Abstract
The objective of the present study was to evaluate whether cefpirome, a member of the latest class of broad-spectrum cephalosporins, sufficiently penetrates subcutaneous adipose tissue in septic patients. After the administration of the drug at 2 g, tissue cefpirome concentrations in septic patients (n = 11) and healthy controls (n = 7) were determined over a period of 4 h by means of microdialysis. To assess the antibacterial effect of cefpirome at the target site, the measured pharmacokinetic profiles were simulated in vitro with select strains of Staphylococcus aureus and Pseudomonas aeruginosa. The tissue penetration of cefpirome was significantly impaired in septic patients compared with that in healthy subjects. For subcutaneous adipose tissue, the area under the concentration-versus-time curve values from 0 to 240 min were 13.11 ± 5.20 g · min/liter in healthy subjects and 6.90 ± 2.56 g · min/liter in septic patients (P < 0.05). Effective bacterial growth inhibition was observed in all in vitro simulations. This was attributed to the significantly prolonged half-life in tissue (P < 0.05), which kept the tissue cefpirome levels above the MICs for relevant pathogens for extended periods in the septic group. By consideration of a dosing interval of 8 h, the values for the time above MIC (T > MIC) in tissue were greater than 60% for pathogens for which the MIC was ≤4 mg/liter in all septic patients. The present data indicate that cefpirome is an appropriate agent for the treatment of soft tissue infections in septic patients. However, due to the high interindividual variability of the pharmacokinetics of cefpirome in tissue, dosing intervals of not more than 8 h should be preferred to ensure that susceptible bacterial strains are killed in each patient.
In septic patients, antibiotic therapy is commonly administered intravenously, and in most cases susceptible bacteria are eliminated from the blood. The eradication of bacteria from the infected tissue is more challenging, because sufficient penetration of an antibiotic to the site of infection is a prerequisite for the successful treatment of patients with soft tissue infections (STIs). However, the pharmacokinetics (PKs) of antimicrobial agents in tissue may differ substantially between critically ill patients, resulting in subinhibitory concentrations in tissue, even though effective levels are detected in the plasma of each individual (12, 27). Such ineffective or subinhibitory concentrations of antibiotics at the target site raise the risk of therapeutic failure, with life-threatening consequences.
Cefpirome, a member of the latest class of broad-spectrum cephalosporins, is characterized by a high degree of stability against hydrolytic bacterial enzymes (8) and is active against numerous gram-negative and gram-positive bacteria. This antibiotic, however, does not harm anaerobic bacteria; hence, it spares the intestinal flora, unlike other antibiotics (8, 31). Due to these favorable properties, cefpirome is frequently used for empirical therapy in severely ill patients in intensive care, oncology, and transplantation units (31).
Against this background we performed the present study to test the ability of cefpirome to penetrate the interstices of soft tissues. Subcutaneous adipose tissue was selected, because in many cases it is the target site of bacterial infection in septic patients. The interstitial space fluid of subcutaneous adipose tissue was collected by use of in vivo microdialysis, and the concentrations of free cefpirome were measured over time. In order to estimate bacterial growth inhibition at the target site, the time-concentration profiles for septic patients were simulated in vitro by use of an established PK-pharmacodynamic (PD) model (21, 22).
MATERIALS AND METHODS
Study protocol.
The study protocol was approved by the Ethics Committee of the Medical University of Vienna and was in accordance with the Declaration of Helsinki in its last revised version (32), with the guidelines for Good Clinical Practice of the European Union (7), and with the Austrian drug law. Healthy volunteers were informed in detail about the purpose, procedures, and risks of the study. All healthy volunteers signed an informed consent prior to inclusion in the study. For comatose septic patients, written consent was sought as soon as it was medically possible. Concomitant therapy was not changed due to the study procedures.
Septic patients.
Since previous PK studies revealed higher degrees of variability in the values of PK parameters in severely ill patients than in healthy volunteers, we decided to include 12 patients and only 8 healthy controls in the present study. All patients required sedation and mechanical ventilation. Sepsis was defined according to the criteria of the American College of Chest Physicians-Society of Critical Care Medicine Consensus Conference Committee (3). Eight male patients and three female patients (n = 11), all Caucasian, were eligible for data analysis. The demographic and clinical characteristics of the study populations are shown in Table 1. The diagnoses at the time of admission to the intensive care unit were coronary heart disease with or without myocardial infarction in seven patients. Decompensated stenosis of the aortic valve, hematothorax linked to surgical complications, carcinoma, and pneumonia were diagnosed in one patient each. Four of 11 patients died during their stays in the intensive care unit. All except one of the patients received additional antibiotic treatment; eight received diuretic therapy, seven received vasopressive drugs, and two patients received glucocorticoid therapy.
TABLE 1.
