Abstract
Introduction
Physical activity (PA) conveys known cardiometabolic benefits to youth, but the contribution of vigorous-intensity PA (VPA) to these benefits is unknown. Therefore, we sought to determine, a) the associations between VPA and cardiometabolic biomarkers independent of moderate-intensity PA (MPA) and time sedentary, and b) the accelerometer cutpoint that best represents the threshold for health-promoting VPA in youth.
Methods
Data from the International Children’s Accelerometry Database (ICAD) were analyzed in 2015. The relationship between cardiometabolic biomarkers and 4 categories of VPA estimated via 3 sets of cutpoints were examined using isotemporal substitution quantile regression modeling at the 10th, 25th, 50th, 75th, and 90th percentile of the distribution of each biomarker, separately. Age, sex, accelerometer wear time, sedentary time, and MPA were controlled for while allowing substitution for light-intensity PA. Data from 11,588 youth (4-18yrs) from 11 ICAD studies (collected 1998-2009) were analyzed.
Results
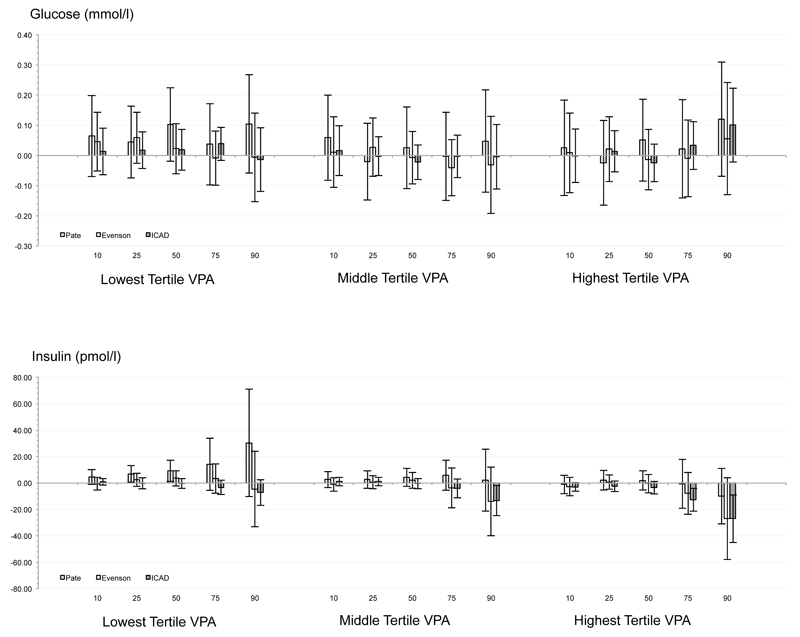
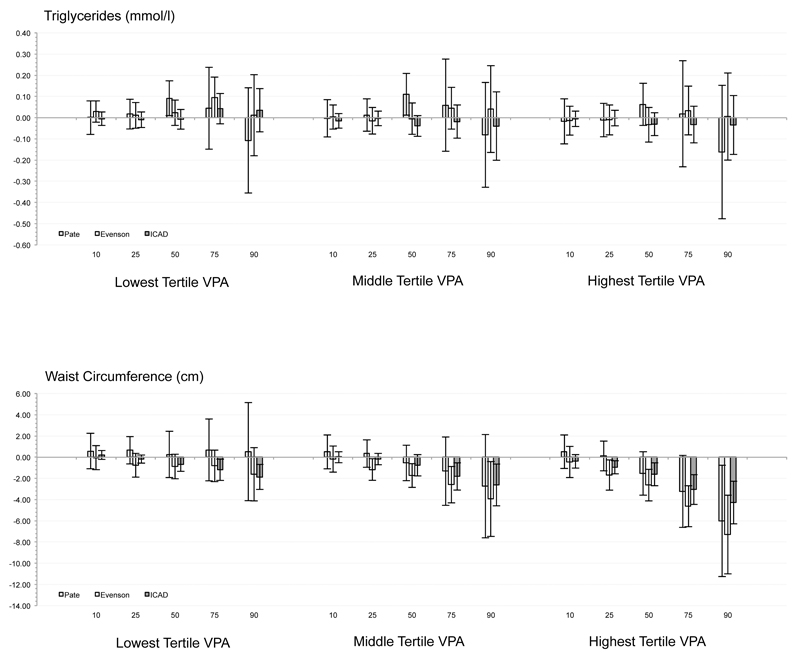
Only 32 of 360 significant associations were observed. Significant, negative relationships were observed for VPA with waist circumference and insulin. Replacing light intensity PA with VPA (corresponding to at the 25th to 90th percentiles of VPA) was associated with a .67 (-1.33, -0.01; P = .048) to 7.30cm (-11.01, -3.58; P < .001) lower waist circumference using Evenson and ICAD cutpoints (i.e., higher CPM). VPA levels were associated with 12.60 (-21.28, -3.92; P = .004) to 27.03 pmol/l (-45.03, -9.03; P = .003) lower insulin levels at the 75th to 90th percentiles using Evenson and ICAD cutpoints when substituted for light PA.
Conclusions
Substituting light PA with VPA was inversely associated with waist circumference and insulin. However, VPA was inconsistently related to the remaining biomarkers after controlling for time sedentary and MPA.
Keywords: Movement, cardiometabolic, adiposity, insulin
Introduction
Emerging research utilizing international samples (7, 17) has indicated that many children globally are spending an insufficient amount of time engaging in physical activity (PA) and an excessive amount of time engaging in sedentary behaviors. Engaging in international guideline recommendations (38) of 60 minutes per day (min/day) of moderate-to-vigorous physical activity (MVPA) is inversely associated with biomarkers of cardiometabolic health (13, 25) including lower rates of obesity (17) independent of time spent sedentary. While the benefits of MVPA are well established cross-sectionally (7) and longitudinally (6), few studies of PA in youth have examined the contribution of specific intensities to the association, despite a growing body of literature that suggests that vigorous-intensity physical activity (VPA) may be more important for the prevention and amelioration of cardiometabolic risk factors (13, 39). A small number of studies have employed an objective measure of PA to examine associations with cardiometabolic biomarkers. These studies suggest that VPA is independently associated with cardiorespiratory fitness (positive) (23), BMI (negative) (17), adiposity (negative) (32), HDL cholesterol (positive) (22), fasting glucose (negative) (31), and fasting insulin (negative) (1). However, an extensive examination of the literature suggests that the relationship between VPA and cardiometabolic biomarkers is inconsistent, potentially due to small samples, definition of VPA, and other methodological limitations (11).
