Abstract
Our coherent perception of external events is enabled by the integration of inputs from different senses occurring within a range of temporal offsets known as the temporal binding window (TBW), which varies from person to person. A relatively wide TBW may increase the likelihood that stimuli originating from different environmental events are erroneously integrated and abnormally large TBW has been found in psychiatric disorders characterized by unusual perceptual experiences. Despite strong evidence of inter-individual differences in TBW, both within clinical and nonclinical populations, the neurobiological underpinnings of this variability remain unclear. We adopted an integrated strategy linking TBW to temporal dynamics in functional magnetic resonance imaging (fMRI)-resting-state activity and cortical excitation/inhibition (E/I) balance. E/I balance was indexed by glutamate/Gamma-AminoButyric Acid (GABA) concentrations and common variation in glutamate and GABA genes in a healthy sample. Stronger resting-state long-range temporal correlations, indicated by larger power law exponent (PLE), in the auditory cortex, robustly predicted narrower audio-tactile TBW, which was in turn associated with lower cognitive-perceptual schizotypy. Furthermore, PLE was highest and TBW narrowest for individuals with intermediate levels of E/I balance, with shifts towards either extreme resulting in reduced multisensory temporal precision and increased schizotypy, effectively forming a neural “tuning curve” for multisensory experience and schizophrenia risk. Our findings shed light on the neurobiological underpinnings of multisensory integration and its potentially clinically relevant inter-individual variability.
Keywords: schizotypy, multisensory perception, functional magnetic resonance imaging (fMRI) resting-state activity, long-range temporal correlations, excitation, inhibition balance, GABA (gamma-amminobutyric acid), glutamate, multilocus genetic score
Introduction
Our coherent and unified perception of external events in everyday life depends upon the ability to correctly bind information across senses.1 Stimuli from different sensory modalities occurring in close temporal proximity have the highest likelihood of being bound together and attributed to the same environmental event. However, we are able to tolerate a certain range of temporal asynchrony between stimuli in order to maintain a unitary perception of multisensory events.2 This range defines the individual’s temporal binding window (TBW3). Within his or her TBW, the individual will perceive multisensory stimuli as synchronous, despite their physical temporal distance. Critically, the width of the TBW shows large inter-individual differences among healthy individuals4 and even greater variability across the continuum from healthy to clinical conditions. For instance, enlarged TBWs have been reported in schizophrenia5–8 and autism,9,10 where they may contribute to abnormal perceptual experiences. Interestingly, multisensory perceptual experiences predicted by the individual TBW11 seem to be abnormal also in sub-clinical populations with high levels of schizotypy.12,13 This suggests that lower temporal resolution in multisensory perception might be a behavioral marker of psychosis proneness. However, no study has investigated the TBW in schizotypy so far. Moreover, notwithstanding the amount of research on the TBW, the neurochemical and genetic origins of its vast inter-individual variability remain unclear.
Spontaneous, or resting-state brain activity, may provide insight into an individual’s propensity to integrate multisensory information over a wide or narrow temporal window. Indeed, spontaneous neural activity exhibits a rich temporal structure14,15 that affects the timescale over which brain regions process information from external stimuli.16,17 More specifically, the temporal structure of spontaneous neural activity can be characterized by long-range temporal correlations (LRTCs)—ie, scale-free dynamics, with a power spectrum following P∝1/fβ, where P is power, f is frequency, and β is the power law exponent (PLE18,19). PLE is an index of the degree of LRTCs20,21: stronger LRTCs, with larger PLE, suggest that the past pattern of a system has a stronger influence on its future dynamics.22 In the human brain this relationship occurs across a range of timescales and frequencies including infra-slow brain amplitude fluctuations (<0.5 Hz) recorded using functional magnetic resonance imaging (fMRI) during resting wakefulness.23,24 Recently, we demonstrated that resting-state LRTCs also affect response amplitude to stimuli delivered at a specific phase of spontaneous brain activity (eg, higher LRTC, with larger PLE, amplify response to stimuli delivered at high-excitability phase24). Interestingly, response to multisensory stimuli and multisensory binding are primarily affected by the phase of spontaneous brain activity when stimuli arrive.25 Based on this evidence, we hypothesized a possible relation between resting-state LRTCs and temporal binding of multisensory events.
Although the molecular and cellular basis of these processes has yet to be elucidated, neuroimaging, neurophysiology, and molecular neurobiology studies strongly suggest that the temporal structure of spontaneous neural activity,26,27 as well as multisensory integration,28,29 may be fine-tuned by the balance between excitatory (ie, glutamatergic) and inhibitory (ie, GABAergic) neurotransmission. Specifically, prior work using cell-based approaches in animal models has shown that pharmacological alterations of the excitation/inhibition (E/I) balance, induced by blockage of either the excitatory NMDA or the inhibitory GABAA receptors, impact on LRTCs and their scale-free dynamics.30 Similarly, animal studies clearly suggest that a strictly held E/I balance, with key contributions from these same receptors, governs multisensory processing.31,32 The role of E/I balance in multisensory integration is further supported by human studies demonstrating that multisensory behavioral performance depends on individual levels of Gamma-AminoButyric Acid (GABA) and glutamate in the brain, measured in vivo by magnetic resonance spectroscopy (MRS33).
Importantly, levels of glutamate, its metabolite glutamine (collectively denoted as Glx), and GABA in the living human brain show considerable inter-individual differences among healthy subjects.34–36 This variability is even greater along the whole spectrum from healthy to clinical conditions, such as schizophrenia and autism,37,38 which are associated with abnormally increased E/I ratio.39–41 Importantly, genetic association studies of schizophrenia and autism42,43 suggest that some of the variability in the E/I balance may be genetically driven. Specifically, both early candidate gene studies44–47 and the most recent genome-wide associated study (GWAS) of schizophrenia48 have associated the disorder with single nucleotide polymorphisms (SNPs) within or near genes known to regulate GABA or glutamatergic signaling.
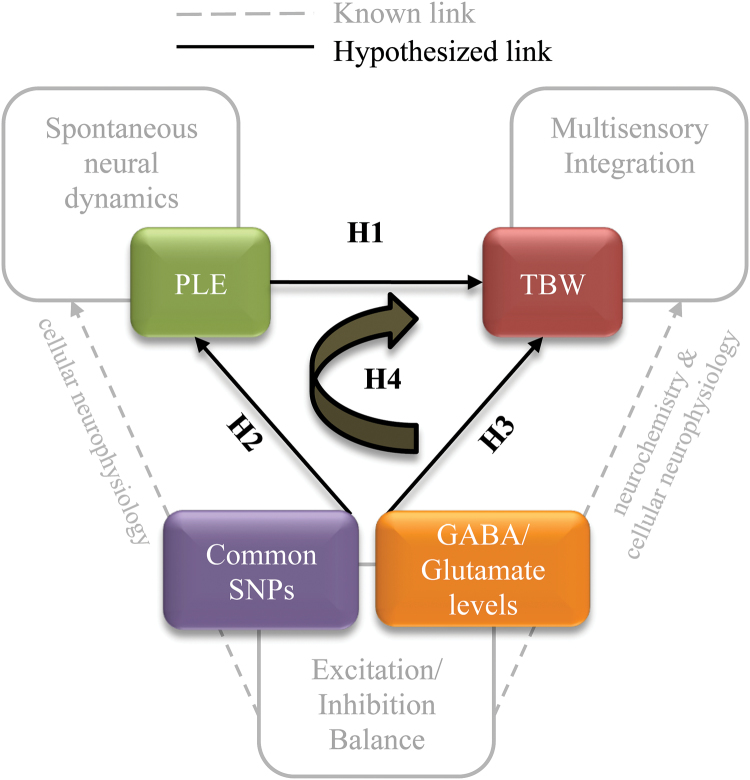
Based on these findings, we hypothesized that functional genetic variation modulating glutamate and GABAergic signaling may affect E/I balance, and consequently resting state neural dynamics and multisensory integration, in the healthy brain, which may in turn create a propensity to unusual perceptual experiences. To test this hypothesis we adopted an integrative strategy (figure 1), wherein first we assessed the relationship between resting state PLE values and individual audio-tactile TBW in the auditory cortex, where audio-tactile integration is known to occur.49 We hypothesized that higher PLE would be associated with a narrower TBW, indicative of higher temporal resolution (ie, more precision) in multisensory perception (H1). We then hypothesized that Glx, and possibly GABA, would modulate both PLE (H2) and TBW (H3), conditional on each individual’s genetically defined E/I balance. We focused our genetic analysis on 4 extensively characterized GABA- and glutamatergic SNPs (table 2), which may stably bias the baseline E/I background against which the more dynamic excitatory and inhibitory neurotransmitter signaling unfolds. Further, we hypothesized a role of PLE as a mediator between the genetically-biased effect of excitatory and inhibitory neurotransmitters and multisensory perception (H4). Finally, we extended this model to schizotypal traits capturing propensity to unusual cognitive/perceptual experiences.
