Abstract
The adverse metabolic risks associated with second generation antipsychotics (SGAs) are well known, and likely contribute to the high rate of premature mortality due to cardiovascular disease in schizophrenia. Female schizophrenia patients appear to be diagnosed with metabolic diseases at higher rates than males, which may reflect disparate adverse responses to SGAs. However, the relationship between sex, metabolic risk, and drug use is less developed. We aimed to explore this relationship further by identifying rates of metabolic disease in community dwelling schizophrenia patients by sex and SGA risk. Schizophrenia participants (N = 287, 40.4% female) were included in this analysis. Oneway-ANOVA and Fisher’s Exact Test were used to compare groups, as appropriate, and Cohen’s d was employed to estimate the effect size of sex. In the group as a whole, the rate of metabolic syndrome was higher than previously reported, but did not differ by sex. For females, greater metabolic disturbances across all medication risk groups were seen in BMI and waist circumference (p < 0.005) but most commonly in those receiving high risk medication (clozapine or olanzapine). Additionally, the number of participants receiving medications for these metabolic disturbances was extremely low (<30%). These results suggest that female schizophrenia patients taking clozapine or olanzapine represent a group at uniquely high risk for metabolic dysfunction and future adverse cardiovascular outcomes, and warrant close monitoring by clinicians to prevent worsening of metabolic risk through proper monitoring and interventions.
Keywords: atypical antipsychotics, antipsychotics, cardiovascular disease, metabolic syndrome, schizophrenia, sex differences
Introduction
Cardiovascular disease (CVD) is a primary cause of mortality among individuals with schizophrenia spectrum disorders, with up to 30 years of life lost compared to the general population.1,2 Although CVD occurrence is undeniably multifactorial, antipsychotic use may increase CVD risk due to greater risk of specific metabolic abnormalities.3-5 In particular, the second generation antipsychotics (SGAs) are associated with weight gain leading to obesity, especially abdominal obesity; dyslipidemias such as hypercholesterolemia, hypertriglyceridemia, low high density lipoprotein (HDL), and elevated low density lipoprotein (LDL); and, impaired glucose homeostasis such as hyperglycemia, insulin resistance, and type 2 diabetes mellitus.
Overall, up to 50% of patients receiving SGAs have been reported to meet metabolic syndrome criteria, which substantially increases the risk of CVD morbidity and mortality.6 In general, metabolic syndrome can be diagnosed when three of five criteria are met that focus on specific cardiovascular risk factors such as abdominal obesity, low high-density lipoprotein (HDL), elevated triglycerides (TG), hypertension, and impaired fasting plasma glucose.7 In looking at the findings from the Clinical Antipsychotics Trials of Intervention Effectiveness (CATIE), the largest clinical trial of antipsychotics to date, specific sex differences were noted in metabolic risk as females had a higher prevalence and risk for metabolic syndrome compared with males (36.6% and 54.2%, respectively).8 Although research fousing on sex related differences for metabolic syndrome in the population have been ongoing, results are mixed.9-11
Most work focusing on sex related differences in metabolic syndrome risk are typically limited to participants within inpatient settings, limiting ‘real world’ generalizability. Therefore, the primary aim of this study was to examine differences in metabolic functioning between community dwelling females and males, investigating specific antipsychotic (AP) medication related risks. We hypothesized that females would have worse metabolic functioning and a greater prevalence of metabolic syndrome compared to males, and that this risk would be commensurate with overall AP metabolic risk profiles.
Materials and Method
Participants
Participants were part of a larger study investigating pharmacogenetics predictors of CVD risk related to antipsychotic use and were recruited from the community and outpatient clinics primarily via advertisements. They were included if they: 1) had a DSM-IV diagnosis of schizophrenia, schizoaffective, or psychotic disorder not otherwise specified (American Psychiatric Association, 2000), 2) aged 18 to 90, and 3) receving antipsychotic treatment for at least six months with no significant recent medication changes. Participants were excluded if they were: 1) unable or unwilling to provide informed consent, 2) diagnosed with Type 2 diabetes mellitus prior to antipsychotic treatment, 3) had a substance dependence disorder, or 4) currently pregnant or nursing. The inclusion criteria were broad to represent ‘real world’ ambulatory practice. The study was approved by the: University of Michigan Medical School Institutional Review Board, Washtenaw County Health Organization, Ann Arbor Veterans Affairs Medical Center, and Detroit-Wayne County Community Mental Health Agency, and is registered at clinicaltrials.gov (NCT00815854). This study was conducted in accordance with the latest version of the Declaration of Helsinki. Participants were assessed at the Michigan Clinical Research Unit at the University of Michigan.