Complicating examinations of the relations between VPA and cardiometabolic biomarkers is an issue of measurement of VPA, or more specifically, the threshold for which VPA occurs. While imperfect, accelerometers are still considered one of the best objective measures available for epidemiological studies of PA (5, 28), but the processing of data generated by accelerometers (e.g., “counts”) lacks uniformity or consistency across studies (18), which can lead to misclassification of exercise intensity (12) and/or lack of comparability across studies (3, 4). Since the choice of cutpoint is a de facto selection of an intensity threshold with all other sources of variability held constant (e.g., monitor brand, epoch), and no standard exists for the VPA cutpoint, it is imperative to consider a range of accelerometer cutpoints for VPA if the relationship between VPA and cardiometabolic biomarkers is to be studied.
The benefits of MVPA in youth are well established, but little research has been conducted to examine the contribution of PA intensity in cardiometabolic health in youth. Therefore, the objective of the present investigation was to determine, a) the associations between VPA and cardiometabolic biomarkers independent of moderate physical activity (MPA) and sedentary time, and b) the accelerometer cutpoint that best represents the threshold for health-promoting VPA in a diverse sample of youth.
Materials/Subjects and Methods
Study Design
Data were utilized from the International Children’s Accelerometry Database (ICAD, http://www.mrc-epid.cam.ac.uk/Research/Studies/), which was established to pool data on PA from studies in youth worldwide. A comprehensive description of the ICAD can be found elsewhere (34). Briefly, in 2008 19 studies were identified from a PubMed search that used an Actigraph (Actigraph, LLC, Pensacola, FL, USA) accelerometer and included a minimum of 400 participants aged 3 to 18 years. Six additional studies were identified through professional colleagues, with 21 studies ultimately contributing data to the final database (7, 34). For the current study, 11 studies were included (7, 34), which are presented in brief with the variables each contributed in Table 1 [details of the Avon Longitudinal Study of Parents and Children (ALSPAC) are available at www.bris.ac.uk/alspac and including the data that are available via a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary)]. Ethical approval for the present study was attained from participating institutions, and data-sharing agreements were established prior to contribution of data.
Table 1.
List of studies contributing data with cardiometabolic biomarkers present in analytical dataset.
| Study ID in the ICAD database | 1 | 4 | 5 | 6 | 9 | 10 | 11 | 12 | 14 | 15 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | ALSPAC | CoSCIS | DEYHS | EEYHS | MAGIC | NHANES (2005-6) | NEYHS | NHANES (2003-4) | Pelotas | PEYHS | KISS | Total # of studies contributing biomarker |
| Biomarker | ||||||||||||
| Diastolic Blood Pressure (mm Hg) | • | • | • | • | • | • | • | • | • | • | 10 | |
| Systolic Blood Pressure (mm Hg) | • | • | • | • | • | • | • | • | • | • | 10 | |
| HDL Cholesterol (mmol/l) | • | • | • | • | • | • | • | • | 8 | |||
| LDL Cholesterol (mmol/l) | • | • | • | • | • | • | • | • | 8 | |||
| Glucose (mmol/l) | • | • | • | • | • | • | • | 7 | ||||
| Insulin (pmol/l) | • | • | • | • | • | • | • | 7 | ||||
| Triglycerides (mmol/l) | • | • | • | • | • | • | • | • | 8 | |||
| Waist Circumference (cm) | • | • | • | • | • | • | • | • | • | • | • | 11 |
| Number of Biomarkers Measured | 3 | 8 | 8 | 8 | 3 | 8 | 6 | 8 | 3 | 8 | 6 | |
Abbreviations: ALSPAC, Avon Longitudinal Study of Parents and Children; CoSCIS, Copenhagen School Child Intervention Study; DEYHS: Denmark European Youth Heart Study; EEYHS: Estonia European Youth Heart Study; ICAD: International Children’s Accelerometry Database; KISS, Kinder Sportstudie; MAGIC, Movement and Activity Glasgow Intervention in Children; NHANES, National Health and Nutrition Examination Survey; NEYHS: Norway European Youth Heart Study; Pelotas: Pelotas 1993 Birth Cohort; PEYHS: Portugal European Youth Heart Study; SPEEDY, Sport, Physical Activity and Eating Behavior: Environmental Determinants in Young People.
Participants
Data from 11,588 youth (4-18yrs), representing 11 studies from Brazil, Europe, and the United States from the ICAD were analyzed. Data from studies conducted between 1998 and 2009 were included in the present analyses if the dataset contained PA, age, sex, and at least one biomarker of a cardiometabolic risk [defined as “A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (2)].
Measurements
Physical activity
A comprehensive description of the measurement of PA has been published previously (34). ICAD data were reanalyzed to allow for comparability across studies by aggregating data to a 60-second epoch. The criterion of 60 minutes of consecutive zeros was utilized to designate non-wear time, with a tolerance for 2 minutes of nonzero epochs (35). Participants with three or more days with 600 minutes of valid wear time were included in analyses. VPA was defined by cutpoints from Pate (26), Evenson (8), and the ICAD workgroup (7, 34). These cutpoints were selected because they represent the most generous, lowest threshold defining VPA [Pate ≥ 3,365 counts/min (CPM)], a medium threshold (Evenson ≥ 4,012 CPM), to the most stringent, highest threshold for VPA (ICAD ≥ 6,000 CPM).
Cardiometabolic biomarkers
Eight cardiometabolic biomarkers reflecting a diverse array of health indices were collected, including; waist circumference [as a proxy for adiposity (30)]; systolic and diastolic blood pressure (hemodynamics); high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting triglycerides (lipid metabolism); fasting glucose and fasting insulin (glucose metabolism). Details of data collection procedures can be found elsewhere (7, 34). Waist circumference (WC) was assessed midway between the lower rib margin and the iliac crest using a metal tape (10), except in the NHANES (National Health and Nutrition Examination Survey) where WC was measured just above the iliac crest at the mid-axillary line using similar equipment (36). Resting blood pressure was measured using standard procedures, reported previously (7). Markers of lipid and glucose metabolism were assessed using standard clinical procedures described in detail elsewhere (36).
