Abstract
Migration status is one of the best-established risk factors for schizophrenia. An increase in risk is observed in both first- and second-generation immigrants, with a varying magnitude depending on the ethnic background of the individuals. The underlying mechanisms for the increased risk are only recently coming into focus. A causal role for social stress has been widely proposed, and recent work indicated altered neural stress processing in the perigenual anterior cingulate cortex (pACC) in migrants. Since previous work shows that social stress may lead to enduring changes in the gray matter volume of vulnerable brain regions, we investigated the impact of migration background on brain structure. We studied healthy young adults (N = 124), native Germans and second-generation migrants, using whole-brain structural magnetic resonance imaging. Groups were matched for a broad range of sociodemographic characteristics including age, gender, urban exposure, and education. We found a significant group by sex interaction effect in pACC gray matter volume, which was reduced in males with migration background only. This mirrors previous findings in urban upbringing, another risk factor for schizophrenia. Our results provide convergent evidence for an impact of environmental risk factors linked to schizophrenia on gray matter volume and extend prior data by highlighting the possibility that the pACC structure may be particularly sensitive to the convergent risk factors linked to schizophrenia.
Keywords: environmental risk, minority status, magnetic resonance imaging, gray matter volume, schizophrenia risk
Introduction
Schizophrenia is a severe neurodevelopmental disorder with a complex genetic and environmental etiology.1,2 Following the scientific evidence regarding the high heritability of schizophrenia during past decades, the focus of research was mostly on the genetic risk factors.1–3 Even though schizophrenia is highly heritable,4 the genetic risk is not the whole story.1–3 Previous research showed that the relative risk of several environmental factors ranges in the order of 2 to 5.5 This points out the importance of causal environmental factors, yet there is only limited research on their mechanisms.5–7
Higher rates of schizophrenia are typically observed in males, immigrants, and individuals brought up in larger cities.5,8–10 To illustrate, the incidence rate ratio of schizophrenia for male:female is about 1.4:1,11 while the ratio for migrant: nonmigrant ranges between 2:1 and 5:1.8,11,12 This shows that migration status is among the strongest environmental risk factors for schizophrenia.5 Importantly, meta-analyses show increased risk for schizophrenia emerges not only in first- but also in second-generation migrants.8,11–13 The latter group was not subjected to adverse experiences in the home country or during the migration process itself, which argues for a role of the post-migratory environment in individuals with a migrant status. Here, accumulating evidence points to a causal role for social adversity and chronic social stress in mediating ethnic minority risk.8,12–16 Social stress is an established environmental risk factor for general and mental health17,18 and has been shown to interfere with the functional organization of neural regulatory circuits,19–21 particularly in vulnerable periods of brain development.22–25
Early life is a vulnerable period during which stress can disrupt normal brain development.25–28 Numerous studies with primates and rodents have shown that prenatal or postnatal exposure to stress can interact with gene expression and disturb neuroplasticity, particularly through alteration of hypo-thalamic pituitary-adrenal (HPA) axis development, and related neurotransmitter systems.25,26,28 The same mechanism has been proposed to mediate vulnerability to psychiatric disorders especially after early exposure to stressful life events in humans.29–31 However, the exact neural mechanisms are still under investigation.
Previous studies32–34 suggest functional alterations in response to social evaluative stress in the perigenual anterior cingulate cortex (pACC), a key neural region for the regulation of negative emotion and stress,35 as a risk mechanism. Using functional magnetic resonance imaging (fMRI) in healthy German participants,32 we found that the pACC activation during stress was associated with urban upbringing.32 This functional alteration also correlated with perceived group discrimination levels of participants with migration background.33 Convergent evidence for the involvement of ACC was obtained by Haddad and colleagues36 who showed a significant interaction between urban exposure during early years of life, another potent environmental risk factor for schizophrenia, and male sex.36 Accordingly, there is extensive literature proposing altered ACC function and structure during the course of schizophrenia. Several studies observed neuroanatomical changes, such as decreased gray matter volume in ACC and other prefrontal structures, in populations with high-risk state for psychosis,37–39 as well as first-episode schizophrenia.40–42 Of those, a recent meta-analysis showed pACC changes, together with insula, to be 1 of 2 regions where both structural and functional changes are found in early psychosis.43
In this study, we hypothesized that structural alterations in pACC might be uncovered in second-generation migrants. Specifically, based on the literature showing earlier age of onset for schizophrenia and poorer illness outcome in males,44–48 and our earlier sex-dependent volumetric findings in pACC in the context of urban upbringing,36 we hypothesized an interaction effect of migration background and sex on pACC volume. Notably, there is no consistent evidence showing that male migrants have an increased risk of schizophrenia compared with female migrants, with some studies reporting such an interaction49–51 and others not.8,12 Based on our previous findings, we assumed that there might be underlying mechanistic differences at the brain level which do not manifest at the clinical behavioral level.
All participants of this study were born-and-raised in Germany. Despite the limited epidemiological evidence on increased schizophrenia risk in migrants living in Germany, the existing research proposes a similar picture for Germany as the rest of Europe.52–55 Even though prevalence of environmental risk factors may still vary according to ethnicity in second-generation migrants,12 we adopted this strategy to minimize possible confounding factors that are related to the country of origin (eg, exposure to environmental toxins) or during the actual migration process (eg, adverse experiences during migration).