Procedure
Participants provided written informed consent after the study procedures had been explained. Participants fasted for 8 hours prior to enrolling in the study and all assessments were completed in one visit.The blood draw assessed glucose and lipids. The physical examination assessed vital signs, height, weight, and hip and waist circumferences. Body mass index (BMI) and waist-to-hip ratio (WHR) were calculated from these measurements. The National Cholesterol Education Program’s Adult Treatment Panel III7 criteria were used to diagnose metabolic syndrome.
The clinical interview included a psychiatric diagnostic assessment using the Structured Clinical Interview for DSM IV Axis I Disorders,12 current and past medication history, and demographics.
Data Analysis
Participants were divided into three AP metabolic risk groups: 1) high – clozapine and olanzapine; 2) moderate – quetiapine, risperidone, paliperidone, and iloperidone; 3) low – all other antipsychotics. This grouping is based on reports in the literature regarding the specific medications’ propensities for metabolic disturbances.13-15
Analyses on categorical variables were conducted using Fisher’s Exact Test and a oneway-ANOVA for continuous variables. Cohen’s d was used to calculate the effect size of sex. Analyses on the prevalence of metabolic syndrome included the entire sample. Participants prescribed a lipid medication were excluded from analyses on lipid variables. Participants receiving treatment for diabetes were excluded from analyses on plasma glucose. Given its weight controlling properties, analyses on BMI, waist circumference, and WHR excluded participants on an oral hypoglycemic (e.g., metformin). Participants receiving treatment for hypertension were excluded from analyses on blood pressure. The exclusions are important methodologically, as these medications may attenuate the metabolically negative impact of the APs and therefore limit efforts to determine medication specific sex based differences. A p-value of <0.05 was considered significant.
Results
Overall, 287 participants were included, comprising 116 (40.4%) females with a mean age of 46.90 ± 11.34 (males: 44.16 ± 11.34 years, p > 0.05). Table 1 lists participant characteristics and rates of medication use. There were no differences in psychotropic exposure between males and females except in the high risk group in which females were on marginally more medications than their male counterparts. Overall antipsychotic exposure was calculated using chlorpromazine equivalents,16 which was not different between sexes.
Table 1. Participant Characteristics.
| WHOLE SAMPLE | HIGH RISK GROUPa | MODERATE RISK GROUPb | LOW RISK GROUPc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MALE (n = 171) | FEMALE (n = 116) | MALE (n = 51) | FEMALE (n = 28) | MALE (n = 72) | FEMALE (n = 40) | MALE (n = 48) | FEMALE (n = 48) | ||||
| Age (M ± SD) | 44.16 ± 11.34 | 46.89 ± 11.34 | 45.90 ± 11.16 | 47.18 ± 12.60 | 41.26 ± 11.41 | 47.30 ± 9.45** | 46.65 ± 10.67 | 46.40 ± 12.22 | |||
| Racial Distribution | |||||||||||
| %White | 50.9 | 56 | 60.8 | 67.9 | 41.7 | 54.2 | 58.3 | ||||
| %Black | 40.9 | 27.6 | 31.4 | 14.3 | 50 | 37.5 | 37.5 | 27.1 | |||
| %Other | 8.2 | 6.4 | 7.8 | 17.3 | 8.3 | 17.5 | 8.6 | 14.6 | |||
| Diagnosis | |||||||||||
| %SCZP/SCZA/NOS | 68.6/27.5/3.9 | 39.3/57.1/3.6 | 45.8/41.7/12.5 | 30.0/57.5/12.5 | 39.6/43.8/16.7 | 27.1/64.6/8.3 | |||||
| % Lipid Medication | 28.7 | 28.4 | 49 | 32.1 | 19.4 | 25 | 20.8 | 29.2 | |||
| % BP Medication | 33.3 | 33.6 | 41.2 | 25 | 26.4 | 35 | 35.4 | 37.5 | |||
| % DM Medication | 19.3 | 22.4 | 19.6 | 21.4 | 15.3 | 17.5 | 25 | 27.1 | |||
| CPZE | 727.5 ± 765.2 | 644.0 ± 746.3 | 791.4 ± 770.7 | 833.0 ± 829.8 | 736.7 ± 711.0 | 656.8 ± 582.7 | 648.2 ± 837.8 | 518.2 ± 805.0 | |||
| Psychotropic Medications (M ± SD) | 2.44 ± 1.13 | 2.85 ± 1.30 | 2.31 ± 1.10 | 2.89 ± 1.29* | 2.53 ± 0.93 | 2.80 ± 1.38 | 2.46 ± 1.41 | 2.85 ± 1.25 | |||
Notes: **p < 0.01.
