Abstract
Objective
To evaluate the association between HIV infection and sexual maturation, and mediation of this association by HIV effects on growth.
Design
Pooled data were analyzed from two longitudinal cohort studies, the IMPAACT P219/219C Study (1993–2007) and the PHACS Adolescent Master Protocol (2007–2015), including perinatally HIV-infected (PHIV) and HIV-exposed uninfected (PHEU) youth.
Methods
We evaluated age at sexual maturity among 2539 PHIV and PHEU adolescents based on annual physician-assessed pubertal staging measures. Interval-censored regression models were used to evaluate associations of HIV infection with age at maturity. Mediation analyses accounting for height and body mass index (BMI) Z-scores at specific ages were used to estimate direct and indirect effects of HIV infection on age at sexual maturity.
Results
Mean ages at sexual maturity for PHIV girls (n=1032) were 15.5 years for both female breast and pubic hair and 15.9 and 15.8 years for PHIV boys (n=1054) for genitalia and pubic hair, respectively. PHIV youth matured approximately 6 months later on average than PHEU (n=221 girls, 232 boys), and this difference persisted after adjustment for race/ethnicity and birth cohort. BMI and height Z-scores mediated the association between HIV infection and later maturation in girls, accounting for up to 74% of the total HIV effect. Only height Z-scores mediated the effect of HIV on male age at maturity, accounting for up to 98% of the HIV effect.
Conclusion
PHIV youth attain sexual maturity later on average than PHEU youth. Much of this difference may be attributable to deficient growth, suggesting directions for future interventions.
Keywords: puberty, growth, HIV, sexual maturity, perinatal, mediation, interval-censoring
Introduction
The transition from a pre-pubertal state to sexual maturity occurs through a complex series of biological transformations known as puberty. This transition plays a critical role in child development, and pubertal timing has significant social and clinical implications [1]. Over the past 15 years, a trend towards earlier onset of puberty has been documented, particularly in girls [2]. This trend has been accompanied by increased rates of childhood obesity and declining physical activity levels, especially in more developed countries [3].
Studies in perinatally HIV-infected (PHIV) children have shown delayed pubertal onset [4–7] and some evidence of delays in attaining sexual maturity [7] compared to HIV-uninfected peers, with greater delay among those with greater disease severity [6,7]. In particular, we previously reported that the adjusted mean age at pubertal onset was delayed by 6 months in PHIV as compared to perinatally HIV-exposed uninfected (PHEU) youth, and by 4 to 13 additional months in PHIV youth with lower CD4 T-lymphocyte percentages (<15%) or higher HIV viral loads (above 10,000 copies/mL) [6]. The biological pathways through which perinatal HIV infection affects the timing of pubertal onset and sexual maturity are mostly unknown, limiting effective interventions for infected youth. As pubertal delay is known to correlate with poor growth in other populations, we hypothesized that growth deficits during late childhood may mediate an association between perinatal HIV infection and delayed sexual maturation [7–14].
Using data from two longitudinal cohort studies of children born to HIV-infected mothers, we evaluated the effect of perinatal HIV infection on the age at sexual maturity and employed novel approaches within a mediation analysis framework to investigate the proportion of this effect that might be explained by deficient growth.
Methods
Study population
This study included youth from two US-based longitudinal cohort studies of children born to HIV-infected mothers, the 219/219C study of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network and the Adolescent Master Protocol (AMP) study of the Pediatric HIV/AIDS Cohort Study (PHACS) Network. The 219/219C study enrolled PHIV and PHEU children at more than 80 sites in the US between 1993–2007 and was designed to evaluate long-term effects of HIV infection and perinatal HIV exposure [6]. AMP was established in 2007 and is a smaller, ongoing cohort study of PHIV and PHEU children who were 7–16 years old at entry [12]. Approximately 70% of AMP youth previously participated in the 219/219C study. Both studies were approved by Institutional Review Boards at Harvard T.H. Chan School of Public Health and at all clinical research sites, and written informed consent was obtained from each parent or legal guardian, with assent from children as appropriate.
Children had scheduled visits every 3 months in the 219/219C study and every 6 (until 2010) or 12 months (2010 and later) in the AMP study. Medical histories and health status were ascertained at each study visit through chart reviews, physical examinations, and laboratory evaluations. Race and ethnicity were self-reported at entry and categorized as white non-Hispanic, black non-Hispanic, Hispanic, or ‘other’. For the current analysis, we excluded children without a study visit after the age of 7 years and children who were at later stages of sexual development (Tanner stages 3 to 5) at their first study visit, yielding a final study population of 2539 children (1253 girls, 1286 boys). Among these, we considered study visits occurring after 7 years through 20 years of age.
Growth and sexual maturity assessments
Height and weight measurements and pubertal staging assessments were recorded at each study visit. Pubertal staging was assessed by visual inspection by study clinicians of breast development and pubic hair in girls and genitalia and pubic hair in boys, according to the criteria of Tanner and Whitehouse, with stages ranging from 1 (pre-pubertal) to 5 (mature) [15].