Demographic characteristics of the study groupsa
| Variable | Patients (n = 11)
|
Volunteers (n = 7)
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Sex (no. of male subjects/no. of female subjects) | 8/3 | 7/0 | ||
| Age (yr) | 66 | 8 | 26 | 5 |
| Ht (cm) | 172 | 5 | 179 | 4 |
| Wt (kg) | 76 | 13 | 71 | 5 |
| Body mass index (kg/m2) | 26 | 4 | 22 | 1 |
| Heart rate (no. of beats/min) | 89 | 14 | 64 | 9 |
| Arterial pressure, systolic (mm Hg) | 112 | 25 | 120 | 6 |
| Arterial pressure, diastolic (mm Hg) | 56 | 6 | 57 | 4 |
| Arterial pressure, mean (mm Hg) | 73 | 9 | 78 | 3 |
| Hematocrit (%) | 31 | 3 | 42 | 3 |
| Hemoglobin concn (g/dl) | 9.6 | 1.1 | 14.8 | 1.3 |
| Leukocyte count (109/liter) | 12 | 5 | 6.6 | 1.7 |
| Protein concn (g/liter) | 55 | 6 | 76 | 2 |
| Serum creatinine level (mg/dl) | 1.3 | 0.7 | 1.1 | 0.1 |
| Blood urea nitrogen level (mg/dl) | 38 | 25 | 13.9 | 2.6 |
| CLCR (ml/min) | 67 | 33 | ||
| Lactate concn (mmol/liter) | 1.5 | 0.9 | ||
| Fibrinogen concn (mg/dl) | 586 | 150 | ||
| Body temp (°C) | 37.5 | 1.0 | ||
| Arterial pH | 7.46 | 0.04 | ||
| Arterial pCO2 (mm Hg) | 38 | 7 | ||
| Arterial pO2 (mm Hg) | 81 | 14 | ||
| Arterial O2 saturation (%) | 96 | 2 | ||
| SOFA score | 5.5 | 2.0 | ||
Values are presented as means ± SDs. Abbreviations: pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SOFA, sepsis-related organ failure assessment.
Healthy controls.
Eight healthy male volunteers of Caucasian race were included in the study as controls, but only seven healthy volunteers were eligible for PK data analysis (Table 1). The volunteers were between 21 and 34 years old and had no relevant abnormalities, as determined from their medical history, a physical examination, electrocardiography, and values of laboratory tests with urine and blood. They received no concomitant medication beginning at least 1 week prior to the start of the trial.
In vivo microdialysis.
Samples for the measurement of tissue cefpirome concentrations were collected by in vivo microdialysis. The principles of microdialysis have previously been described in detail (13, 23). In brief, microdialysis is based on sampling of analytes from the extracellular space of tissues by means of a semipermeable membrane at the tip of a microdialysis probe. The probe is constantly perfused with a physiologic solution. Once the probe is implanted into the tissue, substances present in the extracellular fluid at a certain concentration (Ctissue) diffuse through the membrane into the perfusate, resulting in a concentration (Cdialysate) in the perfusion medium. For most analytes, the equilibrium between the concentration in the extracellular fluid and that in perfusion medium is incomplete, and therefore, Ctissue is greater than Cdialysate. The calibration factor by which the concentrations are interrelated is termed “recovery.” For calibration of the microdialysis probes, in vivo recovery was assessed in each experiment by the retrodialysis method (23). The principle of this method relies on the fact that the diffusion process is quantitatively equal in both directions along the semipermeable membrane. For probe calibration, the investigated agent is added to the perfusate at a defined concentration (Cperfusate). The disappearance rate (delivery) through the membrane is taken as the in vivo recovery. In vivo recovery values for cefpirome were calculated as 100 − [100 · (Cdialysate/Cperfusate)].
Study procedures.
Microdialysis probes with a molecular mass cutoff of 20 kDa (CMA 10; Microdialysis AB, Stockholm, Sweden) were placed in the subcutaneous adipose tissue of the thigh. The microdialysis probes were placed between the middle and lower third of the thigh at its ventrolateral side, as follows: a needle with a catheter was inserted in a skin fold, according to standard procedures for subcutaneous injections. The steel needle was removed from the catheter and was replaced by the microdialysis probe. Then, the catheter was carefully moved backwards about 1.5 cm in a way that the membrane at the tip of the probe remained positioned in the subcutaneous adipose tissue. The probes were fixed and constantly perfused with Ringer solution (Mayerhofer, Linz, Austria) at a flow rate of 1.5 μl/min by means of a precision pump (Precidor; Infors-AG, Basel, Switzerland). After an equilibration period of approximately 1 h, retrodialysis was performed for 30 min. The probes were perfused with Ringer solution containing cefpirome at a concentration of 30 mg/liter to determine the in vivo recovery rate.
Then, 2.0 g of cefpirome (Cefrome; Trockenstechampulle; Usiphar, Compiegne, France) was dissolved in 50 ml of aqua bidest (Fresenius Pharma, Graz, Austria), and the solution was administered intravenously as a single dose to patients and healthy subjects over a period of approximately 15 min. Blood samples were drawn via an intravenous catheter for the determination of the PKs of cefpirome in plasma. Blood and microdialysate samples were collected at 20-min intervals over a period of 240 min and were stored at −80°C until analysis.