Fig. 1.
Overview of the study. TBW = temporal binding window; PLE = power law exponent; SNP = single nucleotide polymorphism; GABA = Gamma-AminoButyric Acid; H = hypothesis.
Table 2.
Characteristics of the SNPs and Distribution of the Genetic Profile Score
| Molecular Characteristics | Phenotype Association Studies | Genotype Distribution | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Protein/Function | SNP | SNP Location | Effect on Expression | Hypothesized Mechanism | Disorders | Endophenotype | Genotypes | N | E/I Profile |
| GRIN1 | Excitatory NMDA receptor (subunit NR1) | rs11146020 | 5′UTR | N/A | C allele may alter consensus binding sequence of p50 subunit of NF-kB transcription factor44 | Schizophrenia44,58 | Depressive symptoms60 | G/C | 8 | Intermediate |
| Bipolar disorder59 | G/G | 29 | High | |||||||
| GRIK3 | Excitatory glutamate kainate receptor (subunit 3) | rs6691840 | Coding | T allele associated with lower expression in brain tissue61 | T allele may decrease expression by facilitating allele- specific imprinting61 | Schizophrenia45,62,63 | Depressive symptoms65 | T/T | 19 | Low |
| Recurrent depression64 | T/G | 16 | Intermediate | |||||||
| G/G | 2 | High | ||||||||
| GAD1 | Glutamate decaroboxylase 1 enzyme— converts Glu to GABA | rs3749034 | 5′UTR | G allele associated with lower expression in brain tissue of schizophrenic patients46 | G allele may alter binding sites of transcription factors AREB6 and MYOD146; may further modulate E/I balance by altering expression of nearby potassium- chloride transporter gene SLC12A5 (KCCN2)66,67 | Schizophrenia,46,68,69 panic disorder (females only)70 | Working memory and PFC function46,69; cortical thickness in parahippocampal gyrus71 | A/A | 2 | Low |
| G/A | 11 | Intermediate | ||||||||
| G/G | 24 | High | ||||||||
| GABRB2 | Inhibitory GABA A receptor (beta 2 subunit) | rs187269 | Intronic | C allele associated with lower expression in brain tissue of sz patients67,72 | C allele may alter an intronic splice site and reduce expression of select splice variants72 | Schizophrenia47,73 | Psychotic symptom severity; altruism/social cognition75 | T/T | 12 | Low |
| Bipolar disorder74 | C/T | 21 | Intermediate | |||||||
| C/C | 4 | High | ||||||||
Note: MPFC, medial prefrontal cortex; CSF, cerebral spinal fluid; GM/WM, grey matter to white matter; PLE, power law exponent; GABA, Gamma-AminoButyric Acid; E/I = excitation/inhibition.
Methods and Materials
Participants
Thirty-seven Caucasian, healthy, right-handed volunteers (12 females, mean age 21.8, age range 20–31 y) participated in the study after providing written informed consent. Participants were undergraduate and postgraduate students recruited via mailing lists at the University “G. D’Annunzio” of Chieti-Pescara. No participant had a history of neurologic, general medical or psychiatric conditions. All subjects received monetary compensation for their participation. The experimental protocol was approved by the University “G. D’Annunzio” of Chieti institutional ethics committee.
Behavioral Session
Temporal Binding Window.
Participants’ TBWs were obtained from 4 different tasks: (1) a multisensory audio-tactile Simultaneity Judgment task (SJ; supplementary figure 1), (2) a unimodal auditory SJ, (3) a multisensory audio-tactile Temporal Order Judgement task (TOJ), and (4) a unimodal auditory TOJ. We adopted well-established procedures50,51 for both data collection and analysis (see supplementary methods and supplementary table 1).
The average TBWs from the SJ tasks were: for the multimodal audio-tactile, 359 ± 135 ms (±SD indicated; range 172–646 ms); for the unimodal auditory, 80 ± 48 ms (range 19–210 ms). The average TBWs from the TOJ tasks were: for the multimodal audio-tactile, 251 ± 181 ms (range 55–700 ms); for the unimodal auditory, 99 ± 55 ms (range 23–294 ms).
MR Session
Spectroscopy (MRS) and functional (fMRI) data were acquired with a 3 Tesla Philips Achieva magnetic resonance scanner. The left primary auditory cortex was selected as the target region for MRS and fMRI investigations, due to its critical role in processing and integration of auditory and tactile stimuli,49,52 and because it has been associated with abnormal concentrations of neurotransmitters and gene expression in schizophrenia.53,54 The medial prefrontal cortex (MPFC), a higher order area characterized by different temporal dynamics,17 was chosen as the control region. In both regions, we quantified Glx and GABA neurotransmitters, as well as the cerebral spinal fluid (CSF), gray matter (GM) and white matter (WM) content. Also, we computed the regional PLE and the SD, indices of the resting-state temporal structural and variance respectively, as previously described24,55 (see supplementary methods, supplementary figure 2 and table 1).
Table 1.
Mean Values ± SD of Neurochemical Variables
| Auditory | MPFC | |
|---|---|---|
| Glx/NAAtot | 0.587 ± 0.096 | 0.684 ± 0.088 |
| GABA/NAAtot | 0.269 ± 0.036 | 0.339 ± 0.040 |
| CSF | 0.115 ± 0.037 | 0.106 ± 0.035 |
| GM/WM | 1.1632 ± 0.352 | 1.744 ± 0.229 |
| PLE (β) | 1.027 ± 0.188 | 1.431 ± 0.233 |
| SD (σ) | 0.390 ± 0.078 | 0.503 ± 0.009 |
Note: MPFC, medial prefrontal cortex; CSF, cerebral spinal fluid; GM/WM, grey matter to white matter; PLE, power law exponent.
Genetic Profile Scores
We compiled individual genetic profile scores56 related to the balance between excitation and inhibition signaling (E/I). The loci of interest were pre-selected based on the most recent published systematic review of cross-disorder (schizophrenia and bipolar disorder) association studies of common genetic variation in the GABA/Glutamate signaling system.43 Out of dozens of loci reviewed therein, we selected the 8 loci showing a positive association with schizophrenia in at least 2 independent studies (namely, GRIN1, GRIN2A, GRIN2B, GRIK3, GRM3, GRM7, GAD1, GABRB243) and followed them up with a targeted literature search in PubMed to identify SNPs consistently driving their association with schizophrenia, along with the SNPs’ functionality, and links to potential biological mechanisms. Thus, we focused on SNPs that are: (1) located within or near genes involved in the maintenance of E/I balance (ie, major GABA and glutamate signaling regulatory proteins); (2) of known or hypothesized molecular functionality; (3) associated with schizophrenia in at least 2 independent studies; (4) associated with at least 1 additional disorder characterized by impairments in E/I balance and/or multisensory integration; and (5) associated with at least 1 behavioral or neural endophenotype of cross-disorder relevance. Only 4 SNPs fulfilled these stringent criteria (see table 2 and supplementary table 2). No additional SNPs were probed in this sample.