Statistical methods
For each participant, the date of the first pubertal assessment at age 7 years or older was used to define the start of follow-up. Measurements of height and weight were used to calculate age- and sex-adjusted Z-scores for height and body mass index (BMI) according to growth standards of the Centers for Disease Control and Prevention (CDC) [16].
Interval-censored approaches for survival outcomes were used to account for the fact that sexual maturity may occur between study visits rather than on a specific study visit date; this approach also allowed for right-censored outcomes, to reflect situations in which sexual maturity had not yet occurred by the last study visit. Interval-censored models were fit using accelerated failure time (AFT) models under a normal distribution assumption for age at sexual maturity [6, 12, 17]. We first estimated the mean age at sexual maturity separately for each pubertal marker, by HIV infection status, race/ethnicity, and birth cohort. We also evaluated the mean age [95% confidence interval (CI)] for each of the four puberty markers by HIV infection status adjusted for race/ethnicity and birth cohort. This final model was replicated in two sensitivity analyses to assess whether results were influenced by the unbalanced distribution of birth years between HIV-infected and uninfected children. First, we restricted the sample to those children born after 1993, thus excluding those categories with a limited number of PHEU children. Next, we repeated the analysis by randomly selecting a sample of children born after 1993 matched for birth cohort. Adjusted mean ages at menarche were also calculated by HIV status among the subset of girls with this information available; an AFT model with an assumed normal distribution was used accounting for right censoring for girls not reaching menarche by their latest assessment.
Mediation analysis was conducted to partition the total effect of HIV infection at birth on the age at sexual maturity into a direct effect of HIV infection, not acting through height and BMI z-scores, and an indirect effect acting through height and BMI Z-scores (mediators). We hypothesized that some proportion of the effect of HIV infection on sexual maturity might be explained by effects of deficient height experienced by PHIV children. Mediation analysis in this context requires modeling age at maturity as a function of HIV infection, repeating this model further adjusting for the mediator(s), and separately modeling the mediators as a function of HIV infection [18,19]. In this study, we applied an extension of the method to jointly evaluate the effect of two mediators (height and BMI Z-scores) [20]. Coefficients corresponding to the HIV infection effects without and with adjustment for the mediators were used to estimate, respectively, the total effect and direct effect of HIV infection, using interval-censored methods as previously described. The indirect effects, acting through differences in growth, were calculated by fitting separate models for the height and BMI Z-score mediators and multiplying the HIV coefficients from these models with the HIV coefficient from the mediator-adjusted maturity model [18,21,22]. We present results in terms of proportion mediated, calculated for each mediator as the ratio between the indirect and the total effect. Because our mediators were continuous, linear models were utilized to model the HIV-mediator relationships for height and BMI Z-scores. All statistical models were further adjusted for race/ethnicity and birth cohort. Possible interactions of the mediators with each other and with HIV were also explored by extending the multiple mediators approach proposed by Vanderweele and Vansteelandt [20]. However, no evidence of such interactions were detected, and all presented results omit interaction terms.
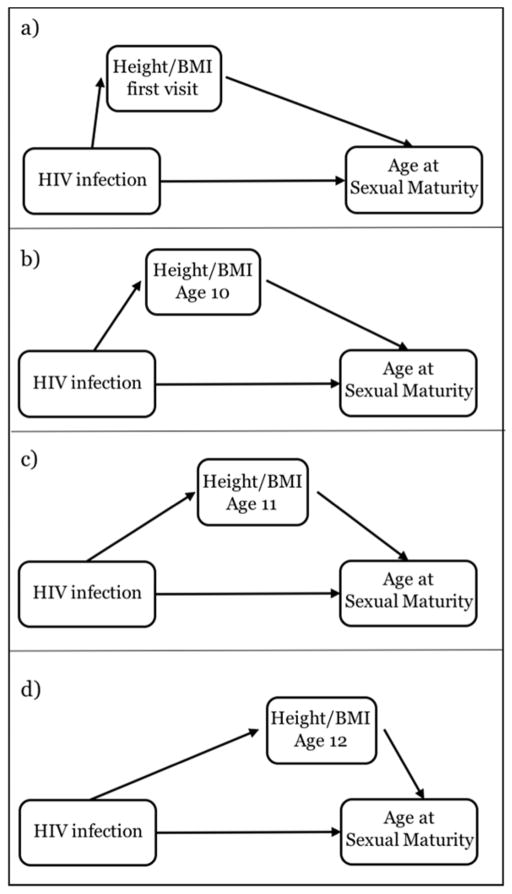
Because height and BMI change over time, their contribution in the causal pathway from HIV infection to sexual maturity might vary by age. Our repeated measures of height and BMI allowed exploration of how the percent mediated effect varied at different time points in adolescence. For each sexual maturity marker, mediation analysis was replicated based on height and BMI Z-scores at four different ages (each in a separate model, as illustrated in Figure 1): at the first study visit and at ages 10, 11, and 12 years. To allow comparison between the four scenarios, the mediation models were restricted to ~500 children of each sex who had a first study visit before age 10 years, at least one visit per year between 10–12 years, and experienced sexual maturity after 12 years. When multiple visits per year were available, the visit closest to the subject’s birthday was used. All statistical analyses were performed with the R package survreg. Statistical tests were two-tailed and p-values <0.05 were considered statistically significant.
