Abstract
Hypertension and chronic kidney disease (CKD) have a significant impact on global morbidity and mortality. The Low Birth Weight and Nephron Number Working Group has prepared a consensus document aimed to address the relatively neglected issue for the developmental programming of hypertension and CKD. It emerged from a workshop held on April 2, 2016, including eminent internationally recognized experts in the field of obstetrics, neonatology, and nephrology. Through multidisciplinary engagement, the goal of the workshop was to highlight the association between fetal and childhood development and an increased risk of adult diseases, focusing on hypertension and CKD, and to suggest possible practical solutions for the future. The recommendations for action of the consensus workshop are the results of combined clinical experience, shared research expertise, and a review of the literature. They highlight the need to act early to prevent CKD and other related noncommunicable diseases later in life by reducing low birth weight, small for gestational age, prematurity, and low nephron numbers at birth through coordinated interventions. Meeting the current unmet needs would help to define the most cost-effective strategies and to optimize interventions to limit or interrupt the developmental programming cycle of CKD later in life, especially in the poorest part of the world.
Keywords: Low birth weight, Nephron number, Intrauterine growth restriction, Small for gestational age, Preterm birth, Programmed risk of hypertension, Programmed risk of kidney disease, Maternal nutrition, Infant and child nutrition, Neonatal acute kidney injury
Preface
This consensus document aims to address the relatively neglected issue of the developmental programming of hypertension and chronic kidney disease (CKD). It emerged from a workshop, entitled The Fault Is Not in Our Stars but May Be in Our Embryos – Glomerular Number in Low Birth Weight Babies, held at the Clinical Research Center for Rare Diseases Aldo e Cele Daccò, IRCCS – Mario Negri Institute for Pharmacological Research, Bergamo, Italy, on April 2, 2016, including eminent internationally recognized experts in the field of obstetrics, neonatology, and nephrology (see Appendix). The goal of the workshop through multidisciplinary engagement was to highlight the association between fetal and childhood development and an increased risk of adult diseases, focusing on hypertension and CKD, and to suggest possible practical solutions for the future. Low birth weight (LBW), growth restriction, and preterm birth are the most consistent clinical surrogates for low nephron numbers and are associated with an increased risk of hypertension, proteinuria, and kidney disease later in life. This relationship is amplified by the development of acute kidney injury (AKI) in preterm infants, which may further reduce nephron numbers soon after birth, as well as by rapid catch-up growth or overfeeding during infancy or childhood in children born small, which may further augment the risk of hypertension and CKD and predispose to obesity and type 2 diabetes later in life. Many questions about the developmental origins of chronic renal disease, possible nutritional and pharmacologic interventions, as well as strategies for optimal follow-up and management of vascular, metabolic, and renal functions remain unanswered. The working group has discussed in depth how to raise awareness about developmental programming and renal disease risk later in life, and practical, locally adaptable preemptive strategies were suggested that could have long-term benefits in terms of future kidney health and cost saving worldwide. The discussion ended with the consensus recommendations presented here. This document is well aligned with the recent emphasis on a “life course” approach outlined by the World Health Organization (WHO) in the Minsk Declaration and the Global Action Plan for the Prevention and Control of Noncommunicable Diseases (NCD) [1, 2]. In both documents, the need to begin to prevent later-life chronic disease even before conception is emphasized, but specific recommendations beyond general nutritional interventions have not yet been made [3]. In turn, the life course approach aligns with the targets proposed by the United Nations 2030 Agenda for Sustainable Development, where a much broader approach is advocated to maintain health, and many goals are highly relevant to renal development and kidney disease [4].
Introduction to a Health Problem
The Global Burden of NCD
The WHO endorsed the Global NCD Action Plan in 2008 in response to growing recognition that NCD have replaced communicable diseases as the predominant causes of premature mortality worldwide [2]. Nevertheless, the global burden of NCD has been relatively neglected by policy makers, major aid donors, and academics until recently, given the global push to address communicable diseases over the past decade which diverted funds from NCD [5]. The NCD Action Plan aims to reduce premature mortality from cardiovascular disease (CVD), diabetes, cancer, and chronic lung disease by 25% by 2020 and emphasizes prevention as a crucial strategy to reduce NCD [2]. A “life course approach” is suggested as 1 of 9 overarching approaches for the prevention of NCD and has been highlighted in the recent Minsk Declaration, reflecting the increasing realization that early development is a determinant of later-life health and disease [1, 2]. Optimizing early development provides the chance for true primary prevention of NCD with major potential multiplier effects on overall health and well- being throughout life [4].
The worldwide prevalence of chronic diseases is projected to increase substantially over the next few decades [6]. For example, according to the International Diabetes Federation, the worldwide prevalence of diabetes is predicted to rise from 415 to 642 million between 2015 and 2040 [7]. In addition, by 2025, more than 75% of the world's diabetic population will reside in low- and middle-income countries (LMIC) [8]. Similarly, the prevalence of ischemic heart disease has almost doubled globally between 1990 and 2013 [9]. Although age-standardized mortality rates attributed to NCD have fallen worldwide, NCD remain the leading cause of death in the world, as shown by the 42% increase in the number of NCD-related deaths from 27 to 39.8 million between 1990 and 2015 [10]. Thus, the social, economic, and public health consequences of the expected increase in most NCD could have devastating consequences especially for LMIC.
CKD: A Global Health Problem
CKD is a key determinant of poor health outcomes for major NCD and has a risk-multiplier effect on CVD [11]. Recent findings from the Global Burden of Disease Study have highlighted CKD as an important cause of global mortality [10]. The number of reported deaths due to CKD was estimated to be 1.2 million, a 32% increase from 2005, with deaths from diabetic and hypertensive kidney disease comprising over 75% of these deaths [10]. The prevalence of end-stage kidney disease (ESKD) patients receiving renal replacement therapy (RRT) with maintenance dialysis has increased 1.7 times from 165 patients per million population in 1990 to 284 patients per million population worldwide in 2010 [12]. The number of people who will receive RRT (dialysis or transplantation) worldwide has been projected to more than double from 2.6 to 5.4 million from 2010 to 2030 [13]. Notably, it has been estimated that between 2.3 and 7.1 million people who could have been kept alive with RRT in 2010 died prematurely because they did not have access to the treatment [13]. Most of these deaths occurred in Asia, Africa, and Latin America, where RRT remains unaffordable [11]. With a population that is aging, steep increases in the worldwide incidence of type 2 diabetes mellitus and hypertension are driving the growth in the CKD burden, putting an enormous pressure on health care resources [11]. ESKD is only the tip of the iceberg. CKD occurs in approximately 10% of the population [11]. While the true prevalence of CKD in many LMIC countries remains ill defined [14], in industrialized countries CKD affects more disadvantaged populations and ethnic minorities and, therefore, causes a disproportionate burden on the poor [11]. Kidney disease is, therefore, a global public health priority. Given the very high individual and societal costs of treatment, prevention is the most effective strategy to sustainably address the growing global burden of kidney disease.
Developmental Programming of Chronic Diseases
The large individual variability in susceptibility to kidney disease and other NCD remains unexplained. Genetic predisposition and environmental exposures are contributory factors, but increasingly it is being recognized that fetal development is also an important modulator of the NCD risk. The quality and quantity of nutrition received during fetal life, exposure to pollutants, drugs, and infections during gestation, as well as the mother's health while she is pregnant, all impact fetal kidney development [15]. Perinatal exposures and nutrition as well as early childhood growth are also important. Since the first observations that adults who were born with LBW (defined as a birth weight <2.5 kg) were at a higher risk of premature cardiovascular death, increasingly compelling epidemiologic and experimental evidence has highlighted the “programming” impact of intrauterine and early childhood stresses on organ development and long-term organ functions [16, 17]. LBW, growth restriction, and preterm birth (defined in Table 1) have been the most accessible surrogate markers for intrauterine stress so far.
Table 1.
Definitions of birth weight categories and preterm birth
| Category | Definition |
|---|---|
| Birth weight categories | |
| Normal birth weight | >2,500 and <4,000 g (usually) |
| Large for gestational age | >2 SD above the mean birth weight for gestational age |
| Low birth weight | <2,500 g |
| Very low birth weight | <1,500 g |
| Appropriate for gestational age | ±2 SD of the mean birth weight for gestational age |
| Small for gestational age | >2 SD below the mean birth weight for gestational age |
| Intrauterine growth restriction | Evidence of fetal malnutrition and growth restriction at any time during gestation |
| Gestational categories | |
| Extremely preterm | <28 weeks’ gestation |
| Very preterm | <32 and >28 weeks’ gestation |
| Moderately preterm | <34 and >32 weeks’ gestation |
| Late preterm | <37 and >34 weeks’ gestation |
| Full term | >37 weeks’ gestation |
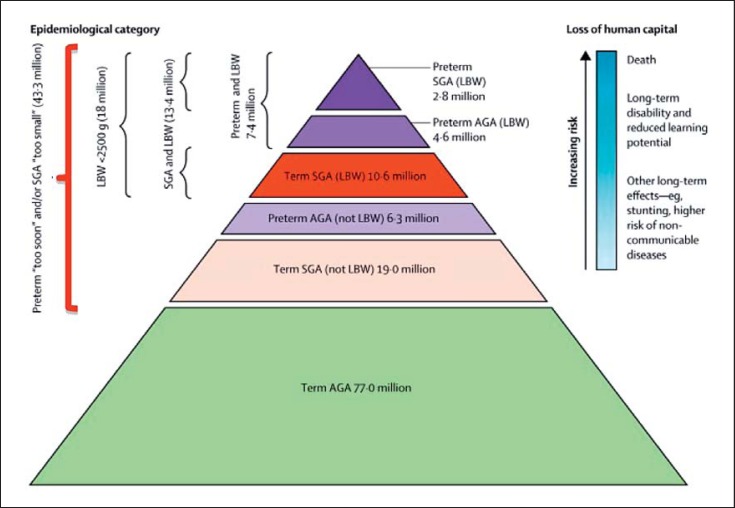
Although programming associations between LBW, growth restriction, preterm birth, and hypertension have been studied the most, evidence pointing to associations between LBW and CKD, CVD, obesity, glucose intolerance, type 2 diabetes, and preeclampsia is also quite convincing [16, 17, 21, 22, 23, 24, 25]. Until recently, research has largely focused on LBW and preterm birth as markers for developmental programming of hypertension and renal disease, but high birth weight (HBW), often as a result of a diabetic pregnancy or maternal obesity, is also emerging as a risk factor [18, 26, 27]. It is important to recognize that many babies who are born yearly with birth weights above 2.5 kg (technically not LBW) still experienced intrauterine growth restriction (IUGR) and may be inappropriately small for gestational age (SGA) (Fig. 1).
Fig. 1.
Number of infants born small for gestational age (SGA) or with low birth weight (LBW), and premature birth in low- and middle-income countries − 2010 (reprinted with permission according to CC Creative Commons Attribution-NonCommercial-noDerivs from Lee et al. [28]). A large number of infants born at term and SGA do not meet the definition of LBW and, therefore, likely experienced programming but may not be identified as at risk.
In addition, preterm infants may also have either an appropriate, although low, birth weight for gestational age (AGA) or may be SGA if they experienced superimposed growth restriction (Fig. 1). Such growth restriction per se is also associated with programming effects in the kidney, emphasizing the continuum of the programming risk and the need for heightened awareness of this risk [29, 30, 31, 32]. Worldwide, the incidence rates of LBW and preterm birth lie at around 15–20% and 11%, respectively [33, 34]. There is, however, significant overlap between LBW, preterm birth, and SGA, with the total reaching around 36% of live births in LMIC in 2010 [28]. Globally, the incidence of HBW is increasing, ranging from 5 to 20%, with many infants probably exposed to maternal diabetes or obesity [18]. Therefore, many infants born every year likely undergo developmental programming, which may be the first in a succession of “hits” that ultimately manifest in overt disease. Consequently, the population impact of developmental programming may be considerable. For example, a population-based study in the US found that of every 13 adolescents born with LBW, or 5 with very low birth weight (VLBW), 1 had elevated systolic blood pressure and 1 had a reduced glomerular filtration rate (GFR) [35]. These numbers would be expected to increase as subjects age.
The Low Nephron Number Hypothesis
Under normal developmental conditions, nephrogenesis continues until the 36th week of gestation in utero, and no new nephrons develop following birth in full-term infants [36].
Building on early epidemiologic evidence linking LBW and adult CVD, Brenner et al. [37] hypothesized that developmental programming in the kidney may result in a reduction in nephron number, which in turn may be a factor contributing to higher blood pressure and increased risk of CKD. The authors hypothesized that a reduction in whole kidney glomerular surface area resulting from a programmed reduction in nephron number would enhance susceptibility to hypertension by limiting sodium (salt) excretory capacity and increase susceptibility to CKD through a reduced capacity to compensate for renal injury. Consistent with this possibility, LBW, hypertension, and CKD, all tend to occur more frequently in poorer populations [38, 39, 40, 41, 42]. In animal studies early on, feeding pregnant rats a low-protein diet induced LBW in the offspring, which subsequently developed spontaneous hypertension that increased with age, chronic renal injury, and premature death [43, 44, 45]. The rat offspring also had smaller kidneys and reduced nephron numbers, which strongly supports the nephron number hypothesis. Although the magnitude of programming effects observed often differs between males and females [46] and between experimental conditions, many diverse animal studies also strongly support the association between adverse intrauterine conditions and a higher risk of hypertension and renal dysfunction with age, as reviewed elsewhere [47, 48].
Nephron Numbers in Humans
The inability to determine nephron number in living humans has been a major obstacle to definitively investigating the nephron number hypothesis. To date, all nephron-counting studies have been performed on autopsy samples. From 7 studies with nearly 500 subjects, we know that the average nephron number is ∼1,000,000 per kidney [49, 50, 51, 52, 53, 54]. Human nephron number is highly variable, however, ranging from 210,000 to1 2.7 million [53]. This 13-fold variability likely contributes to individual susceptibility to hypertension and kidney disease [51, 52, 55]. Significant variability is already present at birth, highlighting the importance of early nephrogenesis [56, 57]. Nearly 60% of nephrons are developed in the third trimester of pregnancy [58]. In preterm infants, nephrogenesis may occur for up to 40 days after birth, but may be abnormal [57, 58]. Nephron numbers have been found to be reduced in infants who were born preterm or of LBW [58, 59, 60, 61]. Importantly, however, it has been observed in some animal models that low nephron numbers may also occur with normal birth weight, so the burden or risk of renal programming may be underestimated if birth weight is the only surrogate marker considered [62]. Over time, nephron numbers decline due to age-related glomerulosclerosis and obsolescence, and thus age is also an important risk modifier of the programmed renal risk [51, 63, 64]. Further clinical surrogates associated with reduced nephron numbers in humans include adult height, female gender, Australian Aboriginal ethnicity, and maternal vitamin A deficiency [65, 66] (Table 2).