Materials and Methods
Participants
A total of 124 right-handed, healthy young volunteers (50 men, mean age = 23.17, SD = 3.98 y) participated in this study. Participants were recruited from communities in and around the city of Mannheim (in the southwestern part of Germany). Sixty-six native Germans (defined as both parents being born and raised in Germany), and 58 individuals with migration background took part in our neuroimaging study. In this study, we examined the effects of migrant status in “second-generation migrants” which was defined to mean that the individuals were born in Germany to non-German parents (both parents were born abroad and moved to Germany later in life). The second-generation migrants possessed a diverse ethnic parental background including Algerian, Egyptian, Italian, Kazakhstani, Polish, Romanian, Russian, Syrian, Turkish, Vietnamese, and former Yugoslavian. Detailed characteristic of the samples is provided in table 1. All participants provided written informed consent to the study protocol approved by the institutional review board of the University of Heidelberg. Following a thorough phone screening, participants were included if only they did not meet any of the following exclusion criteria: a lifetime history of general medical, psychiatric or neurological illness, prior psychopharmacological or psychotherapeutic treatment, drug or alcohol abuse, a history of head trauma and unsuitability for magnetic resonance environment.
Table 1.
Characteristics of Subjects
| Germans | Migrants | Group Comparison (P value) | |||
|---|---|---|---|---|---|
| N | Group Mean (SD) | N | Group Mean (SD) | ||
| Demographics | |||||
| Sex (female/male) | 39/27 | 35/23 | 1.00 | ||
| Age (y) | 66 | 23.47 (3.88) | 58 | 22.83 (4.10) | .372 |
| School education (y) | 66 | 12.55 (1.08) | 58 | 12.31 (1.13) | .239 |
| Early urbanicity | 66 | 34.55 (10.17) | 58 | 36.76 (10.09) | .229 |
| Current urbanicity | 66 | 2.61 (0.68) | 58 | 2.72 (0.62) | .314 |
| Monthly income (€) | 44 | 1847.50 (1666.89) | 55 | 2171.00 (1591.54) | .328 |
| Psychological measures | |||||
| Chronic stress scale (sum score) | 66 | 14.02 (6.74) | 58 | 19.41 (9.05) | <.001 |
| Perceived social support (mean score) | 34 | 3.68 (0.40) | 56 | 3.70 (0.43) | .833 |
| Perceived social status (10 rung social ladder) | 56 | 6.39 (1.59) | 58 | 6.57 (1.35) | .525 |
| Perceived self-discrimination | — | — | 57 | 2.32 (0.95) | — |
| Perceived group-discrimination | — | — | 57 | 3.56 (1.02) | — |
Psychological Measures
Psychometric self-reports were obtained according to previously described32 standard procedures. Chronic Stress Screening Scale (CSSS)56 was used to measure chronic stress by asking the frequency of negative and stressful experiences within the past 3 months. Perceived social support was assessed using subscales of the Berlin Social Support Scales (BSSS)57 which comprise items of emotional and instrumental support. For the assessment of perceived social status in German society, ie, the individual’s perception of his or her social standing relative to other people in German society, we used a previously published58,59 single-item measure presented as a pictorial 10 rung “social ladder,” on which participants marked the rung corresponding to their current perceived standing relative to other individuals residing in Germany. In migrants only, perceived discrimination of the own person and the perceived discrimination of the own ethnic group in German society were evaluated using an adapted version of the discrimination measures detailed in Ruggiero and Taylor.60 As detailed in our previous work,33 this measure is a self-rate instrument quantifying the extent of discrimination that ethnic minority individuals experience in German society, and attribute to their variant ethnic background. In addition to the psychological measures explained above, we have also acquired details about each participant’s places of residence from birth to age 15. Current and early urbanicity scores were quantified using categories as described previously.32,36
MRI Data Acquisition
MRI was acquired on a 3-Tesla whole-body Siemens Magnetom Tim Trio (Siemens) scanner located at the Central Institute of Mental Health, Mannheim. We used a T1-weighted 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence with whole-brain coverage, spatial resolution of 1 mm3 and the following specifications: repetition time = 2300 milliseconds (ms), echo time = 3.00 ms, inversion time = 900 ms, flip angle = 9°, 192 contiguous sagittal slices, 1-mm slice thickness, field of view = 256 mm.
Image Processing
Images were processed using the Voxel-Based Morphometry (VBM) toolbox (VBM8, http://dbm.neuro.uni-jena.de/vbm) implemented in the Statistical Parametric Mapping (SPM) software, (SPM8; Wellcome Trust Centre for Neuroimaging, University College London; http://www.fil.ion.ucl.ac.uk/spm) executed in Matlab R2011a (MathWorks). VBM is an automated whole-brain processing method, which allows the composition of brain tissue to be compared among (between and within) groups,61 being an indirect measure of volume. Image processing followed previously published procedures.36 As it is formerly explained,36 automated image processing included tissue classification into gray matter (GM), white matter, cerebrospinal fluid, and non-cerebral tissue classes. Normalization to Montreal Neurological Institute (MNI) space was done with a diffeomorphic image registration algorithm (DARTEL). Additionally correction for image intensity nonuniformity, cleaning up of GM partitions, the application of a hidden Markov random field model and spatial adaptive nonlocal means denoising was applied. The resulting tissue segments were multiplied by the Jacobian determinants of the deformation field to transform the GM density values into volume equivalents. Images of each subject were corrected for total intracranial volume. The segmented, normalized, noise-corrected, and modulated GM images were then smoothed with a 10-mm full width at half maximum isotropic Gaussian kernel.