*p < 0.05.
aHigh Risk Group = clozapine or olanzapine.
bModerate Risk Group = quetiapine, risperidone, iloperidone, or paliperidone.
cLow Risk Group = all other antipsychotics.
Abbreviations: BP, blood pressure; CPZE, chlorpromazine equivalence; DM, Type 2 diabetes mellitus; M, Mean; NOS, psychosis not otherwise specified; SCZP, schizophrenia; SCZA, sczhioaffective disorder; SD, standard deviation.
Prevalence and Sex Differences of Metabolic Syndrome
The prevalence of metabolic syndrome in the overall sample was 49.1% with a higher prevalence in females compared to males (52.6% and 46.8%, respectively, p = 0.12). This prevalence was highest among participants in the high risk group (64.6%) although there was no sex difference (64.3% females and 64.7% males; p = 0.58). In the moderate risk group, metabolic syndrome prevalence was 41.1%, with a higher rate trend in females (52.5%) than males (34.7%), (p = 0.05). In the low risk group, metabolic syndrome prevalence was 45.8%, with no sex differences, p = 0.58. Table 2 contains values for the overall sample (N = 287) stratified by metabolic risk group.
Table 2. Prevalence of Metabolic Syndrome and its Components.
| WHOLE SAMPLE | HIGH RISK GROUPa | MODERATE RISK GROUPb | LOW RISK GROUPc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| METS VARIABLES | MALE (n = 171) | FEMALE (n = 116) | MALE (n = 51) | FEMALE (n = 28) | MALE (n = 72) | FEMALE (n = 40) | MALE (n = 48) | FEMALE (n = 48) | |||
| % MetS | 46.8 | 52.6 | 64.7 | 64.3 | 34.7 | 52.5* | 45.8 | 45.8 | |||
| % Abdominal Obesity | 60.4 | 91.4*** | 69.4 | 96.4*** | 58.3 | 92.5*** | 54.2 | 87.5*** | |||
| % Hypertension | 43.3 | 37.9 | 54.9** | 32.1 | 31.9 | 42.5 | 47.9 | 37.5 | |||
| % Elevated Triglycerides | 48.2 | 45.7 | 66.7 | 57.1 | 37.7 | 45 | 43.8 | 39.6 | |||
| % Low HDL | 47.3 | 51.7 | 68.6 | 53.6 | 35.7 | 45 | 41.7 | 56.3 | |||
| % Elevated FPG | 45.6 | 45.7 | 52.9 | 53.6 | 36.1 | 45 | 52.1 | 41.7 | |||
Notes: *p < 0.1.
**p < 0.05.
***p < 0.01.
aHigh Risk Group = clozapine or olanzapine.
bModerate Risk Group = quetiapine, risperidone, iloperidone, or paliperidone.
cLow Risk Group = all other antipsychotics.
Abbreviations: FPG, fasting plasma glucose; HDL, high density lipoprotein; MetS, metabolic syndrome.
Sex Differences in Metabolic Syndrome Components
Body Composition, Visceral Adiposity, and Fasting Plasma Glucose
In the overall sample of participants not on an oral hypoglycemic (n = 227), both females and males were obese on average (BMI ≧ 30 kg/m2 (Table 3)). In particular, females had greater BMIs and higher abnormal waist circumference (WC) and waist-to-hip ratio (WHR) compared to males. These BMI sex differences were greatest in the high risk group (F (1, 59) = 8.59, p = 0.005,) and resulted in a fairly robust effect size (d = 0.7). A similar pattern though in smaller magnitude was seen in the low risk group (F(1, 68) = 5.39, p = 0.023, d = 0.5).