We incorporated these pre-selected SNPs into a multilocus genetic score.56 The score represented the total number of variants across the 4 functional polymorphic loci. Across all loci, relatively “High” E/I genotypes were assigned a score of 1, “Low” E/I genotypes a score of 0, and “Intermediate” E/I genotypes a score of 0.556 (table 2). Alleles associated with “High” E/I had either a positive impact on glutamate signaling, or a negative impact on GABA signalling (or both in the case of GAD1 rs3749034). Complementarily, alleles associated with “Low” E/I had either a negative impact on the expression of glutamate-related genes, or a positive impact on the expression of GABA-related genes. These scores at each locus (see table 2 for their distribution) were then summed to create an individual profile score, such that higher total scores reflect a putative shift towards higher excitation coupled with less efficient inhibition. For example, the genetic profile score for an individual with the following 4 genotypes—GRIN1 G/G, GRIK3 T/G, GAD1 A/A, GABRB2 C/C—was 2.5 (1 + 0.5 + 0 + 1). The average E/I score was 2.351 ± 0.551. All observed genotype frequencies were consistent with those reported for Caucasian populations in the 1000 Genomes project.57
Schizotypal Personality Questionnaire
Schizotypy is thought to reflect the subclinical expression of the symptoms of schizophrenia in the general population and to constitute a dynamic continuum ranging from personality variation to psychosis.76,77 Schizotypal traits were assessed using the Italian version of the Schizotypal Personality Questionnaire (SPQ78). It consists of 3 subscales capturing the “Interpersonal,” “Cognitive-Perceptual,” and “Disorganization” aspects of schizotypy. The Cognitive-Perceptual component concerns the disposition to unusual perceptual experiences, such as hallucinations, which are associated with impaired multisensory integration and TBW in patients.7,79 Hence, we specifically focused on this subscale in our analysis. While its distribution was slightly positively skewed, its skewness and kurtosis were within the acceptable range (skewness < 1, kurtosis < 2.5). Thus, we used raw Cognitive-Perceptual SPQ scores (range 0–17).
Statistical Analyses
All individual variable distributions had skewness < 0.82 and kurtosis < 4.3 (absolute value). Thus, no power transformations were applied. Correlations among the main variables are listed in supplementary table 3. Three principal analyses were conducted. (1) Pearson’s correlation coefficients were computed using either PLE or SD values as an independent variable predicting individual TBWs. (2) Moderation analyses (model 1 in the SPSS PROCESS macro80; performed to probe genetic moderation by individual E/I genetic profiles of the effects of the more dynamic81–85 local concentrations of Glx, GABA or their ratio (Glx/GABA) on either PLE or TBW, with and without CSF fraction and grey matter to white matter (GM/WM) ratios as covariates. Significant interactions were further probed using the Johnson–Neyman (J–N) method,86 as implemented in the SPSS MODPROBE macro.87 The J–N method calculates the critical moderator variable values at which the relationship between the focal predictor (Glx, GABA or Glx/GABA) and the dependent variable (PLE or TBW) changes significance. J–N regions of significance are reported using standardized Z scores. (3) A moderated mediation analysis (model 8 in the SPSS PROCESS macro80) was performed to probe any effects of metabolite concentrations on individual TBW, mediated by its effects on PLE in the auditory cortex, conditional on E/I genetic profile. Then, we extended this model to individual schizotypal personality traits (SPQ) to detect any 3-path mediated effect on this variable. All the analyses were carried out with standardized values for all the variables. Consistent with published guidelines88,89 and in light of our relatively small sample size, we report 95% CIs based on 5000 bootstrap iterations (bias-corrected) for all major effects. Cohen’s f2 was computed to quantify effect sizes in individual regression analyses.90
Results
Resting State Temporal Dynamics (PLE) and TBW
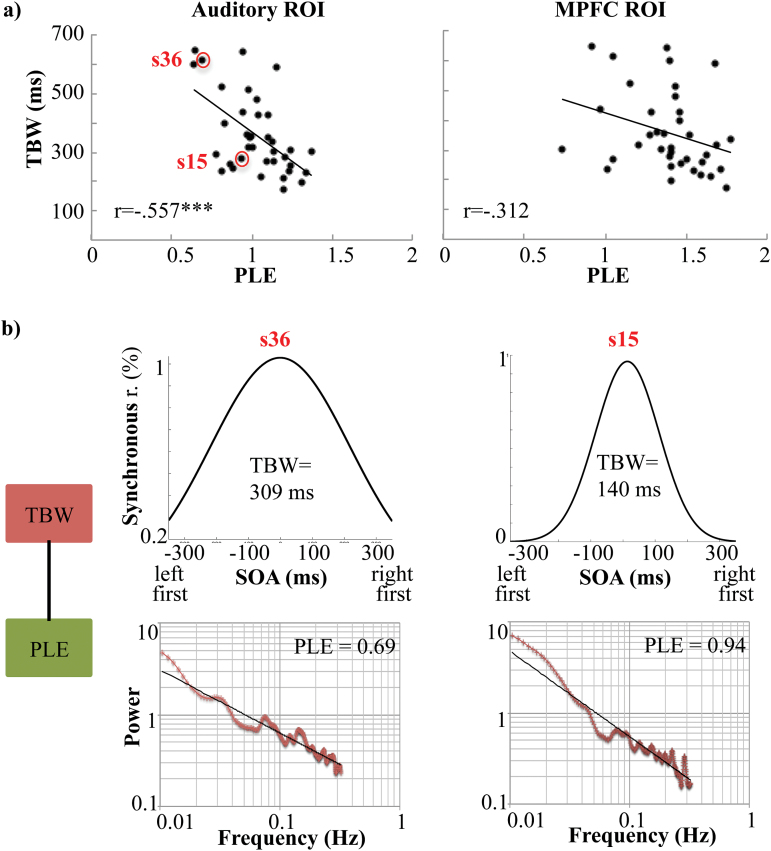
As hypothesized, we found a strong negative correlation between PLE in the auditory region of interest (ROI) and individual audio-tactile TBW for both the SJ (r = −.557, P < .001, 95% CI [−.779, −.261], Cohen’s f2 = .45; figures 2a–b) and the TOJ (r = −.428, P = .008, 95% CI [−.663, −.172], Cohen’s f2 = .22; not shown) tasks: the higher the degree of LRTCs (ie, larger PLE) in auditory cortex, the narrower the audio-tactile TBW. In contrast, the correlation between PLE in the control MPFC ROI and individual audio-tactile TBW did not survive bootstrap correction (SJ, 95% CI [−.664, .021], figure 2a; TOJ, 95% CI [−.417, .240], not shown). This relative region-specificity suggests the observed relationship is confined to the primary cortical area where multisensory integration is known to occur, as compared to higher-order information processing regions. Critically and in contrast to PLE, the SD of the signal recorded from the same auditory ROI did not predict individual TBWs from either task (SJ, 95% CI [−.213, .349]; TOJ, 95% CI [−.121, .391]), suggesting individual audio-tactile TBWs is predicted by the temporal structure (PLE), rather than the mere variance (SD), of resting state temporal dynamics. Finally, demonstrating the specificity of our findings to multi-modal rather than uni-modal sensory phenomena, no significant correlation was found between PLE in the auditory ROI and individual unimodal auditory-auditory TBW for either task (SJ, 95% CI [−.488, .029]; TOJ, 95% CI [−.490, .142]). Given the stronger correlation between PLE and individual audio-tactile TBW measured by SJ—than by TOJ—task, we used only the former in all subsequent analyses.