Table 2.
Clinical associations with low nephron numbers [reproduced with permission from 67]
| Clinical feature | Association with nephron number | Population | Reference |
|---|---|---|---|
| Low birth weight | ↑ of 257,426 glomeruli per kilogram increase in birth weight | USA white and black, children and adults | 60 |
| Prematurity | ↓ glomerular number in preterm vs. term infants | US premature and full-term neonates | 58, 59 |
| Gender | Nephron number is 12% lower in females | USA white and black, Aboriginal Australian | 66 |
| Age | ↓ 3,676 glomeruli per kidney per year of age >18 years | USA white and black, Aboriginal Australian | 66 |
| Adult height | ↑ 28,000 glomeruli per centimeter increase in height | Aboriginal Australian, German, white | 52, 66 |
| Kidney mass | ↑ 23,459 glomeruli per gram of kidney tissue | Infants <3 months of age | 68 |
| Glomerular volume | Inverse correlation between glomerular volume and nephron number | US white and black, Aboriginal Australian, German adults, Cuban infants | 52, 55, 61 |
| Ethnicity | ↓ Aboriginal Australian vs. US white and black | US white and black, Aboriginal Australian | 66 |
Nephron numbers have been shown to correlate with kidney weight, so renal mass has also been used as a surrogate marker for nephron numbers, although this relationship may be confounded by renal hypertrophy [51, 68, 69]. In all studies, glomerular numbers correlate inversely with glomerular volume, largely independent of gender and race, potentially reflecting compensatory glomerular hyperfiltration [55, 61, 70]. Therefore, glomerular volume has also been proposed as a surrogate marker for reduced nephron numbers in the absence of other causes. A promising innovative method for nephron enumeration is the use of cationic ferritin as an MRI-detectable contrast agent, which highlights the glomerular basement membrane of each nephron [71, 72, 73, 74]. This contrast allows for the quantification of all glomeruli in a nondestructive manner and may potentially be useful in vivo. A more definitive quantification of nephron numbers in real time would permit more comprehensive and larger-scale studies of the relationship between nephron number, clinical parameters, and the risk of hypertension and renal disease.
Nephron numbers increase in proportion to birth weight and gestational age [60]. Importantly, there is no known discrete threshold above which a nephron number is “high enough”; nephron numbers occur along a continuum in the population, as does disease risk. It is likely, however, that individuals with nephron numbers on the lower side of the spectrum are those at higher risk of hypertension and kidney disease [52]. The relationship between an individual's body size (metabolic demand) and nephron numbers is probably an important modulator of this risk [75]. Superimposed renal “hits” or other risk modifiers, therefore, likely determine the phenotypic expression of disease along the spectrum of nephron number. It is clear that better biomarkers for the early detection of renal structural changes are needed to help predict which LBW, SGA, or preterm infant will develop hypertension and CKD.
Developmental Programming of Hypertension and Kidney Disease
Programmed Associations with Blood Pressure
LBW and preterm birth are both associated with an increased risk of elevated blood pressure in later life. Meta-analyses have shown that systolic blood pressure levels were higher in preterm or VLBW adolescents than in controls born at term (mean increase of 2.5 mm Hg; 95% confidence interval, CI, 1.7–3.3 mm Hg from 10 studies) and in subjects with birth weights <2.5 kg compared with ≥2.5 kg (mean increase 2.28 mm Hg; 95% CI, 1.24–3.33 from 9 studies). In the latter study, the odds ratio (OR) of overt hypertension was 1.21 (95% CI, 1.13–1.3) for those with birth weights <2.5 kg compared with ≥2.5 kg [22]. A systematic review of the impact of HBW on blood pressure, however, also found a risk ratio of 1.18 (95% CI, 1.05–1.32 from 6 studies) for hypertension in children who had birth weights ≥4 kg compared with birth weights <4 kg, but this effect did not persist in adults [27]. In a further meta-analysis of 13 studies including 1,115 children aged 2–20 years exposed to diabetes during gestation, systolic blood pressure levels were found to be higher compared to controls (mean difference 1.88 mm Hg; 95% CI, 0.47–3.28); however, this effect appeared to predominate in males [76]. Similarly, a systematic review found that systolic blood pressure levels were 2.39 mm Hg (95% CI, 1.74–3.05 from 18 studies) higher among young adults who had been exposed to preeclampsia [77]. Longer-term studies are required to determine the impact of preterm birth, exposure to diabetes, and preeclampsia on blood pressure in older cohorts.
Number of Nephrons in Human Subjects with Primary Hypertension
In white adults aged 35–59 years who died in accidents, nephron numbers were significantly reduced in 10 subjects with known essential hypertension compared with 10 matched normotensive controls [52]. Although birth weights were unknown, this study supports an association between reduced nephron numbers and the risk of essential hypertension. In other studies, nephron numbers were found to be lower in Caucasians, or Aboriginal Australians, with higher blood pressure levels [52, 66, 78]. This relationship was not as strong in African-Americans, although hypertension was more prevalent in those with nephron numbers below the group mean, implying that nephron numbers likely have a modifying effect on hypertension in this population [78]. Hypertension increases with glomerular volume in both white and African-American subjects, although the probability of developing hypertension is universally higher for African-American subjects [55]. A better understanding of the ethnic variability in developmental programming risks is important to refine our understanding of the pathophysiology of the programming of hypertension. The findings that salt sensitivity of blood pressure in humans is associated with LBW and a small kidney size are consistent with altered sodium handling having a role in the pathogenesis of hypertension in LBW subjects, which may at least partially be mediated by a reduction in nephron numbers [79, 80, 81].
Other Programmed Factors Contributing to Increased Blood Pressure
Low nephron numbers alone are not always associated with programmed hypertension, suggesting that additional factors also contribute to this phenotype. Restoring nephron numbers by supplementing a low-protein diet with urea or alanine in pregnant rats did not prevent the programmed rise in blood pressure of the rat offspring, whereas supplementation with glycine did, suggesting that varying amino-acid deficiencies during gestation may have different programming effects on the kidney [82]. Similarly, postnatal hypernutrition in normal rats led to obesity, hypertension, and glomerulosclerosis with age, despite a 20% increase in nephron numbers [83]. Other elegant studies have demonstrated changes in renal tubular sodium handling in all tubule segments and altered vascular function in developmentally programmed animals that likely also contribute to blood pressure and renal function changes later in life [84, 85]. As with nephron number studies, the varying experimental conditions and animals used are associated with variations in the programmed phenotype, which underscores the likely multifactorial nature and ramifications of developmental “hits.”
Clinical Associations of Renal Programming with Renal Function and CKD
LBW has been the best-studied marker for having experienced an adverse intrauterine environment and renal developmental programming [15]. Studies have shown strong associations with fewer and bigger glomeruli, a greater risk of hypertension, proteinuria, salt sensitivity of blood pressure, and progressive CKD [16, 22, 60, 61, 65, 78, 79, 80, 86]. Overall, a meta-analysis of 31 studies, including over 2 million subjects, documented that in LBW offspring the risk of developing CKD (defined as albuminuria, a reduced GFR, or renal failure) in later life is increased by 70% [16]. In a Norwegian population-based study, the odds of a reduced GFR (<100 mL/min) were 1.66 (95% CI, 1.16–2.37) in men and 1.65 (95% CI, 1.17–2.35) in women who were born SGA compared with AGA, which increased further among those who had been very small for gestational age, demonstrating a dose-response effect [32]. Studies examining renal function after preterm birth have thus far been conducted predominantly in children, and many have described an association with reduced GFR and increased urinary albumin excretion among those who had been born preterm [30, 31, 87, 88, 89]. In a cohort of young adults born preterm, birth weight correlated negatively with microalbuminuria and positively with GFR [90]. In addition, those who had been preterm and SGA had a 2.4-fold (95% CI, 0.6–9.3) increase in microalbuminuria, suggesting an additional impact of growth restriction. Although changes in renal function are generally small and may still be within the normal range in children and adolescents, these may progress to overt renal dysfunction with age or superimposed renal insults. Potentially consistent with this hypothesis is a population-based case-control study in subjects with known childhood CKD (<21 years of age); LBW was significantly associated with an increased risk of CKD (OR 2.88; 95% CI, 2.28–3.63), renal dysplasia/aplasia (OR 4.51; 95% CI, 3.47–5.85), and a reduced GFR (OR 6.36; 95% CI, 4.00–10.12) [91] (Table 3).
Table 3.
Prenatal risk factors for childhood CKD [adapted from 91]
| Neonatal factors |
Maternal factors |
|||||
| LBW | HBW | preexisting DM | GDM | overweight | obesity | |
|---|---|---|---|---|---|---|
| Crude OR | 2.41 | 1.17 | 1.97 | 1.40 | 1.19 | 1.27 |
| 95% CI | 2.08 – 2.80 | 1.03 – 1.34 | 1.15 – 3.37 | 1.11 – 1.77 | 1.02 – 1.38 | 1.08 – 1.49 |
| Adjusted OR1 | 2.88 | 0.97 | 1.12 | 1.54 | 1.24 | 1.26 |
| 95% CI | 2.28 – 3.63 | 0.79 – 1.21 | 0.4 – 2.84 | 1.13 – 2.09 | 1.05 – 1.48 | 1.05 – 1.52 |
CI, confidence interval; DM, diabetes mellitus; HBW, high birth weight; OR, odds ratio. Chronic kidney disease (CKD) defined by ICD-9 code 585.x, including obstruction and dysplasia.
Adjustments listed in primary reference included maternal body mass index, smoking, and gestational hypertension.
Exposure to maternal diabetes and overweight/obesity are also increasingly being recognized as risk factors for renal developmental programming. It has long been known that exposure to diabetes during gestation leads to congenital malformations of the kidney, and the risk seems to persist after controlling for maternal body mass index (BMI) [91]. Diabetes during pregnancy is associated with HBW, which in turn has been associated with increased risks of proteinuria and ESKD [26, 39]. It has been suggested that exposure to diabetes during gestation, rather than genetic factors, is a mediator of renal programming in offspring based on the finding that renal functional reserve is lower in young adult offspring of mothers with diabetes during gestation than in those with diabetic fathers [92]. In animals, maternal diabetes exposure (models of type 1 and 2 diabetes) is associated with reduced nephron numbers in offspring, which would be consistent with a reduction in renal functional reserve [93, 94].
As shown in Table 3, the adjusted OR for childhood CKD following exposure to maternal diabetes was increased in unadjusted analyses but was attenuated in those with pregestational diabetes after adjustment for maternal BMI and smoking [91]. Exposure to maternal overweight and obesity was also independently associated with increased odds of childhood CKD [91]. The OR for renal dysplasia or aplasia was significantly increased with maternal pregestational type 1 or 2 diabetes, whereas gestational diabetes mellitus (GDM) was associated with an increased risk of obstructive uropathy [91]. Among a Pima Indian population with type 2 diabetes (aged 12–77 years), the OR for albuminuria in those who were the offspring of mothers with diabetes mellitus compared with mothers with prediabetes was 3.8 (95% CI, 1.7–8.4), and the age- and sex-adjusted incidence rate for ESKD in this population was 4.12 (95% CI, 1.54–11.02) [26, 95]. As maternal diabetes and maternal obesity are both increasing worldwide and are highly correlated with each other, the impact of these conditions on the blood pressure and renal health of future generations is likely to increase [96, 97, 98].
Clinical Associations of Renal Programming with ESKD
Several large studies have demonstrated associations specifically between LBW and the risk of ESKD [16, 39]. The strongest evidence probably comes from a Norwegian birth registry study where birth weight <10th percentile for the population (around 2.8 kg) was associated with a relative risk (RR) of 1.7 (95% CI, 1.4–2.2) for ESKD during the first 38 years of life [86]. In separate analyses with LBW defined as <2.5 kg, even stronger effect estimates were seen [29]. This dose-response relationship suggests that the degree of IUGR is an important programming factor [29] (Table 4).
Table 4.
Risk of ESKD according to birth weight and gestational age category [derived from 29]
| All |
1 – 18 years |
>18 – 42 years |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| LBW | ||||||
| All (BW <10%ile) | 1.63 | 1.29 – 2.06 | 2.72 | 1.88 – 3.92 | 1.23 | 0.9 – 1.68 |
| <2.5 kg | 2.25 | 1.59 – 3.19 | ||||
| SGA (all; <37 weeks) | 1.67 | 1.3 – 2.07 | 1.93 | 1.28 – 2.91 | 1.53 | 1.15 – 2.03 |
| Preterm (<37 weeks) | 1.36 | 0.94 – 1.99 | ||||
| LBW | ||||||
| Term | 1.56 | 1.18 – 2.07 | ||||
| Preterm | 1.89 | 1.25 – 2.86 | 1.42 | 0.82 – 2.48 | ||
| Term SGA Preterm | 1.54 | 1.2 – 1.96 | 1.41 | 1.05 – 1.90 | ||
| AGA | 1.09 | 0.69 – 1.73 | ||||
| SGA | 4.03 | 2.08 – 7.80 | 4.02 | 1.79 – 9.03 | ||
AGA, appropriate for gestational age; BW, birth weight; HR, hazard ratio; LBW, low birth weight; SGA, small for gestational age. All comparisons for term birth, LBW term, AGA term as relevant.