Statistical Analysis
Descriptive analyses of accelerometer-derived estimates of min·day-1 spent in sedentary, MPA, and VPA were computed across all studies using three sets of cutpoints to define PA intensities. To evaluate the cross-sectional association of cardiometabolic biomarkers and time spent in VPA, a series of isotemporal substitution quantile regression models were estimated for each set of cutpoints separately (20, 21, 40). Quantile regression models were employed since biomarkers are often non-normal in their distribution, and quantile regression models are not influenced by normality and are free from distributional assumptions (19). Individual models for each biomarker as the dependent variable were estimated. Time spent in VPA, defined by one of the 3 sets of cutpoints, separately, served as the primary independent variable. Because of its non-normal distribution, min·day-1 spent in VPA was placed into 4 categories – none (0mins/d – reference category), low (lower 33%), middle (middle 33%), and high (upper 33%) – based on the distribution of VPA for each of the 3 sets of cutpoints. The relationship between cardio-metabolic biomarkers and 4 categories of VPA min/d [none (0 min/d – reference category), low (7.2Pate, 4.0Evenson, 1.5ICAD min/d), medium (18.6Pate, 11.0Evenson, 3.5ICAD min/d), and high (42.7Pate, 28.9Evenson, 11.9ICAD min/d)] estimated via 3 sets of cutpoints [Pate: sedentary = 0 - 152 counts/min (CPM), MPA = 1677 – 3364, VPA = ≥ 3,365 CPM; Evenson: sedentary = 0 - 100 CPM, MPA = 2296 – 4011, VPA = ≥ 4,012 CPM; and ICAD: sedentary = 0 - 100 CPM, MPA = 3000 – 6000, VPA = ≥ 6,001 CPM] ––were examined using isotemporal substitution quantile regression modeled at the 10th, 25th, 50th, 75th, and 90th percentiles of the distribution of each biomarker. Included in each model were age (years), sex, average total daily wear time, and min·day-1 in sedentary and MPA distilled using the corresponding cutpoint for VPA. Since light PA (LPA) was the only intensity excluded from the models, all estimates are interpreted as substituting “x” amount of LPA with VPA. Separate models were estimated for each study and for each set of cutpoints used to define VPA within each study. An example of the modeling approach is: insulin serving as the dependent variable, with 3 separate models using VPA levels (i.e., low, middle and high, with no VPA as the referent group) reduced with each of the sets of cutpoints for each study, run separately. Statistical significance was set at P = .05.
Meta-analytical techniques were used to combine the quantile regression model coefficients and standard errors for each biomarker across the 11 studies for each of the 3 sets of cutpoints, separately. Random effects inverse variance weighting was used to pool effects across studies and within study for each set of cutpoints. The study served as the unit of analysis for each quantile and category of VPA. For instance, the VPA estimates representing the lowest 33rd of the distribution of VPA regressed on the 10th quantile of insulin were combined across all studies for a given biomarker. All quantile regression analyses were conducted in 2015 using Stata (v.13.0, College Station, TX) and all meta-analytic analyses were conducted using Comprehensive Meta-Analysis (v2.2, Englewood, NJ).
Results
Descriptive information for each study is presented in Table 2. The average amount of VPA min·day-1 for each set of cutpoints (highest to lowest) by tertile ranged from 1.5 to 7.2 min/day for the lowest tertile, the medium tertile 3.5 to 18.6 min/day, and the highest tertile 11.9 to 42.7 min/day. The results of the pooled meta-analytic effects for each quantile and level of VPA across each cardiometabolic biomarker are presented in the supplemental table (see Table, Supplemental Digital Content 1, Results of meta-analytical combination of quantile regression model coefficients and standard errors for each risk factor across the 11 studies for each of the three sets of accelerometer cutpoints)
Table 2.
Descriptive statistics for demographic, physical activity, and cardiometabolic biomarkers variables by study.
| Study | ALSPAC | CoSCIS | DEYHS | EEYHS | MAGIC | NHANES (2005-6) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | |
| Sex (% male) | 5340 | 52.8% | 263 | 46.4% | 1143 | 55.6% | 436 | 57.8% | 195 | 52.3% | 1619 | 49.8% | ||||||