Cefpirome concentrations.
The free cefpirome concentrations in the plasma and microdialysate samples were measured by micellar electrokinetic chromatography, as described previously (20). In brief, the unbound fraction of cefpirome was separated from the protein-bound fraction by ultrafiltration. Ultrafiltrated plasma samples and microdialysis samples were injected into a capillary electrophoresis instrument (3DCE model 1600A) equipped with a UV detector operating at 270 nm (Agilent Technologies, Waldbronn, Germany). Calibration curves for the plasma and microdialysis samples were generated with spiked drug-free plasma samples and cefpirome in Ringer solution. The accuracy was determined on three different days by analyzing spiked plasma samples and cefpirome in Ringer's solution and ranged from 90 to 103% with a precision lower than 7%. The limits of quantification in plasma and microdialysis samples were 2 and 0.3 mg/liter, respectively.
The concentrations of cefpirome measured in microdialysate samples were adjusted by the following equation to determine the absolute, free concentrations in tissue: Ctissue = (Cdialysate× 100)/recovery rate.
CLCR.
In the septic patients, creatinine clearance (CLCR) was assessed by the equation (Ccreatinine in urine× volumeurine)/(Ccreatinine in plasma× urine collection time). In one patient CLCR was estimated by the formula of Cockroft and Gault (29), [(140 − age) × weight]/(72 × Ccreatinine in plasma), because no measured CLCR value was available.
PK analysis.
The values of the PK parameters for plasma and tissue were calculated by standard noncompartmental analysis by including all datum points without regression analysis or weighting. This was performed by using Kinetica (version 3.0) software (InnaPhase Corporation, Philadelphia, Pa.). The area under the concentration-versus-time curve (AUC) values from 0 to 240 min (AUC0-240) for plasma and the interstitium were calculated from nonfitted data by using the linear trapezoidal rule. The volume of drug distribution (V) was calculated by the equation dose/(AUCtotal× kel), where AUCtotal represents the total AUC and kel represents the elimination rate constant. The total drug clearance (CL) was determined from the equation dose/AUCtotal. The half-life of the terminal slope (t1/2β) was defined as ln 2 × kel−1.
The times during which the antibiotic concentrations exceeded the MICs for relevant pathogens (T > MIC) were determined for individual patients and are expressed as a percentage of the dosing interval of 8 h. For this purpose, the individual concentrations of cefpirome from 4 to 8 h after drug administration were calculated by the equation C = C0 × e−kel × t, whereby C represents the concentration at a defined time point, C0 is the last concentration measured in vivo, kel is the elimination rate constant, and t is the time between the measurement of C0 and the defined time point.
Statistics.
All PK data are presented as means ± standard deviations (SDs). The Mann-Whitney U test was used for comparison of PK and PK-PD parameters between study groups, and the Spearman coefficient of correlation (ρ) was applied for analysis of the relationship between CLCR and cefpirome CL because the variables were nonnormally distributed. SPSS (version 11.5.1) software (SPSS Inc., Chicago, Ill.) was used. A P value <0.05 was considered significant.
Test strains.
For the in vitro experiments, Staphylococcus aureus ATCC 29213, for which the cefpirome MIC is 1 mg/liter, and a clinical isolate of Pseudomonas aeruginosa for which the cefpirome MIC is 8 mg/liter were chosen, since these values have frequently been reported as the MICs at which 50% of isolates are inhibited (MIC50s) or the MIC90s for these pathogens (1, 2, 4, 16, 19, 26, 30). The susceptibilities of the bacteria to cefpirome were determined by the broth microdilution method, according to the criteria of the NCCLS (24).
In vitro PDs.
An established in vivo PK-in vitro PD model was used (6, 33) to evaluate the antibacterial effects of the measured cefpirome concentrations. The PK profiles for those patients with the lowest levels of tissue penetration (AUC0-240, 3.271 g · min/liter) and the highest levels of tissue penetration (AUC0-240, 11.257 g · min/liter) and the mean curve for the septic population (AUC0-240, 6.899 g · min/liter) were simulated.
Briefly, 3 ml of Mueller-Hinton broth (Merck, Darmstadt, Germany) was inoculated with test strains in order to achieve a concentration of 5 × 105 to 1 × 106 CFU/ml. The strains were then incubated at 37°C for 8 h and were exposed to cefpirome concentrations that changed dynamically in broth, according to the PK profile measured over time in tissue in vivo.
The drug concentrations in the culture tubes containing the test strains were adjusted at 20-min intervals during the first 4 h and were then adjusted at 60-min intervals. Increasing antibiotic concentrations were simulated by the addition of cefpirome. Decreasing concentrations were attained by adding Mueller-Hinton broth without antibiotic at appropriate volumes, according to the equation V2 = (C1/C2) × V1, where C1 and V1 are the current cefpirome concentration and the current volume in the test tube, respectively; C2 is the desired cefpirome concentration; and V2 is the volume in the test tube after the addition of adequate broth. The test tubes were vortexed after the addition of cefpirome or pure broth.