Analysis of Psychological Measures
Data analysis of psychological and demographic measures was performed using the SPSS Predictive Analytics Software (SPSS 22, IBM Inc). Group differences for categorical variables were examined using Chi-square tests, those for continuous variables were examined using t tests for independent samples. The level of statistical significance for psychological variables was defined as P < .05 (2-tailed).
MRI Data Analysis
Raw images were visually inspected to confirm that they had not been affected by scanner artifacts. Pre-processed GM volume segments were analyzed in SPM8 using a general linear model (GLM) with whole-brain random-effects group statistics. Effects of migration background,33 male sex relating to schizophrenia risk36,62,63 and interaction of early urbanicity with sex36 were tested in an ANOVA model with migrant status and sex as factors, and early urbanicity along with CSSS score as predictors allowing factor by covariate interaction. Additionally, age, education, and current urbanicity were introduced as covariates of no interest in the model. In brief, statistical testing of all hypotheses was done within the same statistical model to account for confounding effects of other predictors on the contrast of interest.
Consistent with prior work from our laboratory32,33,64 and other groups,65 we expected social risk-associated structural effects to map to the pACC, a crucial brain region for the top-down regulation of neural responses in emotion and stress processing circuitries.66,67 As in previous work,32,33,64 all imaging analyses were family-wise error (FWE) corrected at a threshold of P < .05 for multiple comparisons over an a priori defined region of interest (ROI), namely pACC, derived from the Harvard Oxford Atlas (HO, http://www.cma.mgh.harvard.edu), which was modified to cover the rostral-ventral divisions of the ACC (see previous publications32,33 for details). Outside this pre-hypothesized ROI, effects were considered significant only if they survived FWE multiple comparisons correction across the whole brain.
Results
Psychological Measures
Groups did not show any significant difference in sociodemographic variables (all P > .200). Individuals with migration background reported significantly higher perceived chronic stress levels (CSSS: T = 3.721; P < .001; table 1). There were no significant differences in any of the demographic variables between females and males with migration background (all P > .100). However, within the migrant group, males indicated lower perceived support (BSSS: T = 2.232; P = .034), compared with females (table 2).
Table 2.
Comparison of Female and Male Migrants
| Female Migrants | Male Migrants | Group Comparison (P value) | |||
|---|---|---|---|---|---|
| N | Group Mean (SD) | N | Group Mean (SD) | ||
| Demographics | |||||
| Age (y) | 35 | 22.57 (3.54) | 23 | 23.33 (4.88) | .561 |
| School education (y) | 35 | 12.40 (1.06) | 23 | 12.17 (1.23) | .460 |
| Early urbanicity | 35 | 37.86 (10.21) | 23 | 35.09 (9.89) | .310 |
| Current urbanicity | 35 | 2.77 (0.55) | 23 | 2.65 (0.71) | .475 |
| Psychological measures | |||||
| Chronic stress scale (sum score) | 35 | 19.91 (8.65) | 23 | 18.65 (9.78) | .608 |
| Perceived social support (mean score) | 33 | 3.82 (0.26) | 23 | 3.54 (0.56) | .034 |
| Perceived social status (10 rung social ladder) | 35 | 6.80 (1.35) | 23 | 6.22 (1.31) | .109 |
| Perceived self-discrimination | 34 | 2.21 (0.98) | 23 | 2.48 (0.90) | .291 |
| Perceived group-discrimination | 34 | 3.56 (1.13) | 23 | 3.57 (0.84) | .982 |
Gray Matter Volume
For all the results we report here, we introduced age, sex, education, chronic stress, and urban exposure as covariates in our models. Therefore, we propose that these demographic variables cannot be the explanation for the findings we report here.
Main Effect of Group and Main Effect of Sex.
We detected no significant differences in GM volume between second-generation immigrants and German participants in pACC (Tmaximum = 2.30; Pminimum = .418, FWE corrected in ROI) or elsewhere in the brain including the dorsolateral prefrontal cortex (DLPFC). This was also true for the comparison of female vs male GM volume (Tmaximum = 2.56; Pminimum = .277, FWE corrected in ROI).
Group by Sex Interaction.