LBW was associated with an increased risk of ESKD due to any cause. The association was, however, stronger in the first 15 years of life and was strongest for congenital malformations/hereditary diseases [48]. Taking this further, an investigation into the programmed risk in a subgroup aged 18–42 years, excluding subjects with congenital renal disease, found that LBW per se was not significantly associated with developing ESKD, but being SGA was [29]. In these studies, LBW, SGA, and preterm birth were overlapping groups. When using the definition of <10th percentile of birth weight for LBW and <10th percentile weight for gestational age for SGA, among the 10% with LBW, 61.0% had SGA, and 31.7% were preterm. When using the 2.5-kg cutoff for LBW, 52% were also considered SGA, and 65.6% were preterm [29]. Although LBW can be explained simply by short gestational age in prematurity, SGA is more often explained by intrauterine nutritional restriction. It is possible, therefore, that being SGA and/or being preterm are better markers for an adverse intrauterine environment. Previous studies have suggested LBW, SGA, and preterm birth are all associated with hypertension, proteinuria, and a reduced GFR [21, 22, 30, 31, 32, 87]. Indeed, in the Norwegian study cited above, among those 18–42 years old, being SGA (birth weight <10th percentile for gestational age) was significantly associated with the risk of ESKD, and the effect was much stronger in those born preterm with SGA than those born at term with SGA (RRs of ESKD of 4.02 [95% CI, 1.79–9.03] and 1.41 [95% CI, 1.05–1.9], respectively; Table 4) [29]. These population level data suggest that both SGA and prematurity are important risk factors and likely potentiate each other's effects, with preterm SGA infants being at highest risk.
Maternal Nutrition and Health, Pregnancy Outcomes, and the Intergenerational Impact of Programming
Maternal health and nutrition are important determinants of healthy pregnancies and impact kidney development [15, 99]. These factors are strongly impacted by socioeconomic and structural factors [65, 100] (Table 5).
Table 5.
Maternal factors that modify a healthy pregnancy and comments [reprinted with permissions from 15]
| Developmental factors |
| Maternal birth weight <2.5 or >4.0 kg |
| Short stature, stunting (height <145 cm) |
| Behavioral factors |
| Cigarette smoking |
| Alcohol consumption |
| Substance and/or drug abuse |
| Demographic factors |
| Age <18 or >40 years |
| Ethnicity |
| Health-related factors |
| Undernutrition, low maternal body mass index |
| Iron deficiency |
| Malaria |
| Diabetes mellitus or gestational diabetes mellitus |
| Hypertension |
| Preeclampsia, eclampsia |
| Chronic kidney disease, transplant, dialysis |
| Birth before term |
| Multiple gestations |
| Multiparous (≥3) |
| Assisted reproduction |
| Infections |
| Obesity |
| Social factors |
| Highly active antiretroviral therapy for HIV |
| Prenatal care |
| Unplanned pregnancy, birth spacing |
| Teenage pregnancy |
| Marriage during childhood |
| Conflict, war, stress |
| Education level |
| Poverty |
| Environmental factors |
| Seasonal variations in nutrient availability |
| Toxin or pollutant exposure |
Throughout life, maternal nutrition is an important determinant of pregnancy outcome and offspring birth weight (Table 6).
Table 6.
Global distribution of maternal nutritional indices
| Obesity | Anemia (defined as hemoglobin <110 g/L) (2011) | Vitamin A deficiency (1995 – 2005) | |
|---|---|---|---|
| Global prevalence | 11% | 38% (34 – 43) | 15.3% (7.4 – 23.2) |
| HIC | USA (2011 – 2012): 31.8% (28.3 – 35.5) European region (2009): 7.1 – 25.2% |
22% (16 – 29) | |
| LMIC | European region (2003 – 2012): 5.0 – 21.2% Eastern Mediterranean region (2003 – 2013): 9.7 – 31.0% African region (2004 – 2012): 0.7 – 26.8% American region (2008 – 2012): 6.4 – 26.3% Southeast Asian region (2006 – 2011): 0.9 – 12.1% |
Central and Eastern Europe: 24% (14 – 40) East and Southeast Asia: 25% (17 – 38) Oceania: 36% (18 – 59) South Asia: 52% (40 – 63) Central Asia, Middle East, and North Africa: 31% (22 – 42) Central and West Africa: 56% (46 – 62) East Africa: 36% (30 – 41) South Africa: 31% (20–48%) Andean and Central Latin America and Caribbean: 27% (21 – 34) Southern and Tropical Latin America: 31% (13 – 56) |
|
| Reference | 101 | 102 | 103 |
HIC, high-income country. Numbers in parentheses are study durations and 95% confidence intervals. Vitamin A deficiency was defined as serum retinol <70 µmol/L.
Short maternal stature is a risk factor for offspring SGA or preterm birth, and may result from the mother herself having been born preterm or SGA [104, 105]. In animal studies, deficiencies in total calorie, protein/amino acid, iron, vitamin A, and zinc intake in pregnancy have been associated with reduced nephron numbers in offspring [reviewed in [15]. In humans, mothers being underweight or iron deficient during pregnancy have an increased risk of having an LBW infant [103]. Maternal vitamin A levels have been shown to correlate with offspring kidney size and nephron number [15, 106]. Supplementation of iron, micronutrients, balanced energy, calcium, zinc, and iodine in pregnant women have all been associated with reductions in LBW or preterm birth and, therefore, may have a positive impact on developmental programming in the kidney [103, 107]. Maternal intake of alcohol, caffeine, as well as tobacco consumption are also known to be associated with an increased risk of LBW, preterm birth, as well as programming of childhood blood pressure, kidney size, and function [108, 109, 110, 111, 112, 113, 114, 115]. Interventions to reduce smoking in pregnancy have been associated with reductions in the risk of LBW and preterm birth [107].
Chronic maternal illness and acute infections increase the maternal risk of LBW, SGA, preterm birth, and preeclampsia [15, 116, 117]. Acute infections such as malaria are an important cause of LBW, SGA, and preterm birth, which was estimated to contribute to 900,000 LBW deliveries in sub-Saharan Africa in 2010 [118]. Registry data from Denmark reported an increase in maternal chronic disease in pregnancy from 3.71 to 15.76% between 1989 and 2013 [119]. A population survey in Germany reported 20% of pregnant women having at least 1 chronic disease, which was associated with an increased risk of preterm delivery [120]. Specifically, women with all stages of CKD in pregnancy have increased risks of preterm birth, SGA, and LBW, which increase with worsening renal function [121, 122]. The major maternal risk factors for preeclampsia identified in a secondary analysis of the WHO Global Survey on Maternal and Perinatal Health included chronic hypertension, GDM, cardiac disease, renal disease, urinary tract infections, pyelonephritis, and severe anemia [123]. Among these, chronic hypertension had the highest OR (7.75; 95% CI, 6.77–8.87) followed by cardiac/renal disease (OR 2.3; 95% CI, 1.86–3.05), and GDM (OR 2.00; 95% CI, 1.63–2.45). In turn, the odds of offspring preterm birth (2.86; 95% CI, 2.68–3.06) and LBW (OR 2.32; 95% CI, 2.16–2.50) were significantly increased in pregnancies complicated by preeclampsia.
A mother's own birth history and circumstances impact her risk of pregnancy complications. The risk of GDM or gestational hypertension including preeclampsia or eclampsia was significantly increased in women who themselves were born preterm [124]. The risk increased with decreasing gestational age and with superimposed SGA, again demonstrating a dose-response relationship with the degree of prematurity and the impact of growth restriction on long-term risk (OR for ≥1 complication 1.95; 95% CI, 1.54–2.47, if the mother was born <32 weeks, and 1.14; 95% CI, 1.03–1.25, if the mother was born between 32 and 36 weeks). Preeclampsia is associated with an increased risk of LBW, SGA, and preterm birth [123]. Based on the programming paradigm, the offspring of these pregnancies in turn would be at increased risk of pregnancy complications, perpetuating the intergenerational cycle of developmental programming. Similarly, maternal LBW or prematurity are risk factors for LBW or preterm infants. Interestingly, the risk of offspring prematurity was significantly increased if the mother was premature, in inverse proportion to her gestational age, but was not increased if the father was born preterm, suggesting a direct programming effect in the mother [125]. Maternal obesity is a risk factor for both HBW and LBW, and maternal diabetes increases the risk for HBW in the offspring. These outcomes associate with renal developmental programming [100]. Importantly, the risk of LBW was highest in mothers who had been born preterm but became obese before pregnancy, again indicating the compounding hazard of obesity after being born small [126]. Both maternal LBW or HBW was also associated with an increased risk of GDM [127]. Whether all of these intergenerational risks transmitted through developmental programming and alterations in offspring phenotype are mediated directly or via epigenetic mechanisms is not yet clear and requires further study [46, 128, 129, 130].
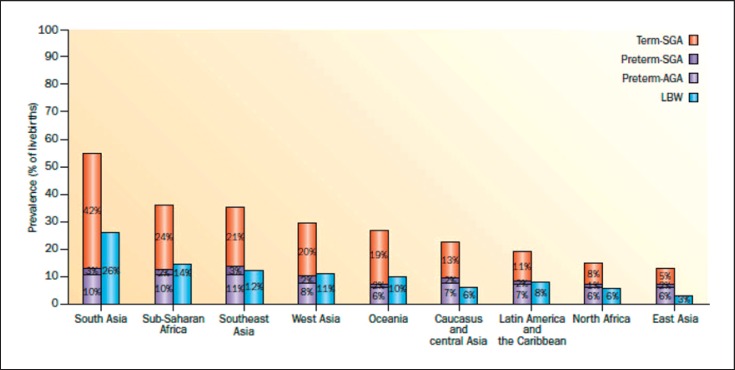
The majority of maternal factors impacting LBW and prematurity do not exist in isolation. Their developmental effects on the kidney are highly relevant for women in developing countries, where the prevalence of SGA infants, preterm birth, and LBW infants is known to be higher than in developed countries, but also remain highly relevant in developed countries with increasing maternal age, more frequent maternal chronic disease, and use of assisted reproduction technologies (ART) (Fig. 2).
Fig. 2.
Prevalence of SGA, preterm birth, and LBW infants by United Nations Millennium Development Goal regions in 2010 (reprinted with permissionaccording to CC Creative Commons Attribution-NonCommercial-noDerivs from Lee et al. [28]).
Renal Programming and Congenital Anomalies of the Kidneys and Urinary Tract
Congenital anomalies of the kidneys and urinary tract (CAKUT) account for 50% of pediatric kidney transplants, with obstructive nephropathy and hypoplasia/dysplasia constituting the majority of these [131]. Monogenic mutations have been established in approximately 17% of CAKUT, but, in most cases, the etiology remains undetermined and is likely the result of multiple genetic, epigenetic, and fetal environmental factors. In a recent population-based case-control study of children <21 years of age with CKD, LBW (OR 4.51; 95% CI, 3.47–5.85) and maternal pregestational diabetes (OR 7.52; 95% CI, 3.97–14.24) were significantly associated with the risk of renal dysplasia or aplasia [91]. Similarly, maternal GDM (OR 1.50; 95% CI, 1.07–2.09), maternal overweight (OR 1.27; 95% CI, 1.05–1.52), maternal obesity (OR 1.27; 95% CI, 1.05–1.55), and LBW (OR 2.53; 95% CI, 1.95–3.29) were all significantly associated with childhood obstructive uropathy [91]. Many gestational stress factors can, therefore, potentially impact renal development.
An important but underrecognized clinical correlate of reduced nephron numbers is congenital urinary tract obstruction. Animal models have been developed to examine the relationship of kidney development to injury resulting from urinary tract obstruction. In contrast to humans, in whom all nephrons are formed before birth, nephrogenesis continues in the first postpartum week in rats and mice. Surgical unilateral ureteral obstruction (UUO) in the early postnatal period, therefore, models obstruction in the human third-trimester pregnancy. Complete UUO in the newborn rat reduced nephron number by 40%: release of obstruction after 5 days normalized GFR at 1 month of age, but did not restore nephron number [132]. When these rats were followed to 1 year of age, nephron number remained 40% of normal, but GFR of the postobstructed kidney decreased by 80%, and glomerular sclerosis and interstitial fibrosis were increased in both kidneys [133]. There is a linear correlation between the duration of UUO and the nephron number reduction in the neonatal rat [134]. In contrast to the neonate, however, release of complete UUO in the adult rat does not result in a decreased nephron number [134]. These studies suggest that the developing kidney is particularly susceptible to obstructive injury, and that early surgical release of urinary tract obstruction can improve long-term nephron number.
In most children requiring RRT for CAKUT, the onset of renal failure is delayed until adulthood [135]. Nephron number at birth may, therefore, be an important determinant of outcome after relief of congenital obstruction and a modulator of the decline in renal function over time. Consistent with this possibility, the risk of ESKD was found to be significantly higher among SGA subjects with CAKUT or inherited causes of renal disease compared to those with normal birth weights (OR 2.5; 95% CI, 1.6–3.7) [86].
Neonatal AKI and Perinatal Drug Exposure
AKI occurs in 16–70% of neonatal populations [136, 137, 138]. Some of this variability comes from reports of neonates and preterm infants with varying comorbidities (e.g., congenital diaphragmatic hernia, cardiac surgery, and asphyxia) but also reflects the challenge of diagnosing AKI in the neonate and the lack, until recently, of a uniform diagnostic classification [136]. A neonatal KDIGO classification has been proposed, but serum creatinine may not be reliable as it reflects maternal creatinine and is also dependent on maturity of renal tubule function [136]. Cystatin C levels may reflect renal function better than creatinine, and various biomarkers are being investigated as a tool to detect AKI early [139, 140]. The major risk factors for neonatal AKI are preterm birth, LBW, reduced nephron numbers, critical illness, and nephrotoxin exposure [58, 136, 141, 142, 143]. All of these factors in turn may also reduce the potential for postnatal nephrogenesis, which can occur for a limited period following preterm birth [58].