Fig. 2.
Predictive power of PLE for individual TBWs in Auditory and medial prefrontal cortex (MPFC) ROIs. (a) PLE was strongly negatively correlated with TBW (r = −.557) in the auditory ROI (left panel), but not the MPFC (r = −.312, right panel). (b) TBW predicted by PLE in the Auditory ROI for 2 single representative subjects showing either a wide (s36, left) or a narrow (s15, right) TBW. ***P ≤ .001. TBW = temporal binding window; PLE = power law exponent.
E/I Balance and Resting State Temporal Dynamics (PLE)
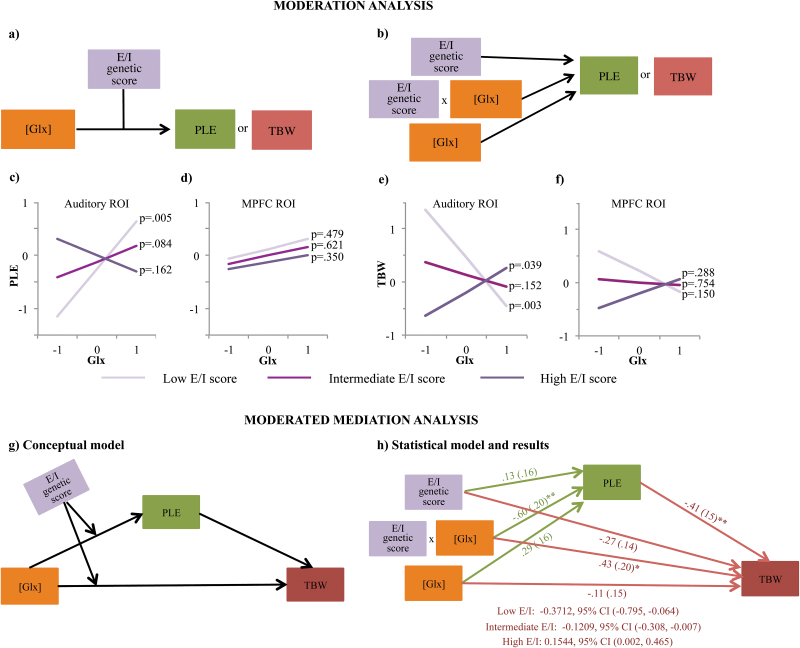
A significant interaction emerged between individual E/I genetic profile scores and concentrations of Glx in the Auditory ROI predicting PLE (ΔR2 = .2065, b = −.6063, 95% CI [−.976, −.218], P = .0056, R2 = .226, Cohen’s f2 = .29), wherein higher concentrations of Glx were associated with higher PLE values only for participants with relatively low E/I genetic profile scores (1 SD below the mean; figure 3c)—ie, participants showing E/I balance shifted towards inhibition. Specifically, J–N region of significance analyses indicated that higher concentrations of Glx were associated with higher PLE in participants with E/I genetic profile scores below −0.09 (mean-centered around 0), but with lower PLE in participants with E/I genetic profile score above 1.47 ie, participants showing E/I balance shifted towards excitation.
Fig. 3.
Genetic moderation of Glx effects on PLE and TBW. (a) Conceptual and (b) statistical depiction of the effects tested, ie, the main effect of each of the 2 factors and their interaction. (c–d) Glx was positively correlated with PLE in individuals with relatively low excitation/inhibition (E/I) genetic score (1 SD below mean; light purple line), but not in those with relatively high E/I genetic score (1 SD above mean; dark purple line) in the auditory cortex (c). Notably, Glx became a significant positive predictor of PLE at E/I score values >1.47 SD above the mean (not visualized). This interaction did not emerge for the MPFC (d). Analogously, auditory cortex Glx was negatively correlated with TBW width in those with low E/I scores (light purple line), but negatively correlated with TBW in those with high E/I scores (dark purple line, e). E/I score did not moderate a relationship between Glx and TBW in the MPFC (f). Statistical analyses were carried out with standardized values for all the variables. Notably, for visualization purposes, slope estimates are presented at discrete values of the moderator (mean levels and ±1SD away from the mean). However, these estimates are based on the linear trends present in the entire sample (ie, no arbitrary splitting of the sample was performed). (g) Conceptual depiction of the moderated mediation model tested. (h) Results from a path analysis testing the moderated mediation model presented in (g). PLE mediated a significant relationship between Glx concentrations and TBW at Low (mean - 1 SD), Intermediate (Mean) and High (mean + 1.1 SD) values of E/I scores. There was also a conditional direct interaction effect on TBW. Numbers represent standardized parameter estimates (bootstrapped standard errors in parentheses). Indirect (ie, mediated) effects are represented as parameter estimates along with 95% bias-corrected bootstrapped confidence intervals computed at representative values of the moderator (E/I genetic scores). As the model was saturated, only individual paths were tested for significance and no overall model fit statistics were produced.*P ≤ .05; **P ≤ .01. TBW = temporal binding window; PLE = power law exponent.
Importantly, the interaction term explained significant PLE variance above and beyond the main effects of individual E/I genetic profile (b = .1285, P = .4302) and Glx concentration (b = .2929, P = .0842). Furthermore, the interaction effect remained significant after controlling for either GM/WM ratios or the fraction of CSF (P values < .006). Consistent with prior work,56 the additive effects of all the polymorphisms were crucial to explaining a significant proportion of inter-individual variance in PLE. Indeed, after removing each of the polymorphisms from the E/I genetic profile, the moderation model was never significant (all P values > .12).
In contrast to auditory cortical Glx concentration, no significant interaction was found between the individual E/I genetic profile score and the concentration of GABA (supplementary figure 3) or the Glx/GABA ratio in the auditory ROI (P values > .19). Likewise, no significant interactions were found between the individual E/I genetic profile score and the concentration of either Glx or GABA in the MPFC ROI (P values > .25; figure 3d).
E/I Balance and TBW
In addition to predicting PLE, the interaction between individual E/I genetic profile scores and concentrations of Glx in the Auditory ROI also significantly predicted individual audio-tactile TBW (ΔR2 = .2590, b = .6790, 95% CI [.283, 1.075], P = .0014, R2 = .30, Cohen’s f2 = .42). The interaction effect was such that higher concentrations of Glx were associated with narrower TBW for participants with relatively low E/I score (1 SD below the mean; J–N: Z < −0.17), but wider TBW for those with high E/I score (1 SD above the mean; J–N: Z > 0.94) (figure 3e).
As with PLE, the interaction effect remained significant after controlling for either GM/WM or the fraction of CSF (P values < .002), and was not present when we performed the same analyses using the unimodal auditory-auditory task (P = .3527). In contrast to auditory cortical Glx concentration, no significant interaction was found between the individual E/I genetic profile score and the concentration of GABA in the Auditory ROI (P = .4201; supplementary figure 3). Similarly, no significant interactions were found between the individual E/I genetic profile score and the concentration of either Glx or GABA in the MPFC ROI (P values > .1; figure 3f and supplementary figure 3). However, the Glx/GABA ratio interacted with E/I genetic profile score to predict TBW (ΔR2 = .2656, b = .6445, 95% CI [.276, 1.013], P = .0011, R2 = .31, Cohen’s f2 = .45), such that higher Glx/GABA ratios were associated with narrower TBW for participants with relatively low E/I score (1 SD below the mean; J–N: Z < −0.12), but not for participants with relatively high E/I score (1 SD above the mean; J–N: Z > 1.9).