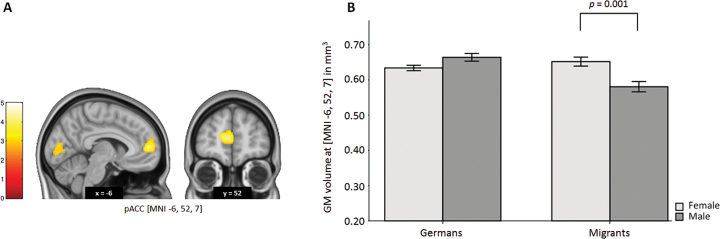
We observed a significant group (migrant status) by sex interaction in pACC (MNI: x = −6, y = 52, z = 7; T = 4.17, PFWE = .004, corrected within ROI). Post hoc analyses revealed a significant reduction of pACC GM volume only in males with migration background (P = .001, uncorrected, Cohen’s d = 0.98) compared with female migrants (figure 1). This effect was specific to pACC, and not observed in the German group (Tmaximum = 2.77; Pminimum = 0.214, FWE corrected in ROI). Additional analysis did not reveal any significant association between brain and BSSS or CSSS scores. Moreover, in order to investigate whether the heterogeneous ethnic background of the migrant sample might have influenced our findings, we conducted a post hoc analysis using the same statistical model on the group of Germans compared with the group of second-generation migrants with Turkish parental background only. Our results were consistent, suggesting a significant decrease in GM volume in pACC in migrant males (MNI: −14, 50, 10; T = 3.39, PFWE = .045, FWE corrected in ROI).
Fig. 1.
Migrant status and sex interaction on ACC GM volume. (A) T-map of interaction effects between migrant status and sex on GM volume. A significant interaction effect is seen in the pACC (T = 4.17, P = .004, FWE-corrected within ROI). A significant decrease in pACC GM volume in migrant males compared with females and nonmigrants is observed. (B) Mean GM volumes of the most significant voxel (MNI: x = −6, y = 52, z = 7). Error bars indicate standard error of the mean. Coordinates refer to the MNI standard space. T-maps are displayed at P < .005 uncorrected for presentation purposes. The color bar represents T-values. GM, gray matter; pACC, perigenual anterior cingulate cortex; MNI, Montreal Neurological Institute; FWE, family-wise error correction for multiple comparisons; ROI, region of interest.
Replication of Sex by Early Urbanicity Interaction.
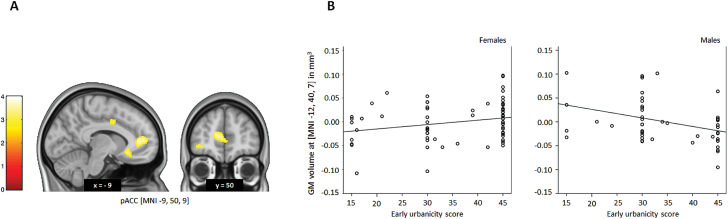
In our whole sample we replicated the results by Haddad and colleagues36 suggesting a decrease in pACC GM volume in males with high early urbanicity (MNI: x = −12, y = 40, z = 7; T = 3.78, PFWE = .014; and MNI: x = −9, y = 50, z = 9; T = 3.49, PFWE = .033 corrected within ROI; figure 2). Post hoc analyses revealed a significantly negative correlation between early-life urbanicity and pACC GM volume specifically in males (r = −.318, P = .026) after corrected for age, which was absent in females (r = .220, P = .061). This effect was specific to pACC. Since 5.6% of our sample was overlapping with the sample of Haddad and collegues36 we additionally retested our replication of the urban upbringing by sex interaction effect on pACC gray matter volume in a nonoverlapping subsample of N = 117 adults. None of the participants from this subsample was also part of the sample reported by Haddad and colleagues.36 The results were consistent (MNI: x = −14, y = 50, z = 10; T = 3.76, PFWE = .016 corrected within ROI).
Fig. 2.
Early life urbanicity and sex interaction on ACC GM volume. (A) T-map of interaction effects between early-life urbanicity and sex on GM volume. A significant interaction effect is seen in the pACC (T = 3.49, P = .033, FWE-corrected within ROI). (B) Scatterplots of early-life urbanicity and adjusted GM volumes of the most significantly correlated voxel for the interaction effect (MNI: x = −12, y = 40, z = 7) illustrate a negative correlation in males and no correlation in females. The scatter plot is adjusted for demographic covariates. Coordinates refer to the MNI standard space. T-maps are displayed at P < .005 uncorrected for presentation purposes. The color bar represents T-values. GM, gray matter; pACC, perigenual anterior cingulate cortex; MNI, Montreal Neurological Institute; FWE, family-wise error correction for multiple comparisons; ROI, region of interest.
Discussion
The goal of our study was to examine the hypothesis that migration background, an established environmental risk for schizophrenia, impacts brain structure, specifically in pACC. Our main result is a significant reduction in pACC GM volume in migrant males. As previous literature suggests, we also observed significantly higher chronic stress in the second-generation migrant group compared with nonmigrants. Additionally, we were able to replicate the finding of Haddad and colleagues36 indicating a significant association between early urbanicity and ACC GM volume in males. In line with our hypothesis, we provide evidence for an adverse effect on a neurobiological system where social environmental risk seems to converge. To our knowledge, this is the first study that demonstrates a link between brain morphology and migration background, which is a well-established risk factor for schizophrenia that has been associated with exposure to stress in social environment.18,33 Following epidemiological studies showing the increased risk of schizophrenia in both first- and second-generation migrants, many researchers proposed a causal role of social stress and chronic social defeat in migrants.5,8,14 Exposure to chronic social defeat and discrimination starting early in life might result in a condition where social stress is persistent and inevitable, and consequently psychological well-being is disturbed.14,68–70 Considering that early childhood and adolescence are critical developmental periods where neuroplasticity is at its peak level, the brain is particularly sensitive to environmental input during these first decades of life.27,71,72 Therefore, it can be suggested that exposure to chronic social stress in early life can alter particular brain structures, such as ACC and thereby increasing the risk for schizophrenia in certain groups. How these neural mechanisms exactly work and lead to increased risk for schizophrenia in immigrant populations, however, is not yet completely clear.