The kidney is vulnerable to the toxic effects of many drugs [144]. Preterm neonates are often exposed to potentially nephrotoxic drugs during ongoing renal development [141]. Aminoglycosides are frequently prescribed in the neonatal intensive care unit (ICU) and can lead to tubular injury and AKI [144, 145, 146, 147]. Furthermore, in animals, aminoglycosides have been shown to lead to reduced nephron numbers [148, 149]. Nonsteroidal anti-inflammatory drugs (NSAID) are used to treat patent ductus arteriosus (a congenital defect of the heart) in the postnatal period, and, particularly in preterm infants, this can potentially impact ongoing nephrogenesis and negatively influence short-term renal function [150]. The true risk of AKI in neonates exposed to nephrotoxic medications is not well described, however, as the toxicity cannot merely be extrapolated from knowledge in older children and adults. A prospective study of 269 infants exposed to medication perinatally (i.e., medication prescribed to mothers during late pregnancy or administered to the infant within the first 7 days of life) and stratified according to whether they had a GFR below or above the group median on day 7 found that ibuprofen administration before day 7 was associated with an OR of 2.6 (95% CI, 1.2–5.3) for having a lower GFR [151]. The lower GFR in infants administered ibuprofen persisted for the month of follow-up. Importantly, aminoglycoside serum concentrations were higher in infants receiving ibuprofen, suggesting potentially enhanced toxicity [151]. Exposure to aminoglycosides was not associated with a lower GFR in this study although 7 days could be too soon to detect an effect. Others have reported higher serum creatinine values at 2 months of age in preterm infants born SGA who received aminoglycosides compared with those who did not [152]. Given that many infants receive multiple medications, and that infants with the lowest birth weights tend to receive more nephrotoxic medications per day, increased awareness of risks and of potential interventions to minimize the risk of toxicity are crucial [136, 141, 146, 147]. A medication that is frequently used in the neonatal ICU that may be protective against AKI is caffeine, but more study is required to better determine the true effect [153]. Current guidelines recommend prophylactic administration of theophylline, pharmacologically similar to caffeine but with a greater side effect profile, to infants at high risk of AKI after perinatal asphyxia [154].
Medications given to mothers before delivery have also been associated with an impact on neonatal renal function. Tocolytic therapy administered to the mother until the day of delivery was significantly associated with a lower GFR in the infant on day 7, and administration of the COX-2 inhibitor nimusulide as a tocolytic has been reported to induce renal failure and ESKD in neonates in multiple case reports [151, 155, 156]. In animals, multiple medications that could be prescribed during pregnancy have been found to impact offspring kidney development, including β-lactam antibiotics, cyclosporine, and long-term steroids, although their renal impact in humans is largely not known and long-term follow-up is needed [48, 65, 157]. In humans, antibiotic treatment during pregnancy has also been associated with LBW, although the effect was strongest for the nonpenicillins [158].
The risk of neonatal AKI increases with increasing degree of prematurity, demonstrating a dose-response effect in the susceptibility of the developing kidney to injury [159]. Neonatal AKI is associated with poor short-term outcomes, such as increased mortality and longer hospital stays [159, 160]. In addition to the association between neonatal AKI and short-term outcomes, AKI is linked to the development of CKD both in epidemiology studies and in studies of LBW subjects (weighing <1.5 or 1.0 kg), an effect that may be modulated by the development of obesity [20, 161, 162, 163, 164]. There is academic debate surrounding the pathway linking AKI and CKD. Some believe AKI permanently damages nephrons, and this reduction in nephron numbers causes CKD. Others believe AKI is a “red flag” or a harbinger for patients at risk, with a reduced number of nephrons, and these patients were destined to develop CKD. In either case, AKI may be a potentially modifiable risk factor for later-life CKD [165]. Importantly, it has been reported that episodes of AKI occurring during neonatal hospitalization are often not recorded in hospital discharge letters [159]. Such information is crucial to communicate as ongoing follow-up of infants with AKI is necessary.
LBW Is Associated with More Rapid Progression of CKD
It is unlikely that developmental changes in the kidney associated with LBW, prematurity, or other developmental stressors alone are enough to lead directly to renal disease except in severe cases, but a kidney with fewer nephrons would plausibly be less able to withstand additional “hits” such as AKI, glomerulonephritis, or renal injury imposed by other developmentally programmed conditions such as diabetes, CVD, and obesity, which all exacerbate the risk of renal injury [20, 23, 166, 167] (Fig. 3).
Fig. 3.
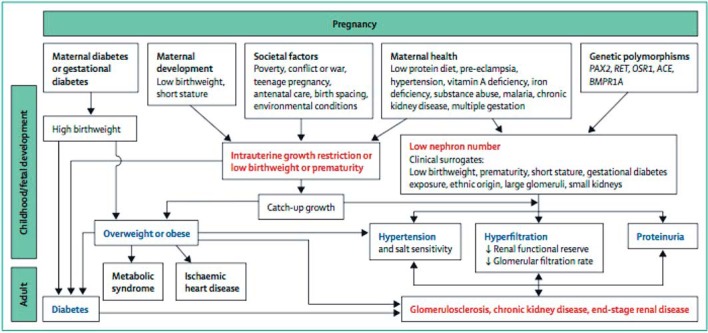
Multi-hit nature of renal disease programming (reprinted with permission from Luyckx et al. [65]).
IgA nephropathy (IgAN), for example, is the most frequent primary idiopathic glomerulonephritis worldwide [168, 169, 170]. Patients with IgAN tend to be younger and have fewer confounding conditions than other CKD patients, but they are at risk of rapid disease progression. IgAN is therefore a good model to study the impact of renal programming. Lower glomerular density has been shown to predict the long-term prognosis of IgAN [171]. In children with IgAN, LBW was associated with higher rates of progressive disease [172]. In a further analysis of a Norwegian population-based study, LBW and SGA were independently associated with an increased risk of reaching ESKD in adult males (OR 2.2; 95% CI, 1.1–4.4, and OR 2.7; 95% CI, 1.4–5.5) compared to controls; however, the risk was further increased among those with both LBW and SGA (OR 3.6; 95% CI, 1.6–8.2) [173]. There were no associations found between birth parameters and ESKD among females, but the numbers were small and statistical power was limited. Preterm birth alone was also not associated with ESKD risk in this study, and among those born SGA, those born preterm had a higher risk of developing ESKD (OR 10.8; 95% CI, 2.6–4.5) than those born at term [173]. In this study, IgAN patients with LBW/SGA had lower estimated GFR at the time of diagnosis, and, after adjustments for this, the association was no longer significant [173]. Exactly how LBW/SGA modulates the risk of renal disease progression in IgAN is thus not yet clear, although unpublished data show that the patients with LBW/SGA had larger glomerular volumes, potentially consistent with reduced nephron numbers. Other studies have also shown more rapid progression of other primary renal diseases in humans associated with LBW [174, 175, 176, 177, 178, 179, 180, 181] (Table 7).
Table 7.
Examples of primary kidney diseases that progress more rapidly in patients with low birth weight (LBW)
| Clinical findings |
|---|
|
IgA nephropathy [172, 173] Increased hypertension and glomerulosclerosis in LBW children Increased progression to end-stage renal disease if LBW and/or small for gestational age, especially among males |
|
Membranous nephropathy [181] LBW associated with steeper decline in glomerular filtration rate |
|
Minimal change disease [172, 174, 177] More relapses and steroid dependence in LBW children |
|
Chronic pyelonephritis [179] Patients with progressive deterioration in renal function had lower birth weight |
|
Autosomal dominant polycystic kidney disease [178] Earlier onset of end-stage renal disease with lower birth weight |
|
Focal-segmental glomerulosclerosis [180] Very LBW and preterm birth are risk factors for focal-segmental glomerulosclerosis |
|
Alport syndrome [182] More rapid progression in LBW identical twin |
Aging and Programming of Renal Disease
Observational data in humans show that GFR normally declines with age, usually beginning after about 30 years of age, but at variable rates [183, 184]. Such decreases in GFR are seen in the healthiest of the healthy (living kidney donors) [185]. In normal subjects studied longitudinally, the distribution of the slopes of change in renal function over time is nearly Gaussian, with an increased rate of decline in the “tail” [183]. The changes in GFR with aging can be dissociated from blood pressure and cardiovascular function [186]. The variability in the rate of “renal senescence” might be traceable to renal endowment. If renal senescence, whatever the mechanism, is a programmed phenomenon, then it is reasonable to postulate that the number of nephrons present at the beginning of life will directly influence the rate of GFR decline with aging. Indeed, experimental data in animals have suggested that inbred strains with impaired nephrogenesis develop glomerulosclerosis later in life [187, 188]. Experimentally induced LBW and low nephron numbers are associated with the acquisition of an accelerated “renal senescence” phenotype, especially after catch-up growth [189, 190, 191]. Premature renal senescence may, therefore, be a programmed phenotype.
The association between LBW and ESKD has not been studied in subjects older than 50 years apart from 1 Japanese study suggesting that diabetic nephropathy was more common among elderly patients on hemodialysis who had been born with LBW [16, 192]. Whether this finding suggests an effect of programming on diabetes, renal disease, or both, is not known [29]. As the impact of prenatal programming is expected to be compounded with age, the association of LBW and SGA with the risk of ESKD seen in younger adults may become greater with age [29] (Table 4).
Potential Effects of Programming on Kidney Transplantation
Kidney donation involves the loss of one half of existing nephrons. Donors having a single remaining kidney with a reduced number of nephrons per kidney may be at increased risk of loss of renal function over time [193]. Animal studies have demonstrated that a reduction in renal mass in rats with congenitally reduced nephron numbers leads to accelerated loss of renal function compared to similar renal mass reduction in genetically identical rats with normal nephron numbers [187]. These data are relevant to both transplant recipients and kidney donors. In recipients, mismatch of kidney size to donor size, i.e., smaller kidneys transplanted into larger donors, is associated with accelerated loss of renal function over time [194, 195]. It is conceivable that kidneys from donors with low nephron numbers would be at the highest risk of failure [196]. Nephron numbers in donated kidneys have not been studied, but smaller kidneys, by weight or volume, which are proportional to nephron number, have been shown to have shorter graft survival [197, 198, 199]. Similarly, a donor with a reduced nephron number may also be at increased risk of loss of renal function over time with a single kidney [193] (Table 8).
Table 8.
Hypertension and renal function in living kidney donors at risk of renal programming
| Population | US [200] |
Australia [201] |
Canada [202] |
Germany [203] |
||||
|---|---|---|---|---|---|---|---|---|
| black donor/nondonor | white donor/nondonor | indigenous donors | nonindigenous donors | aboriginal donors | white donors | BW ≤2.5 kg | BW <2.5 kg | |
| Donor number | 12,387 | 71,769 | 22 | 28 | 38 | 76 | 18 | 73 |
| Population programming risk factors | LBW prem. | LBW | Ref | HBW (offspring DM pregnancies) | Ref | LBW | Ref | |
| HT | – | – | 50% | 6% | 42% | 19% | 39% | 15% |
| Proteinuria | – | – | 81% | 6% | 21% | 4% | 81% | 35% |
| ↓ GFR | – | – | 81% | 38% | Not different | Not different | ||
| ESKD | 74.7 vs. 23.9/10,000 | 22.7 vs. 0.0/10,000 | 19% | 0% | 1 | 0 | 0 | 0 |
| Follow-up, years (IQR) | 7.6 (3.9 – 11.5) | 16.1 (1.27 – 20.2) | 6.37 (2.54 – 21.2) | 14.6 ± 9.3 | 13.4 ±9.5 | ≥5 | ≥1 – 3 | |
BW, body weight; DM, diabetes mellitus; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; HBW, high birth weight; HT, hypertension; LBW, low birth weight; Ref, referring group.
Similarly, women who have experienced preeclampsia are themselves at increased risk of developing ESKD, a risk which may increase after the donation of 1 kidney [204]. Indeed, women with preeclampsia have a 4- to 15-fold increased risk of all-cause ESKD compared to women without preeclampsia [86, 205]. The risk was highest in women who also gave birth to offspring with LBW, in women with only 1 lifetime pregnancy and in women with recurrent preeclampsia.
Programmed Risk of Hypertension and Kidney Disease May Be Different for Different Ethnic Groups and Socioeconomic Environments
Hypertension and renal disease prevalence vary between populations from different ethnic backgrounds, with very high rates being observed among Aboriginal Australians, Native Americans, and people of African descent [26, 40, 206]. Renal programming has largely been studied in western Caucasian populations; therefore, the impact of developmental programming of hypertension and kidney disease in high-risk populations, although suggestive, has not been comprehensively studied [66, 207]. The incidence rates of major risk factors for developmental programming of CKD in LMIC are highlighted in Table 9[20].
Table 9.
Prevalence of LBW, prematurity, maternal diabetes, and obesity in low- and middle income countries [18, 28, 96, 97]
| Fetal/maternal circumstances | Proportions in LMIC |
|---|---|
| LBW (2010) | 15% (138 LMICs) |
| Prematurity (2010) | 11.3% (138 LMIC) |
| HBW (2004 – 2008) | 0.5 – 14.9% (24 countries) |
| Gestational diabetes (2013) | 0.4 – 24.3% (15 countries) |
| Maternal overweight (2003 – 2009) | 13.7% (27 sub-Saharan countries) |
| Maternal obesity (2003 – 2009) | 5.3% (27 sub-Saharan countries) |
HBW, high birth weight; LMIC, low- and middle-income countries. In gestational diabetes, rates vary in part related to differences in cutoff values for diagnosis.
For example, only 3.3% of subjects in a Norwegian study on the association of birth weight with ESKD had birth weight under 2.5 kg, whereas LBW in sub-Saharan Africa has an incidence of 13–15% [3, 29]. In lower-income countries, maternal undernutrition is a significant contributor to IUGR, whereas in higher-income countries multiple gestations, ART use, and placental insufficiency are more frequent causes [117, 208, 209]. It is not known whether the varying causes of IUGR affect nephron development similarly or not. Importantly, however, maternal undernutrition in lower-income countries may be a frequent cause of impaired nephron development and may impact the future risk of renal disease and high blood pressure in these populations [15, 210].
Hoy et al. [211, 212] have described a strong and consistent association between LBW, reduced nephron numbers, hypertension, susceptibility to renal disease, and premature death in the Australian Aboriginal population, in which LBW is more prevalent and socioeconomic disadvantage is greater than in their white counterparts. How observations of developmental programming apply from one population to another, however, has not been well studied and may be different. In India, for example, LBW is common and has also been associated with higher blood pressure in some studies, but the programming effects appear to be more consistent for insulin resistance and type 2 diabetes in this population, possibly modulated by the “thin-fat” phenotype [213]. As discussed above, the inverse relationship between nephron numbers and blood pressure observed in Aboriginal Australians was similar to that seen among Caucasian Americans, but not as evident in African-Americans [66]. Similarly, the relationship between LBW and blood pressure is more consistently shown in Caucasian than African-American children [214, 215].