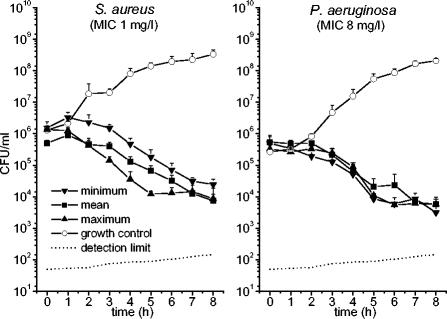
After the test tubes were vortexed, samples of 200 μl were drawn at 60-min intervals to determine the bacterial counts. The samples were serially diluted with 0.9% sodium chloride solution, and 20 μl of each dilution was plated onto Mueller-Hinton agar plates (Biomerieux, Marcy l'Etoile, France). The agar plates were cultured overnight, and the bacterial counts were determined and backextrapolated to the original volume to account for the respective dilution. Each simulation was performed in triplicate. Bacterial growth control experiments were performed in culture tubes without antibiotic. Fifty CFU was considered the minimum accurately countable number of bacteria in 1 ml of broth. The detection limit, plotted in Fig. 4, rises throughout the test period due to admixture of the broth and backextrapolation to the original volume.
FIG. 4.
Growth-inhibition curves for S. aureus and P. aeruginosa. The strains were exposed in vitro to the cefpirome concentrations measured in subcutaneous adipose tissue of the septic patients with the lowest and the highest levels of penetration into tissue. In addition, the mean concentration-versus-time profile in the tissue of septic patients was simulated.
RESULTS
After intravenous administration of 2 g of cefpirome, the concentrations in plasma and in the interstitium of subcutaneous adipose tissue were measured over a period of 4 h.
Tolerability and dropouts.
Cefpirome administration was well tolerated by the healthy volunteers and the septic patients. No drug-related adverse effects were detected in the study population. The microdialysis probes provided inaccurate volumes of dialysate in one patient and in one healthy volunteer. After removal of the probes, visible damage of the semipermeable membrane was detected in both probes, and the PK data derived from these subjects were abandoned.
For two healthy volunteers and two septic patients, an initial dose of less than 200 mg of cefpirome (less than 10% of the total dose) was erroneously administered before the microdialysis experiments were started. We subsequently postponed the experiments in these subjects for several hours, but unfortunately, the concentrations did not reach levels below the limit of detection, as the sensitivity of the assay was very high. Thus, the baseline levels in the plasma and interstitium of these subjects were very low but resulted in a mean baseline cefpirome level of greater than the detection limit of 2 mg/liter in the patients and the healthy controls. These low baseline concentrations were not considered relevant to the overall calculations, results, and conclusions drawn in the present study.
PKs in plasma.
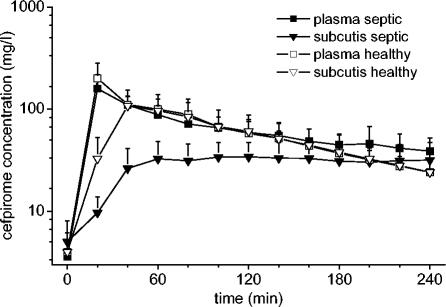
The mean concentration-versus-time profiles of cefpirome in plasma and subcutaneous adipose tissue are shown in Fig. 1. The main PK parameters are presented in Table 2. The maximum concentration of drug in plasma (Cmax) and the time to reach Cmax (Tmax) for cefpirome were not significantly different between septic patients and healthy volunteers (P = 0.82 and 0.83, respectively). However, the t1/2β of cefpirome in plasma was significantly longer for patients than for healthy controls (P < 0.05).
FIG. 1.
Concentration-versus-time curves for plasma and subcutaneous adipose tissue for septic patients (n = 11) and healthy subjects (n = 7) after administration of 2 g of cefpirome. Values are presented as means ± SDs.
TABLE 2.
Values of PK parameters for cefpirome in plasma and interstitium of subcutaneous adipose tissuea
| Compartment | Population | Cmax (mg/liter) | Tmax (min) | AUC0-240 (g · min/liter) | t1/2β (min) | CL (ml/min) | V (liters) |
|---|---|---|---|---|---|---|---|
| Plasma | Septic (n = 11) | 166 ± 50 | 24 ± 8 | 16.19 ± 4.08 | 183 ± 54b | 80 ± 26 | 21.9 ± 4.5b |
| Healthy (n = 7) | 188 ± 82 | 23 ± 8 | 16.50 ± 5.20 | 95 ± 30 | 105 ± 31 | 15.8 ± 5.6 | |
| Subcutis | Septic (n = 11) | 41 ± 17b | 129 ± 63b | 6.90 ± 2.56b | 310 ± 145b,c | NDd | ND |
| Healthy (n = 7) | 116 ± 48 | 51 ± 16 | 13.11 ± 5.20 | 93 ± 22 | ND | ND |
Values are presented as means ± SDs.