| Age (years) | 5340 | 11.74 | (0.23) | 263 | 9.54 | (0.38) | 1143 | 12.22 | (2.95) | 436 | 12.42 | (3.00) | 195 | 4.17 | (0.31) | 1619 | 12.54 | (3.34) |
| Height (cm) | 5312 | 150.68 | (7.23) | 262 | 139.78 | (5.94) | 1143 | 152.36 | (16.59) | 436 | 152.81 | (17.45) | 195 | 102.89 | (4.26) | 1618 | 152.00 | (17.44) |
| Weight (kg) | 5317 | 43.40 | (9.84) | 262 | 33.25 | (5.59) | 1143 | 45.30 | (15.63) | 436 | 44.56 | (15.72) | 195 | 17.26 | (2.58) | 1619 | 51.01 | (20.72) |
| Physical Activity (min/day) | ||||||||||||||||||
| ICAD cutpoints | ||||||||||||||||||
| Sedentary time | 5340 | 370.8 | (71.3) | 263 | 352.9 | (74.6) | 1143 | 412.0 | (121.3) | 436 | 373.5 | (106.0) | 195 | 226.4 | (65.5) | 1619 | 443.7 | (127.5) |
| Moderate PA | 5340 | 31.8 | (17.9) | 263 | 30.2 | (14.6) | 1143 | 25.1 | (18.0) | 436 | 33.4 | (23.0) | 195 | 23.1 | (13.2) | 1619 | 21.4 | (16.0) |
| Vigorous PA | 5340 | 4.0 | (5.3) | 263 | 4.7 | (4.6) | 1143 | 4.6 | (6.3) | 436 | 3.7 | (5.9) | 195 | 3.1 | (3.3) | 1619 | 4.1 | (6.5) |
| Evenson cutpoints | ||||||||||||||||||
| Sedentary time | 5340 | 370.8 | (71.3) | 263 | 352.9 | (74.6) | 1143 | 412.0 | (121.3) | 436 | 373.5 | (106.0) | 195 | 226.4 | (65.5) | 1619 | 443.7 | (127.5) |
| Moderate PA | 5340 | 40.5 | (17.1) | 263 | 42.3 | (16.1) | 1143 | 33.4 | (19.9) | 436 | 41.1 | (24.5) | 195 | 37.8 | (16.7) | 1619 | 30.1 | (17.5) |
| Vigorous PA | 5340 | 16.9 | (13.1) | 263 | 16.5 | (10.9) | 1143 | 14.8 | (13.7) | 436 | 17.9 | (16.3) | 195 | 11.3 | (8.6) | 1619 | 12.7 | (12.8) |
| Pate cutpoints | ||||||||||||||||||
| Sedentary time | 5340 | 412.3 | (71.0) | 263 | 390.0 | (75.8) | 1143 | 450.8 | (120.7) | 436 | 414.6 | (108.0) | 195 | 259.3 | (69.5) | 1619 | 483.0 | (127.8) |
| Moderate PA | 5340 | 58.4 | (20.3) | 263 | 66.5 | (21.7) | 1143 | 52.2 | (27.9) | 436 | 60.5 | (33.0) | 195 | 67.9 | (24.2) | 1619 | 49.7 | (24.7) |
| Vigorous PA | 5340 | 27.5 | (17.5) | 263 | 26.5 | (14.7) | 1143 | 22.9 | (18.1) | 436 | 28.3 | (20.7) | 195 | 19.2 | (12.6) | 1619 | 19.6 | (16.7) |
| Diastolic Blood Pressure (mm Hg) | 5250 | 58.69 | (6.55) | 262 | 61.36 | (5.45) | 1143 | 60.79 | (6.19) | 436 | 61.50 | (7.27) | 195 | 60.69 | (6.53) | 1402 | 57.75 | (10.60) |
| Systolic Blood Pressure (mm Hg) | 5250 | 105.43 | (9.71) | 262 | 103.66 | (8.19) | 1143 | 104.85 | (9.99) | 436 | 106.14 | (10.80) | 195 | 97.04 | (7.63) | 1411 | 107.06 | (10.20) |
| HDL Cholesterol (mmol/l) | – | – | – | 211 | 1.61 | (0.35) | 1088 | 1.51 | (0.35) | 430 | 1.43 | (0.29) | – | – | – | 1482 | 1.42 | (0.34) |
| LDL Cholesterol (mmol/l) | – | – | – | 211 | 2.33 | (0.57) | 1088 | 2.39 | (0.64) | 430 | 2.94 | (0.70) | – | – | – | 404 | 2.30 | (0.67) |
| Triglycerides (mmol/l) | – | – | – | 211 | 0.53 | (0.23) | 1088 | 0.82 | (0.41) | 430 | 0.78 | (0.34) | – | – | – | 404 | 0.90 | (0.48) |
| Glucose (mmol/l) | – | – | – | 214 | 4.82 | (0.46) | 1088 | 5.08 | (0.39) | 430 | 5.07 | (0.38) | – | – | – | 411 | 5.18 | (0.46) |
| Waist Circumference (cm) | 5314 | 67.96 | (9.19) | 262 | 61.89 | (6.39) | 1141 | 65.37 | (9.16) | 436 | 62.29 | (7.94) | 195 | 51.09 | (4.07) | 1602 | 73.35 | (14.75) |
| Insulin (pmol/l) | – | – | – | 206 | 5.74 | (3.01) | 1086 | 57.17 | (36.76) | 426 | 54.42 | (31.83) | – | – | – | 404 | 78.17 | (56.57) |
| Study | NEYHS | NHANES (2005-06) | Pelotas | PEYHS | KISS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | Number | M | (SD) | |
| Sex (percent male) | 274 | 51.1% | 1507 | 48.8% | 360 | 46.4% | 807 | 51.2% | 567 | 52.7% | |||||
| Age (years) | 274 | 9.69 | (0.32) | 1507 | 12.71 | (3.28) | 360 | 13.32 | (0.31) | 807 | 11.28 | (3.13) | 567 | 9.94 | (2.08) |
| Height (cm) | 273 | 139.37 | (6.39) | 1496 | 153.52 | (17.40) | 360 | 158.18 | (8.25) | 806 | 144.24 | (14.91) | 564 | 139.81 | (13.37) |
| Weight (kg) | 273 | 33.20 | (5.68) | 1497 | 52.51 | (20.68) | 359 | 51.18 | (12.11) | 807 | 41.36 | (14.43) | 564 | 34.86 | (10.38) |
| Physical Activity (min/day) | |||||||||||||||
| ICAD cutpoints | |||||||||||||||
| Sedentary time | 274 | 346.5 | (108.4) | 1507 | 449.7 | (134.5) | 360 | 777.9 | (107.5) | 807 | 418.6 | (105.6) | 567 | 631.8 | (181.6) |
| Moderate PA | 274 | 36.2 | (21.2) | 1507 | 23.7 | (17.4) | 360 | 21.9 | (16.1) | 807 | 25.8 | (19.2) | 567 | 37.8 | (18.9) |
| Vigorous PA | 274 | 7.6 | (10.4) | 1507 | 4.4 | (6.2) | 360 | 0.6 | (1.5) | 807 | 2.2 | (3.5) | 567 | 5.5 | (6.1) |
| Evenson cutpoints | |||||||||||||||
| Sedentary time | 274 | 346.5 | (108.4) | 1507 | 449.7 | (134.5) | 360 | 777.9 | (107.5) | 807 | 418.6 | (105.6) | 567 | 631.8 | (181.6) |