Resting State Temporal Dynamics (PLE) Mediate the Effects of E/I Balance on the TBW
To integrate these findings, we used a moderated mediation model to examine possible effects of Glx concentrations on TBW, mediated by PLE and conditional on individual E/I genetic profiles (figure 3g). We found that PLE mediated the relationship between Glx concentrations and TBW, such that for those with low E/I scores (≤mean), higher Glx was associated with higher PLE and, subsequently, narrower TBW, whereas for those with high E/I scores (> +1.1 SD), higher Glx was associated with lower PLE and, subsequently, wider TBW. Notably, there was also a conditional direct effect of Glx concentration on TBW (b = .4287, P = .040) in the model including PLE as a covariate, suggesting PLE was only a partial mediator of the relationship among these variables (figure 3h).
TBW and Schizotypal Personality Traits
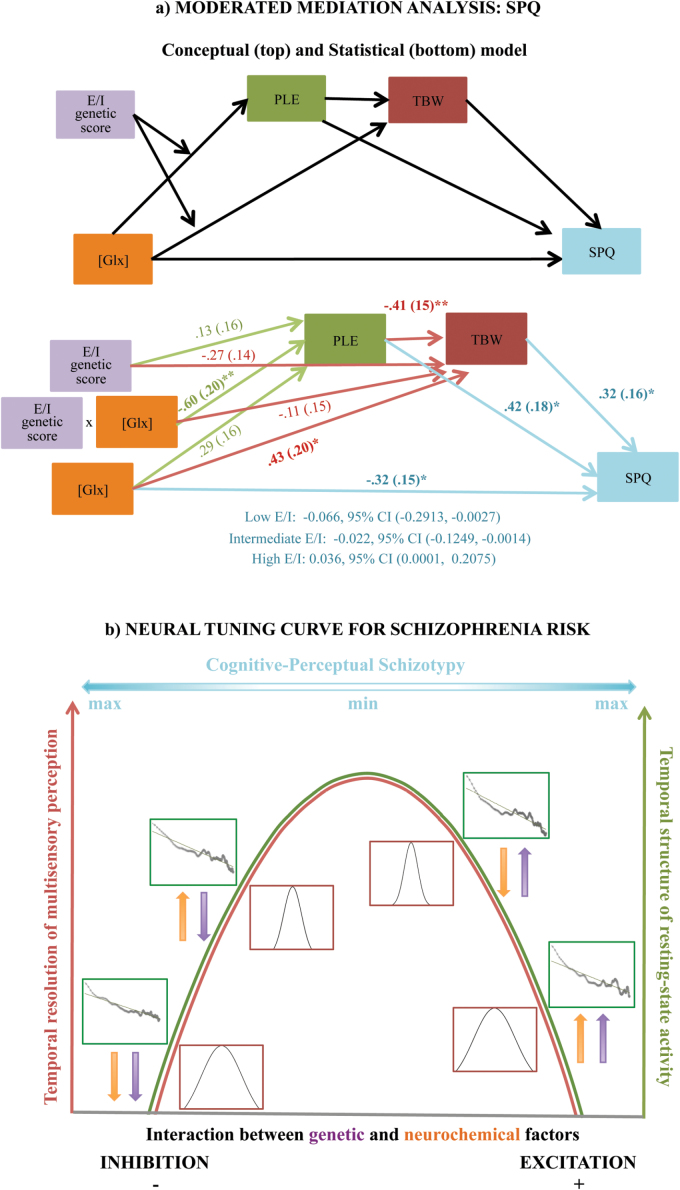
Despite relatively low power to detect a 3-path mediated effect, we sought to extend this model to schizotypal personality, consistent with prior work suggesting a link between multisensory phenomena and psychosis proneness.91 Thus, we found that PLE and TBW serially mediated a relationship between Glx and Cognitive-Perceptual SPQ, conditional on individual E/I scores. Specifically, higher Glx was associated with higher PLE, narrower TBW and consequently lower SPQ in those with relatively low E/I genetic score, but with lower PLE, wider TBW and ultimately higher SPQ in those with relatively high E/I genetic score (figure 4a). Furthermore, this effect was amplified when limiting the analysis to participants reporting any (as opposed to no) degree of cognitive-perceptual schizotypy (ie, SPQ > 0, n = 32), such that TBW accounted for 10% of all variance in SPQ Cog Perc (Cohen’s f2 = .11) and 26% variance (Cohen’s f2 = .35) in non-zero SPQ responses.
Fig. 4.
Proposed integrated model of the results. (a—top) A conceptual depiction of the moderated mediation model tested. PLE and TBW were tested as serial mediators of a genetically moderated indirect link between Glx and Cognitive-Perceptual SPQ. (a—bottom) Path analysis showed that, via its genetically moderated effects on PLE and TBW, higher Glx was associated with lower SPQ in those with relatively low E/I scores (mean or lower), but with higher SPQ in those with relatively high E/I score (>1.3 SD above the mean). Notably, the mediation effect was partial, as it emerged in the context of a significant negative direct path from Glx to SPQ. Numbers represent standardized parameter estimates (bootstrapped standard errors in parentheses). Indirect (ie, serially mediated) effects are represented as parameter estimates along with 95% bias-corrected bootstrapped confidence intervals computed at representative values of the moderator (E/I genetic scores). As the model was saturated, only individual paths were tested for significance and no overall model fit statistics were produced. (b) Our results support an inverted-U shape relationship between excitation/inhibition (E/I) balance and both the temporal structure of resting state activity (Green curve) as well as the temporal resolution of multisensory perception (Red curve). Individual TBW and power spectra are depicted for subjects representative of the 4 main combinations of E/I genetic score (Purple arrows) and Glx levels (Orange arrows). Subjects for whom both are low or both are high fall on the nonoptimal left and right side of the curve, respectively, whereas those with low–high or high–low combinations are near the putative optimal level of E/I on top of the curve. TBW = temporal binding window; PLE = power law exponent; SPQ = schizotypal personality questionnaire.
Discussion
In the current study, we adopted an integrative strategy (figure 1) to identify the neurobiological and molecular underpinnings of inter-individual differences in multisensory perception and propensity to unusual perceptual experiences. We found that a larger power law scaling exponent (PLE) in the resting-state temporal dynamics of the auditory cortex was associated with narrower audio-tactile TBW; ie, the higher the degree of LRTCs in the resting state auditory cortex activity, the higher the temporal resolution or precision in audio-tactile perception. Furthermore, this effect accounted for more than one-third of the variance in TBW and emerged specifically for the temporal structure, rather than the variance (SD), of spontaneous activity in the auditory cortex. Notably and as predicted, this association was not observed in the MPFC, a control brain area that is not directly involved in multisensory integration.
These results are in line with prior fMRI studies showing a functional link between the multiple timescales of neural dynamics in different brain regions and the multiple timescales within which the same regions reliably process unimodal sensory information.17 They are also consistent with fMRI studies associating individual spontaneous brain temporal dynamics with behavioral accuracy during event-timing tasks.19,92 Here, for the first time we extend this prior work into the multisensory domain of human cognition and identify a novel task-free assessment and biomarker (ie, PLE) of individual ability to integrate signals across sensory systems that may in turn predict propensity to unusual perceptual experiences.