Figure 1.
Illustration of mediation analysis of the role of height and BMI Z-scores in explaining the effect of perinatal HIV infection on the age at sexual maturity. The four scenarios were investigated in four separated statistical models by selecting different time points for the intermediate variables. For simplicity, the figure does not include potential confounders.
Results
Characteristics of the study population at baseline (first visit at 7 years or older) are presented by HIV infection status and sex in Table 1. The 453 PHEU children were on average younger and had greater height and BMI Z-scores than the 2086 PHIV youth. The distribution of sex and race were similar between PHIV and PHEU youth, with high percentages of black non-Hispanic and Hispanic youth in both groups.
Table 1.
Characteristics of the IMPAACT 219/219C and PHACS AMP study population, by HIV status, at the first study visit at age 7 years or older (n=2539)
| Characteristics | Perinatally HIV-exposed uninfected (PHEU) (n=453) | Perinatally HIV- infected (PHIV)(n=2086) | ||
|---|---|---|---|---|
|
| ||||
| Boys (n=232) | Girls (n=221) | Boys (n=1054) | Girls (n=1032) | |
| Age at baselinea (years), median (IQR) | 7.6 (7.2–8.5) | 7.5 (7.2–8.1) | 8.0 (7.4–9.9) | 7.9 (7.3–9.5) |
| Study | ||||
| AMP and 219/219C | 45 (19%) | 56 (25%) | 139 (13%) | 157 (15%) |
| 219/219C | 133 (57%) | 132 (60%) | 872 (83%) | 846 (82%) |
| AMP only | 54 (24%) | 33 (15%) | 43 (4%) | 29 (3%) |
| Birth cohort | ||||
| Before 1990 | 0 (0%) | 0 (0%) | 272 (21%) | 228 (22%) |
| 1990–1992 | 2 (9%) | 19 (9%) | 322 (27%) | 332 (32%) |
| 1993–1996 | 63 (27%) | 87 (39%) | 325 (30%) | 319 (31%) |
| 1997 or later | 147 (63%) | 115 (52%) | 135 (13%) | 153 (15%) |
| Race/ethnicity | ||||
| White non-Hispanic | 26 (11%) | 27 (12%) | 131 (12%) | 135 (13%) |
| Black non-Hispanic | 115 (50%) | 115 (52%) | 599 (57%) | 598 (58%) |
| Hispanic | 89 (38%) | 73 (33%) | 305 (29%) | 276 (27%) |
| Other/unknownb | 2 (1%) | 6 (3%) | 19 (2%) | 23 (2%) |
| Pubertal onset at baselinec | 29 (6%) | 48 (11%) | 138 (7%) | 198 (9%) |
| Height Z-score at baseline, mean (SD) | 0.26 (1.08) | 0.13 (1.07) | −0.71 (1.23) | −0.58 (1.16) |
| BMI Z-score at baseline, mean (SD) | 0.83 (1.25) | 0.68 (1.21) | 0.31 (1.07) | 0.25 (1.01) |
BMI=body mass index; IQR=interquartile range; SD=standard deviation
Baseline was defined as first pubertal assessment at age 7 years or older
Other race or multiracial:1,1,9,5; Unknown: 1,5,12,18.
Pubertal onset defined as achieving either stage 2 breast or stage 2 pubic hair development for girls, and as either stage 2 genitalia or stage 2 pubic hair development in boys
During a median follow-up of 4 years, 338 boys (27%) and 385 girls (31%) achieved sexual maturity according to at least one pubertal marker. Mean ages at sexual maturity were 15.5 years (95% CI: 14.5, 16.5) and 15.5 years (14.4, 16.6) for breast and pubic hair among PHIV girls, and 15.9 years for genitalia (14.8, 17.1) and 15.8 years for pubic hair (14.7, 16.9) among PHIV boys (Table 2).
Table 2.
Estimated mean ages in years at sexual maturity [with 95% confidence intervals]a by perinatal HIV infection status, race/ethnicity, and birth cohort, for perinatally HIV-exposed and perinatally HIV-infected youth.