| Moderate PA | 274 | 45.6 | (22.5) | 1507 | 32.3 | (17.8) | 360 | 33.2 | (20.7) | 807 | 37.2 | (21.9) | 567 | 51.8 | (18.7) |
| Vigorous PA | 274 | 22.5 | (17.5) | 1507 | 14.0 | (13.6) | 360 | 7.7 | (7.9) | 807 | 11.8 | (11.5) | 567 | 20.3 | (14.4) |
| Pate cutpoints | |||||||||||||||
| Sedentary time | 274 | 385.7 | (109.0) | 1507 | 488.5 | (135.6) | 360 | 818.0 | (108.8) | 807 | 454.9 | (104.5) | 567 | 672.8 | (184.6) |
| Moderate PA | 274 | 66.5 | (29.5) | 1507 | 51.6 | (23.9) | 360 | 49.5 | (27.5) | 807 | 57.8 | (28.3) | 567 | 78.9 | (23.7) |
| Vigorous PA | 274 | 34.0 | (22.3) | 1507 | 21.6 | (18.1) | 360 | 15.7 | (12.9) | 807 | 20.7 | (17.2) | 567 | 32.8 | (18.9) |
| Diastolic Blood Pressure (mm Hg) | 273 | 62.52 | (5.96) | 1299 | 57.87 | (11.51) | 360 | 68.40 | (11.03) | 807 | 55.74 | (6.46) | – | – | – |
| Systolic Blood Pressure (mm Hg) | 274 | 102.91 | (7.76) | 1315 | 106.20 | (10.18) | 360 | 110.71 | (13.98) | 807 | 98.23 | (9.85) | – | – | – |
| HDL Cholesterol (mmol/l) | 65 | 1.55 | (0.32) | 1420 | 1.42 | (0.33) | – | – | – | 788 | 1.57 | (0.33) | 566 | 1.63 | (0.36) |
| LDL Cholesterol (mmol/l) | 58 | 2.88 | (0.96) | 686 | 2.31 | (0.69) | – | – | – | 788 | 2.19 | (0.60) | 567 | 2.11 | (0.61) |
| Triglycerides (mmol/l) | 48 | 0.92 | (0.33) | 687 | 0.95 | (0.54) | – | – | – | 788 | 0.71 | (0.33) | 521 | 0.62 | (0.27) |
| Glucose (mmol/l) | – | – | – | 457 | 4.98 | (0.48) | – | – | – | 788 | 5.19 | (0.44) | 499 | 4.59 | (0.39) |
| Waist Circumference (cm) | 273 | 60.23 | (5.42) | 1485 | 74.18 | (14.81) | 359 | 68.71 | (8.53) | 806 | 64.28 | (8.41) | 559 | 59.57 | (6.95) |
| Insulin (pmol/l) | – | – | – | 448 | 70.75 | (59.96) | – | – | – | 787 | 36.24 | (23.17) | 500 | 7.39 | (4.28) |
Abbreviations: ALSPAC, Avon Longitudinal Study of Parents and Children; CSCIS, Copenhagen School Child Intervention Study; DEYHS: Denmark European Youth Heart Study; EEYHS: Estonia European Youth Heart Study; ICAD: International Children’s Accelerometry Database; KISS, Kinder Sportstudie; MAGIC, Movement and Activity Glasgow Intervention in Children; NA, not available; NHANES, National Health and Nutrition Examination Survey; NEYHS: Norway European Youth Heart Study; PEACH, Personal and Environmental Associations with Children’s Health; Pelotas: Pelotas 1993 Birth Cohort; PEYHS: Portugal European Youth Heart Study; SPEEDY, Sport, Physical Activity and Eating Behavior: Environmental Determinants in Young People.
Relationship of volume of VPA with cardiometabolic biomarkers
Substituting LPA with VPA was inconsistently related to systolic/diastolic blood pressure, fasting triglycerides, HDL, or LDL after controlling for time sedentary and MPA at all tertiles of VPA volume, with only 32 of a possible 360 associations statistically significant (P < .05). Independent of min·day-1 spent sedentary and in MPA, substituting LPA with VPA was associated with a .67 to 7.30 cm smaller waist circumference at the 50th to 90th percentiles. Relationships were observed for all three tertiles of VPA, but relationships at the lowest tertile of VPA volume were significant at only the highest cutpoint value (i.e., ICAD). Substituting LPA with VPA was associated with 12.6 to 27.0 pmol/l lower insulin values at the 75th to 90th percentiles. Relationships were observed for all three tertiles of VPA, but relationships at the lowest tertile of VPA were significant at only the highest tertiles of VPA volume for the highest cutpoint value (i.e., ICAD).
Influence of cutpoint
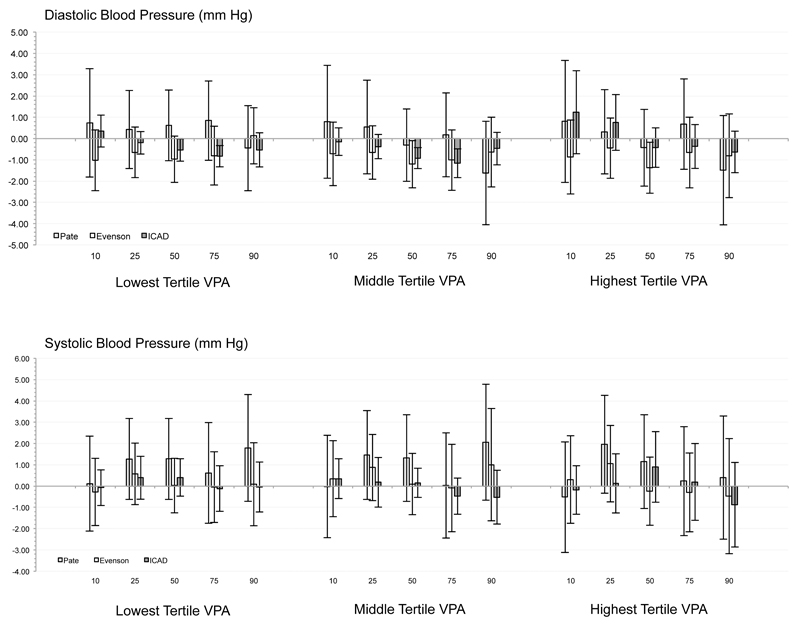
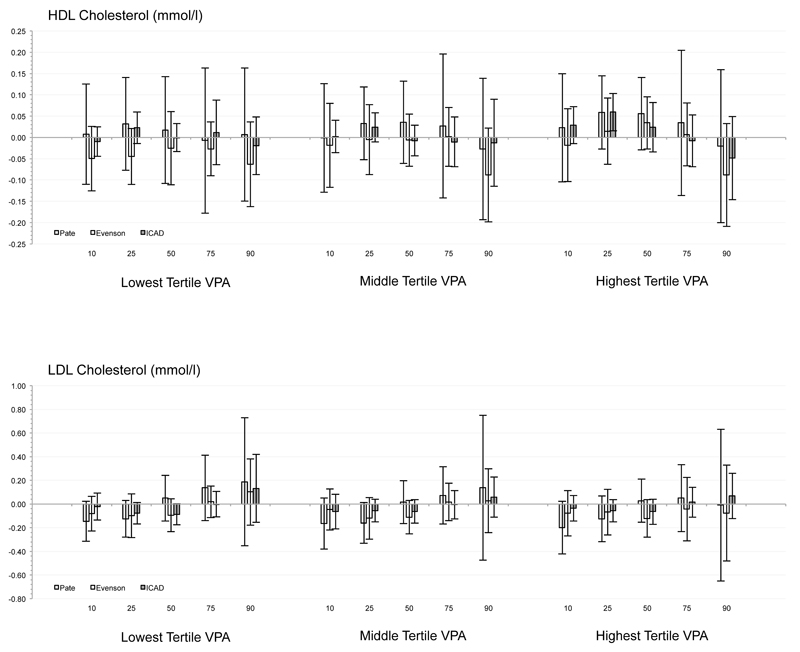
Independent of min·day-1 spent sedentary and in MPA, substituting LPA with the high volume of VPA defined via Pate cutpoints was associated with a smaller waist circumference only at the 90th percentile. For VPA determined via Evenson cutpoints, substituting LPA for medium and high VPA levels were associated with a smaller waist circumference at the 25th to 90th centiles. Substituting LPA with the lowest, medium, and highest volumes of VPA reduced via ICAD cutpoints was associated with a smaller waist circumference at the 50th to 90th, the 75th and 90th, and the 25th to 90th, respectively. Across all other biomarkers (i.e., SBP, DBP, HDL, LDL, glucose, and triglycerides), no consistent associations or patterns were observed, with only 9 significant associations observed from a possible 270 tested (<5%; see Figure).