A wider TBW indicates that stimuli originating from different environmental events are more likely to be bound together, which may give rise to unusual sensory phenomena, possibly continuous with the positive symptoms of schizophrenia. Consistent with this notion, we found that greater TBW width was associated with increased levels of schizotypy, specifically in the Cognitive-Perceptual domain. While prior work has shown abnormally wide TBW in individuals with schizophrenia,5,6,8 here, for the first time, we extend these results to a dimensional measure of psychosis proneness. Indeed, even if most individuals with schizotypal traits are not expected to develop schizophrenia, increasing levels of schizotypy are robustly associated with heightened risk for the development of psychotic disorders.93 Accordingly and consistent with the current results, substantial overlap has been found between schizotypy and schizophrenia in terms of etiological factors at the genetic, biological, and psychosocial levels,94 but also concerning a wide range of perceptual, cognitive, and motor impairments.95
We further show that inter-individual variability in auditory resting state dynamics, audio-tactile TBW, and perceptual schizotypy were accounted for by local concentrations of glutamatergic compounds (Glx) conditional on individual E/I genetic profiles. Specifically, for relatively lower values of the E/I score, indicating a putative genetic shift towards greater inhibition, higher concentrations of Glx were associated with higher values of PLE, narrower TBW and, ultimately, lower schizotypy. In contrast, for relatively greater values of the E/I score, indicating a putative shift towards greater excitation, higher concentrations of Glx were associated with lower PLE, wider TBW and higher schizotypy.
This interaction pattern is consistent with recent work, which describes the relationship between E/I balance and information processing efficiency as an inverted U-shaped curve that may differ per brain region and individual.96 Importantly, the distribution of E/I balance in the healthy population is skewed with the majority showing a peak shifted towards inhibition. A transcranial direct current stimulation (tDCS) treatment, which reduces inhibition (ie, shifts the imbalance towards the optimum), thus, typically improves an individual’s performance.96 According to this model, increased Glx in our participants is expected to be associated with improved neural and behavioral efficiency for everyone who is genetically predisposed to be on the nonoptimal side of the balance closer to the inhibition end of the spectrum (ie, low E/I genetic scores; figure 4b). In contrast, higher levels of Glx might push those who are already genetically predisposed towards excitation (ie, with high E/I genetic scores) over and/or further away from the peak of the curve, toward the nonoptimal side closer to excitation. In effect, this might worsen neural and behavioral efficiency and increase risk for schizotypal traits in the cognitive/perceptual domain, effectively forming a neural “tuning curve” for multisensory experience and perceptual risk for schizophrenia. It is worth clarifying here that we do not believe individual genetic profiles are sufficient to determine the position of an individual on the E/I spectrum; rather, they represent a predisposing, or conditioning, factor. Through context-dependent interaction with excitatory and inhibitory neurotransmitters, individual genetic profiles would allow a range of possible dynamics of E/I balance and potentially clinically relevant gene-brain-behavior relationships.
Our results are further consistent with prior work, which has demonstrated the importance of glutamatergic neurotransmission for both resting state brain dynamics and audio-tactile integration. Specifically, previous studies in humans have suggested that Glx (or Glu) concentrations may exert a critical role in the synchronization of spontaneous neural activity.97 However, a possible role of excitatory neurotransmission for LRTCs30 and integration of auditory and somatosensory stimuli in the auditory system has only been previously demonstrated in animal models.98 Thus, our study is the first to directly demonstrate the importance of glutamatergic neurotransmission, as constrained by common genetic variation, for LRTC and audio-tactile perception in humans. Most importantly, the degree of LRTC and the precision in audio-tactile perception were partial mediators of the link between Glx and schizophrenia proneness, in the presence of a direct negative link between Glx and SPQ in our sample. Our results are thus also broadly consistent with prior evidence showing that altered glutamatergic neurotransmission, due to either increased99 or reduced100 glutamatergic metabolites, and altered activity of glutamate receptors101 are associated with subclinical and clinical manifestations of psychosis. In addition, they suggest one possible mechanism that may specifically mediate this hypothesized link in the context of perceptual disturbance. This may further explain the relative specificity of our findings to glutamate as opposed to GABA levels in the brain. Moreover, a possible reason why we did not find significant effect of GABA levels on multisensory perception might be related to the fact that we did not quantify the levels of GABA while participants were performing the task, rather during resting state. Prior cellular studies, indeed, suggested that a crucial role played by inhibitory transmission in the healthy brain is to sharpen the response properties of excitatory neurons. By putting a brake on cortical excitability, inhibitory neurons provide temporal precision to cortical firing in response to sensory inputs and enhance their saliency.102
The current study is not without limitations. First, even though we selected biological predictors of TBW based on a strong a priori rationale, many additional factors not accounted for by our study are likely to contribute to this complex perceptual phenotype. Along the same lines, we do not assume the SNPs included in our E/I genetic profile exhaustively represent all genetically driven variability in E/I balance. Rather, they represent genotypes of convenience, which were chosen for this proof-of-principle study because of their known or hypothesized functionality on the molecular level, and their prior association with neuropsychiatric disorders characterized by perceptual disturbances. Future work incorporating additional genetic variability in a more exhaustive way is warranted. Our study is also limited by its relatively small sample size of 37 participants. However, published power analyses suggest a sample size of 34 is sufficient to detect a large 2-path mediated effect when using bootstrapped bias-corrected confidence interval statistics.103 The large effect sizes, directions of effect and conceptual links at each step of our investigation are in turn broadly consistent with prior work,79,91,104–107 which gives us confidence we did not overestimate effect sizes due to sampling error. While 3-path mediation models generally require slightly larger sample sizes to test (n = ~50 for medium to large 3-path mediated effects108) this consistency of effect sizes and directionality in our current model supports its conceptual validity. Future studies in larger samples of healthy individuals and psychiatric patients will help to further generalize our findings, confirm effect size, and extend our proposed model. Finally, our results are correlational. Thus, while we propose PLE as a mediator between E/I balance and TBW, it is possible that the effects of GABA/Glx and E/I genetic score on TBW may also contribute to shaping LRTC phenomena. This is a particularly intriguing possibility, given that all our mediation effects were partial, suggesting the presence of additional factors. Future work in preclinical models of multisensory integration would be required to determine true causality in the proposed pathway.