An increase in blood pressure among adults exposed to famine during gestation and early development was, however, found to be similar among Nigerians exposed to the Biafran famine (1967–1970) and those exposed to the Dutch famine (1944–1945) [216, 217]. Among Biafran subjects, studied at age 37–43 years, fetal and infant exposure to famine was associated with an increased risk of hypertension (OR 2.87; 95% CI, 1.9–4.34) compared to those born after the famine [216]. Among Dutch subjects, studied at age 59 years, the risk of hypertension was increased after exposure to famine for 10 weeks or more (OR 1.44; 95% CI, 1.04–2.0) compared to unexposed subjects [217]. Earlier analysis in the Dutch subjects between ages 48–53, however, did not find significant differences in blood pressure among those exposed or not exposed to famine [218]. The effect of famine on blood pressure may, therefore, be accelerated in the African compared with the European populations, which suggests that additional factors likely contribute to hypertension in African populations. In both studies, exposed compared with nonexposed subjects also had increased risks of obesity and glucose intolerance in adulthood, demonstrating the multisystem impact of developmental programming [216, 218].
Although the prevalence of childhood undernutrition is declining, the global estimate for childhood wasting in 2011 was still 8%, of whom 70% lived in Asia [103]. The long-term consequences of infant malnutrition on blood pressure and renal function have been scarcely studied. Among African-Caribbeans aged 28 years who survived Kwashiorkor or Marasumus, exposure to infant malnutrition was associated with alterations in cardiac function, higher systemic vascular resistance, and increased diastolic blood pressure [219]. These data emphasize the importance of early childhood nutrition in modulating CVD risk and highlight the need for further studies to understand the pathophysiology and determine how best to intervene.
At present, there is also compelling evidence of an association between variants in the apolipoprotein L1 (APOL1) gene and CKD in African-Americans and in West Africans [220, 221, 222]. A key question is whether these variants interact with LBW in a way that influences the development of CKD given that LBW is prevalent in sub-Saharan Africa and among African-Americans compared to their Caucasian counterparts [34, 223]. The relationship between nephron number and birth weight in subjects of African origin has been found to be consistent with that seen in Caucasian subjects; therefore, despite studies in adults with unknown birth weights showing a large variation in nephron numbers among African-Americans, with the mean being similar between Caucasians, African-Americans, and Senegalese, LBW is likely associated with reduced nephron numbers [60, 61, 224, 225]. One study reported that African-Americans with 1–2 APOL1 variant alleles did not have fewer glomeruli or larger glomeruli than African-Americans without risk alleles, but kidneys from subjects with 1–2 APOL1 risk variants experienced accelerated loss of nephrons after age 38 years, which was further increased by concurrent obesity [226]. This possible interaction between APOL1 risk variants and the effect of LBW on kidney disease in these ethnic groups needs further investigation.
Catch-Up Growth and Nutrition in Early Childhood as Modulators of Developmental Programming
Postnatal nutrition also has potential programming consequences. Especially in preterm infants or those born SGA, optimal early nutrition is important for growth and survival [227]. Through experimental and human studies, it has been shown that postnatal nutrition in terms of calories, protein content, and micronutrients can impact nephron numbers and long-term renal function [15, 20, 31, 82, 83, 228, 229, 230]. Animal data suggest some reversal of programmed renal changes can occur with the restoration of normal dietary composition, but overfeeding leads to obesity and hypertension independently of nephron numbers and may therefore be harmful [83, 228]. In preterm children studied at age 7 years, both intra- and extrauterine growth restriction were associated with reduced GFR (although still within the normal range), suggesting an impact of postnatal growth restriction on kidney development [31]. Optimizing postnatal nutrition in preterm infants is a challenge.
Evidence is mounting to show that rapid “catch-up” growth (i.e., upward crossing of weight centiles) or increase in BMI leads to the development of higher blood pressure, insulin resistance, and cardiovascular risk already in childhood [231, 232, 233]. These findings are most marked in those who were born small and became relatively larger [213, 231, 234, 235]. In resource-limited countries, catch-up growth is necessary as it improves child survival, stunting, and malnutrition [227]. The timing of catch-up growth appears to modulate the risk/benefit ratio, as early catch-up seems beneficial and later catch-up appears to be more harmful [227, 233, 234, 236]. The effects of catch-up growth may be different if the catch-up occurs predominantly in height (linear growth) or in weight, and, in most studies, the adverse effects were most marked among those who had been LBW or preterm and became overweight or obese [233, 234, 235, 236, 237]. Effects of catch-up growth may also differ between developed and developing countries [238]. HBW and exposure to GDM are also risk factors for childhood overweight and obesity [239].
It has been suggested that in individuals born small (LBW, SGA, or preterm), the superimposition of a high metabolic demand from a large body on a relatively small kidney may be a factor leading to hypertension and kidney disease over time [75], termed the “capacity load” model. Indeed, in a pediatric renal clinic population, children who were preterm and became obese had more rapid progression of renal disease compared to similar preterm children who were not obese [240]. In a separate cohort, in a follow-up of extremely LBW preterm children who had experienced neonatal AKI, GFR were lower at age 7.5 years among those with elevated BMI [241]. In a population study where birth weights were unknown, obesity in adolescents was found to be a risk factor for later-life ESKD [242]. Finding the inflection point where postnatal nutrition is optimal to improve short-term survival and not increase the long-term risk of CVD is an ongoing challenge. It would seem that close monitoring of growth trajectories in early life and life-long prevention of overweight and obesity through education, diet, and exercise in those born small is a safe and achievable principle [243, 244].
Nature versus Nurture in the CKD Developmental Programming Debate
There has been ongoing debate as to the underlying causes of the associations between LBW and later hypertension and kidney disease [245, 246, 247]. The relationship between birth weight and nephron number and the associations between intrauterine malnutrition and cardiovascular risk factors suggest a direct programming effect signaled by growth restriction [59, 82, 248, 249, 250]. On the other hand, LBW, CVD, and CKD do aggregate in families, suggesting possible genetic or environmental factors determining or confounding the association [247, 251, 252, 253, 254, 255]. The association between LBW and hypertension has been studied the most. A meta-analysis of small twin studies suggested that family factors do confound the relationships, but a large Swedish twin study suggested fetal growth was the most dominant programming factor [245, 256, 257]. In another study, higher blood pressure, BMI, and dyslipidemia in the father were found to be associated with LBW, which may also support genetic or environmental causes [258]. A recent follow-up Norwegian study examined the potential familial confounding of the association between LBW and ESKD risk [29]. In this study, the positive association between being LBW or SGA and later ESKD risk was not significantly modified by having a sibling with LBW or SGA. This study, therefore, argues that LBW or SGA per se have a greater impact compared to familial factors. Twin studies of renal function have also shown a lower GFR and more rapid progression of inherited renal disease in the lower birth weight twin in both dizygotic and monozygotic twins, which argues for a greater impact of fetoplacental over genetic factors in renal developmental programming [182, 259].
Consensus Recommendations
What follows is the approach adopted by the Low Birth Weight and Nephron Number Working Group.
Recommendation 1: On Maternal Preconception Health (Including Social Factors and Maternal Chronic Diseases)
Preconception Care and Embryonic Health
• Rationale
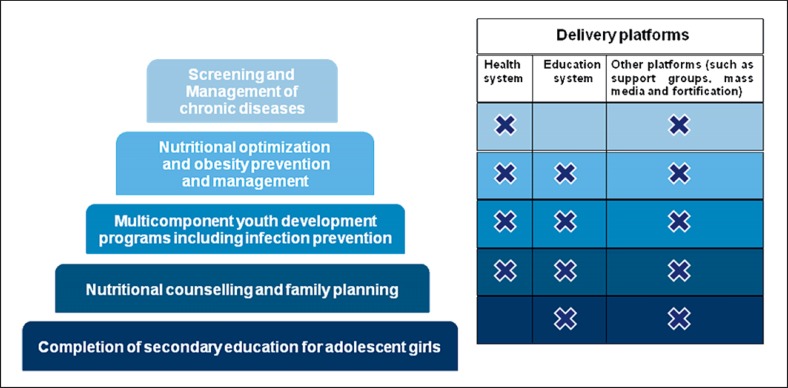
In addition to impacting fetal growth, maternal characteristics, and to some extent paternal determinants, also affect gametogenesis and embryonic development [3, 260], with a lasting impact on offspring health [261, 262]. Prepregnancy underweight is associated with an increased risk of offspring SGA (OR 1.81; 95% CI, 1.76–1.87) and LBW (OR 1.47; 95% CI, 1.27–1.71) [263]. Another study found a 32% increased risk of preterm birth in women who were underweight before conception [264]. Overweight before pregnancy is associated with an increased risk of macrosomia (OR 1.67; 95% CI, 1.42–1.97) [263]. The risk of preeclampsia and GDM increases around 2-fold with maternal overweight before pregnancy [264]. Weight loss before pregnancy has in some studies been associated with a reduced pregnancy risk in overweight women [264]. Preconception care has been shown to improve pregnancy outcomes [264, 265, 266], although it is important to recognize that up to 65% of preterm births remain unexplained [267]. Counseling and optimization of maternal weight and nutrition, and avoidance of alcohol, tobacco, and caffeine before pregnancy, all have a positive impact on pregnancy outcomes. The risk of having a preterm birth or an SGA infant is increased in mothers with chronic diseases [268]. Preconception care for women with underlying chronic diseases is crucial to plan a healthy pregnancy in terms of maximization of maternal health, making medication adjustments and timing of pregnancy [269]. The prevalence of chronic diseases in women of reproductive age is not comprehensively described globally, but it has increased in recent years and may be compounded by increasing maternal age [119]. Significant regional differences exist in the prevalence of maternal diseases, for example diabetes mellitus, sickle-cell disease, thyroid disease, and obesity, and, therefore, care should be tailored to regional needs [270, 271]. In some cases, chronic diseases, e.g., diabetes or CKD, may also be associated with reduced fertility, which may delay pregnancy or increase the risk of complications [90, 121]. A particular problem in many countries is teenage pregnancy, which, in many cases, is unplanned and may be associated with socioeconomic factors, poverty, lack of education for girls, lack of access to family planning services, and child marriage. Interventions targeting birth spacing have been shown to reduce LBW and preterm birth [272]. The rates of teenage pregnancy vary globally, with the highest rates of pregnancies in women/girls under age 19 years being reported in Latin America (peak 288 per 1,000 live births in Nicaragua) [273]. Teenage pregnancies are associated with higher risks of preeclampsia, eclampsia, infections, anemia, LBW, and preterm birth [272, 273, 274]. First or recurrent teenage pregnancy can be reduced by 15–37% through implementation of comprehensive targeted strategies [272]. Preconception health is, therefore, not only related to obstetric and medical risk factors, such as chronic diseases, but also to lifestyle, education, working conditions, experience of violence, geography, and the socioeconomic status of women [265, 266, 275]. Preconception care, therefore, encompasses a multisectoral approach as highlighted in the Sustainable Development Goals (SDGs) to improve overall health, life choices, and opportunities for women, and can be delivered successfully at all tiers of the health system [266] (Fig. 4).
Fig. 4.
Different packages of preconception care interventions (reprinted with permission according to CC Creative Commons Attribution-NonCommercial-noDerivs from Lassi et al. [266]).
• Recommendations for action
Implement comprehensive programs for general and specialist preconception care and education starting from school age girls, as the periconception period is one of the most critical periods in the life course [97, 261].
Deliver preconception counseling regarding dietary modification, weight management, physical exercise, and lifestyle choices to optimize future maternal and neonatal outcomes [3, 276].
Identify and treat diseases and complications (e.g., preexisting diabetes, renal insufficiency, hypertension, anemia, and infections) that may affect maternal, fetal, and neonatal health before conception [121, 269, 277, 278, 279, 280, 281].
-
Implement routine preconception care in the immediate postpartum period following every delivery or pregnancy loss (interconception) [282]. This approach would have the following advantages/aims:
(i) Most (relevant) women would be accessed.
(ii) Health education would improve the health of young mothers but would also extend to their infants and families.
(iii) Emphasize the importance of regaining pregestational weight as a simple and achievable goal, with proven benefits for future pregnancies and offspring [283], which can also be used as a justification for long-term follow-up.
(iv) Health care workers can approach women from the perspective of “offering help” instead of “blaming them for their mistakes”.
Institutions and governments should take nonmedical risk factors, such as those related to poverty (i.e., reducing teenage marriage and pregnancies, access to education for girls, ensuring access to family planning to space pregnancies, and experience of violence) into account.
Societal valorization programs of new knowledge to improve perinatal health should be initiated and supported by both universities and governmental bodies [284].
Local and WHO guidelines should be followed.
Assisted Reproductive Technology
• Rationale
There is increasing evidence that infertility or subfertility per se are independent risk factors for obstetrical complications and adverse perinatal outcomes, even without the addition of ART [285]. Unadjusted analyses suggest a 2-fold increased risk of preeclampsia in spontaneous singleton pregnancies in women with a history of infertility compared with women in the general population [286]. Women requiring ART, therefore, appear to have an increased baseline risk for pregnancy-related complications. ART has been associated with preterm birth, LBW, and SGA [287]. ART is becoming an increasingly relevant cause of these complications in high-income countries where, for example in Australia and Denmark, 4–5% of all births result from ART [288]. Multiple gestation is the most powerful predictive factor for adverse maternal, obstetrical, and perinatal outcomes in ART pregnancies [289]. However, ART is also associated with an increased risk of preterm birth and LBW in singleton pregnancies [290, 291]. Several studies report an increased risk of preeclampsia with ART. The odds of preeclampsia is significantly increased in women undergoing ART (OR 2.2; 95% CI, 1.03–4.72) after controlling for factors such as multiple gestations [292]. A retrospective population-based study of singleton pregnancies conceived through in vitro fertilization (IVF) and ovulation induction compared to spontaneously conceived pregnancies showed a significant linear association in the incidence of severe preeclampsia in the ART groups (2.7% in IVF, 1.8% in ovulation induction, and 1.1% in the comparison group, p < 0.001) [293]. However, in another study, using propensity score matching analysis, the association between IVF and preeclampsia was found to be weaker than when conventional adjustments were made, suggesting potential confounding of the association between IVF and preeclampsia by multiple factors [294]. A recent meta-analysis found that the risk of preeclampsia is 3-fold higher in pregnancies achieved by IVF with oocyte donation compared to with a woman's own oocytes [295]. The pathophysiological relationship between oocyte donation and preeclampsia remains unclear. An immunological theory based on the allogenicity of the fetus to the mother has been postulated, while other authors hypothesize that a patient needing oocyte donation might also have an immunologically based condition that predisposes to preeclampsia [295]. Given the inherent risks of ART, preconception counseling and optimization of maternal health and nutrition prior to conception are crucial, and “judicious use” of ART, including reduction in the number of embryos transferred, is proposed as a strategy to reduce preterm births by as much as 63% [209, 288].