Figure.
Combination of quantile regression model coefficients and standard errors for each risk factor across the 11 studies for each of the three sets of accelerometer cutpoints.
Discussion
The present study is the first of this scope (e.g., sample size, diversity of national origin) to examine the relationship between VPA and cardiometabolic biomarkers in youth. The results are consistent with previous studies using more homogeneous samples, such as Carson et al. (6) where no association was found between diastolic blood pressure and VPA, but a significant negative association was reported between waist circumference and VPA in children of the 2nd and 3rd quartiles (relative to the 1st). The more nuanced analyses presented here, taken with those of Carson et al. (6), provide additional insight into the complex relationship between VPA and cardiometabolic biomarkers (11, 25). The results suggest that substituting modest amounts of LPA for VPA may have cardiometabolic benefits above and beyond those conveyed by MPA and avoidance of sedentary behavior (24). Of potentially greater importance, the current results suggest that these health supportive associations are most pronounced in those who have undesirable levels of these biomarkers, specifically those with relatively large waist circumference or fasting insulin levels. If these relationships are found to be robust in longitudinal and experimental studies, then a specific frequency and duration of VPA could be incorporated as a distinct component of a PA “prescription” for youth (24). However, it must be noted that VPA was independently associated with only two of the markers examined. Therefore, while VPA may relay meaningful health benefits, the number of markers exhibiting those benefits may be few relative to less intense movement.
These results, taken with a growing body of literature demonstrating the independent health benefits of VPA for youth (6, 11, 14, 16, 17, 23, 24, 37), support the assertion that this intensity should be considered when setting policy recommendations for PA of youth. For example, it has been shown previously that as little as 9 (15) to 14 minutes (17) of VPA per day is associated with less adiposity in Canadian (15) and multinational samples of youth (17). These previous findings, derived from independent samples, are consistent with the present findings showing an association of substituting 11.9 to 42.7 min/day of LPA for VPA. While this is a considerable range, with the top end (42.7 min/day) potentially impractical, consistent benefits were seen for VPA defined by the ICAD cutpoints, which even in the high volume category represented 11.9 min/day of VPA, is potentially achievable for most youth (39). Therefore, the present findings suggest a modest duration (e.g., approximately 10 min) of high intensity PA may be related to health benefits in youth who exhibit undesirable levels of insulin or waist circumference.
While the present study has a number of strengths, including an objective measure of PA, a large sample size, a diverse and international sample, and an advanced analytical approach, the present results should be considered in light of a number of limitations. First, all data were cross-sectional in nature, therefore causality cannot be assumed. For example, it is possible that children with smaller waist circumference are more vigorously active because it is less cumbersome for them to do so. However, the nature of our analyses, which examined the relationship of VPA and waist circumference at different quantiles of waist circumference, is less supportive of this possibility. Second, while these cross-sectional results are supportive of VPA specific PA recommendations for youth, it is unknown if changes in youth VPA levels will result in meaningful changes in diastolic blood pressure, HDL, cholesterol, insulin or adiposity. While a recent study is supportive of the latter three (29), the literature is mixed on the relationship between increased VPA and blood pressure (9, 11, 27, 33), and very few studies have examined the responsiveness of insulin or other markers of glucose metabolism (11, 13). Third, the database we utilized lacks standardized dietary data or genetic data that might confound the observed relationships. For example, children with higher levels of VPA may consume fewer calories, or possess a genetic make-up supportive of a positive biomarker profile. This possibility cannot be ruled out using the currently available data. Despite these limitations, this study represents one of the largest to date that examined VPA in relation to cardiometabolic biomarkers in youth.
In summary, the present results suggest few significant or clinically meaningful associations between VPA and most cardiometabolic biomarkers studied in youth, but health promoting associations were observed between VPA and select cardiometabolic biomarkers (i.e., insulin, waist circumference), with the associations observed at higher levels of the biomarkers and higher volumes of VPA. As such, VPA may have unique metabolic health benefits beyond those conveyed by MPA or minimizing time spent sedentary. The present results also suggest that higher VPA cutpoints represent an intensity that is associated with healthier insulin levels and waist circumference. Future longitudinal and intervention studies are needed to determine the temporal relationship between these variables, the modifiability of VPA, and the effect of increased VPA on biomarkers in youth. If these results are indeed robust, then a less time consuming, more intense dose of PA may be a viable option for youth seeking to achieve or maintain cardiovascular health.