Table 3. Results from Analyses on Lipid, Anthropometric, Glucose Dysregulation, and Blood Pressure Variables.
| WHOLE SAMPLE | HIGH RISK GROUPa | MODERATE RISK GROUPb | LOW RISK GROUPc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MALE (n = 118) | FEMALE (n = 80) | MALE (n = 26) | FEMALE (n = 18) | MALE (n = 54) | FEMALE (n = 29) | MALE (n = 38) | FEMALE (n = 33) | ||||
| Total Cholesterol (mg/dL) | 172.46 ± 39.46 | 190.74 ± 38.41** | 173.12 ± 37.54 | 196.39 ± 37.22** | 170.13 ± 36.5 | 195.59 ± 43.46** | 175.32 ± 45.23 | 183.39 ± 34.04 | |||
| HDL (mg/dL) | 50.14 ± 15.88 | 61.31 ± 18.78*** | 45.96 ± 15.4 | 61.06 ± 17.89*** | 50.91 ± 14.68 | 63.14 ± 19.83*** | 51.92 ± 17.68 | 59.85 ± 18.75* | |||
| LDL (mg/dL) | 109.67 ± 34.78 | 122.19 ± 35.03* | 109.85 ± 31.41 | 127.83 ± 38.56* | 108.85 ± 28.09 | 121.35 ± 35.32* | 110.71 ± 45.01 | 119.85 ± 33.52 | |||
| Triglycerides (mg/dL) | 125.70 ± 91.13 | 118.24 ± 94.29 | 147.77 ± 120.42 | 113.33 ± 40.20 | 110.69 ± 73.36 | 134.59 ± 117.91 | 131.92 ± 89.67 | 106.55 ± 92.36 | |||
| MALE (n = 138) | FEMALE (n = 89) | MALE (n = 40) | FEMALE (n = 21) | MALE (n = 54) | FEMALE (n = 29) | MALE (n = 38) | FEMALE (n = 33) | ||||
| BMI (kg/m2) | 30.40 ± 6.50 | 34.03 ± 8.79*** | 30.17 ± 4.83 | 35.44 ± 9.28*** | 31.09 ± 7.27 | 32.88 ± 6.64 | 29.48 ± 6.75 | 34.33 ± 10.35** | |||
| % WC | 58.1 | 88.8*** | 64.9 | 90** | 58.1 | 91.2*** | 50 | 81.8*** | |||
| % WHR | 77.8 | 97.7*** | 83.8 | 100* | 74.2 | 100*** | 77.8 | 93.9* | |||
| FPG (mg/dL) | 98.07 ± 31.20 | 93.80 ± 13.62 | 94.65 ± 10.61 | 97.48 ± 15.89 | 100.34 ± 44.83 | 94.41 ± 14.39 | 98.17 ± 15.80** | 90.88 ± 10.79 | |||
| MALE (n = 29) | FEMALE (n = 21) | MALE (n = 54) | FEMALE (n = 29) | MALE (n = 38) | FEMALE (n = 33) | ||||||
| Diastolic BP (mm/Hg) | 75 ± 11.13 | 69.88 ± 11.01** | 76.28 ± 11.21* | 69.33 ± 13.2 | 72.81 ± 10.2 | 72.65 ± 11.52 | 77.5 ± 12.16*** | 67.87 ± 8.48 | |||
| Systolic BP (mm/Hg) | 122.10 ± 14.84 | 118.50 ± 17.70 | 121.93 ± 16.54 | 119.38 ± 22.31 | 120.91 ± 17.62 | 122.12 ± 17.62 | 124.29 ± 13.42*** | 114.73 ± 13.54 | |||
Notes: ***p < 0.001.
**p < 0.01.
*p < 0.05.
aHigh Risk Group = clozapine or olanzapine.
bModerate Risk Group = quetiapine, risperidone, iloperidone, or paliperidone.
cLow Risk Group = all other antipsychotics. %WC, percent of participants with waist circumference ≧ 102cm for males and ≧ 90 cm in females; %WHR, percent of participants with waist-to-hip ratio ≧ 0.90 in males and ≧ 0.80 in females.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; BMI, body mass index; FPG, fasting plasma glucose; BP, blood pressure.