• Recommendations for action
Women of reproductive age undergoing ART procedures must be informed that these techniques are associated with an increased risk of preterm birth, LBW, and preeclampsia [289, 290, 291].
Routine preconception counseling is necessary to optimize maternal nutrition, weight, and lifestyle before conception.
Women undergoing assisted reproduction procedures should receive more intensive monitoring during pregnancy.
Reduction in embryo transfers as a mechanism is necessary to reduce the risk of preterm birth [209, 288].
Local and WHO guidelines should be followed.
Advanced Maternal Age
• Rationale
Advanced reproductive age, commonly defined as maternal age of 35 years or older, is a risk factor for fetal chromosomal abnormalities as well as medical complications [274]. Advanced maternal age, compared with maternal age under 35, is associated with worse pregnancy outcomes and maternal morbidity, with a higher incidence of GDM, gestational hypertension, and preterm labor [296]. Pregnancies resulting from ART conferred further additional risk with advancing maternal age [297]. Older women are more prone to developing chronic illnesses, particularly obesity, hypertension, and diabetes mellitus, although preexisting disease does not fully explain all the adverse events associated with age and obstetric outcomes [298]. Physiologic changes in pregnancy challenge aging organ systems that might be well compensated in a nonpregnant state but may ultimately be overwhelmed by the increases in blood volume, cardiac output, and insulin resistance that accompany pregnancy.
• Recommendations for action
Women over 35 years should be counselled concerning the increased risk of infertility, miscarriage, fetal anomalies, obstetric complications, and preterm birth and should know that their pregnancy is considered “high risk” due to their age.
Local and WHO guidelines should be followed.
Recommendation 2: On Fetal Exposure to Maternal Diabetes and Obesity
• Rationale
Hyperglycemia is one of the most common medical conditions women encounter during pregnancy. The prevalence of diabetes during pregnancy varies widely, but it is estimated that globally 17% of pregnancies are complicated by hyperglycemia [96, 98]. Concerningly, in LMIC, around one half of women with hyperglycemia in pregnancy are undiagnosed. Around one sixth of hyperglycemia in pregnancy may be due to preexisting diabetes, the prevalence of which appears similar across the globe, around 2% between ages 20 and 24 years and rising to 4% between ages 30 and 34 years [98]. GDM, however, contributes the major portion of hyperglycemia in pregnancy, and the prevalence varies widely across the globe, in part due to varying diagnostic thresholds but also in part due to variability in the prevalence of maternal obesity in pregnancy, advancing maternal age, and weight gain in pregnancy [1, 299]. In LMIC, the rates vary with reports ranging from 0.4% in a cohort in Brazil to 24.3% in a cohort in Vietnam when defined by WHO criteria [96] (Table 9). Screening for GDM is not universal, and, therefore, the generalizability of these data is uncertain. GDM is associated with a higher incidence of maternal morbidity including cesarean deliveries, shoulder dystocia, birth trauma, hypertensive disorders of pregnancy (including preeclampsia), and subsequent development of type 2 diabetes [299, 300]. Perinatal and neonatal morbidities also increase; the latter include macrosomia, birth injury, hypoglycemia, polycythemia, and hyperbilirubinemia [299, 300]. Exposure to hyperglycemia in early pregnancy has also been associated with IUGR [300]. Maternal and neonatal mortality are also increased [299]. In 2013, it was estimated that 21.4 million live births were exposed to hyperglycemia during gestation [299].
Macrosomia is a major complication of both hyperglycemia in pregnancy and elevated maternal BMI [18]. Increasing maternal age has also been found to be a risk factor [18]. In developed countries, macrosomia occurs in 5–20% of pregnancies, but the incidence is increasing [18]. In 23 developing countries, the incidence of macrosomia (defined as a birth weight >4.0 kg) ranged from 0.5% in India to 14.9% in Algeria [18]. Long-term sequelae in offspring with in utero exposure to maternal hyperglycemia and/or HBW include higher risk for obesity and diabetes later in life as well as renal disease [26, 28, 39, 300, 301]. Dietary counseling for diabetes and interventions to prevent GDM in pregnancy were found in randomized controlled trials to reduce the RR of having large for gestational age (LGA) infants by 0.37 (95% CI, 0.20–0.66) and 0.09 (95% CI, 0.01–0.69) [107].
In 2008, around 70% of adult American and Caribbean women were either overweight or obese, as were around 40% of adult women in Africa [103]. Women who gain excess weight in pregnancy tend to retain some of the weight gained with each pregnancy [302]. A recent meta-analysis found that, compared with women who gain the recommended amount of weight during pregnancy, those with a gestational weight gain above the recommendations retained, on average, an additional 3.06 kg after 3 years and 4.72 kg 15 years postpartum [303]. Close monitoring of weight gain during pregnancy is important, especially in women who begin pregnancy overweight [3], not only to improve maternal health in the current pregnancy but also to prevent future maternal obesity.
Maternal obesity is a growing problem worldwide, including low-income countries [97]. Recent data from sub-Saharan Africa showed that 19.1% of mothers were overweight and 5.3% were obese [97, 284]. Importantly, women who were born LBW themselves but became obese have an increased risk of preterm birth compared to women who are just obese, illustrating the intergeneration impact of developmental programming [126]. Maternal obesity is a strong risk factor for GDM, which increases with increasing maternal weight (OR 2.14, 95% CI, 1.82–2.53 for overweight; OR 3.56, 95% CI, 3.05–4.21 for obese; OR 8.56, 95% CI, 5.07–16.04 for severely obese) compared with normal-weight mothers [304]. Overweight or obesity in pregnancy are also associated with many adverse obstetric and perinatal outcomes, such as hypertension, preeclampsia, a higher incidence of cesarean deliveries, preterm birth, macrosomia, and perinatal mortality [97, 103, 303]. Women who were obese and experienced GDM have an increased risk of later-life diabetes and CVD. Offspring of obese mothers have increased long-term risks of obesity, diabetes, and CVD [305]. Interestingly, in a population level study of Caucasian and First-Nation Canadians with 24 years of follow-up, initiation of breastfeeding prior to hospital discharge was associated with a significant reduction in the risk of subsequent maternal diabetes in both mothers who had and not had GDM [306]. Similarly, the incidence of youth-onset type 2 diabetes was reduced by 17% in breastfed offspring, demonstrating the importance of breastfeeding and the programming impact of good early nutrition [306]. The mechanisms underlying these observations are unknown, but changes in insulin sensitivity, risk of obesity, gut microbiome, and maternal and infant metabolism have been suggested [306].
• Recommendations for action
-
All pregnant women should be screened for hyperglycemia during pregnancy, and all countries should promote strategies to ensure this [279].
(i) The WHO criteria for the diagnosis of diabetes mellitus in pregnancy [307] and the WHO and the International Association of Diabetes in Pregnancy Study Group criteria for diagnosis of GDM [307, 308] should be used when possible.
(ii) Given resource constraints in many low-income countries, alternative strategies should also be considered, which are equally acceptable [301]. They include at least the possibility to measure glucose in the urine to identify the presence of glycosuria as a risk factor for the subsequent detection of gestational diabetes.
GDM should be managed in accordance with the available national guidelines [309]. Achieve normoglycemia with avoidance of hypoglycemia through pregnancy [277, 279].
Nutritional counseling and physical activity should be the primary tools for managing GDM, which should be practical, affordable, and empower pregnant women to choose the right quantity and quality of food and level of physical activity.
If lifestyle modification alone fails to achieve glucose control, metformin, glyburide, or insulin are safe and effective treatment options for gestational diabetes.
Most normal gestational weight gain occurs after 20 weeks of gestation, and the definition of “normal” is subject to regional variations but should take prepregnant BMI into consideration. Women who are underweight at the start of pregnancy (i.e. BMI <18.5) should aim to gain 12.5–18 kg, women who are normal weight at the start of pregnancy (i.e., BMI 18.5–24.9) should aim to gain 11.5–16 kg, overweight women (i.e., BMI 25–29.9) should aim to gain 7–11.5 kg, and obese women (i.e., BMI >30) should aim to gain 5–9 kg [3, 310, 311].
Women should be counseled repeatedly during pregnancy to continue the same healthy lifestyle after delivery to reduce the risk of future obesity, type 2 diabetes, CVD, and renal diseases [276].
GDM should be documented in the mother's and infant's health record.
Breastfeeding should be initiated before hospital discharge, as this is associated with a reduced risk of incident diabetes in mother and offspring [306].
Following a pregnancy complicated by GDM, the postpartum period is an important time to introduce beneficial health practices for both mother and child to reduce the future burden of several NCD.
Obstetricians should establish links with family physicians, internists, pediatricians, and other health care providers to support postpartum follow-up of gestational diabetic mothers and their children.
A follow-up program linked to the child's vaccination and regular health checkup visits provide an opportunity for continued engagement with the high-risk mother-child pair.
Recommendation 3: On Maternal Nutrition Early in and during Pregnancy
• Rationale
Pregnancy is a period of increased nutritional needs due to both the mother's adaptation to pregnancy and to fetal and placental demands. Health outcomes associated with maternal nutrition range from infertility, miscarriage, fetal malformations, pregnancy diseases related to inadequate placental adaptation, such as IUGR, and preeclampsia to gestational diabetes [107, 312, 313]. Imbalances in both nutritional intake and status during pregnancy may have long-lasting effects both on maternal health outcomes and the long-term health and development of offspring through fetal programming of chronic diseases such as kidney diseases [314].
Maternal undernutrition (defined as a BMI <18) is still prevalent worldwide, being most severe in South Asia where up to 40% of women are undernourished, which contributes to 25–50% of IUGR in the region [103, 208]. South Asia also has the highest incidence of LBW globally at 28% [3]. Short maternal stature (defined as height <155 cm) is a marker of chronic malnutrition in women and may also result from remaining stunted after been born LBW, preterm, or SGA [105]. It has been estimated that maternal short stature is associated with 6.5 million SGA and/or preterm birth annually in LMIC [104]. Important barriers to adequate maternal nutrition include female illiteracy, poverty, and gender inequality [208]. Malnutrition during pregnancy ranges from total calorie inadequacy to micronutrient deficiencies, which may be acute or chronic and exacerbated by intercurrent illness, infection, stressfull work conditions, and environmental factors [208]. Importantly, even obese mothers are at risk of micronutrient deficiency, which can have important programming effects [3, 210]. WHO surveys conducted between 1993 and 2005 found anemia to be present in up to 40% of pregnant women, of which half was due to iron deficiency [103, 208]. Iron supplementation in pregnancy has been associated with a 19% decrease in LBW [107]. WHO data from 2006 estimated around 9.8 million women were vitamin A deficient in pregnancy, placing their offspring at risk for reduced nephron numbers [208]. Some micronutrients, e.g., vitamin A, may be harmful if supplemented at high doses, and, therefore, supplementation is not routinely recommended, but a recent Cochrane review concluded that multiple-micronutrient supplementation with iron and folic acid is likely beneficial [310, 315]. Multiple-micronutrient supplementation of maternal nutrition with iron and folic acid compared to iron and/or folic acid in LMIC resulted in a reduced risk of LBW (RR 0.88; 95% CI, 0.85–0.90) and SGA (RR 0.91; 95% CI, 0.74–0.97), although no effect was seen on preterm birth [315]. Routine use of multiple-micronutrient preparations is not, however, recommended in the latest WHO Antenatal Care Guidelines, as there may be some risk that has not yet been fully determined. The current recommendations are, therefore, that pregnant women receive combined iron and folate supplementation as an effective means to reduce LBW and SGA [310].
Healthy (traditionally based) dietary patterns during pregnancy, such as the New Nordic Diet [316] and Mediterranean diets [317], are associated with a lower risk of having an SGA infant, while a potential causal link between maternal consumption of “junk food” and having a large newborn has been identified [318]. The degree of Mediterranean diet adherence was positively associated not only with fetal size, but also with plasma folate and serum vitamin B12 concentrations, and inversely correlated with uteroplacental vascular resistance, plasma homocysteine, and high-sensitivity C-reactive protein levels [317]. Inverse relationships were also reported between adherence to the Mediterranean diet [319], and Prudent and Traditional diets [320], and preterm delivery risk. The consumption of specific food item(s)/substances, such as fulfilling the fish intake criteria, resulted in a lower risk of preterm delivery [320], consistent with a meta-analysis indicating that never consuming fish in pregnancy could be an extremely strong risk factor [321].
The importance of all components of the maternal diet before and during pregnancy are reviewed in the recent WHO publication “Good Maternal Nutrition. The Best Start in Life” [3]. The benefits of multiple nutrition supplementation strategies which have the potential to impact developmental programming in the kidney are highlighted in Table 10.
Table 10.
Impact of nutritional interventions on birth weight and preterm birth and programming of blood pressure and kidney disease [103, 107, 315, 322, 323, 324, 325, 326, 327, 328, 329]
| LBW/SGA | Prematurity | Preeclampsia/eclampsia | HBW/LGA | Child blood pressure | Child GFR | Child microalbuminuria | |
|---|---|---|---|---|---|---|---|
| Iron and folate supplementation | ↓ | ↓ | ↓ | ↑ | |||
| Micronutrient supplementation | ↓ | ↑↓1 | |||||
| Calcium supplementation | ↓ | ↓ | ↓ | ||||
| Protein supplementation | ↓ | No effect | |||||
| Vitamin A supplementation | No effect | Possible ↓ | |||||
| Folate supplementation | ↓ | ||||||
| Zinc supplementation | ↓ | No effect | |||||
| Iodine supplementation | ↓ | ||||||
| Malaria prevention and treatment | ↓ | ||||||
| Treatment of genital infections | ↓ | ↓ | |||||
| Treatment of asymptomatic bacteriuria | ↓ | ||||||
| Magnesium sulfate | ↓ | ||||||
| Antiplatelet agents | ↓ | ↓ | ↓ | ||||
| Diabetes education | ↓ | ||||||
| Smoking cessation | ↓ | ↓ | |||||
No effect vs. iron/folate.
