil et al., 2000) and both cortical and subcortical structural connectivity with the NAcc. The NAcc is a central hub of reward processing in the brain, integrating diverse cortical and subcortical input to modulate reward processing and goal-directed behavior (Haber and Knutson, 2010; Floresco, 2015). In line with predictions, elevated hypo/mania proneness, as indexed by the HPS (Eckblad and Chapman, 1986), was associated with elevated structural connectivity between the NAcc and both the mOFC (cortical connectivity) and the amygdala (subcortical connectivity). Furthermore, a multiple regression analyses in which HPS scores were regressed onto both NAcc-mOFC and NAcc-amygdala structural connectivity indicated that these two pathways were uniquely related to hypo/mania proneness.
Our observation of a relationship between NAcc-centered connectivity and individual differences in hypo/mania proneness has important implications for understanding the pathophysiology of bipolar spectrum disorders. First, it suggests that elevated NAcc-centered connectivity may be a pre-existing risk factor for bipolar disorder. Identifying pre-existing risk factors is critical for understanding the pathophysiology of an illness because it helps to dissociate mechanisms involved in the initial onset of an illness from markers that are a consequence or ‘scar’ of an illness. Second, our design rules out any effects of psychotropic medication, which have been shown to directly modulate reward-related brain function in bipolar individuals (Chase et al., 2013), as none of our participants were taking psychotropic medication at the time of this study.
The mOFC plays an important role in encoding the value of a potential reward, assessing the probability of reward receipt, and facilitating reward-directed behavior (Haber and Knutson, 2010; Samanez-Larkin and Knutson, 2015). Both non-human animal and human research document the importance of excitatory white matter tracts from the mOFC to the NAcc in modulating reward processing (Haber and Knutson, 2010; Sesack and Grace, 2010). In line with the Reward Hypersensitivity Model, fMRI research indicates that both individuals with a hypo/manic temperament who have not yet developed a bipolar spectrum disorder (Harada et al., 2013) and individuals with diagnosable bipolar disorder (Nusslock et al., 2012, 2014) display abnormally elevated activation in the mOFC during reward processing. This suggests that elevated mOFC activation to reward cues reflects a trait like marker of bipolar risk that is observed along the entire spectrum, from subsyndromal hypo/manic temperament to syndromal bipolar disorder. However, as discussed, this work typically examines the OFC in isolation, neglecting the network of relationships among reward-related neural regions. This study reports, for the first time, that elevated hypo/mania proneness is associated with elevated structural connectivity between the mOFC and the NAcc. This suggests that individuals at risk for bipolar disorder may engage the mOFC in a manner that amplifies or up-regulates NAcc-based reward processing in the presence of reward-relevant cues. In the extreme, this cortical amplification of subcortical reward processing may contribute to the onset of hypo/manic symptoms.
The amygdala is also involved in reward/salience processing and goal-directed behavior (see Wassum and Izquierdo, 2015, for review). Excitatory input from the basolateral amygdala to the NAcc modulates reward prediction in learning paradigms and the central nucleus of the amygdala marks reward outcome to guide the modulation of behaviors through the NAcc (Haber and Knutson, 2010; Sesack and Grace, 2010; Janak and Tye, 2015). Like the mOFC, the amygdala is abnormally activated to positive or rewarding stimuli in individuals with bipolar disorder (Bermpohl et al., 2009). Also like the mOFC, this work focuses on syndromal bipolar disorder and typically examines the mOFC in isolation. This study reports, for the first time, that elevated subsyndromal hypo/mania proneness is associated with elevated structural connectivity between the amygdala and the NAcc. Furthermore, a multiple regression model indicated that both the NAcc-amygdala and NAcc-mOFC pathways each contributed unique variance to hypo/mania proneness. Collectively, this suggests a two-hit model in which reward-relevant tracts from both cortical (via the mOFC) and subcortical (via the amygdala) neural regions amplify NAcc-centered reward processing among individuals at elevated risk for bipolar disorder. This cortical/subcortical reward amplification circuit may represent an important mechanism underlying the elevated self-report, neurophysiological, and neurobiological indices of reward sensitivity observed among individuals with, and at risk for, bipolar disorder (see Nusslock et al., 2014, for review). These findings also provide support to a growing literature suggesting that the NAcc, amygdala, and OFC play a critical role in the pathophysiology of risk for hypo/mania (see Nusslock et al., 2014, for review). Finally, these results may have important treatment implications. Growing evidence documents the ability of noninvasive neuromodulation techniques, such as transcranial magnetic stimulation (van Schouwenburg et al., 2012) and transcranial direct current stimulation (Chib et al., 2013), to remotely modulate subcortical reward processing via white matter tractography from the cortex. Thus, NAcc-centered connectivity may provide a unique site for interventions for managing bipolar risk/symptoms via the modulation of reward-related neural circuitry. Future research is needed to examine this possibility.