There is no conclusive evidence showing that male migrants have an increased risk of schizophrenia compared with female migrants. Yet, previous animal research indicates that there are clear sex differences in the neurobiological outcome of exposure to chronic stress. The effects of early life stress seem to operate differently for male and female sexes on the brain level.73,74 While the exact rodent homologues of prefrontal structures such as DLPFC and pACC are debatable,75,76 the evidence indicates sex specific differences in stress processing in ACC in humans.77,78 Besides, it seems that defeat is a major stressor that particularly affects males,79 and chronic stress induces long-term neural plasticity (such as dendritic atrophy) specifically in male rats and not in female rats.80 Likewise, Brydges and colleagues81 demonstrated differential long-term effects of prepubertal stress in male and female rats.81 Once more, similar findings were observed in humans as well.82 The reasons for the difference between sexes in neurobiological responses to stress can be explained by the distinctive level of gonadal hormones, estrogen and testosterone in the developing brain particularly during prenatal development.74,83 This is in clear accordance with the previous finding of a sex-specific effect of urban upbringing on brain structure.36 Therefore, we conclude that this might also be the case for males with migration background. Furthermore, our results suggesting a sex specific pACC GM volume reduction in migrant males were still consistent in the subsample of second-generation migrants with a homogenous ethnic background. This deems the possibility of our results being driven by the heterogeneity of the ethnic background unlikely. Our post hoc analysis of the sociodemographic differences between females and males revealed no demographic differences between sexes in migrant group. However, males with migration background declared lower perceived social support. Lack of social support is suggested to be one of the most important social risk factors for the development of several mental health problems.9,84–87 Even though the feeling of loneliness is not a cause of mental illness per se, isolation increases the neurobiological stress reaction88 and affects striatal dopamine transporter binding following defeat in animal models.89 Moreover, lack of social support is suggested to be one of the possible psychological components of increased schizophrenia risk related to high urbanicity and minority status.14,70,90–92 We did not find any direct correlation of our social support measure with the brain; however, this does not eliminate the possibility of its involvement in the psychological mechanisms of increased risk associated with having a migration background.
Existing literature on the neurobiological basis of schizophrenia risk and social stress conform to the localization of our results. Numerous studies highlight the involvement of ACC in schizophrenia, including detectable structural and functional changes revealing itself already at disease onset.93–96 Additionally, volumetric alterations in this region have been associated with both genetic and environmental risk factors for the illness.1,3,9,20,37 Smaller ACC volume has been previously shown to relate cumulative adversity over the lifetime97 and early life stress.98 Our ROI, pACC, is a fundamental brain region in stress processing and emotion regulation.19,34,99,100 PACC activation correlates with cardiovascular markers of the stress response101–103 and has been repeatedly shown to relate to several social environmental risk factors for schizophrenia, such as urban upbringing,32,36 low socioeconomic status,58 unstable social hierarchies,104 migration,33 perceived discrimination,33 and urban violence.105 Therefore, in line with our data, the previous literature supports the idea that social environmental risk factors converge on pACC in humans.1,20,106,107 Consequently, it is plausible that long-lasting structural and functional changes in this neural system might eventually increase the risk for psychiatric disorders in humans.19,108,109
Here, we present the first neuroimaging study of gray matter volume in migrants. Our study has several limitations. Firstly, our cross-sectional study design does not allow for a causal interpretation of the observed neural effects. Yet, experimental investigation of epidemiological findings is essential for the examination of the underlying mechanisms of environmental risk factors for mental health. Secondly, we examined only volunteers who have contacted us following our advertisements. Therefore, further research is needed to replicate these findings in diverse ethnic minority groups with different sociodemographic characteristics. Third, participants were screened via a thorough telephone interview for exclusion criteria, including history of mental and neurological illness. Even though this approach is an established screening method in neuroimaging research,110 a face-to-face interview should be considered for future studies. Next, we cannot rule out the possibility that the observed results in gray matter volume are influenced to some extend by possible group differences in other risk-related factors such as diet, childhood socioeconomic status, or childhood adversity. It is important to note that evidence showing that male migrants have an increased risk of schizophrenia compared with their female counterparts is inconclusive. Even though numerous studies showed that migrant patients were more likely to be male,49 and psychosis risk was more pronounced in males in Moroccan,111 Northwest African,51 and refugee50 migrant samples, studies exist that found no difference between female and male migrants.8,12 However, neural-level effects may be more sensitive to underlying risk factors than clinical diagnoses.112 Ideally, future studies should involve comparison of several homogenous samples with different ethnic backgrounds and control samples of subjects of the same ethnicity who did not migrate. Further, we have not measured early life stress directly. Our proposition that having a migrant status leads to high social stress is well-accepted but not directly verified in this specific sample. Lastly, unlike our findings for urban upbringing,36 we did not observe a structural main effect of migrant status on DLPFC volume in our sample. We speculate that although urban upbringing and migrant status may share some neural effects (eg, in pACC), there are also risk factor-specific effects on local gray matter volume that are nonoverlapping at the brain system level.