Abdominal obesity (WC ≧ 102 cm for males and ≧ 90 cm in females and WHR ≧ 0.90 in males and ≧ 0.80 in females)(NHLBI, 1998), was seen in 69.4% of participants without oral hypoglycemic treatment, comprising 87.4% of females and 57.8% of males, p < 0.001. Higher rates of abdominal obesity in females compared to males were seen across the three metabolic risk groups (Table 3; Figure 1).
Figure 1.
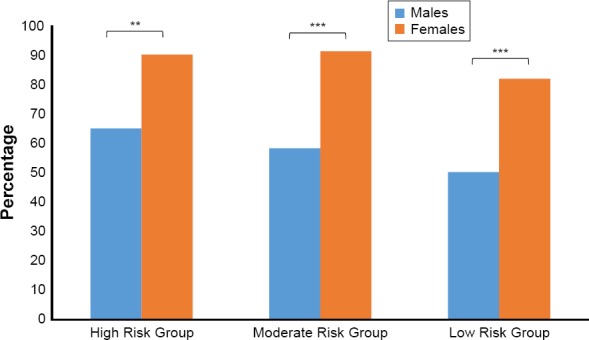
Percentage of Participants Meeting Criteria for Abdominal Obesity by Sex
For WHR, 97.7% of females and 77.8% of males (n = 227; p < 0.001) met or exceeded this criterion. Notably, 100% of females in the high and moderate metabolic risk groups and 93.9% of females in the low risk group had abnormal WHR compared to 74.2%–83.8% of males across the metabolic risk groups. These striking results trended towards significance (p ≤ 0.06 for all), and are highlighted for their clinical relevance. Although few sex differences in fasting plasma glucose were seen overall, males in the low risk group alone had higher fasting plasma glucose compared to females (F(1, 67) = 4.92, p = 0.03), resulting in a moderate effect size (d = 0.5). The average fasting plasma glucose for all groups was <100 mg/dL, suggesting that these differences may not be clinically significant. In terms of clinical significance, however, more than twice as many males (36.1%) than females (17.6%), p = 0.04, had fasting plasma glucose levels at or above the risk level.
Hypertension
For hypertension, in the overall sample of participants not on an antihypertensive (n = 190), males had higher diastolic BP (dBP) compared to females (F(1, 188) = 9.76, p = 0.02), with a moderately sized sex effect, d = 0.46 (Table 3). Stratified by metabolic risk group, a larger effect of sex was found, showing higher dBP in males than females in the high (F(1, 48) = 4.03, p = 0.05), d = 0.57 and low (F(1, 59) = 12.93, p = 0.001), d = 0.92 risk groups (Table 3). Males in the low risk group alone also had higher systolic BP (sBP) compared to females, (F(1, 59) = 7.67, p = 0.008), d = 0.71. However, these differences were considered clinically non-significant as dBP and sBP values were below the threshold for hypertension diagnosis.
Lipids
The lipids analyses stratified by metabolic risk group examined total cholesterol (TC), triglycerides, LDL, and HDL. As lipid levels may be impacted by age,18 and slight sex differences in age were identified in the moderate risk group, a series of regressions were conducted to determine the impact of age and sex on predicting lipid laboratory values. No significant differences were noted and analyses were carried out as planned.
In the overall sample of participants not on dyslipidemia medication (n = 198), females had higher TC than males, (F(1, 196) = 10.45, p = 0.001), with a medium sized effect, d = 0.50. Stratified by metabolic risk group, higher TC in females than males carried across the high (F(1, 42) = 4.12, p = 0.049, d = 0.6) and moderate (F (1, 81) = 8.02, p = 0.006, d = 0.94) risk groups, with a larger sex effect. Although average TC values fell below the risk threshold (<200 mg/dL), and males’ average TC across the three risk groups were normal and relatively similar (170.13–175.32 mg/dl), females in the high and moderate risk groups (195.59–196.39 mg/dl), were much closer to the risk threshold. Additionally, the prevalence of high TC (≧200 mg/dL) was more than twice as high among females (41.4%–50.0%) compared to males (18.5%–19.2%) in the high and moderate risk groups (p < 0.03 for all).
In addition to TC, higher LDL levels in females compared to males were found in the overall sample, (F(1, 196) = 6.14, p = 0.01), with a small sex effect, d = 0.36. Average LDL levels were within normal range across groups but females’ levels approached the risk threshold (≧130 mg/dL) whereas males’ did not, specifically in the high, and to a lesser extent, moderate risk groups (Table 3).