| Girls (n=1253)b | ||||
|---|---|---|---|---|
| Breast Maturity | Pubic Hair | |||
|
| ||||
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |
| HIV infection: | ||||
| HIV-exposed uninfected | 14.9 (14.4–15.4) | 15.0 (14.5–15.5)c | 15.0 (14.5–15.6) | 15.1 (14.6–15.6) |
| HIV-infected | 15.5 (14.5–16.5)* | 15.3 (15.1–15.6) | 15.5 (14.4–16.6) | 15.4 (15.1–15.6) |
| Race/ethnicity | ||||
| White non-Hispanic | 15.8 (15.4–16.3) | 15.6 (15.2–16.1) | 15.8 (15.3–16.3) | 15.6 (15.1–16.1) |
| Black non-Hispanic | 15.3 (14.4–16.3)* | 15.2 (14.9–15.4) | 15.3 (14.3–16.3) | 15.2 (14.9–15.4) |
| Hispanic | 15.5 (14.5–16.5) | 15.3 (15.0–15.6) | 15.7 (14.7–16.6) | 15.5 (15.1–15.8) |
| Other/unknown | 15.5 (14.1–16.9) | 15.4 (14.4–16.4) | 15.6 (14.1–17.0) | 15.5 (14.4–16-5) |
| Birth cohort | ||||
| Pre-1990 | 15.8 (15.5–16.1) | 15.7 (15.4–16.0) | 15.8 (15.5–16.1) | 15.7 (15.4–16.0) |
| 1990–1992 | 15.3 (14.7–15.9)* | 15.3 (15.0–15.6)* | 15.3 (14.7–16.0)* | 15.3 (15.0–15.6)* |
| 1993–1996 | 15.0 (14.4–15.6)* | 15.0 (14.7–15.4)* | 15.1 (14.5–15.8)* | 15.2 (14.8–15.5)* |
| 1997 or later | 15.1 (14.3–15.9)* | 15.2 (14.5–15.9) | 15.2 (14.3–16.0) | 15.3 (14.6–16.0) |
|
| ||||
| Boys (n=1286) | ||||
|
| ||||
| Genitalia | Pubic Hair | |||
|
| ||||
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |
|
| ||||
| HIV infection: | ||||
| HIV-exposed uninfected | 15.3 (14.7–15.9) | 15.3 (14.7–15.9) | 15.3 (14.7–15.9) | 15.4 (14.8–16.0) |
| HIV-infected | 15.9 (14.8–17.1) | 15.8 (15.6–16.1) | 15.8 (14.7–16.9) | 15.7 (15.4–15.9) |
| Race/ethnicity | ||||
| White non-Hispanic | 15.9 (15.5–16.3) | 15.7 (15.3–16.2) | 15.9 (15.5–16.3) | 15.7 (15.3–16.1) |
| Black non-Hispanic | 15.8 (14.9–16-7) | 15.7 (15.4–15.9) | 15.7 (14.9–16.6) | 15.6 (15.3–15-8) |
| Hispanic | 16.1 (15.1–17.0) | 15.9 (15.6–16.2) | 15.8 (14.9–16.7) | 15.6 (15.3–15.9) |
| Other/unknown | 16.2 (14.6–17.9) | 16.1 (14.7–17.5) | 16.4 (14.8–18.1) | 16.2 (14.8–17.7) |
| Birth cohort | ||||
| Pre-1990 | 16.1 (15.8–16.3) | 16.0 (15.7–16.2) | 15.9 (15.7–16.2) | 15.9 (15.6–16.1) |
| 1990–1992 | 15.9 (15.4–16.4) | 15.8 (15.5–16.1) | 15.8 (15.3–16.3) | 15.8 (15.5–16.0) |
| 1993–1996 | 15.5 (15.0–16.1)* | 15.5 (15.2–15.8)* | 15.5 (14.9–16.0)* | 15.5 (15.1–15.8)* |
| 1997 or later | 15.7 (14.9–16.4) | 15.8 (15.1–16.5) | 15.3 (14.6–16.1) | 15.4 (14.8–16.1) |
Estimated from interval-censored AFT regression models assuming a normal distribution
Mean ages are adjusted for all other characteristics in the table at their average level
385 (34 PHEU, 351 PHIV) girls, and 338 (15 PHEU, 323 PHIV) boys achieved maturity
Statistical comparisons with the reference level (the first within each group) are marked by * P-value <0.05
Compared to PHEU youth, PHIV children showed consistently later attainment of sexual maturity by ~6 months for all puberty indicators (Table 2). These differences attained statistical significance only for breast maturity in females, but exhibited consistent trends for other maturity indicators. In the overall sample, race was significantly associated with age at sexual maturity only among females, with black non-Hispanic girls experiencing maturity 5 months earlier on average than white non-Hispanic girls (Table 2). Among black non-Hispanic youth, the estimated mean ages at sexual maturity among PHEU youth were 14.2 (13.5, 14.9) and 14.4 (13.7, 15.1) years for breast and pubic hair among girls, respectively, and 15.1 years (14.3, 15.9) for both markers for boys. A temporal trend was also documented, with a significant decrease in mean age for younger birth cohorts (i.e., born more recently) for all maturity markers. This trend remained significant after adjusting for HIV infection status and race/ethnicity. After adjustment for race/ethnicity and birth cohort, the mean age at sexual maturity was consistently older for PHIV than for PHEU youth, but significant differences were no longer observed (Table 2). Our two sensitivity analyses (excluding children born before 1993 and matching by birth cohort) showed negligible differences from our original findings, suggesting minimal influence of the imbalance in birth years between HIV-infected and uninfected children (Supplementary Tables S1 and S2). The mean age at menarche in a subset of 390 girls with available information was 12.5 years for PHIV girls and 12.0 years for PHEU girls, reflecting a delay of 5 months in both the unadjusted (4.9 months; 95% CI: 1.3, 8.5) and adjusted models (5.2 months; 95% CI: 1.4, 9.1).