In conclusion, our study points out a potentially useful target for the prevention of the severe neurodevelopmental disorder, namely schizophrenia. By identifying a convergent risk circuit involving pACC, we propose the indisputable role of chronic social stress as a mediator which may present itself as an objective for future prevention and intervention programs. In the long term, the better understanding of these neural circuits will enable the comprehension of the causes of observed differences in risk ratios between sexes and in minority groups for the development of schizophrenia. For this reason, it should be noted that experimental studies based on epidemiological findings, such as this one, are useful to better understand the components and mechanisms of risk, and to translate social and biological determinants of risk for schizophrenia into daily life.
Funding
This work was supported by the European Community’s Seventh Framework Programme under grant agreement No. HEALTH-F2-2010-241909 (Project EU-GEI). H.T. acknowledges grant support by the German Federal Ministry of Education and Research, BMBF (01GQ1102).
Acknowledgments
The Authors have declared that there are no conflicts of interest in relation to the subject of this study. We would like to thank Dagmar Gass, Ilka Alexi, Oliver Grimm, Michael Schneider, Urs Braun, and Carolin Mössnang for their help. A.M-L. has received consultant fees and travel expenses from Alexza Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Defined Health, Decision Resources, Desitin Arzneimittel, Elsevier, F. Hoffmann–La Roche, Gerson Lehrman Group, Grupo Ferrer, Les Laboratoires Servier, Lilly Deutschland, Lundbeck Foundation, Outcome Sciences, Outcome Europe, PriceSpective, and Roche Pharma and has received speaker’s fees from Abbott, AstraZeneca, BASF, Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Pfizer Pharma, and Servier Deutschland. No other disclosures were reported.
References
- 1. Tost H, Meyer-Lindenberg A. Puzzling over schizophrenia: schizophrenia, social environment and the brain. Nature Medicine. 2012;18:211–213. [DOI] [PubMed] [Google Scholar]
- 2. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 3. Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. [DOI] [PubMed] [Google Scholar]
- 4. McGuffin P, Gottesman Risk factors for schizophrenia. N Engl J Med. 1999;341:370–371; author reply 372. [PubMed] [Google Scholar]
- 5. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 6. van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–608. [DOI] [PubMed] [Google Scholar]
- 8. Bourque F, van der Ven E, Malla A. A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychol Med. 2011;41:897–910. [DOI] [PubMed] [Google Scholar]
- 9. Akdeniz C, Tost H, Meyer-Lindenberg A. The neurobiology of social environmental risk for schizophrenia: an evolving research field. Social Psychiatry Psychiatr Epidemiol. 2014;49:507–517. [DOI] [PubMed] [Google Scholar]
- 10. Picchioni MM, Murray RM. Schizophrenia. BMJ. 2007;335:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. [DOI] [PubMed] [Google Scholar]
- 13. Veling W. Ethnic minority position and risk for psychotic disorders. Current Opin Psychiatry. 2013;26:166–171. [DOI] [PubMed] [Google Scholar]
- 14. Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Brit J Psychiatry. 2005;187:101–102. [DOI] [PubMed] [Google Scholar]
- 15. Mondelli V, Dazzan P, Hepgul N, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veling W, Susser E. Migration and psychotic disorders. Expert Rev Neurother. 2011;11:65–76. [DOI] [PubMed] [Google Scholar]
- 17. Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatry. 2007;20:158–162. [DOI] [PubMed] [Google Scholar]
- 18. Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18:1421–1431. [DOI] [PubMed] [Google Scholar]
- 19. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Baram TZ. Toward understanding how early-life stress reprograms cognitive and emotional brain networks [published online ahead of print June 24, 2015]. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niwa M, Jaaro-Peled H, Tankou S, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burghy CA, Stodola DE, Ruttle PL, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010;119:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bock J, Wainstock T, Braun K, Segal M. Stress in utero: prenatal programming of brain plasticity and cognition. Biol Psychiatry. 2015;78:315–326. [DOI] [PubMed] [Google Scholar]
- 26. McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis. 2010;39:66–72. [DOI] [PubMed] [Google Scholar]
- 27. Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci Biobehav Rev. 2009;33:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–723. [DOI] [PubMed] [Google Scholar]
- 29. McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. J R Soc Med. 2012;105:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. [DOI] [PubMed] [Google Scholar]
- 31. Khan A, McCormack HC, Bolger EA, et al. Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front Psychiatry. 2015;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. [DOI] [PubMed] [Google Scholar]
- 33. Akdeniz C, Tost H, Streit F, et al. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. [DOI] [PubMed] [Google Scholar]