Although sex differences in HDL levels were found in the overall sample as well as the metabolic risk groups (Table 3), these were expected due to known sex differences (Grundy et al., 2004). No sex differences were found in the prevalence of low HDL but notably, 28.6% of the sample (47% female and 53.0% males) met the criterion.
Discussion
This study investigated medication related sex differences in metabolic measures in individuals with schizophrenia spectrum disorders on long term AP treatment within the community. Within the whole sample (i.e., no treatment exclusions) almost 50% of participants met criteria for metabolic syndrome, with higher rates seen in the high risk medication group regardless of sex (Table 2). These findings contrast CATIE which showed a lower prevalence of metabolic syndrome overall (42.7%; McEvoy et al., 2005), and a higher prevalence in females compared to males. However approximately 14% of our participants were receiving clozapine relative to none of the participants in the CATIE trial. The higher incidence of metabolic syndrome within the high risk groups is also higher than that found in other studies of individuals on clozapine or olanzapine10,19 and suggest that long term use of such drugs may pose a higher metabolic risk than previously reported.
Results excluding those diagnosed and receiving treatment for glucose, blood pressure, or lipid dysregulation revealed more nuanced sex differences. To our knowledge this investigation is the first to conduct analyses specifically excluding those receiving current treatment for these conditions, which is important, as these medications may attenuate the metabolically negative impact of the APs and therefore limit efforts to determine medication specific sex based differences. Overall, 70% of our participants were not receiving an oral hypoglycemic (n = 227) and compared to males, females had greater BMI, higher rates of abnormal WC and WHR, and greater dysregulation of TC and LDL. Importantly, high risk group females had the greatest risk across all metabolic factors considered, whereas there was more variability in the type and severity of risk factors among females in the moderate and low risk groups.Thus, females in the high risk group appear to be at greatest risk for metabolic syndrome and overall CVD. In general, these findings were in line with expectations. Males, however, had higher fasting plasma glucose levels as well as higher diastolic and systolic blood pressure, with the greatest sex effect seen in the low risk group, contrary to expectations. Our findings regarding the females were somewhat expected given the widely known weight gain propensity of clozapine and olanzapine (Klemettilä et al., 2014; Taylor and McAskill, 2000). However, higher BMI in females than males in the low risk group has not been previously reported.
Results in WC and WHR also aligned with predictions, lending further credence to reports showing that visceral adiposity markers are predictive of AP associated CVD risk, especially compared to BMI.22 More females had elevated WC and WHR compared to males across the three risk groups which parallels results from other groups10,23 and provides further evidence that females are at greater risk for AP associated visceral adiposity,7 insulin resistance, diabetes, and coronary artery disease risk17,24-26 and is a predictor of CVD mortality.27,28 The striking finding from this analysis is that 100% of females in the high and moderate risk groups met central adiposity criterion (WHR > 0.8) which is clinically significant, suggesting an important sex-specific risk to consider in long term AP therapy. Overall, our findings confirm that an overwhelmingly large proportion of individuals with schizophrenia spectrum disorders prescribed antipsychotic medication are at risk for obesity and visceral adiposity, which may be worsening despite available monitoring guidelines that recommend routine BMI measurements.29 Though not specifically outlined in the monitoring guidelines, obtaining routine waist and hip circumferences may be useful for medication management in the context of cardiovascular health and should be considered, especially for females.
Sex differences were also seen in fasting plasma glucose, where low risk group males had higher values compared to female counterparts. Although the average was below the current diabetes risk threshold (<100 mg/dL), it is important to note that twice as many males (36.1%) than females (17.6%) met the criterion. This finding may also point to a nontrivial lack of preventive screening and treatment for elevated glucose within the low risk group. As 40.5% of those in the high risk group and 31.1% in the moderate risk group had an elevated glucose, adhering to metabolic monitoring guidelines, which recommends annual checks is critical. Since only 21% of participants were receiving an oral hypoglycemic agent at study assessment, our findings of widespread non-specific glucose dysregulation are clinically significant and also highlight the need for continued metabolic monitoring.