Effectively addressing maternal and fetal nutrition requires a life course and multisectoral approach, including agriculture, education, and social safety nets [330].
• Recommendations for action
Maternal nutrition during pregnancy should be evaluated carefully for the correct relationship between the quality of food intake and the characteristics of the mother, and for gestational weight gain.
Appropriate maternal nutrition should consider intake not only of macronutrients (e.g., proteins, carbohydrates, and fats) but also of micronutrients (e.g., iron, folate, and iodine), for which it may be easier to incur deficiencies or inadequacies [3, 99, 310]. Importantly, obese women may have important nutritional deficiencies [3].
Efforts should be made to ensure women start pregnancy with an appropriate nutritional status, with a healthy and balanced diet, and to keep gestational weight gain within the recommended ranges. mHealth applications have been shown to be effective in achieving this [331].
Healthy dietary patterns (in particular the Mediterranean diet) and micronutrient supplementation, particularly during the periconception period, as well as throughout pregnancy, should be encouraged, although nutritional recommendations should remain culturally sensitive.
Efforts to improve nutrition of women and girls require advocacy for a multisectoral approach, consistent with the SDGs to reduce poverty, improve nutrition, increase gender equality, education for girls, access to family planning, reduce teenage pregnancy, and access to antenatal care [4].
Recommendation 4: On Maternal Consumption of Tobacco and Alcohol and Caffeine Intake
• Rationale
Alcohol and tobacco exposure during development affect the expression of genes involved in cell cycle control, apoptosis, and transcriptional regulation, mostly through epigenetic mechanisms [332, 333]. Alcohol, tobacco, and caffeine exposure are associated with adverse maternal and child health outcomes [269]. Such consumptions are modifiable risk factors for LBW and preterm birth, and, therefore, they are important targets for prevention strategies.
Alcohol. Alcohol is a toxic substance during pregnancy, particularly due to its teratogenicity [334, 335, 336, 337, 338]. There are no safe limits for alcohol consumption in pregnancy [339]. The first weeks of pregnancy are the most vulnerable to teratogenicity; however, risks have also been reported in the second and the third trimester, particularly for preterm deliveries and LBW [340], as well as for abnormal cognitive development [341]. Maternal alcohol consumption prior to, or during pregnancy, is associated with dose-dependently increased risks of preterm birth and offspring LBW and SGA [109, 342]. Interventions to reduce maternal alcohol consumption during pregnancy were associated with a 202.1 g (95% CI, 60.85–343.35) increase in offspring birth weight [Appendix in 107]. Renal hypoplasia has been reported in association with fetal alcohol syndrome [113], and, in animals, acute prenatal alcohol exposure was associated with reduced nephron numbers and subsequently increased blood pressure levels [343]. The long-term consequences in humans require more study.
Tobacco. Both active and passive smoking prior to conception and during pregnancy are associated with adverse reproductive outcomes, ranging from delayed conception to spontaneous abortion and reduced birth weight [344, 345, 346]. Dose-dependent effects have been demonstrated, with both maternal and paternal exposure associated with infertility and the risk of spontaneous abortion [344]. LBW and preterm birth have been strongly associated with smoking during pregnancy, particularly during the third trimester [347]. The relationship between smoking, birth weight, and preterm birth is subject to many confounders, but the strength of the associations is significant and therefore likely valid [347]. Offspring of women smoking over 10 cigarettes per day weigh approximately 200 g less at birth than offspring of nonsmokers, and the risk of preterm birth is increased by around 25% in smokers [347]. These risks are likely related to chronic fetal hypoxemia, potentially due to both nicotine levels decreasing uterine vascularization and increased carbon monoxide transferred to the fetal circulation. In a population-based study among women who had a previous preterm delivery, smoking was found to be a risk factor for subsequent moderate or very preterm delivery, although this risk was eliminated if women stopped smoking [110]. Nicotine replacement strategies have been found to reduce preterm birth (RR 0.7; 95% CI, 0.61–0.97); introduction of incentives to stop smoking were associated with a reduced risk of LBW (RR 0.45; 95% CI, 0.22–0.93) [Appendix in 107]. The impact of nicotine replacement strategies on fetal and longer-term outcomes requires further study.
It is estimated that although smoking rates have declined in many developed countries over the past decades, 11–13% of women still smoke throughout their pregnancies and around 22–30% of nonsmoking women are passively exposed to smoke [347]. In LMIC, smoking rates are increasing with a recently reported pool prevalence of 2.6% [348]. Smoking and exposure to passive smoke during pregnancy are modifiable, and it has been estimated that combined current tobacco control policies and an increase in cigarette tax could prevent 227,300 LBW births and 351,100 preterm births between 2015 and 2065 [349].
In mice, exposure to maternal smoking was associated with reduced birth weights and spontaneous development of albuminuria at 13 weeks of age [350]. These findings were associated with evidence of increased oxidative stress and changes in mitochondrial structure and function in offspring kidneys [350]. Similarly, in rats exposed to maternal smoking, birth weights and nephron numbers were reduced, proinflammatory proteins were upregulated in offspring kidneys, and albuminuria and blood pressure increased over time [111, 351]. A prospective cohort study found that exposure to maternal smoking during pregnancy was associated with a dose-dependent reduction in fetal and infant kidney volumes after adjustment for multiple variables, suggesting an independent impact of smoking on fetal kidney development [114, 352]. In children studied at a mean age of 6 years, continued maternal smoking during pregnancy was associated with a dose-dependent decrease in kidney volumes and estimated GFR [114]. Albumin/creatinine ratios were increased among children exposed to smoking during the first trimester [114]. Importantly, among those whose fathers, but not mothers, smoked during pregnancy, renal volumes were also reduced, suggesting that even intermittent and environmental exposure to tobacco impacts kidney development [114]. Exposure to maternal smoking during gestation is also associated with an increased risk of gestational diabetes in daughters, which may contribute to the intergenerational impact of programming through smoking [353].
Caffeine. Caffeine intake was consistently associated with a lower birth weight and higher odds of SGA, not only when consumption exceeds the WHO recommendation (300 mg/day) [310], but even with the intake recommended in Nordic countries and the USA (maximum 200 mg/day) [108], which suggests a risk of SGA even at very low caffeine intake levels.
• Recommendations for action
Pregnant women should be asked about alcohol and tobacco use. Tobacco and alcohol should not be used in pregnancy [109, 342].
Women should be advised that both alcohol and tobacco may also have an impact on the gametes in the periconception phase, so women planning pregnancy should avoid alcohol and tobacco.
Tobacco control policies should be implemented and impact monitored to determine cost and effectiveness of strategies in local contexts.
Exposure to passive smoke during pregnancy should be avoided; therefore, women should be asked and educated about household or occupational smoke exposure.
Smoking reduction efforts must be directed at household members of pregnant women as well as the pregnant woman herself.
Offspring of mothers who used alcohol or smoked during pregnancy should be followed long term.
Women should be advised to reduce coffee intake to a minimum [310].
Recommendation 5: On Screening, Risk Factors, Detection, and Monitoring of Hypertensive Disorders in Pregnancy
• Rationale
A recent systematic review including 39 million women from 40 countries estimated that 4.6 and 1.4% of pregnancies were affected by preeclampsia and eclampsia between 2002 and 2010, respectively, although most studies included came from North America and Europe [354]. A subsequent cross-sectional study of 357 higher-volume health facilities across 29 LMIC found that 2.73% of women had hypertensive disorders during pregnancy: 2.16% had preeclampsia, 0.28% eclampsia, and 0.29% chronic hypertension [355]. Preeclampsia increases the risk of LBW and preterm birth, and is associated with higher childhood and young adult blood pressures in offspring [77, 355, 356]. In prior decades, it has been estimated that 12% of SGA infants and 20% of those born preterm result from preeclampsia [357]. Women born with LBW or SGA themselves are at increased risk of pregnancy-induced hypertension in their own pregnancies [358]. Women who were born with SGA (<5th percentile z-score) were 2- to 3-fold more likely to have preeclampsia before 34 weeks of gestation compared with those with birth weights between the 25th and 75th percentiles [359]. Women who develop preeclampsia are more likely to have high blood pressure and dyslipidemia several years before pregnancy, factors which may be associated with the women themselves having been born with LBW [360]. From a cross-sectional study including 313,030 women from 29 countries, maternal renal disease (OR 4.52; 95% CI, 3.63–4.54) and chronic hypertension (OR 8.32; 95% CI, 7.13–9.72) were most strongly associated with the odds of preeclampsia, and these odds increased 1.5- to 2-fold for eclampsia. Preexisting maternal diabetes is associated with an RR for preeclampsia of 3.56 (95% CI, 2.54–4.99) [361]. Consistent with either a programmed or genetic risk, previous preeclampsia, especially early in gestation, increases the risk of developing preeclampsia in later pregnancies [362, 363]. Women who experience preeclampsia have a 2-fold increased risk of long-term CVD and an approximately 10-fold increased risk of ESKD [204, 364, 365, 366]. Indeed, many studies in women with previous preeclampsia, but not all, have shown an increased risk of developing hypertension later in life and increased mean blood pressure compared to women without preeclampsia [367, 368, 369]. Furthermore, 2 meta-analyses have shown a greater than 2-fold increased risk of ischemic heart disease/cardiac disease later in life, and an increased risk of later hypertension, stroke, and venous thromboembolism, but no significant association with peripheral arteriosclerosis [364, 370] (Table 11).
Table 11.
Maternal long-term risks after adverse pregnancy outcomes
| Clinical event | Maternal outcome1 | Relative risk (95% CI) | Reference |
|---|---|---|---|
| Preeclampsia | Hypertension | 3.7 (2.7 – 5.05) | 364 |
| Cardiovascular disease | 2.16 (l.86 – 2.52) | 364 | |
| Stroke | 1.81 (l.45 – 2.27) | 364 | |
| Death | 1.49 (1.05 – 2.14) | 364 | |
| Microalbuminuria | 4- to 8-fold increase | 365 | |
| End-stage renal disease | 4.7 (3.6 – 6.1)2 | 204 | |
| Kidney biopsy | 3.3 (2.5 – 4.2)2 | 371 | |
| LBW infant | End-stage renal disease | 2.7 (1.8 – 3.8)2 | 204 |
| Kidney biopsy | 2.0 (1.5 – 2.7)2 | 371 | |
| LBW + preeclampsia | End-stage renal disease | 6.8 (3.9 – 12.0) | 204 |
| Kidney biopsy | 4.8 (2.8 – 8.2)2 | 371 | |
| Preterm birth | End-stage renal disease | 3.8 (2.9 – 4.9) | 204 |
| Gestational diabetes | Diabetes type 2 | 7.43 (4.79 – 11.51) | 372 |
LBW, low birth weight.
Follow-up times vary, see individual studies.
1st pregnancy; see references for risks with more pregnancies/episodes of preeclampsia.
Several studies have also found an association between preeclampsia and future kidney disease [204, 365, 371] (Table 11). A recent meta-analysis of 7 studies showed that microalbuminuria was present in 31% of women 7 years after a preeclamptic pregnancy [365, 373, 374]. In a thoroughly performed Norwegian study of otherwise healthy women, preeclampsia 10 years earlier was not associated with an increased risk of persisting microalbuminuria, suggesting lower absolute risks than anticipated in otherwise healthy women [375].
A recent study from the US also suggested a possible modulation of the association of preeclampsia with later-life ESKD by obesity [376]. Effective screening for early detection and management of cardiovascular and renal risk factors may, therefore, have an important impact on long-term morbidity in these women.
Major challenges remain to determine the risk of preeclampsia in the individual patient and to detect it early and manage it optimally to safely delay delivery and improve maternal and fetal outcomes. For example, the use of antihypertensives for moderate maternal hypertension has not been associated with reductions in the rates of preterm birth or infant SGA [Appendix in 107]. The use of antiplatelet agents in women at risk of preeclampsia, however, was associated with risk reductions in preeclampsia (RR 0.83; 95% CI, 0.77–0.89), preterm birth (RR 0.92; 95% CI, 0.88–0.97), and SGA (RR 0.90; 95% CI, 0.83–0.98) [Appendix in 107]. Supplementation of calcium during pregnancy in populations with low calcium intake, before 20–32 weeks of gestation and continued until delivery, was also associated with risk reductions in preeclampsia (RR 0.48; 95% CI, 0.34–0.67) and severe preeclampsia (RR 0.75; 95% CI, 0.57–0.98), as well as gestational hypertension (RR 0.65; 95% CI, 0.53–0.81) [Appendix in 107; 310].
• Recommendations for action
Women at increased risk of preeclampsia (including hypertension, renal disease, diabetes, obesity, prior preeclampsia, advanced maternal age, or having themselves been LBW, SGA, or preterm) should undergo preconception care to optimize any modifiable risk factors or conditions.
National guidelines adopted for the prevention and treatment of preeclampsia should be followed [377].
All women should be screened for hypertensive disorders of pregnancy at regular antenatal clinic visits starting from 20 weeks of gestation for prompt treatment and close follow-up. Simple screening is possible in communities if community health workers are adequately trained. There are promising new biomarkers (s-Flt, endoglin, and platelet-derived growth factor) that could be used as screening tools where available [378, 379].
Early detection of hypertensive disorders of pregnancy can enable appropriate antihypertensive treatment, lifestyle modification, and close monitoring of maternal and fetal health. National guidelines adopted for hypertensive disorders of pregnancy should be followed.
A possible tool to diagnose/monitor hypertensive disorders could be the presence of an abnormal Doppler waveform in the uterine arteries which has been accepted as a sign of severity of preeclampsia and a sign that might predict the appearance of hypertensive disorders in pregnancy when found in asymptomatic patients. However, the positive predictive value might change according to the population screened (50/70%) [380].