- 34. Streit F, Haddad L, Paul T, et al. A functional variant in the neuropeptide S receptor 1 gene moderates the influence of urban upbringing on stress processing in the amygdala. Stress. 2014;17:352–361. [DOI] [PubMed] [Google Scholar]
- 35. Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haddad L, Schafer A, Streit F, et al. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia [published online ahead of print June 3, 2014]. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. [DOI] [PubMed] [Google Scholar]
- 38. Nakamura K, Takahashi T, Nemoto K, et al. Gray matter changes in subjects at high risk for developing psychosis and first-episode schizophrenia: a voxel-based structural MRI study. Front Psychiatry. 2013;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. [DOI] [PubMed] [Google Scholar]
- 40. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. [DOI] [PubMed] [Google Scholar]
- 42. Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65:746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–2333. [DOI] [PubMed] [Google Scholar]
- 44. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. [DOI] [PubMed] [Google Scholar]
- 45. Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pulver AE, Brown CH, Wolyniec P, et al. Schizophrenia: age at onset, gender and familial risk. Acta Psychiatr Scandinavica. 1990;82:344–351. [DOI] [PubMed] [Google Scholar]
- 47. Nasrallah HA, Schwarzkopf SB, Olson SC, Coffman JA. Gender differences in schizophrenia on MRI brain scans. Schizophr Bull. 1990;16:205–210. [DOI] [PubMed] [Google Scholar]
- 48. Angermeyer MC, Kuhn L, Goldstein JM. Gender and the course of schizophrenia: differences in treated outcomes. Schizophr Bull. 1990;16:293–307. [DOI] [PubMed] [Google Scholar]
- 49. Mitter P, Reeves S, Romero-Rubiales F, Bell P, Stewart R, Howard R. Migrant status, age, gender and social isolation in very late-onset schizophrenia-like psychosis. Int J Geriatr Psychiatry. 2005;20:1046–1051. [DOI] [PubMed] [Google Scholar]
- 50. Hollander AC, Dal H, Lewis G, Magnusson C, Kirkbride JB, Dalman C. Refugee migration and risk of schizophrenia and other non-affective psychoses: cohort study of 1.3 million people in Sweden. BMJ. 2016;352:i1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Ven E, Veling W, Tortelli A, et al. Evidence of an excessive gender gap in the risk of psychotic disorder among North African immigrants in Europe: a systematic review and meta-analysis [published online ahead of print July 2, 2016]. Soc Psychiatry Psychiatr Epidemiol. [DOI] [PubMed] [Google Scholar]
- 52. Schouler-Ocak M, Schepker R, Bretz HJ, et al. [Patients of immigrant origin in inpatient psychiatric facilities. Differences between first and second generation: nationwide questionnaire of the Psychiatry and Migration Working Group of the German Federal Conference of Psychiatric Hospital Directors]. Der Nervenarzt. 2010;81:86–94. [DOI] [PubMed] [Google Scholar]
- 53. Schouler-Ocak M, Bretz HJ, Penka S, et al. Patients of immigrant origin in inpatient psychiatric facilities. A representative national survey by the Psychiatry and Migration Working Group of the German Federal Conference of Psychiatric Hospital Directors. Eur Psychiatry. 2008;23(suppl 1):21–27. [DOI] [PubMed] [Google Scholar]
- 54. Haasen C, Lambert M, Mass R, Krausz M. Impact of ethnicity on the prevalence of psychiatric disorders among migrants in Germany. Ethn Health. 1998;3:159–165. [DOI] [PubMed] [Google Scholar]
- 55. Salentin K. Diskriminierungserfahrungen ethnischer Minderheiten in der Bundesrepublik. In: Groenemeyer A, Wieseler S, eds. Soziologie sozialer Probleme und sozialer Kontrolle: Realitäten, Repräsentationen und Politik. Festschrift für Günther Albrecht. Wiesbaden, Germany: VS Verlag für Sozialwissenschaft; 2008:515–526. [Google Scholar]
- 56. Schulz P, Schlotz W, Becker P. Trierer inventar zum chronischen stress. Göttingen, Germany: Hogrefe Verlag; 2004. [Google Scholar]
- 57. Schwarzer R, Schulz U. Soziale Unterstützung bei der Krankheitsbewältigung: Die Berliner Social Support Skalen (BSSS). Diagnostica. 2003;49:73–82. [Google Scholar]
- 58. Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. [DOI] [PubMed] [Google Scholar]
- 60. Ruggiero K, Taylor DM. Coping with discrimination: how disadvantaged group members perceive the discrimination that confronts them. J Pers Soc Psychol. 1995;68:826–838. [DOI] [PubMed] [Google Scholar]
- 61. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- 62. Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. [DOI] [PubMed] [Google Scholar]
- 63. Eranti SV, MacCabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol Med. 2013;43:155–167. [DOI] [PubMed] [Google Scholar]
- 64. Haddad L, Schäfer A, Streit F, et al. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr Bull. 2015;41:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. D’Aguiar C. What stress does to your brain: a review of neuroimaging studies. Can J Psychiatry. 2009;54:6. [DOI] [PubMed] [Google Scholar]
- 68. Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: shame, social self-esteem, and cortisol activity. Psychosom Med. 2004;66:915–924. [DOI] [PubMed] [Google Scholar]
- 69. Selten JP, Cantor-Graae E, Kahn RS. Migration and schizophrenia. Current Opin Psychiatry. 2007;20:111–115. [DOI] [PubMed] [Google Scholar]