For our hypertension analysis, 66% (n = 190) of study participants were included, as 34% were currently receiving hypertension treatment. Given the APs’ intrinsic antagonistic action on adrenergic receptors, we expected higher values in all medication groups for males than females due to known sex differences in regulation (for a review, see Reckelhoff, 2001).30 However, although males had higher dBP in the high risk group and higher dBP and sBP in the low risk group the average dBP and sBP values seen in both males and females fell within normal range, suggesting that blood pressure dysregulation may not be a sex-specific concern associated with AP maintenance therapy. Regardless, blood pressure should be routinely assessed, especially for those on SGAs in the high and moderate risk groups given their weight gain propensity.
Sex differences were also found in regards to lipids, with females having higher TC and LDL and males having lower HDL. This analysis included 70% (n = 189) of our total study participants, as the others were currently receiving a lipid lowering agent. Nervertheless, these findings are similar to our group’s previous work,31 except this current study further explores sex differences stratified by AP risk for metabolic disturbances. Specifically, within the high and moderate risk groups, females had higher TC than males. Most notably, the prevalence of abnormal TC (≧ 200 mg/dL) was much more common in females (41.4–50%) compared to males (18.5–19.2%) in the high and moderate risk groups.
Similarly, higher LDL in females was found in the high and moderate risk groups with females approaching the threshold of borderline high whereas values in males were closer to the “normal” range. This clinically significant finding parallels other research showing increased LDL associated with AP use especially in clozapine and olanzapine therapy.
Overall males had lower HDL levels than females in the three groups which has been previously reported,20 although conflicting literature does exist.8,10,23 Therefore, comparisons between findings here and those in other studies must be done cautiously.
Altogether, our results suggest that both females and males are at risk for medication specific lipid dysregulation and that these findings may be especially beneficial for clinicians to consider when initiating and maintaining antipsychotic therapy.
Limitations
Although this study revealed important findings, there are some limitations to be acknowledged. Firstly, the cross-sectional design of the study limits a definitive causal relationship on sex, AP use, and metabolic parameters. Secondly, APs were analyzed in groups rather than in isolation due to sample size constraints. However, APs in each group have very similar metabolic risk propensity and participants had been using these medications chronically and were stable on them. Future research with large sample sizes should examine these APs individually. Lastly, p-value corrections for multiple comparisons were not used given a priori hypotheses and in order to decrease the likelihood of committing Type II error.
Conclusion
Based on our data we present three primary conclusions. Firstly, the prevalence of metabolic syndrome among those on maintenance AP therapy is notably high, especially among those in the high risk medication group, and this estimate may be higher than previously reported. Secondly, with respect to sex, females on APs considered to confer high or moderate metabolic risk appear to have the highest levels of metabolic dysfunction, specifically dyslipidemia and central adiposity, both of which increase risk for metabolic syndrome and overall CVD. Importantly, females on clozapine or olanzapine appear to represent a very high risk group for metabolic dysfunction and CVD, which has been previously reported. Conversely, males on these medications appear to be at highest risk for low HDL only. Clinicians should take note of these findings and consider the benefit-risk tradeoff when prescribing APs. In addition, preventative screening and adjunctive interventions aimed at decreasing risk for metabolic dysfunction should be utilized to help attenuate risks. As approximately 35% of participants were prescribed medications for the treatment of hypertension, dyslipidemia, or glucose dysregulation, this study suggests a striking lack of metabolic monitoring, which unfortunately parallels other research which concludes that adherence to the monitoring guidelines is poor.32,33
Acknowledgments
The authors thank the staff and consumers at the Washtenaw County Health Organization, Ann Arbor Veterans Affairs Medical Center, and the Detroit-Wayne County Community Mental Health Agency for their assistance and participation, respectively, in the current study. In addition, this study was supported by grants from The National Institute of Mental Health (R01MH082784), the National Center for Advancing Translational Sciences of the National Institutes of Health (2UL1TR000433-06), and the Chemistry Core of the Michigan Diabetes Research and Training Center (DK020572) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Role of Funding Source