These limitations notwithstanding, the current study is the first empirical demonstration that neural, neurochemical and genetic factors related to excitation-inhibition mechanisms contribute to inter-individual differences in multisensory perception and propensity to unusual sensory experiences in healthy humans. Further, our postulated tuning curve model might improve our understanding of clinical conditions associated with impaired multisensory perception and open the door to innovative neuromodulation interventions (eg, TMS, tDCS) informed by individual genetic and neurochemical profiles. In individuals with profiles shifted towards inhibition, excitatory neuromodulation treatment would bring cortical neurotransmission closer to an optimal E/I balance. The same would hold true for inhibitory neuromodulation treatment in individuals with profiles shifted towards excitation. In sum, using a multimodal approach within an integrative framework, we delineate a promising novel mechanism of perceptual variability, and identify directions for future research with potential clinical implications.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by the University of Ottawa Brain and Mind Research Centre, and the Canada Institute of Health Research (CIHR) to G.N. Y.S.N. is supported by a Banting Postdoctoral Fellowship (CIHR).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Murray M, Wallace M. The Neural Bases of Multisensory Processes. Boca Raton, FL: CRC Press; 2011. [PubMed] [Google Scholar]
- 2. Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci. 1987;7:3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace MT, Stevenson RA. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. 2014;64C: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevenson RA, Zemtsov RK, Wallace MT. Individual differences in the multisensory temporal binding window predict susceptibility to audiovisual illusions. J Exp Psychol Hum Percept Perform. 2012;38:1517–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foucher JR, Lacambre M, Pham BT, Giersch A, Elliott MA. Low time resolution in schizophrenia Lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res. 2007;97:118–127. [DOI] [PubMed] [Google Scholar]
- 6. Martin B, Giersch A, Huron C, van Wassenhove V. Temporal event structure and timing in schizophrenia: preserved binding in a longer “now”. Neuropsychologia. 2013;51: 358–371. [DOI] [PubMed] [Google Scholar]
- 7. Stevenson RA, Park S, Cochran C, et al. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tseng HH, Bossong MG, Modinos G, Chen KM, McGuire P, Allen P. A systematic review of multisensory cognitive-affective integration in schizophrenia. Neurosci Biobehav Rev. 2015;55:444–452. [DOI] [PubMed] [Google Scholar]
- 9. Foss-Feig JH, Kwakye LD, Cascio CJ, et al. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res. 2010;203:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevenson RA, Siemann JK, Schneider BC, et al. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014;34:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costantini M, Robinson J, Migliorati D, Donno B, Ferri F, Northoff G. Temporal limits on rubber hand illusion reflect individuals’ temporal resolution in multisensory perception. Cognition. 2016;157:39–48. [DOI] [PubMed] [Google Scholar]
- 12. Kallai J, Hegedus G, Feldmann A, et al. Temperament and psychopathological syndromes specific susceptibility for rubber hand illusion. Psychiatry Res. 2015;229:410–419. [DOI] [PubMed] [Google Scholar]
- 13. Thakkar KN, Nichols HS, McIntosh LG, Park S. Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS One. 2011;6:e27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He BJ. Scale-free brain activity: past, present, and future. Trends Cogn Sci. 2014;18:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray JD, Bernacchia A, Freedman DJ, et al. A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci. 2014;17:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honey CJ, Thesen T, Donner TH, et al. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. 2012;76:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stephens GJ, Honey CJ, Hasson U. A place for time: the spatiotemporal structure of neural dynamics during natural audition. J Neurophysiol. 2013;110:2019–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chialvo DR. Emergent complex neural dynamics. Nat Phys. 2010;6:744–750. [Google Scholar]
- 19. Palva JM, Zhigalov A, Hirvonen J, Korhonen O, Linkenkaer-Hansen K, Palva S. Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. Proc Natl Acad Sci U S A. 2013;110:3585–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bullmore E, Long C, Suckling J, et al. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linkenkaer-Hansen K, Nikouline VV, Palva JM, Ilmoniemi RJ. Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci. 2001;21:1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He BJ. Scale-free properties of the functional magnetic resonance imaging signal during rest and task. J Neurosci. 2011;31:13786–13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Z, Zhang J, Longtin A, et al. Is there a nonadditive interaction between spontaneous and evoked activity? Phase-dependence and its relation to the temporal structure of scale-free brain activity. Cereb Cortex. 2015. [DOI] [PubMed] [Google Scholar]
- 25. Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deco G, Jirsa VK. Ongoing cortical activity at rest: criticality, multistability, and ghost attractors. J Neurosci. 2012;32:3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deco G, Ponce-Alvarez A, Hagmann P, Romani GL, Mantini D, Corbetta M. How local excitation-inhibition ratio impacts the whole brain dynamics. J Neurosci. 2014;34:7886–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meredith MA. On the neuronal basis for multisensory convergence: a brief overview. Brain Res Cogn Brain Res. 2002;14:31–40. [DOI] [PubMed] [Google Scholar]
- 29. van Atteveldt N, Murray MM, Thut G, Schroeder CE. Multisensory integration: flexible use of general operations. Neuron. 2014;81:1240–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazzoni A, Broccard FD, Garcia-Perez E, Bonifazi P, Ruaro ME, Torre V. On the dynamics of the spontaneous activity in neuronal networks. PLoS One. 2007;2:e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Binns KE, Salt TE. Importance of NMDA receptors for multimodal integration in the deep layers of the cat superior colliculus. J Neurophysiol. 1996;75:920–930. [DOI] [PubMed] [Google Scholar]
- 32. Populin LC. Anesthetics change the excitation/inhibition balance that governs sensory processing in the cat superior colliculus. J Neurosci. 2005;25:5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balz J, Keil J, Roa Romero Y, et al. GABA concentration in superior temporal sulcus predicts gamma power and perception in the sound-induced flash illusion. Neuroimage. 2015;125:724–730. [DOI] [PubMed] [Google Scholar]
- 34. Bai Y, Nakao T, Xu J, et al. Resting state glutamate predicts elevated pre-stimulus alpha during self-relatedness: a combined EEG-MRS study on “rest-self overlap”. Soc Neurosci. 2016;11:249–263. [DOI] [PubMed] [Google Scholar]
- 35. Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31:16556–16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. [DOI] [PubMed] [Google Scholar]
- 37. Gaetz W, Bloy L, Wang DJ, et al. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev. 2015;51:276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophr Bull. 2014;40:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yizhar O, Fenno LE, Prigge M, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen J, Yu S, Fu Y, Li X. Synaptic proteins and receptors defects in autism spectrum disorders. Front Cell Neurosci. 2014;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cherlyn SY, Woon PS, Liu JJ, Ong WY, Tsai GC, Sim K. Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci Biobehav Rev. 2010;34:958–977. [DOI] [PubMed] [Google Scholar]
- 44. Begni S, Moraschi S, Bignotti S, Fumagalli F, Rillosi L, Perez J, Gennarelli M. Association between the G1001C polymorphism in the GRIN1 gene promoter region and schizophrenia. Biol Psychiatry. 2003;53:617–619. [DOI] [PubMed] [Google Scholar]
- 45. Begni S, Popoli M, Moraschi S, Bignotti S, Tura GB, Gennarelli M. Association between the ionotropic glutamate receptor kainate 3 (GRIK3) ser310ala polymorphism and schizophrenia. Mol Psychiatry. 2002;7:416–418. [DOI] [PubMed] [Google Scholar]
- 46. Straub RE, Lipska BK, Egan MF, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. [DOI] [PubMed] [Google Scholar]
- 47. Zhao C, Xu Z, Chen J, et al. Two isoforms of GABA(A) receptor beta2 subunit with different electrophysiological properties: differential expression and genotypical correlations in schizophrenia. Mol Psychiatry. 2006;11:1092–1105. [DOI] [PubMed] [Google Scholar]
- 48. Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kayser C, Petkov CI, Augath M, Logothetis NK. Integration of touch and sound in auditory cortex. Neuron. 2005;48:373–384. [DOI] [PubMed] [Google Scholar]
- 50. Keetels M, Vroomen J. Perception of synchrony between the senses. In: Murray MM, Wallace MT, eds. The Neural Bases of Multisensory Processes. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 51. Vroomen J, Keetels M. Perception of intersensory synchrony: a tutorial review. Atten Percept Psychophys. 2010;72:871–884. [DOI] [PubMed] [Google Scholar]