Supplementary Material
Acknowledgements
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and the results of the present study do not constitute endorsement by ACSM. We would like to thank all participants and funders of the original studies that contributed data to ICAD. We also gratefully acknowledge the contribution of Professor Chris Riddoch, Professor Ken Judge, and Dr. Pippa Griew to the development of ICAD.
Funding source: The pooling of the data was funded through a grant from the National Prevention Research Initiative (Grant Number: G0701877) (http://www.mrc.ac.uk/research/initiatives/national-prevention-research-initiative-npri/). The funding partners relevant to this award are: British Heart Foundation; Cancer Research UK; Department of Health; Diabetes UK; Economic and Social Research Council; Medical Research Council; Research and Development Office for the Northern Ireland Health and Social Services; Chief Scientist Office; Scottish Executive Health Department; The Stroke Association; Welsh Assembly Government and World Cancer Research Fund. This work was additionally supported by the Medical Research Council [MC_UU_12015/3; MC_UU_12015/7], Bristol University, Loughborough University, and Norwegian School of Sport Sciences.
Footnotes
ICAD Collaborators
The ICAD Collaborators include: Prof LB Andersen, University of Southern Denmark, Odense, Denmark (Copenhagen School Child Intervention Study (CoSCIS)); Prof S Anderssen, Norwegian School for Sport Science, Oslo, Norway (European Youth Heart Study (EYHS), Norway); Prof G Cardon, Department of Movement and Sports Sciences, Ghent University, Belgium (Belgium Pre-School Study); Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Hyattsville, MD USA (National Health and Nutrition Examination Survey (NHANES)); Prof A Cooper, Centre for Exercise, Nutrition and Health Sciences, University of Bristol, UK (Personal and Environmental Associations with Children's Health (PEACH)); Dr. R Davey, Centre for Research and Action in Public Health, University of Canberra, Australia (Children’s Health and Activity Monitoring for Schools (CHAMPS)); Prof U Ekelund, Norwegian School of Sport Sciences, Oslo, Norway & MRC Epidemiology Unit, University of Cambridge, UK; Dr. DW Esliger, School of Exercise and Health Sciences, Loughborough University, UK; Dr. K Froberg, University of Southern Denmark, Odense, Denmark (European Youth Heart Study (EYHS), Denmark); Dr. P Hallal, Postgraduate Program in Epidemiology, Federal University of Pelotas, Brazil (1993 Pelotas Birth Cohort); Prof KF Janz, Department of Health and Human Physiology, Department of Epidemiology, University of Iowa, Iowa City, US (Iowa Bone Development Study); Dr. K Kordas, School of Social and Community Medicine, University of Bristol, UK (Avon Longitudinal Study of Parents and Children (ALSPAC)); Dr. S Kriemler, Institute of Social and Preventive Medicine, University of Zürich, Switzerland (Kinder-Sportstudie (KISS)); Dr. A Page, Centre for Exercise, Nutrition and Health Sciences, University of Bristol, UK; Prof R Pate, Department of Exercise Science, University of South Carolina, Columbia, US (Physical Activity in Pre-school Children (CHAMPS-US) and Project Trial of Activity for Adolescent Girls (Project TAAG)); Dr. JJ Puder, Service of Endocrinology, Diabetes and Metabolism, Centre Hospitalier Universitaire Vaudois, University of Lausanne, Switzerland (Ballabeina Study); Prof J Reilly, Physical Activity for Health Group, School of Psychological Sciences and Health, University of Strathclyde, Glasgow, UK (Movement and Activity Glasgow Intervention in Children (MAGIC)); Prof J Salmon, School of Exercise and Nutrition Sciences, Deakin University, Melbourne, Australia (Children Living in Active Neigbourhoods (CLAN)); Prof LB Sardinha, Exercise and Health Laboratory, Faculty of Human Movement, Technical University of Lisbon, Portugal (European Youth Heart Study (EYHS), Portugal); Dr. LB Sherar, School of Sports, Exercise and Health Sciences, Loughborough University, UK; Dr. A Timperio, Centre for Physical Activity and Nutrition Research, Deakin University Melbourne, Australia (Healthy Eating and Play Study (HEAPS)); Dr. EMF van Sluijs, MRC Epidemiology Unit, University of Cambridge, UK (Sport, Physical activity and Eating behaviour: Environmental Determinants in Young people (SPEEDY)).
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Alhassan S, Robinson TN. Objectively measured physical activity and cardiovascular disease risk factors in African American girls. Ethn Dis. 2008;18(4):421–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein DB, Beets MW, Byun W, Welk G, Bottai M, Dowda M, et al. Equating accelerometer estimates of moderate-to-vigorous physical activity: In search of the Rosetta Stone. J Sci Med Sport. 2011;14(5):404–10. doi: 10.1016/j.jsams.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazendale K, Beets MW, Bornstein DB, Moore JB, Pate RR, Weaver RG, et al. Equating accelerometer estimates among youth: The Rosetta Stone 2. J Sci Med Sport. 2016;19(3):242–9. doi: 10.1016/j.jsams.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butte NF, Ekelund U, Westerterp KR. Assessing Physical Activity Using Wearable Monitors: Measures of Physical Activity. Med Sci Sports Exerc. 2012;44(1S):S5–S12. doi: 10.1249/MSS.0b013e3182399c0e. [DOI] [PubMed] [Google Scholar]
- 6.Carson V, Rinaldi RL, Torrance B, Maximova K, Ball GD, Majumdar SR, et al. Vigorous physical activity and longitudinal associations with cardiometabolic risk factors in youth. Int J Obes (Lond) 2014;38(1):16–21. doi: 10.1038/ijo.2013.135. [DOI] [PubMed] [Google Scholar]