- 70. Selten JP, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 2013;39:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biological psychiatry. 1999;46:1509–1522. [DOI] [PubMed] [Google Scholar]
- 72. Montaron MF, Koehl M, Lemaire V, Drapeau E, Abrous DN, Le Moal M. Environmentally induced long-term structural changes: cues for functional orientation and vulnerabilities. Neurotox Res. 2004;6:571–580. [DOI] [PubMed] [Google Scholar]
- 73. Lu J, Wu X-Y, Zhu Q-B, et al. Sex differences in the stress response in SD rats. Behav Brain Res. 2015;284:231–237. [DOI] [PubMed] [Google Scholar]
- 74. Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nature Neurosci. 2015;18:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cognitive Neuroscience. 1995;7:1–24. [DOI] [PubMed] [Google Scholar]
- 76. Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. [DOI] [PubMed] [Google Scholar]
- 77. Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Map. 2011;32:1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goldstein JM, Lancaster K, Longenecker JM, et al. Sex differences, hormones, and fMRI stress response circuitry deficits in psychoses. Psychiatry Res. 2015;232:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haller J, Fuchs E, Halász J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull. 1999;50:33–39. [DOI] [PubMed] [Google Scholar]
- 80. Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brydges NM, Wood ER, Holmes MC, Hall J. Prepubertal stress and hippocampal function: sex-specific effects. Hippocampus. 2014;24:684–692. [DOI] [PubMed] [Google Scholar]
- 82. Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. [DOI] [PubMed] [Google Scholar]
- 83. Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. [DOI] [PubMed] [Google Scholar]
- 84. Kawachi I, Berkman LF. Social ties and mental health. J Urban Health. 2001;78:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bovier PA, Chamot E, Perneger TV. Perceived stress, internal resources, and social support as determinants of mental health among young adults. Qual Life Res. 2004;13:161–170. [DOI] [PubMed] [Google Scholar]
- 86. Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–387. [DOI] [PubMed] [Google Scholar]
- 87. Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. [DOI] [PubMed] [Google Scholar]
- 88. Niesink RJ, van Ree JM. Involvement of the pituitary-adrenal axis in socio-behavioral disturbances after short-term isolation. Physiol Behav. 1983;30:825–830. [DOI] [PubMed] [Google Scholar]
- 89. Isovich E, Engelmann M, Landgraf R, Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. Eur J Neurosci. 2001;13:1254–1256. [DOI] [PubMed] [Google Scholar]
- 90. March D, Hatch SL, Morgan C, et al. Psychosis and place. Epidemiol Rev. 2008;30:84–100. [DOI] [PubMed] [Google Scholar]
- 91. Weiser M, Werbeloff N, Vishna T, et al. Elaboration on immigration and risk for schizophrenia. Psychol Med. 2008;38:1113–1119. [DOI] [PubMed] [Google Scholar]
- 92. Das-Munshi J, Becares L, Boydell JE, et al. Ethnic density as a buffer for psychotic experiences: findings from a national survey (EMPIRIC). Brit J Psychiatry. 2012;201:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hall MH, Smoller JW, Cook NR, et al. Patterns of deficits in brain function in bipolar disorder and schizophrenia: a cluster analytic study. Psychiatry Res. 2012;200:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rish I, Cecchi G, Thyreau B, et al. Schizophrenia as a network disease: disruption of emergent brain function in patients with auditory hallucinations. PLoS One. 2013;8:e50625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bakhshi K, Chance SA. The neuropathology of schizophrenia: a selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience. 2015;303:82–102. [DOI] [PubMed] [Google Scholar]
- 97. Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. [DOI] [PubMed] [Google Scholar]
- 99. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. [DOI] [PubMed] [Google Scholar]
- 100. Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. [DOI] [PubMed] [Google Scholar]
- 101. Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rocha-Rego V, Pereira MG, Oliveira L, et al. Decreased premotor cortex volume in victims of urban violence with posttraumatic stress disorder. PLoS One. 2012;7:e42560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Holz NE, Laucht M, Meyer-Lindenberg A. Recent advances in understanding the neurobiology of childhood socioeconomic disadvantage. Current Opin Psychiatry. 2015;28:365–370. [DOI] [PubMed] [Google Scholar]
- 107. Holz NE, Boecker R, Jennen-Steinmetz C, et al. Positive coping styles and ACC volume - two related mechanisms for conferring resilience? [published online ahead of print January 7, 2016]. Soc Cogn Affect Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shtasel DL, Gur RE, Mozley PD, et al. Volunteers for biomedical research. Recruitment and screening of normal controls. Arch Gen Psychiatry. 1991;48:1022–1025. [DOI] [PubMed] [Google Scholar]
- 111. Veling W, Selten J-P, Veen N, Laan W, Blom JD, Hoek HW. Incidence of schizophrenia among ethnic minorities in the Netherlands: a four-year first-contact study. Schizophr Res. 2006;86:189–193. [DOI] [PubMed] [Google Scholar]
- 112. Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. [DOI] [PubMed] [Google Scholar]