- 52. Schurmann M, Caetano G, Hlushchuk Y, Jousmaki V, Hari R. Touch activates human auditory cortex. Neuroimage. 2006;30:1325–1331. [DOI] [PubMed] [Google Scholar]
- 53. Atagun MI, Sikoglu EM, Can SS, et al. Investigation of Heschl’s gyrus and planum temporale in patients with schizophrenia and bipolar disorder: a proton magnetic resonance spectroscopy study. Schizophr Res. 2015;161:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hugdahl K, Loberg EM, Specht K, Steen VM, van Wageningen H, Jorgensen HA. Auditory hallucinations in schizophrenia: the role of cognitive, brain structural and genetic disturbances in the left temporal lobe. Front Hum Neurosci. 2007;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang Z, Wang Z, Zhang J, et al. Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum Brain Mapp. 2014;35:5368–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Genomes Project C , Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galehdari H. Association Between the G1001C Polymorphism in the GRIN1 Gene Promoter and Schizophrenia in the Iranian Population. J Mol Neurosci. 2009;38:178–181. [DOI] [PubMed] [Google Scholar]
- 59. Mundo E, Tharmalingham S, Neves-Pereira M, et al. Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol Psychiatry. 2003;8:241–245. [DOI] [PubMed] [Google Scholar]
- 60. Georgi A, Jamra RA, Klein K, et al. Possible association between genetic variants at the GRIN1 gene and schizophrenia with lifetime history of depressive symptoms in a German sample. Psychiatr Genet. 2007;17:308–310. [DOI] [PubMed] [Google Scholar]
- 61. Schiffer H, Swanson G, Masliah E, Heinemann F. Unequal expression of allelic kainate receptor GluR7 mRNAs in human brains. J Neurosci. 2000;20:9025–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahmad Y, Bhatia MS, Mediratta PK, Sharma KK, Negi H, Chosdol K, Sinha S. Association between the ionotropic glutamate receptor kainate3 (GRIK3) Ser310Ala polymorphism and schizophrenia in the Indian population. World J Biol Psychiatry. 2009;10:330–333. [DOI] [PubMed] [Google Scholar]
- 63. Kilic G, Ismail Kucukali C, Orhan N, Ozkok E, Zengin A, Aydin M, Kara I. Are GRIK3 (T928G) gene variants in schizophrenia patients different from those in their first-degree relatives? Psychiatry Res. 2010;175:43–46. [DOI] [PubMed] [Google Scholar]
- 64. Schiffer HH, Heinemann SF. Association of the human kainate receptor GluR7 gene (GRIK3) with recurrent major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:20–26. [DOI] [PubMed] [Google Scholar]
- 65. Luciano M, Houlihan LM, Harris SE, et al. Association of existing and new candidate genes for anxiety, depression and personality traits in older people. Behav Genet. 2010;40:518–532. [DOI] [PubMed] [Google Scholar]
- 66. Hyde TM, Lipska BK, Ali T, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tao R, Li C, Newburn EN, et al. Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders. J Neurosci. 2012;32:5216–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Addington AM, Gornick M, Duckworth J, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–588. [DOI] [PubMed] [Google Scholar]
- 69. Lett TA, Kennedy JL, Radhu N, et al. Prefrontal white matter structure mediates the influence of GAD1 on working memory. Neuropsychopharmacology. 2016;41:2224–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weber H, Scholz CJ, Domschke K, et al. Gender differences in associations of glutamate decarboxylase 1 gene (GAD1) variants with panic disorder. PLoS One. 2012;7:e37651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brauns S, Gollub RL, Walton E, et al. Genetic variation in GAD1 is associated with cortical thickness in the parahippocampal gyrus. J Psychiatr Res. 2013;47:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao C, Xu Z, Wang F, et al. Alternative-splicing in the exon-10 region of GABA(A) receptor beta(2) subunit gene: relationships between novel isoforms and psychotic disorders. PLoS One. 2009;4:e6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lo WS, Lau CF, Xuan Z, et al. Association of SNPs and haplotypes in GABAA receptor beta2 gene with schizophrenia. Mol Psychiatry. 2004;9:603–608. [DOI] [PubMed] [Google Scholar]
- 74. Chen J, Tsang SY, Zhao CY, et al. GABRB2 in schizophrenia and bipolar disorder: disease association, gene expression and clinical correlations. Biochem Soc Trans. 2009;37:1415–1418. [DOI] [PubMed] [Google Scholar]
- 75. Tsang SY, Zhong S, Mei L, et al. Social cognitive role of schizophrenia candidate gene GABRB2. PLoS One. 2013;8:e62322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lenzenweger MF. Schizotaxia, schizotypy, and schizophrenia: Paul E. Meehl’s blueprint for the experimental psychopathology and genetics of schizophrenia. J Abnorm Psychol. 2006;115:195–200. [DOI] [PubMed] [Google Scholar]
- 77. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291–326. [DOI] [PubMed] [Google Scholar]
- 78. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 79. Williams LE, Light GA, Braff DL, Ramachandran VS. Reduced multisensory integration in patients with schizophrenia on a target detection task. Neuropsychologia. 2010;48:3128–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hayes A. Introduction to Mediation. Moderation and Conditional Process Analysis: A Regression-based Approach. New York, NY: Guildford Press; 2013. [Google Scholar]
- 81. Maddock RJ, Casazza GA, Buonocore MH, Tanase C. Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): a dynamic 1H-MRS study. Neuroimage. 2011;57:1324–1330. [DOI] [PubMed] [Google Scholar]
- 82. Harris RE, Sundgren PC, Pang Y, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. [DOI] [PubMed] [Google Scholar]
- 83. Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic Action in the Autistic Brain. Curr Biol. 2016;26:80–85. [DOI] [PubMed] [Google Scholar]
- 84. Chellappa SL, Gaggioni G, Ly JQ, et al. Circadian dynamics in measures of cortical excitation and inhibition balance. Sci Rep. 2016;6:33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab. 2012;32:1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Johnson PO, Fay LC. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15:349–367. [DOI] [PubMed] [Google Scholar]
- 87. Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. [DOI] [PubMed] [Google Scholar]
- 88. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82:171–185. [Google Scholar]
- 89. Preacher K, Rucker D, Hayes A. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivar Behav Res. 2007;42:185–227. [DOI] [PubMed] [Google Scholar]
- 90. Cohen J. A power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 91. Germine L, Benson TL, Cohen F, Hooker CI. Psychosis-proneness and the rubber hand illusion of body ownership. Psychiatry Res. 2013;207:45–52. [DOI] [PubMed] [Google Scholar]
- 92. Smit DJ, Linkenkaer-Hansen K, de Geus EJ. Long-range temporal correlations in resting-state alpha oscillations predict human timing-error dynamics. J Neurosci. 2013;33:11212–11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J Abnorm Psychol. 2013;122:807–815. [DOI] [PubMed] [Google Scholar]
- 94. Barrantes-Vidal N, Grant P, Kwapil TR. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull. 2015;41(suppl 2):S408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ettinger U, Mohr C, Gooding DC, et al. Cognition and brain function in schizotypy: a selective review. Schizophr Bull. 2015;41(suppl 2):S417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krause B, Marquez-Ruiz J, Cohen Kadosh R. The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci. 2013;7:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-a review of multimodal imaging studies. Neurosci Biobehav Rev. 2014;47:36–52. [DOI] [PubMed] [Google Scholar]
- 98. Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009;29:4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–674. [DOI] [PubMed] [Google Scholar]
- 100. Thakkar KN, Rosler L, Wijnen JP, et al. 7T proton magnetic resonance spectroscopy of gamma-aminobutyric acid, glutamate, and glutamine reveals altered concentrations in patients with schizophrenia and healthy siblings. Biol Psychiatry. 2016. [DOI] [PubMed] [Google Scholar]
- 101. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Scheyltjens I, Arckens L. The current status of somatostatin-interneurons in inhibitory control of brain function and plasticity. Neural Plast. 2016;2016:8723623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stone DB, Urrea LJ, Aine CJ, Bustillo JR, Clark VP, Stephen JM. Unisensory processing and multisensory integration in schizophrenia: a high-density electrical mapping study. Neuropsychologia. 2011;49:3178–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Capa RL, Duval CZ, Blaison D, Giersch A. Patients with schizophrenia selectively impaired in temporal order judgments. Schizophr Res. 2014;156:51–55. [DOI] [PubMed] [Google Scholar]
- 106. Enzi B, Duncan NW, Kaufmann J, Tempelmann C, Wiebking C, Northoff G. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex—a combined fMRI-MRS study. Neuroscience. 2012;227:102–109. [DOI] [PubMed] [Google Scholar]
- 107. Zhang X, Tang Y, Maletic-Savatic M, et al. Altered neuronal spontaneous activity correlates with glutamate concentration in medial prefrontal cortex of major depressed females: an fMRI-MRS study. J Affect Disord. 2016;201:153–161. [DOI] [PubMed] [Google Scholar]
- 108. Taylor A, MacKinnon D, JY T. Tests of the three-path mediated effect. Organ Res Method. 2008;11:241–269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.