Significantly different compared with the results for healthy subjects (P < 0.05).
n = 8, because in three patients the plasma-to-tissue distribution process was not completed within the observation period of 4 h.
ND, not determined.
PKs in tissue.
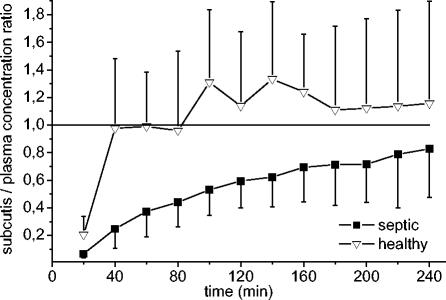
In the microdialysis experiments the mean recovery rate from subcutaneous adipose tissue was 25.9% ± 10.7%. Individual recovery rates were used to convert the concentrations in the dialysates into absolute concentrations in tissue. For tissue, the Cmax was significantly lower in patients than in the healthy subjects (P < 0.05). In the subcutaneous adipose tissue of patients, Cmax was reached significantly later than it was in healthy controls (P < 0.05 for Tmax). Thus, the penetration of cefpirome from plasma into subcutaneous adipose tissue occurs rapidly in healthy subjects, whereas it is strongly delayed in septic patients. This is also illustrated in Fig. 2, which presents the ratios of the concentrations in tissue to the concentrations in plasma over time. In three patients, the equilibration from plasma to tissue was not completed within the observation period of 4 h, and thus, t1/2β was not determined.
FIG. 2.
Ratios of concentrations in subcutis/concentrations in plasma in septic patients (n = 11) and healthy controls (n = 7) over 240 min. Values are presented as means ± SDs.
To assess the antimicrobial and clinical efficacies of cefpirome, T > MIC, which is an adequate surrogate parameter for beta-lactam antibiotics, was calculated for subcutaneous adipose tissue and plasma. By using the individual kel values, the measured concentrations in tissue were extrapolated from 4 to 8 h, because 8 h is the dosing interval frequently applied for cefpirome. The PK extrapolation to 8 h was not performed for the three patients in whom the process of distribution from plasma to tissue was not completed within the observation period. The values of T > MIC were calculated for three relatively high MICs, namely, 4 and 8 mg/liter (susceptible) and 16 mg/liter (intermediately resistant) (24). Table 3 presents the range of values of the T > MIC and the numbers of patients assigned to three categories of T > MIC: less than 60, 60 to 90, or more than 90%. Apparently, for plasma and the subcutis, the values of T > MIC tended to be higher in patients than in healthy subjects, although statistical significance was not reached due to the relatively small sample size (Table 3).
TABLE 3.
T > MICa in plasma and interstitium of subcutaneous adipose tissue of septic patients and healthy subjects after administration of 2 g of cefpirome
| MIC (mg/liter) | Populationb | Plasma
|
Subcutisc
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| T > MIC range (%) | No. of subjects with T > MIC of:
|
T > MIC range (%) | No. of subjects with T > MIC of:
|
||||||
| <60% | 60-90% | >90% | <60% | 60-90% | >90% | ||||
| 4 | Septic | 100 | 0 | 0 | 11 | 90-100 | 0 | 1 | 7 |
| Healthy | 75-100 | 0 | 2 | 5 | 77-100 | 0 | 2 | 5 | |
| 8 | Septic | 81-100 | 0 | 1 | 10 | 55-100 | 1 | 1 | 6 |
| Healthy | 60-100 | 0 | 4 | 3 | 59-85 | 1 | 6 | 0 | |
| 16 | Septic | 57-100 | 1 | 3 | 7 | 13-100 | 2 | 2 | 4 |
| Healthy | 45-91 | 3 | 3 | 1 | 37-81 | 4 | 3 | 0 | |
T > MIC categories in percent refer to a dosing interval of 8 h.
Eleven septic patients and seven healthy subjects were tested.
Data for 3 of 11 patients were excluded from the T > MIC calculation, because the distribution from plasma to tissue was not completed within the observation period.
CLCR and CL.
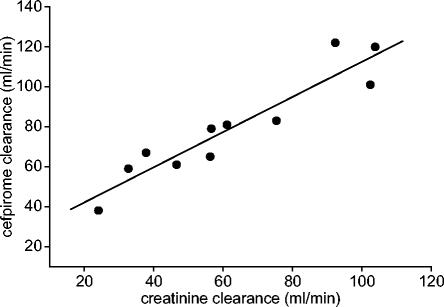
CLCR ranged from 24.1 to 103.8 ml/min and the CL of cefpirome ranged from 38.1 to 122.5 ml/min in septic patients. The Spearman ρ for these parameters was 0.945 (P < 0.05) (Fig. 3).