Table 3 shows the effect of perinatal HIV infection status on growth measures in a subsample of the study population with annual visits between ages 9–12 years, stratified by sex. After adjusting for race/ethnicity and birth cohort, PHIV youth had significantly lower height Z-scores at all ages evaluated, with the largest difference at age 11 for girls and age 12 for boys. While BMI Z-scores were also lower among PHIV than PHEU youth at all ages, differences were not significant.
Table 3.
Adjusted differences in height and BMI Z-scores at different ages between perinatally HIV-infected youth as compared to perinatally HIV-exposed but uninfected youth, within the subset having annual follow-up at ages 10 through 12 years
| Age (years) | Height Z-score | BMI Z-score | ||
|---|---|---|---|---|
|
| ||||
| Estimated Difference | 95% CI for difference | Estimated Difference | 95% CI for difference | |
| Girls (n=491)a | ||||
| Baseline | −0.66 | (−1.06, −0.27) | −0.12 | (−0.50, 0.27) |
| Age 10 | −0.62 | (−1.04, −0.20) | −0.20 | (−0.58, 0.17) |
| Age 11 | −0.65 | (−1.14, −0.16) | −0.25 | (−0.65, 0.14) |
| Age 12 | −0.56 | (−0.99, −0.13) | −0.13 | (−0.52, 0.26) |
| Boys (n=526)b | ||||
| Baseline | −0.51 | (−0.91, −0.10) | −0.02 | (−0.39, 0.34) |
| Age 10 | −0.60 | (−0.98, −0.22) | −0.10 | (−0.48, 0.28) |
| Age 11 | −0.62 | (−0.99, −0.25) | −0.09 | (−0.61, 0.43) |
| Age 12 | −0.75 | (−1.15, −0.39) | −0.19 | (−0.61, 0.22) |
CI=confidence interval; BMI=body mass index (kg/m2). Estimates are adjusted for race/ethnicity and birth cohort.
32 PHEU, 459 PHIV
36 PHEU, 490 PHIV
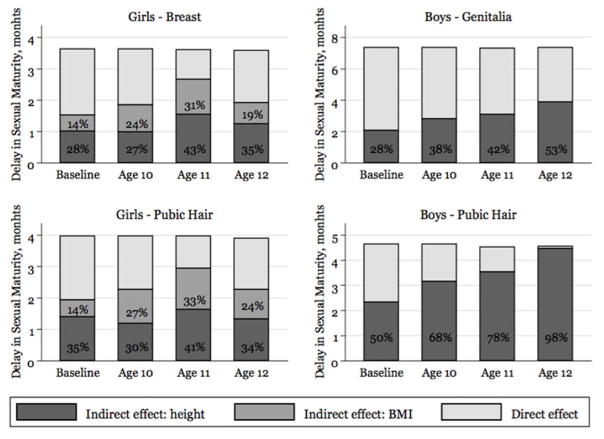
Results from the mediation models are presented in Figure 2. Growth as reflected by height and BMI z-scores mediated a large proportion of the total effect of HIV infection on age at sexual maturity for all pubertal markers, with considerable differences between boys and girls. Among females, both BMI and height Z-scores played a substantial role, with the strongest association at age 11: 74% of total effect was mediated by the two growth measures for both breast and pubic hair maturity. Among males, the proportion mediated by growth progressively increased with age, explaining up to 53% of the lag in genitalia maturation and 98% of the lag in pubic hair maturation at age 12 years. However, in contrast to girls, indirect effects were entirely attributable to height Z-score, with no evidence that BMI Z-score mediated the effect of HIV infection on timing of sexual maturity.
Figure 2.
Proportions of the total effect of perinatal HIV infection on the age at sexual maturity mediated by height Z-scores (dark grey) and BMI Z-scores (light grey) (“indirect effect”), according to four indicators of sexual maturity. Results were obtained by fitting mediation analysis models on ~500 children of each sex (breast: n=491; female pubic hair: n=487; genitalia: n=522; male pubic hair: n=526) with assessments at all 4 time points, corresponding to the four panels of Figure 1. No significant results were observed in this analysis.
Discussion
This study is one of the first to estimate age at sexual maturity among youth with perinatal HIV infection and to evaluate differences between infected and uninfected youth within the context of a large prospectively-followed cohort. We observed a delay in the age at sexual maturity among PHIV youth compared to PHEU youth. Moreover, we found that this effect was partly mediated by deficient height experienced by PHIV children. The impact of growth differed by sex, with both lower height and BMI among girls contributing to later age at maturity while only lower height appeared to mediate effects of HIV on timing of sexual maturity among boys. The contribution of growth as a mediator of the effect of HIV on sexual maturity varied with age, with the strongest contribution at age 11 for girls and age 12 for boys. This finding is not unexpected given the typically earlier pubertal onset of girls as compared to boys [6].