There was no relationship between hypo/mania proneness and whole-brain white matter integrity, indicating that the relationship between hypo/mania and NAcc-centered connectivity was not simply an artifact of the overall structural integrity of the brain. There was also no relationship between hypo/mania proneness and structural integrity within the uncinate fasciculus, a white matter tract between the mOFC and the amygdala that is implicated in more general emotion modulation, including both threat- and reward-valuation (Wassum and Izquierdo, 2015, for review). Growing evidence highlights the involvement of the uncinate fasciculus in the top-down regulation of amygdala activity, particularly in the context of threat-processing (Wassum and Izquierdo, 2015). Furthermore, reduced structural connectivity within the uncinate fasciculus is associated with emotion regulation deficits of negative affect, and elevated symptoms of general distress in unipolar depression (Aghajami et al., 2014) and anxiety (Phan et al., 2009). Collectively, this suggests that hypo/mania proneness may not be associated with emotion regulation deficits of negative or threat-related affect, but rather uniquely related to NAcc-centered reward processing. Future research is needed to test this prediction.
We predicted that individuals with syndromal bipolar disorder (cyclothymia, bipolar II disorder, bipolar I disorder) would display a profile of elevated NAcc-centered connectivity comparable to, or greater than, individuals with elevated hypo/mania proneness. This prediction was based on considerable evidence for a dimensional model of reward processing abnormalities along the entire bipolar spectrum. For example, the majority of studies indicate that non-clinical samples and individuals with cyclothymia, bipolar II disorder, and full-blown bipolar I disorder display a comparable profile of reward hypersensitivity using multiple methodologies (i.e. psychosocial, neurophysiological, neural; see Johnson et al., 2012; Nusslock et al., 2014; Alloy et al., 2015, for reviews). Furthermore, elevated reward sensitivity has been shown to prospectively predict onset of first bipolar episode (Alloy et al., 2012a) and, among individuals with a bipolar spectrum disorder, predict a worsening of course (i.e. conversion from cyclothymia/bipolar II disorder to bipolar I; Alloy et al., 2012b). Finally, particularly relevant to this study, data indicate that both non-clinical and clinical samples of bipolar participants display comparable profiles of structural and functional reward-related neural profiles (see Nusslock et al., 2014 for review). An alternative hypothesis is that individuals with syndromal bipolar disorder display a unique profile of NAcc-centered connectivity, perhaps as a consequence or ‘scar’ of the illness. Future research directly comparing clinical and at-risk populations is needed to determine whether this is the case.