FIG. 3.
Relationship between CLCR and CL of cefpirome in plasma of septic patients (ρ = 0.945).
In vitro PDs.
Strains of P. aeruginosa (MIC, 8 mg/liter) and S. aureus (MIC, 1 mg/liter) were exposed in vitro to the cefpirome concentrations measured in tissue in vivo. To evaluate the relevance of the variability in PKs in tissue on bacterial growth inhibition, the PK profiles were simulated for those patients with the lowest level of tissue penetration (AUC0-240, 3.271 g · min/liter) and the highest level of tissue penetration (AUC0-240,11.257 g · min/liter) and the mean curve for the septic population (AUC0-240, 6.899 g · min/liter). The PK-PD growth inhibition curves are shown in Fig. 4, which indicates that all experiments resulted in effective antimicrobial activity against both strains.
DISCUSSION
The rapid penetration of cefpirome into the subcutaneous adipose tissue of healthy subjects (10, 14, 23) and into the lung tissue of patients with tumors (9) was reported previously. The present study shows that the equilibration of cefpirome from plasma to tissue is considerably delayed in septic patients compared with that in healthy controls (Fig. 2), a finding that closely resembles the findings of previous investigations with piperacillin (12), levofloxacin (33), and cefpirome (13) in muscle tissue.
The low concentrations of cefpirome in the interstitium of subcutaneous adipose tissue of severely ill patients might be explained by systemic inflammation with subsequent changes in the permeability of the vascular wall (15). The loss of capillary integrity is associated with a shift of fluid and albumin to the extravascular space, resulting in edema. This increase in the extracellular fluid volume is augmented by attempts to keep the mean arterial blood pressure sufficiently high for organ perfusion by the therapeutic restoration of the intravascular volume. Since cefpirome is a highly hydrophilic compound and the V of cefpirome approximates the volume of extracellular water (18, 31), the PKs of cefpirome in tissue are substantially affected by interstitial edema (Table 2). The increase in the amount of albumin in the interstitium is expected to be of minor relevance, because the level of binding of cefpirome to plasma proteins was reported to range only from 5 to 10% (31).
In addition, septic shock patients commonly receive vasopressive catecholamines, which reduces the number of capillaries available for the distribution of drugs from plasma to tissue. Thus, pathophysiological changes and subsequent therapeutic interventions are expected to account, at least in part, for the delayed or incomplete process of equilibration of antimicrobial agents from plasma to tissue observed in septic patients.
For the physician, it is relevant to know whether the impaired penetration of cefpirome into tissues, in combination with given susceptibility data, affects the efficacy of the antibiotic at the target site in septic patients. Therefore, the cefpirome concentrations in the subcutis were simulated in vitro by use of an established PK-PD model. Importantly, effective bacterial growth inhibition was observed in all simulations (Fig. 4), despite the high level of interindividual variability in Cmaxs and AUC0-240s in septic patients.
This finding is probably due to the fact that beta-lactams are most effective when T > MIC is maximized (5, 11, 25, 28). In the present study the individual T > MICs tended to be higher in the septic patients than in the healthy controls (Table 3), although this trend was statistically not significant due to the relatively small sample size. Apparently, the values of T > MIC for the tissue of septic patients were sufficient to achieve effective bacterial growth inhibition. Thus, these PK-PD data suggest that effective bacterial growth inhibition is achieved with cefpirome for the treatment of STIs in septic patients, despite the impaired tissue penetration, and can be interpreted as the net effect of delayed total drug elimination in patients.
All these thoughts are based on the assumption that a dosing regimen of cefpirome, along with T > MICs of more than 60% of the dosing interval, is effective for the treatment of STIs in septic patients. In order to avoid underdosing and the low trough levels in plasma and tissue that might occur upon twice-daily dosing, the shortening of the intervals of cefpirome dosing appears to be advisable (10, 17, 18). By using our data to calculate the average concentration at steady state [Cav(ss)] for cefpirome by the equation AUCtotal/dosing interval and given a dosing regimen of 2 g every 8 h, the values of free Cav(ss) of cefpirome in plasma would be about 57 ± 21 mg/liter (range, 34 to 109 mg/liter) and 41 ± 12 mg/liter (range, 26 to 63 mg/liter) for patients and healthy controls, respectively. Accordingly, the values of Cav(ss) for cefpirome in subcutaneous adipose tissue would be 36 ± 30 mg/liter (range, 12 to 109 mg/liter) for patients and 34 ± 14 mg/liter (range, 15 to 55 mg/liter) for controls. Thus, given a dosing regimen of 2 g of cefpirome three times a day, the calculated values of Cav(ss) indicate that cefpirome is adequate for the treatment of infections caused by clinically relevant pathogens in the tissue and plasma of critically ill patients. In agreement with previous studies, the ranges of values of Cav(ss) and T > MIC show that there is a higher interindividual variability in patients. These data support the present thinking that twice-daily dosing of cefpirome may be insufficient (18) and that the administration of a loading dose of cefpirome may be advisable to effectively kill bacteria at the target site in critically ill patients.