- 7.Ekelund U, Luan Ja, Sherar LB, Esliger DW, Griew P, Cooper A. Moderate to Vigorous Physical Activity and Sedentary Time and Cardiometabolic Risk Factors in Children and Adolescents. JAMA. 2012;307(7):704–12. doi: 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1557–65. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- 9.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54(25):2396–406. doi: 10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Golding J, Pembrey M, Jones R, Team AS ALSPAC--the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 11.Gralla MH, McDonald SM, Breneman C, Beets MW, Moore JB. Associations of Objectively Measured Vigorous Physical Activity With Body Composition, Cardiorespiratory Fitness, and Cardiometabolic Health in Youth: A Review. Am J Lifestyle Med. 2016 doi: 10.1177/1559827615624417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinhouya CB, Lemdani M, Vilhelm C, Durocher A, Hubert H. Actigraph-defined moderate-to-vigorous physical activity cut-off points among children: statistical and biobehavioural relevance. Acta Paediatrica. 2009;98(4):708–14. doi: 10.1111/j.1651-2227.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 13.Gutin B, Owens S. The influence of physical activity on cardiometabolic biomarkers in youths: a review. Pediatr Exerc Sci. 2011;23(2):169–85. doi: 10.1123/pes.23.2.169. [DOI] [PubMed] [Google Scholar]
- 14.Gutin B, Yin Z, Humphries MC, Barbeau P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am J Clin Nutr. 2005;81(4):746–50. doi: 10.1093/ajcn/81.4.746. [DOI] [PubMed] [Google Scholar]
- 15.Hay J, Maximova K, Durksen A, Carson V, Rinaldi RL, Torrance B, et al. Physical activity intensity and cardiometabolic risk in youth. Arch Pediatr Adolesc Med. 2012;166(11):1022–9. doi: 10.1001/archpediatrics.2012.1028. [DOI] [PubMed] [Google Scholar]
- 16.Irving HM, Adlaf EM, Allison KR, Paglia A, Dwyer JJM, Goodman J. Trends in vigorous physical activity participation among Ontario adolescents, 1997-2001. Can J Public Health. 2003;94(4):272–4. doi: 10.1007/BF03403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Barreira TV, Broyles ST, Champagne CM, Chaput JP, Fogelholm M, et al. Physical Activity, Sedentary Time, and Obesity in an International Sample of Children. Med Sci Sports Exerc. 2015;47(10):2062–9. doi: 10.1249/MSS.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Beets MW, Welk GJ. Everything you wanted to know about selecting the "right" Actigraph accelerometer cut-points for youth, but…: a systematic review. J Sci Med Sport. 2012;15(4):311–21. doi: 10.1016/j.jsams.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Koenker R, Bassett G. Regression Quantiles. Econometrica. 1978;46(1):33–50. [Google Scholar]
- 20.Koenker R, Hallock KF. Quantile Regression. J Econ Perspect. 2001;15(4):143–56. [Google Scholar]
- 21.Koenker R, Xiao ZJ. Inference on the quantile regression process. Econometrica. 2002;70(4):1583–612. [Google Scholar]
- 22.Martinez-Gomez D, Eisenmann JC, Moya JM, Gomez-Martinez S, Marcos A, Veiga OL. The role of physical activity and fitness on the metabolic syndrome in adolescents: effect of different scores. The AFINOS Study. J Physiol Biochem. 2009;65(3):277–89. doi: 10.1007/BF03180580. [DOI] [PubMed] [Google Scholar]
- 23.Moore JB, Beets MW, Barr-Anderson DJ, Evenson KR. Sedentary time and vigorous physical activity are independently associated with cardiorespiratory fitness in middle school youth. J Sports Sci. 2013;31(14):1520–5. doi: 10.1080/02640414.2013.793378. [DOI] [PubMed] [Google Scholar]
- 24.Owens S, Galloway R, Gutin B. The Case for Vigorous Physical Activity in Youth. Am J Lifestyle Med. 2015 doi: 10.1177/1559827615594585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens S, Gutin B. Physical Activity and Cardiometabolic Biomarkers in Youths: A 2013 Update. Curr Cardiovasc Risk Rep. 2014;8(2):1–8. [Google Scholar]
- 26.Pate RR, Almeida MJ, McIver KL, Pfeiffer KA, Dowda M. Validation and calibration of an accelerometer in preschool children. Obesity (Silver Spring) 2006;14(11):2000–6. doi: 10.1038/oby.2006.234. [DOI] [PubMed] [Google Scholar]
- 27.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia. 2006;49(9):2078–85. doi: 10.1007/s00125-006-0320-6. [DOI] [PubMed] [Google Scholar]
- 28.Rachele JN, McPhail SM, Washington TL, Cuddihy TF. Practical physical activity measurement in youth: a review of contemporary approaches. World J Pediatr. 2012;8(3):207–16. doi: 10.1007/s12519-012-0359-z. [DOI] [PubMed] [Google Scholar]
- 29.Racil G, Ben Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113(10):2531–40. doi: 10.1007/s00421-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 30.Reilly JJ, Kelly J, Wilson DC. Accuracy of simple clinical and epidemiological definitions of childhood obesity: systematic review and evidence appraisal. Obes Rev. 2010;11(9):645–55. doi: 10.1111/j.1467-789X.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo NS, Ruiz JR, Oja L, Veidebaum T, Sjostrom M. Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am J Clin Nutr. 2008;87(3):586–92. doi: 10.1093/ajcn/87.3.586. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz JR, Rizzo NS, Hurtig-Wennlof A, Ortega FB, Warnberg J, Sjostrom M. Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am J Clin Nutr. 2006;84(2):299–303. doi: 10.1093/ajcn/84.1.299. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz KH, Jacobs DR, Jr, Hong CP, Steinberger J, Moran A, Sinaiko AR. Association of physical activity with insulin sensitivity in children. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(10):1310–6. doi: 10.1038/sj.ijo.0802137. [DOI] [PubMed] [Google Scholar]
- 34.Sherar LB, Griew P, Esliger DW, Cooper AR, Ekelund U, Judge K, et al. International Children's Accelerometry Database (ICAD): design and methods. BMC Public Health. 2011;11:485. doi: 10.1186/1471-2458-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 36.US Department of Health and Human Services. Anthropometry and physical activity monitor procedures manual. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2005. p. 132. [Google Scholar]
- 37.Vankim NA, Nelson TF. Vigorous physical activity, mental health, perceived stress, and socializing among college students. Am J Health Promot. 2013;28(1):7–15. doi: 10.4278/ajhp.111101-QUAN-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: WHO Press; 2010. [PubMed] [Google Scholar]
- 39.Yin Z, Moore JB, Johnson MH, Vernon MM, Gutin B. The impact of a 3-year after-school obesity prevention program in elementary school children. Childhood Obesity. 2012;8(1):60–70. doi: 10.1089/chi.2011.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu KM, Lu ZD, Stander J. Quantile regression: applications and current research areas. J Roy Stat Soc D-Sta. 2003;52:331–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.