While previous studies addressed differences between PHIV and PHEU children in timing of pubertal onset [4–7], few studies have evaluated sexual maturity among PHIV youth [7]. The shift in mean age at maturity of approximately 6 months in PHIV as compared to PHEU adolescents is similar to that previously observed for age at pubertal onset [6], suggesting that once pubertal onset has begun the pace of pubertal progression remains similar in PHIV and PHEU youth.
Clinical effects of a shift of 6 months in the age at sexual maturity are anticipated to be subtle, and may not reflect “clinical delay” for most PHIV youth, but may nevertheless translate to a higher than expected percentage of PHIV youth with clinically delayed maturity. Based on age at Tanner stage 5 more than 2 standard deviations above sex- and race-specific means (from Susman et al [23]), 9.9% of PHIV boys and 10.7% of PHIV girls met the criteria for clinically delayed sexual maturation, as compared to an expected 2.5% in the general US population. In addition, there may be other public health implications of alterations in timing of sexual maturity, including reduced self-esteem and decreased bone mineral density. Previous research has focused predominantly on associations of early puberty on adverse health outcomes [1, 24], but recent findings have indicated associations of delayed puberty with increased risk of specific cancers. For example, Lope and colleagues recently reported a 6% increase in risk of prostate cancer for each 1-year delay in pubertal onset in boys [25].
A recent African study of PHIV youth reported mean ages of 16.1 and 16.5 years for breast and pubic hair maturity in girls and 16.9 and 16.8 years for genitalia and pubic hair maturity in boys [7]. These later mean ages at maturity than those we observed may be partially explained by greater height and weight deficits in HIV-infected children in Africa relative to those of the US [26, 27]. Consistent with our findings of strong effects of growth on pubertal maturation, this African study found that lower height was associated with additional pubertal delays among HIV-infected boys and girls, and also observed effects of BMI on pubertal maturation only in girls [7, 14]. Other diseases known to affect growth in children, such as type 1 diabetes and cystic fibrosis, have also been shown to affect sexual maturation [28, 29].
Growth delays in HIV-infected children, including those receiving combination ART, are common and not typically associated with growth hormone deficiency. However, low IGF-I levels may be present, possibly mediated by chronic inflammation causing increased production of cytokines or by under-nutrition, either of which may lead to a state of growth hormone (GH) resistance [30, 31]. Evidence also exists for concomitant GH and IGF-I resistance in association with HIV infection [32, 33]. In the present study, height had differential effects on genitalia and pubic hair maturation in boys; height explained about half of the HIV effect on genital maturation, but almost 100% of the effect on pubic hair. The pulsatile secretion of gonadotropin-releasing hormone (GnRH) triggers pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) which, in boys, drive testicular growth and spermatogenesis, respectively [34, 35]. In contrast, the start of pubic hair maturation is linked to adrenal maturation (adrenarche), the exact hormonal controller of which is unknown. Thus, it is not entirely unexpected that the role linear growth plays in affecting subsequent maturity may vary between these two pathways.
Our analysis represents a novel approach for evaluating the contributions of both HIV infection and growth on timing of sexual maturity. Using recently developed mediation analysis frameworks, we were able to evaluate multiple mediators (height and BMI) and their interactions on age at maturity [18,20]. Mediation analysis approaches for survival outcomes have only been recently developed and, to our knowledge, have not specifically been applied to interval-censored outcomes [19]. However, the framework of AFT models allows parametric models to be specified for the interval-censored outcomes of interest, and identification of indirect and direct effects of the primary exposure of interest (in this case, perinatal HIV infection) follows directly from previous assertions [19].
The large size of our cohort, longitudinal follow-up over almost 4 years, inclusion of an uninfected comparison group from a similar racial and socioeconomic background, and annual physician-assessed evaluations of pubertal staging are key strengths of our study; however, we recognize that our study has some limitations. Our observational cohort of perinatally infected youth represents a survivor cohort, particularly for youth born in the earliest years when effective antiretroviral therapy was not available. In addition, youth without HIV infection tended to be born in later years as a result of shifts in the HIV epidemic and improved management of HIV-infected pregnant women; thus, distinguishing trends over time within this subgroup is restricted to later birth cohorts. In addition, due to the generally younger age at study entry for PHEU youth, a much smaller percentage had been followed until an age at which sexual maturity would be expected, despite a similar duration of overall follow-up from study entry. Finally, the effect of HIV infection on timing of sexual maturity via its effect on growth may also be partially attributable to the previously demonstrated association of HIV with later pubertal onset, which in turn affects subsequent growth. Our approach evaluated the overall association between HIV infection and growth through multiple pathways, including alterations in the timing of pubertal onset.