When the excellent correlation of the CLCR and CL of cefpirome (ρ = 0.945) in septic patients (Fig. 3) is considered, optimization of cefpirome therapy for an individual can easily be performed (18). In the circumstance of substantially impaired renal function, as indicated by a CLCR less than 50 ml/min in critically ill patients, the total daily dose of cefpirome should be tailored accordingly. For that purpose, the formula DR = DP · {1 − [fr · (1 − RF)]} can be applied, where DR is the dose in the patient with impaired renal function, DP is the daily dose recommended in the present study (6 g), fr is the ratio of the renal clearance to CL, and RF is the ratio of the CL in the patient with renal impairment to the average CL in our critically ill subjects (67 ml/min). As cefpirome is eliminated primarily by glomerular filtration and the CL of cefpirome is approximately equal to CLCR (31), the value of fr equals 1. Thus, the previous formula can be simplified to DR = 6 · [1 − (1 − RF)] and confirms that dose adjustment is exclusively dependent on RF, i.e., the extent of renal function impairment. This may be done only under the assumption that extrarenal CL remains unaffected if the renal clearance of cefpirome decreases.
In conclusion, the present study showed that the concentrations of cefpirome in the subcutaneous adipose tissue of septic patients were lower than those in the subcutaneous adipose tissue of healthy controls. However, on the basis of PK-PD calculations, the measured concentrations of cefpirome appeared to be appropriate for the treatment of STIs in septic patients due to the long t1/2β in the tissues of critically ill patients. Nevertheless, the dosing intervals should not exceed 8 h, i.e., cefpirome dosing should be at 2 g three times daily, to ensure that infections in plasma and subcutaneous adipose tissue caused by susceptible bacterial strains are adequately treated in each patient. Independently of the renal function, an initial loading dose of cefpirome should be administered.
Acknowledgments
This project was supported, in part, by grant 9346 from Oesterreichische Nationalbank Jubiläumsfonds.
REFERENCES
- 1.Biedenbach, D. J., D. M. Johnson, R. N. Jones, et al. 1999. In vitro evaluation of cefepime and other broad-spectrum beta-lactams in Taiwan medical centers. Diagn. Microbiol. Infect. Dis. 35:299-305. [DOI] [PubMed] [Google Scholar]
- 2.Biedenbach, D. J., M. T. Lewis, R. N. Jones, et al. 1999. In vitro evaluation of cefepime and other broad-spectrum beta-lactams for isolates in Malaysia and Singapore medical centers. Diagn. Microbiol. Infect. Dis. 35:277-283. [DOI] [PubMed] [Google Scholar]
- 3.Bone, R. C., W. J. Sibbald, and C. L. Sprung. 1992. The ACCP-SCCM Consensus Conference on Sepsis and Organ Failure. Chest 101:1481-1483. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo, J. D., R. Fabre, F. Leblanc, M. H. Nicolas-Chanoine, and A. Thabaut. 2000. Antibiotic susceptibility and mechanisms of beta-lactam resistance in 1310 strains of Pseudomonas aeruginosa: a French multicentre study (1996). J. Antimicrob. Chemother. 46:133-136. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 6.Delacher, S., H. Derendorf, U. Hollenstein, M. Brunner, C. Joukhadar, S. Hofmann, A. Georgopoulos, H. G. Eichler, and M. Muller. 2000. A combined in vivo pharmacokinetic-in vitro pharmacodynamic approach to simulate target site pharmacodynamics of antibiotics in humans. J. Antimicrob. Chemother. 46:733-739. [DOI] [PubMed] [Google Scholar]
- 7.European Agency for the Evaluation of Medicinal Products. 2002. Guideline for good clinical practice. ICH Topic E6. [Online.] http://www.emea.eu.int/pdfs/human/ich/013595en.pdf.