In its original conceptualization, the Reward Hypersensitivity Model proposed that reward hypersensitivity can lead to both hypo/manic and bipolar depressive symptoms in response to positive and negative reward-relevant events, respectively (e.g. Depue et al., 1987). The basis for this prediction is that reward hypersensitivity should make individuals hyper-responsive to cues signaling both the possible attainment and loss of reward. To date, however, there is not strong support for a link between reward hypersensitivity and bipolar depression, as reward hypersensitivity appears to be uniquely related to risk for hypo/mania (see Nusslock et al., 2014, for review). Accordingly, we predict that NAcc-centered reward connectivity may be more relevant for understanding the structural pathophysiology of hypo/manic symptoms, as opposed to bipolar depression. Future research with bipolar spectrum participants is needed to test this hypothesis.
Conclusions
This is the first study to report that elevated hypo/mania proneness, a temperamental risk factor for bipolar disorder onset (Kwapil et al., 2000), is associated with elevated structural connectivity within a NAcc-centered reward neural network. Elevated hypo/mania proneness was associated with elevated structural connectivity between the NAcc and both the mOFC (cortical connectivity) and the amygdala (subcortical connectivity). NAcc-centered connectivity may represent an important mechanism underlying abnormally elevated reward processing observed among individuals with bipolar disorder (see Nusslock et al., 2014, for review), and for understanding the structural pathophysiology underlying bipolar disorder, more generally. Future research is needed to examine profiles of NAcc-centered connectivity among individuals with syndromal bipolar disorder, and the extent to which NAcc-centered connectivity predicts bipolar onset, course, and severity. Finally, research on NAcc-centered connectivity in bipolar disorder may help facilitate the development of neuromodulation interventions for managing bipolar risk/symptoms via the modulation of reward-related neural circuitry. Future research is needed to test this possibility.
Acknowledgements
We would like to thank Todd Parrish, Mutahir Rauf, Sandra Shi and Rita Taylor for their assistance with MRI data acquisition.
Conflict of interest. None declared.
Funding
This work was supported by the National Institute of Mental Health [2T32MH067564 to K.S.D., R01 MH100117-01 to R.N., R01 MH077908-01A1 to R.N.]; the Ryan Licht Sang Bipolar Foundation [Young Investigator Grant and Independent Investigator Grant to R.N.]; the Chauncey and Marion D. McCormick Family Foundation [Young Investigator Grant and Independent Investigator Grant to R.N.]; and the National Science Foundation Graduate Research Fellowship Program [DGE-0824162 to C.B.Y.].
Footnotes
Although we directly asked participants whether they had a lifetime history of a psychiatric or neurological disorder, we did not conduct a formal psychiatric interview. Thus, there is a possibility that participants with a history of psychiatric symptoms that we were not aware of were included in the final sample.
We conducted exploratory analyses to assess for laterality effects in both the NAcc-mOFC and NAcc-amygdala pathways. Mean fractional anisotropy scores were extracted separately for each tract from each hemisphere, and were related to HPS scores. Elevated proneness to hypo/mania was associated with increased fractional anisotropy (i.e. elevated structural connectivity) in both hemispheres of the NAcc-mOFC tract [Left: r(55) = 0.23, P = 0.03; Right: r(55) = 0.32, P = 0.01] and the NAcc-amygdala tract [Left: r(55) = 0.21, P = 0.03; Right: r(55) = 0.33, P = 0.02]. Furthermore, there were no significant differences in the relationship between HPS and fractional anisotropy scores across hemispheres for either the NAcc-mOFC [t(55) = −0.6047, df = 51, P-value = 0.55] or NAcc-amygdala tracts [t(55) = −0.7680, df = 51, P-value = 0.45]. Collectively, this indicates there were no laterality effects for the relationship between nucleus accumbens structural connectivity and HPS scores.