To consider the role of growth measures at different ages while limiting the effects of selection bias, we restricted our mediation analysis to a smaller subset of youth with consistent annual follow-up measures, which reduced our power for detecting statistically significant associations. As such, results from the mediation analyses models should be interpreted with caution and need to be replicated in larger cohorts. Like all observational cohorts, there is also the potential for unmeasured confounding, but our findings were relatively robust after adjustment for race/ethnicity and birth cohort. However, the presence of unmeasured confounders, especially of the mediator-outcome associations, cannot be excluded, and may limit the causal interpretation of our results [36]. The limited number of participants achieving maturity by the end of follow-up may also limit the interpretation of our results. However, these two prospective cohorts are characterized by a high retention rate, with less than 5% of study participants lost to follow-up each year [37]. Censoring was primarily due to study closure or administrative censoring and thus was unlikely to bias analysis results. Another possible limitation is the imbalance in age at baseline between PHIV and PHEU children, which might increase the risk of selection bias due to exclusion of a higher proportion of PHIV children who were sexually mature at baseline.
Finally, given the relatively limited number of youth who attained maturity during the course of the study, we were unable to address the impact of specific ART regimens or markers of immune status on timing of sexual maturity. The benefits of combination ARV regimens have led to improvements in growth, at least in more developed countries, which may translate into reductions in previously observed delays in pubertal maturation. Future studies to evaluate the relationships between age of combination ARV initiation (and specific ARV regimens), height and BMI, and sexual maturation are warranted as more perinatally infected children transition to young adulthood.
In conclusion, PHIV youth experience delays in pubertal maturation that may have adverse long-term consequences, including reduced self-esteem and decreased bone mineral density, along with possible increased risks of some cancers [1,24,25,35]. The deficient growth experienced by PHIV children was found to be an important contributor to this negative effect. Effects mediated through growth differed by growth measures (BMI vs. height) and sex and between markers of maturity for boys. The role of growth in altering pubertal development of PHIV children indicates a potential for interventions (including early ART) that should ideally be initiated prior to the adolescent growth spurt.
Supplementary Material
Acknowledgments
Funding Statement:
The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases [U01 AI068632] and by the Statistical and Data Analysis Center at Harvard T.H. Chan School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group.
We thank the children and families for their participation in PHACS and in IMPAACT 219C, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigators: Kenneth Rich, Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases [U01 AI068632] and by the Statistical and Data Analysis Center at Harvard T.H. Chan School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group.
We thank the children, youth, and their families for their participation in PHACS and IMPAACT 219/219C, and the sites and site staff who conducted the AMP study (see www.phacsstudy.org/About-Us/AMP-AMPUp.Acknowledgements) and the IMPAACT 219/219 study (see www.phacsstudy.org/About-Us/219_219C.Acknowledgements)
Footnotes
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Contributions: AB and PLW were the primary authors who conceived and designed the study, conducted all statistical analyses, and led the writing of the manuscript. RVD, PLW, MJA, RH, and GRS provided leadership and oversight of the PACTG 219C study; RVD, GRS, PLW, and RH provided leadership and oversight of the PHACS AMP study; and LAD, KP, DLJ and MEG provided leadership for the PHACS Nutrition/Growth/Metabolism Working Group. All authors provided input on the study design, interpretation of analyses, and revisions to manuscript.
Conflict of interest: All authors state that they have no conflicts of interest related to this manuscript.
References
- 1.Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–S230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- 2.Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. 2012;77:137–145. doi: 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- 3.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121 S:S208–217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 4.De Martino M, Tovo P-A, Galli L, Gabiano C, Chiarelli F, Zapp M, et al. Puberty in perinatal HIV-1 infection: a multicenter longitudinal study of 212 children. AIDS. 2001;15:1527–1534. doi: 10.1097/00002030-200108170-00010. [DOI] [PubMed] [Google Scholar]