- 8.Hancock, R. E., and F. Bellido. 1992. Factors involved in the enhanced efficacy against gram-negative bacteria of fourth generation cephalosporins. J. Antimicrob. Chemother. 29(Suppl. A):1-6. [DOI] [PubMed] [Google Scholar]
- 9.Herkner, H., M. R. Muller, N. Kreischitz, B. X. Mayer, M. Frossard, C. Joukhadar, N. Klein, E. Lackner, and M. Muller. 2002. Closed-chest microdialysis to measure antibiotic penetration into human lung tissue. Am. J. Respir. Crit. Care Med. 165:273-276. [DOI] [PubMed] [Google Scholar]
- 10.Hollenstein, U., M. Brunner, B. X. Mayer, S. Delacher, B. Erovic, H. G. Eichler, and M. Muller. 2000. Target site concentrations after continuous infusion and bolus injection of cefpirome to healthy volunteers. Clin. Pharmacol. Ther. 67:229-236. [DOI] [PubMed] [Google Scholar]
- 11.Hyatt, J. M., P. S. McKinnon, G. S. Zimmer, and J. J. Schentag. 1995. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin. Pharmacokinet. 28:143-160. [DOI] [PubMed] [Google Scholar]
- 12.Joukhadar, C., M. Frossard, B. X. Mayer, M. Brunner, N. Klein, P. Siostrzonek, H. G. Eichler, and M. Muller. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385-391. [DOI] [PubMed] [Google Scholar]
- 13.Joukhadar, C., N. Klein, B. X. Mayer, N. Kreischitz, G. Delle-Karth, P. Palkovits, G. Heinz, and M. Muller. 2002. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit. Care Med. 30:1478-1482. [DOI] [PubMed] [Google Scholar]
- 14.Kavi, J., J. M. Andrews, J. P. Ashby, G. Hillman, and R. Wise. 1988. Pharmacokinetics and tissue penetration of cefpirome, a new cephalosporin. J. Antimicrob. Chemother. 22:911-916. [DOI] [PubMed] [Google Scholar]
- 15.Kinzig, M., F. Sorgel, B. Brismar, and C. E. Nord. 1992. Pharmacokinetics and tissue penetration of tazobactam and piperacillin in patients undergoing colorectal surgery. Antimicrob. Agents Chemother. 36:1997-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, M. T., K. Yamaguchi, D. J. Biedenbach, R. N. Jones, et al. 1999. In vitro evaluation of cefepime and other broad-spectrum beta-lactams in 22 medical centers in Japan: a phase II trial comparing two annual organism samples. Diagn. Microbiol. Infect. Dis. 35:307-315. [DOI] [PubMed] [Google Scholar]
- 17.Lipman, J., S. C. Wallis, and R. J. Boots. 2003. Cefepime versus cefpirome: the importance of creatinine clearance. Anesth. Analg. 97:1149-1154. [DOI] [PubMed] [Google Scholar]
- 18.Lipman, J., S. C. Wallis, C. M. Rickard, and D. Fraenkel. 2001. Low cefpirome levels during twice daily dosing in critically ill septic patients: pharmacokinetic modelling calls for more frequent dosing. Intensive Care Med. 27:363-370. [DOI] [PubMed] [Google Scholar]
- 19.Mathai, D., P. R. Rhomberg, D. J. Biedenbach, and R. N. Jones. 2002. Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against recent clinical isolates from India: a survey of ten medical center laboratories. Diagn. Microbiol. Infect. Dis. 44:367-377. [DOI] [PubMed] [Google Scholar]
- 20.Mayer, B. X., U. Hollenstein, M. Brunner, H. G. Eichler, and M. Muller. 2000. Micellar electrokinetic chromatography for the analysis of cefpirome in microdialysis and plasma samples obtained in vivo from human volunteers. Electrophoresis 21:1558-1564. [DOI] [PubMed] [Google Scholar]
- 21.Mueller, M., A. de la Pena, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob. Agents Chemother. 48:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller, M., A. dela Pena, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48:1441-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Odenholt, I. 2001. Pharmacodynamic effects of subinhibitory antibiotic concentrations. Int. J. Antimicrob. Agents 17:1-8. [DOI] [PubMed] [Google Scholar]
- 26.Pierard, D., K. Emmerechts, S. Lauwers, et al. 1998. Comparative in-vitro activity of cefpirome against isolates from intensive care and haematology/oncology units. J. Antimicrob. Chemother. 41:443-450. [DOI] [PubMed] [Google Scholar]
- 27.Tegeder, I., A. Schmidtko, L. Brautigam, A. Kirschbaum, G. Geisslinger, and J. Lotsch. 2002. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 71:325-333. [DOI] [PubMed] [Google Scholar]
- 28.Turnidge, J. D. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 29.Verhave, J. C., C. P. Balje-Volkers, H. L. Hillege, D. de Zeeuw, and P. E. de Jong. 2003. The reliability of different formulae to predict creatinine clearance. J. Intern. Med. 253:563-573. [DOI] [PubMed] [Google Scholar]
- 30.Wise, R., J. M. Andrews, and N. Brenwald. 1996. In vitro activity of the tricyclic beta-lactam GV104326. Antimicrob. Agents Chemother. 40:1248-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiseman, L. R., and H. M. Lamb. 1997. Cefpirome. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy in the treatment of severe nosocomial infections and febrile neutropenia. Drugs 54:117-140. [DOI] [PubMed] [Google Scholar]
- 32.World Medical Association. 6. October 2002. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. [Online.] http://www.wma.net/e/policy/b3.htm.
- 33.Zeitlinger, M. A., P. Dehghanyar, B. X. Mayer, B. S. Schenk, U. Neckel, G. Heinz, A. Georgopoulos, M. Muller, and C. Joukhadar. 2003. Relevance of soft-tissue penetration by levofloxacin for target site bacterial killing in patients with sepsis. Antimicrob. Agents Chemother. 47:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]