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 3.Asmal L, Flegar SJ, Wang J, Rummel-Kluge C, Komossa K, Leucht S. Quetiapine versus other atypical antipsychotics for schizophrenia. In: Asmal L, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2013. p. CD006625. [DOI] [PubMed] [Google Scholar]
- 4.Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: Prospective data from phase 1. Schizophr Res. 2008;101(1-3):273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70(1):1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Fan X, Liu EY, Freudenreich O, et al. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res. 2010;118(1-3):211–217. doi: 10.1016/j.schres.2010.02.1028. [DOI] [PubMed] [Google Scholar]
- 10.Hägg S, Lindblom Y, Mjörndal T, Adolfsson R. High prevalence of the metabolic syndrome among a Swedish cohort of patients with schizophrenia. Int Clin Psychopharmacol. 2006;21(2):93–98. doi: 10.1097/01.yic.0000188215.84784.17. [DOI] [PubMed] [Google Scholar]
- 11.Lamberti JS, Olson D, Crilly JF, et al. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry. 2006;163(7):1273–1276. doi: 10.1176/appi.ajp.163.7.1273. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, Administration Booklet. American Psyciatric Press; 1997. [Google Scholar]
- 13.Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17(2):97–107. doi: 10.1016/j.molmed.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 15.Ventriglio A, Gentile A, Stella E, Bellomo A. Metabolic issues in patients affected by schizophrenia: Clinical characteristics and medical management. Front Neurosci. 2015 Sep;9 doi: 10.3389/fnins.2015.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHLBI Obesity Education Initiative Expert Panel on the Identification E and T of O in A (US) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. 1998 [Google Scholar]
- 18.Upmeier E, Lavonius S, Heinonen P, et al. Longitudinal changes in serum lipids in older people the Turku Elderly Study 1991-2006. Age Ageing. 2011;40(2):280–283. doi: 10.1093/ageing/afq180. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemettilä JP, Kampman O, Seppälä N, et al. Cytokine and adipokine alterations in patients with schizophrenia treated with clozapine. Psychiatry Res. 2014;218(3):277–283. doi: 10.1016/j.psychres.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Taylor DM, McAskill R. Atypical antipsychotics and weight gain-a systematic review. Acta Psychiatr Scand. 2000;101(6):416–432. doi: 10.1034/j.1600-0447.2000.101006416.x. [DOI] [PubMed] [Google Scholar]
- 22.de Vegt F, Dekker JM, Jager A, et al. Relation of Impaired Fasting and Postload Glucose With Incident Type 2 Diabetes in a Dutch Population. Jama. 2001;285(16):2109. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 23.Bobes J, Arango C, Aranda P, Carmena R, Garcia-Garcia M, Rejas J. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: Results of the CLAMORS Study. Schizophr Res. 2007;90(1-3):162–173. doi: 10.1016/j.schres.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46(3):456–462. doi: 10.2337/diabetes.46.3.456. [DOI] [PubMed] [Google Scholar]
- 25.Burghardt K, Goodrich J, Dolinoy D, Ellingrod V. DNA methylation, insulin resistance and second-generation antipsychotics in bipolar disorder. Epigenomics. 2015;7(3):343–352. doi: 10.2217/epi.15.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellingrod VL, Taylor SF, Brook RD, et al. Dietary, lifestyle and pharmacogenetic factors associated with arteriole endothelial-dependent vasodilatation in schizophrenia patients treated with atypical antipsychotics (AAPs) Schizophr Res. 2011;130(1-3):20–26. doi: 10.1016/j.schres.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folsom AR, Stevens J, Schreiner PJ, McGovern PG. Body mass index waist/hip ratio and coronary heart disease incidence in African Americans and whites. Atherosclerosis Risk in Communities Study Investigators. Am J Epidemiol. 1998;148(12):1187–1194. doi: 10.1093/oxfordjournals.aje.a009608. [DOI] [PubMed] [Google Scholar]
- 28.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 179(11-12):580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists & NAA for the S of O. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 30.Reckelhoff JF, Reckelhoff JF. Gender Differences in the Regulation of Blood Pressure. Hypertenson. 2001;37:1199–1208. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 31.Vassas TJ, Burghardt KJ, Ellingrod VL. Pharmacogenomics of sterol synthesis and statin use in schizophrenia subjects treated with antipsychotics. Pharmacogenomics. 2014;15(1):61–67. doi: 10.2217/pgs.13.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatana SAM, Kane J, Taveira TH, Bauer MS, Wu W-C. Monitoring and Prevalence Rates of Metabolic Syndrome in Military Veterans with Serious Mental Illness. Mitchell AJ, editor. PLoS One. 2011;6(4):e19298. doi: 10.1371/journal.pone.0019298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: Data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1):15–22. doi: 10.1016/j.schres.2006.06.026. [DOI] [PubMed] [Google Scholar]