References
- Abler B., Erk S., Walter H. (2007). Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology, 191, 823–33. [DOI] [PubMed] [Google Scholar]
- Abler B., Greenhouse I., Ongur D., Walter H., Heckers S. (2008). Abnormal reward system activation in mania. Neuropsychopharmacology, 33(9), 2217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M., Veer I.M., van Lang N.D., et al. (2014). Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med, 44(11), 2287–98. [DOI] [PubMed] [Google Scholar]
- Alloy L.B., Abramson L.Y., Walshaw P.D., et al. (2008). Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disorders, 10(2), 310–22. [DOI] [PubMed] [Google Scholar]
- Alloy L.B., Bender R.E., Whitehouse W.G., et al. (2012a). High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. Journal of Abnormal Psychology, 121(2), 339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy L.B., Nusslock R., Boland E.M. (2015). The development and course of bipolar spectrum disorders: an integrated reward and circadian rhythm dysregulation model. Annual Review of Clinical Psychology, 11, 213–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy L.B., Urosevic S., Abramson L.Y., et al. (2012b). Progression along the bipolar spectrum: a longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology, 121(1), 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst F., Stassen H.H., Clayton P.J., Angst J. (2002). Mortality of patients with mood disorders: Follow-up over 34-38 years. Journal of Affective Disorder, 68, 167–81. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Woolrich M.W., Jenkinson M., et al. (2003). Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–88. [DOI] [PubMed] [Google Scholar]
- Bermpohl F., Dalanay U., Kahnt T., et al. (2009). A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disorder, 11(1), 70–5. [DOI] [PubMed] [Google Scholar]
- Bermpohl F., Kahnt T., Dalanay U., et al. (2010). Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping, 31(7), 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Axelson D., Goldstein B., et al. (2009). Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry, 166(7), 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X., Lawrence N.S., Murphy K., Wise R.G., Phillips M.L. (2013). Ventral striatum activity in response to reward: differences between bipolar I and II disorders. American Journal of Psychiatry, 170(5), 533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.W., Nusslock R., Almeida J.R., Forbes E.E., LaBarbara E.J., Phillips M.L. (2013). Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorder, 15(8), 839–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chib V.S., Yun K., Takahashi H., Shimojo S. (2013). Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Translational Psychiatry 3, 268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S.G., Aiken L.S. (2003). Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd edn.Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Cohen M.X., Schoene-Bake J.C., Elger C.E., Weber B. (2009). Connectivity-based segregation of the human striatum predicts personality characteristics. Nature Neuroscience, 12(1), 32–4. [DOI] [PubMed] [Google Scholar]
- Costumero V., Barros-Loscertales A., Bustamante J.C., Ventura-Campos N., Fuentes P., Avila C. (2013). Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. European Journal of Neuroscience, 38(3), 2399–407. [DOI] [PubMed] [Google Scholar]
- Depue R.A., Krauss S.P., Spoont M.R. (1987). A two-dimensional threshold model of seasonal bipolar affective disorder In: Maggnusson D., Ohman A., editors. Psychopathology: An Interactional Perspective, pp. 95–123. New York: Academic Press. [Google Scholar]
- Eckblad M., Chapman L.J. (1986). Development and validation of a scale for hypomanic personality. Journal of Abnormal Psychology, 95(3), 214–22. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S.B. (2015). The nucleus accumbens: an interface between cognition, emotion, and action. Annual Review of Psychology, 66, 25–52. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Behrens T.E.J. (2014). The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron, 83(5), 1019–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Hoaki N., Terao T., et al. (2013). Hyperthymic temperament and brightness judgment in healthy subjects: involvement of left inferior orbitofrontal cortex. Journal of Affective Disorder, 151(1), 143–8. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Abramson L.Y., Nusslock R., et al. (2008). Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry, 63(7), 693–8. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Abramson L.Y., Sigelman J., Bohlig A., Hogan M.E., Harmon-Jones C. (2002). Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology, 82(4), 610–8. [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.L., Edge M.D., Holmes M.K., Carver C.S. (2012). The behavioral activation system and mania. Annual Review of Clinical Psychology, 8, 243–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knosche T.R., Turner R. (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage, 73, 239–54. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Whalen P.J. (2009). The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience, 29(37), 11614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Grochocinski V.J., Cluss P.A., Houck P.R., Stapf D.A. (2002). Demographic and clinical characteristics of individuals in a bipolar disorder case registry. Journal of Clinical Psychiatry, 63, 120–5. [DOI] [PubMed] [Google Scholar]