- 5.Buchacz K, Rogal AD, Lindsey JC, Wilson CM, Hughes MD, Seage GR, et al. Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr. 2003;33:56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Williams PL, Abzug MJ, Jacobson DL, Wang J, Van Dyke RB, Hazra R, et al. Pubertal onset in children with perinatal HIV infection in the era of combination antiretroviral treatment. AIDS. 2013;27:1959–1970. doi: 10.1097/QAD.0b013e328361195b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szubert AJ, Musiime V, Bwakura-Dangarembizi M, Nahirya-Ntege P, Kekitiinwa A, Gibb DM, Nathoo K, Prendergast AJ, Walker AS for the ARROW trial team. Pubertal development in African children on first-line antiretroviral therapy. AIDS. 2015;29:609–618. doi: 10.1097/QAD.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim RJ, Rutstein RM. Impact of antiretroviral therapy on growth, body composition and metabolism in pediatric HIV patients. Paediatr Drugs. 2010;12:187–199. doi: 10.2165/11532520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Arpadi S. Growth failure in children with HIV Infection. J Acquir Immune Def Syndr. 2000;25:S37–S42. doi: 10.1097/00042560-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 10.Guillén S, Ramos JT, Resino R, Bellón JM, Muñoz MA. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- 11.Nachman SA, Lindsey JC, Pelton S, Mofenson L, McIntosh K, Wiznia A, et al. Growth in human immunodeficiency infected children receiving ritonovir-containing antiretroviral therapy. Arch Pediatr Adolesc Med. 2002;156:497–503. doi: 10.1001/archpedi.156.5.497. [DOI] [PubMed] [Google Scholar]
- 12.Van Dyke RB, Patel K, Siberry GK, Burchett SK, Spector SA, Chernoff MC, et al. for the Pediatric HIV/AIDS Cohort Study. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic response. J Acquir Immune Defic Syndr. 2011;57:165–173. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr Rev. 2009;67:343–359. doi: 10.1111/j.1753-4887.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willemsen RH, Dunger DB. Normal Variation in Pubertal Timing: Genetic Determinants in Relation to Growth and Adiposity. Endocr Dev. 2016;29:17–35. doi: 10.1159/000438957. [DOI] [PubMed] [Google Scholar]
- 15.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat. 2002:1–190. [PubMed] [Google Scholar]
- 17.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 18.MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. [Google Scholar]
- 19.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22:582–585. doi: 10.1097/EDE.0b013e31821db37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWeele T, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Method. 2013;2:95–135. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelfand LA, MacKinnon DP, DeRubeis RJ, Baraldi AN. Mediation Analysis with Survival Outcomes: Accelerated Failure Time vs. Proportional Hazards Models. Front Psychol. 2016;7 doi: 10.3389/fpsyg.2016.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulcher IR, Tchetgen ET, Williams PL. A Cautionary Tale: Mediation Analysis Applied to Censored Survival Data. 2016 Aug 17; arXiv preprint arXiv:1608.04958. [Google Scholar]
- 23.Susman EJ, Houts RM, Steinberg L, Belsky J, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 91/2 and 151/2 years. Arch Pediatr Adolesc Med. 2010;164:166–73. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol. 2001;37:608–619. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- 25.Lope V, Garcia-Esquina E, Perez-Gomez B, Altzibar JM, et al. Perinatal and childhood factors and risk of prostate cancer in adulthood: MCC-Spain case-control study. Cancer Epidemiol. 2016;43:49–55. doi: 10.1016/j.canep.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Bakeera-Kitaka S, McKellar M, Snider C, Kekitiinwaa A, Piloya T, Musoke P, et al. Antiretroviral therapy for HIV-1 infected adolescents in Uganda: assessing the impact on growth and sexual maturation. J Pediatr Infect Dis. 2008;3:97–104. [Google Scholar]
- 27.Lundeen EA, Norris SA, Martorell R, et al. Early Life Growth Predicts Pubertal Development in South African Adolescents. J Nutr. 2016;146:622–629. doi: 10.3945/jn.115.222000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao L, Lu W, Ji F, Lv S. Development and linear growth in diabetic children receiving insulin pigment. J Pediatr Endocrinol Metab. 2011;24:433–436. doi: 10.1515/jpem.2011.204. [DOI] [PubMed] [Google Scholar]
- 29.Elamin A, Hussein O, Tuvemo T. Growth, puberty, and final height in children with Type 1 diabetes. J Diabetes Complications. 2006;20:252–256. doi: 10.1016/j.jdiacomp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Jain S, Desai N, Bhangoo A. Pathophysiology of GHRH-growth hormone-IGF1 axis in HIV/AIDS. Rev Endocr Metab Disord. 2013;14:113–118. doi: 10.1007/s11154-013-9245-9. [DOI] [PubMed] [Google Scholar]
- 31.Geffner M, Patel K, Miller T, Hazra R, Silio M, Van Dyke R, et al. for the Pedatric HIV/AIDS Cohort Study. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the Pediatric HIV/AIDS Cohort Study. Horm Res Paediatr. 2011;76:386–91. doi: 10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rondanelli M, Caselli D, Maccabruni A, Maghnie M, Bachella L, et al. Involvement of hormonal circadian secretion in the growth of HIV-infected children. AIDS. 1998;12:1845–1850. doi: 10.1097/00002030-199814000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Viganò A, Mora S, Brambilla P, Schneider L, Merlo M, Monti LD, Manzoni P. Impaired growth hormone secretion correlations with visceral adiposity in highly active antiretroviral treated HIV-infected adolescents. AIDS. 2003;17:1435–1441. doi: 10.1097/00002030-200307040-00003. [DOI] [PubMed] [Google Scholar]
- 34.Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:677–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 35.Ojeda SR, Lomniczi A. Puberty in 2013: unravelling the mystery of puberty. Nat Rev Endocrinol. 2014;10:67–69. doi: 10.1038/nrendo.2013.233. [DOI] [PubMed] [Google Scholar]
- 36.VanderWeele TJ. Explanation in causal inference: methods for mediation and interaction. Oxford University Press; 2015. Feb 13, [Google Scholar]
- 37.Williams PL, Van Dyke R, Eagle M, Smith D, et al. Association of site-specific and participant-specific factors with retention of children in a long-term pediatric HIV cohort study. Am J Epidemiol. 2008;167:1375–1386. doi: 10.1093/aje/kwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.