- Kwapil T.R., Miller M.B., Zinser M.C., Chapman L.J., Chapman J., Eckblad M. (2000). A longitudinal study of high scorers on the hypomanic personality scale. Journal of Abnormal Psychology, 109(2), 222–6. [PubMed] [Google Scholar]
- Lopez-Larson M.P., DelBello M.P., Zimmerman M.E., Schwiers M.L., Strakowski S.M. (2002). Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry, 52(2), 93–100. [DOI] [PubMed] [Google Scholar]
- Mason L., O'Sullivan N., Bentall R.P., El-Deredy W. (2012). Better than I thought: positive evaluation bias in hypomania. PLOS One, 7, 1e8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C., Bullmore E.T., Sham P.C., et al. (2004). Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Archives of General Psychiatry, 61(10), 974–84. [DOI] [PubMed] [Google Scholar]
- Meyer B., Johnson S.L., Carver C.S. (1999). Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment, 21(4), 275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Johnson S.L., Winters R. (2001). Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment, 23(3), 133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Almeida J.R., Forbes E.E., et al. (2012). Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorder, 14(3), 249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Young C.B., Damme K.S. (2014). Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behaviour and Research Therapy, 62, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- O’Sullivan N., Szczepanowski R., El-Deredy W., Mason L. (2011). fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia, 49, 2825–35. [DOI] [PubMed] [Google Scholar]
- Peterson C.K., Harmon-Jones E. (2008). Proneness to hypomania predicts EEG coherence between left motor cortex and left prefrontal cortex. Biology and Psychology, 78(2), 216–9. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Orlichenko A., Boyd E., et al. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry, 66(7), 691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 833–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R., Dohm K., Grotegerd D., et al. (2015). Reward processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology, 40(11), 2623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavert J., Caseras X., Torrubia R., et al. (2007). The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personality and Individual Differences, 42, 1323–31. [Google Scholar]
- Samanez-Larkin G.R., Knutson B. (2015). Decision making in the ageing brain: changes in affective and motivational circuits. Nature Review of Neuroscience, 16(5), 278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Kuhnen C.M., Yoo D.J., Knutson B. (2010). Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. Journal of Neuroscience, 30(4), 1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Kable J.W., Vandekar L., et al. (2015). Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology, 40, 2258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack S.R., Grace A.A. (2010). Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology, 35(1), 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S.M., Adler C.M., DelBello M.P. (2002). Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder?. Bipolar Disorder, 4(2), 80–8. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., MacFall J.R., Gerig G., Krishnan R.R. (2007). Structural integrity of the uncinate fasciculus in geriatric depression: relationship with age of onset. Journal of Neuropsychiatric Disease and Treatment, 3(5), 669–74. [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg M.R., O'Shea J., Mars R.B., Rushworth M.F., Cools R. (2012). Controlling human striatal cognitive function via the frontal cortex. Journal of Neuroscience, 32(16), 5631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.A., DeGeorge D.P., Barrantes-Vidal N., Kwapil T.R. (2015). A 3-year longitudinal study of risk for bipolar spectrum psychopathology. Journal of Abnormal Psychology, 124(3), 486.. [DOI] [PubMed] [Google Scholar]
- Wassum K.M., Izquierdo A. (2015). The basolateral amygdala in reward learning and addiction. Neuroscience and Biobehavioral Reviews, 57, 271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A., Panneck P., Srinivasan P., et al. (2011). Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics, 5, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H., McHugo M., Ray K.L., Glahn D.C., Eickhoff S.B., Laird A.R. (2014). Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cerebral Cortex, 24(1), 232–48. [DOI] [PMC free article] [PubMed] [Google Scholar]