Given the lack of specific therapy to treat preeclampsia or eclampsia when floridly manifest, close monitoring of the mother and fetus could permit timely interventions to maximize the safety and survival of both [361].
Women who experience preeclampsia/eclampsia should receive life-long screening and follow-up for CVD and renal disease and receive immediate lifestyle education.
Recommendation 6: On Fetal Growth (Detection, Management, and Possible Interventions)
• Rationale
The strict definition of being SGA is an infant that is born under the 10% of birth weight for gestational age in a population [20]. Growth restriction may, however, be more subtle and is often overlooked, although it remains highly relevant in developmental programming. It is not possible to predict what the birth weight of each infant should be, but tracking an individual infant's growth rate through pregnancy would permit detection of a slowing growth rate as a signal for growth restriction and may prompt intervention. Such growth would most accurately be monitored through repeated ultrasound, but evaluation of fundal height in low-resource regions is very useful.
Fetal growth standards have recently been published [381, 382], but there is much discussion whether these standards should be customized for ethnicity, maternal features, and parity [383, 384]. Indeed, it has been shown that customized standards are more reliable than population standards in predicting adverse outcomes of SGA fetuses [385]. There is, however, no consensus regarding the role of ultrasound in the evaluation of fetal growth in the third trimester and even at what exact gestational age this should be conducted in low-risk pregnancies [386, 387]. The best parameter identified for the assessment of growth is measuring abdominal circumference, and, according to recent publications, the gestational age with the highest predictive value is between 34 and 36 weeks [388]. Furthermore, the evaluation of Doppler velocimetry of uterine, umbilical, and cerebral arteries, as well as the ductus venosus, can improve monitoring of fetal growth and planning timing of delivery [389]. An assessment of fetal size could at least flag the fetus/pregnancy as high risk early on, and appropriate arrangements can be made for the safest possible delivery and maximal support for the baby, if available. A recent study, for example, described maternal work conditions during pregnancy as a risk factor for LBW [390]. Therefore, some conditions could be modified to improve outcomes.
Strategies that have been found beneficial for the prevention of SGA include: multiple micronutrient supplementation (RR 0.87; 95% CI, 0.83–0.92), balanced energy protein supplementation (RR 0.68; 95% CI, 0.49–0.89), intermittent preventive therapy for malaria in pregnancy (RR 0.65; 95% CI, 0.55–0.77), use of insecticide-treated bed nets (RR 0.65; 95% CI, 0.55–0.77), and antiplatelet agents for preeclampsia (RR 0.90; 95% CI, 0.83–0.98) [Appendix in 107]. No intervention has yet been successful in improving growth when growth restriction is detected, although maternal rest, treatment of hypertension, and maternal anemia may improve growth restriction [391]. Depending on gestational age, one strategy is to try to postpone delivery in order to improve fetal maturity, until it shows a good capacity for adaptation. This approach has been studied extensively for early severe growth restriction [392]. There is no consensus regarding when to deliver growth-restricted babies detected late in the third trimester.
• Recommendations for action
Growth restriction can be prevented or reduced through good maternal nutrition, antibiotic treatment of asymptomatic bacteriuria, and prompt treatment of malaria [Appendix in 107; 310].
Monitoring fetal growth would enable early detection of fetal growth restriction. Measuring uterine size using fundal height should be part of the routine assessment of low-risk pregnant women according to local practice [310].
All pregnant women with lower-than-expected uterine size, as well as all women who are not at low risk, should undergo ultrasound evaluation of fetal growth and, where possible, Doppler velocimetry of uterine, umbilical, and cerebral arteries, and the ductus venosus.
Once growth restriction is detected, emphasize maternal rest and treatment of hypertension and anemia.
In growth-restricted fetuses, the best timing for delivery in order to avoid in utero fetal acidosis can be identified by using cardiotocography and fetal Doppler velocimetry [393].
Recommendation 7: On the Detection and Documentation of Birth Weight and Gestational Age
• Rationale
LBW has been defined by the WHO as weight at birth of less than 2.5 kg [20, 34] (Table 1). VLBW is defined as less than 1.5 kg, extremely low birth weight (ELBW) as less than 1.0 kg. LGA is defined as a birth weight >2 standard deviations above the mean birth weight for gestational age, and macrosomia is defined as a birth weight above 4 or 4.5 kg. SGA is defined as a birth weight <10th percentile for gestational age or <2 standard deviations below the mean birth weight for gestational age. IUGR reflects the failure of normal fetal growth. There is significant overlap between preterm birth and LBW as infants born at lower gestational ages are smaller than term infants [28] (Fig. 1). Preterm infants can either be born with a birth weight AGA or SGA. Importantly, growth restriction may be present in infants not meeting criteria for LBW or SGA; therefore, a clinical challenge exists how to identify such children who are also at risk of the effects of developmental programming. The WHO estimated that in 2014 globally 15–20% of live births per year are LBW, representing over 20 million infants [34]. Global estimates of preterm birth in 2010 estimated that 11.1% of all live births were preterm, representing around 14.9 million babies [33]. Using 22 birth cohort studies and the WHO Global Survey on Maternal Health and Perinatal Health (reflecting data from 138 LMIC), Lee et al. [28] estimated that in 2010, 32.4 million infants were born SGA representing 27% of live births (Fig. 1; Table 12).
Table 12.
| Studies reporting the proportion of infants born LBW, AGA, SGA, preterm, or macrosomic |
||||||||
| 2010 138 LMIC [28] |
WHO LBW policy brief [34] |
2010, systematic analysis, 184 countries [33] |
data from the WHO global survey on maternal and perinatal health [18], % of total |
|||||
|---|---|---|---|---|---|---|---|---|
| n (million) | % of total | n (million) | % of total | n (million) | % of total | developed countries | developing countries | |
| Term (all) | 106.6 | 88.6 | ||||||
| Preterm (all) | 13.7 | 11.4 | 14.9 | 11.1 | ||||
| Min | 12.3 | 9.1 | ||||||
| Max | 18.1 | 13.4 | ||||||
| Term AGA | 77.0 | 64 | ||||||
| LBW (all) | 37.0 | 30.8 | >20 | 15 – 20 | ||||
| SGA (all) | 32.4 | 26.9 | ||||||
| Term SGA | ||||||||
| No LBW | 19.0 | 15.8 | ||||||
| LBW | 10.6 | 8.8 | ||||||
| Preterm AGA | ||||||||
| No LBW | 6.3 | 5.2 | ||||||
| LBW | 4.6 | 3.8 | ||||||
| Preterm SGA with LBW | 2.8 | 2.3 | ||||||
| Macrosomia | 5 – 20 | 0.5 – 14.9 | ||||||
AGA, appropriate for gestational age; LBW, low birth weight; LMIC, low- and middle-income countries; SGA, small for gestational age; WHO, World Health Organization.
In this study, over 50% of term SGA babies weighed >2.5 kg; therefore, such infants may not be identified as having been growth restricted if LBW is the only parameter considered [28]. The proportions of infants born with more subtle forms of growth restriction is unknown. Macrosomia at birth ranges from 5–20% in developed countries to 0.5–15% in developing countries [18]. Many infants in LMIC are born at home and never weighed, and, therefore, the current rates may underestimate the true proportions of infants born LBW or preterm [34]. Given the varying global distributions of birth weight and preterm birth [34] (Fig. 2), specific reference charts should be adapted for different populations to permit study of long-term associations with birth weight in local contexts and to increase accuracy in predicting the programming risk in infants with LBW and SGA [15]. While in industrialized countries the epidemiology of LBW has been extensively studied, in less-developed countries reliable data on LBW and its consequences remain limited [394].
Initially attributed solely to IUGR and a deprived fetal environment, the phenomenon of fetal programming has been expanded to include preterm infants [20]. Some authors have argued that preterm birth is the predominant risk factor, and others have argued that IUGR is the relevant factor. Evidence supports preterm birth, IUGR, and LBW as robust markers for intrauterine programming of hypertension and renal disease, and, as shown in Table 4, hazard ratios for ESKD are highest when preterm birth and SGA coexist [29]. HBW is also emerging as a risk factor for kidney disease and diabetes in later life. Such birth parameters must be documented to highlight an individual's risk. It is important to realize that the risk does not mean disease; it may be modified through good nutrition and weight control as well as screening and early management of blood pressure and proteinuria.
• Recommendations for action
-
The WHO and UNICEF recommend that all infants be weighed at birth.
Birth weight and gestational age at birth should be recorded on the newborn's health record for use in monitoring the infant's growth and as part of the individual's lifetime health record.
A birth weight <2.5 kg, gestational age <37 weeks, or an SGA or HBW birth should be documented and become a prominent part of the person's medical record.
Such documentation could be encouraged in concert with the UN-SDG goal that each child should have a birth certificate.
Better strategies are required to identify growth-restricted infants that do not fall under the traditional categories of LBW, SGA, or preterm birth.
Recommendation 8: On the Risk Factor of Preterm Birth
• Rationale
In 2010, it was estimated that around 14.9 million infants were born preterm worldwide [33]. In a cross-country analysis of high human development index countries, the cause of preterm birth remained unknown in up to two thirds of cases [267]. Such data are not reported for LMIC, but it is known that over 60% of preterm babies globally are born in Asia and sub-Saharan Africa [33], possibly related to the high prevalence of maternal undernutrition in these regions [208]. Known risk factors for preterm birth are multiple, including maternal diabetes, kidney disease, preeclampsia, infections, nutritional deficiencies, and use of some medications [143]. Many of these factors have been shown to reduce nephron numbers and kidney size, or increase blood pressure in experimental animals exposed during gestation [15]. As 60% of nephrons are formed during the third trimester, children born preterm have a significantly lower number of nephrons at birth, which do not catch up adequately postnatally [395, 396]. Preterm birth itself, as well as many risk factors for preterm birth, may, therefore, have compounding effects on nephrogenesis in preterm infants. In addition, preterm infants are at increased risk for AKI postnatally, which may further disrupt nephrogenesis. Progressive kidney disease in preterm individuals is multidimensional, with genetic and environmental events (hypoxia, hyperoxia, nephrotoxic drugs, hypotension, AKI, thrombosis, bleeding, free radical toxicity, and other injuries) contributing to disease risk [15, 20, 137].
Successful strategies in women with prior preterm births have been estimated to reduce subsequent preterm birth in high-income countries by: cessation of smoking (10–20%), reducing multiple embryo transfers during ART (63%), cervical cerclage (20%), progesterone supplementation (45%), avoidance of nonmedically indicated cesarean section (55%), and close follow-up in preterm birth prevention clinics (13%) [209, 288]. Many of these interventions are easily accessible in LMIC. Other strategies that have proven beneficial include: calcium supplementation (RR 0.76; 95% CI, 0.60–0.97), syphilis screening and treatment (RR 0.36; 95% CI, 0.27–0.47), screening and treatment of lower genital tract infections (RR 0.55; 95% CI, 0.41–0.75); antiplatelet agents for preeclampsia (RR 0.92; 95% CI, 0.88–0.97), and nicotine replacement therapy (RR 0.77; 95% CI, 0.61–0.97) [Appendix in 107]. The use of prenatal antidepressants was found to increase the risk of preterm birth (RR 1.55; 95% CI, 1.38–1.74) [Appendix in 107]. Optimization of maternal weight (avoidance of under- or overweight), reduction of maternal stress, and treatment of periodontal disease are also potential strategies to reduce preterm birth [288].
• Recommendations for action
Women should be screened before conception or early in pregnancy for known risks factors for preterm birth.
Simple preventive strategies should be routinely implemented (nutrition, prevention, and treatment of infections).
In women at high risk of preterm birth (especially those with a prior history of preterm birth), preventive strategies should be implemented if possible [209]. Measurement of the length of the uterine cervix at 22–24 weeks of gestation and prevention of vaginal infections should be considered.
Recommendation 9: On Neonatal AKI and Drug-Induced Renal Damage
• Rationale
AKI occurs in 16–70% of preterm infants [136, 137], 40% of neonates with severe perinatal asphyxia, and 60% of newborns who undergo cardiopulmonary bypass for congenital heart disease [138]. AKI is an important risk factor for death in neonates [136, 138]. The risk factors for AKI include lower gestational age and birth weight, SGA, renal hypoperfusion, nephrotoxin exposure, sepsis, obstruction, and renal arterial or venous thrombosis [142, 159]. Preterm and critically ill newborns are predisposed to developing AKI because of renal function immaturity and incomplete nephrogenesis in the early postnatal period, which can be irreversibly impaired by drug exposure, and cellular injury to glomeruli or tubules, which may impair repair capacity and increase susceptibility to renal disease later in life [147, 160]. The management of neonates in intensive care often requires a combination of various therapeutic agents, frequently unlicensed or off-label, with many of them potentially inducing renal tissue injury [397, 398]. Antibiotics (aminoglycosides and vancomycin), antifungals (amphotericin B), antivirals (acyclovir), angiotensin-converting enzyme inhibitors, and NSAID can induce nephrotoxic damage through several concomitant mechanisms of action on different segments of the nephron and may directly impair any ongoing nephrogenesis in a preterm infant [136, 141, 143, 146]. A retrospective review found that 87% of neonates in ICU were exposed to at least 1 nephrotoxic medication for an average duration of around 2 weeks [141]. In this study, greater nephrotoxin exposure was associated with lower gestational age, lower birth weights, being SGA, and an increased risk of AKI [141].
Serum creatinine levels are widely used in the diagnosis of AKI in adults and older children, but its utility in preterm infants is limited by maternal creatinine, immature renal tubule function, and, until recently, the lack of a clear definition of AKI [136, 142, 399]. A neonatal AKI classification which should improve uniformity of diagnosis and comparability of data across institutions has recently been proposed [136, 142, 147] (Table 13).
Table 13.
Neonatal AKI KDIGO classification [reprinted with permission from 136]
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 0 | No change in serum creatinine or rise <0.3 mg/dL | ≥0.5 mL/kg/h |
| 1 | Serum creatinine rise ≥0.3 mg/dL within 48 h or serum creatinine rise ≥1.5 – 1.9 × reference serum creatinine1 within 7 days | <0.5 mL/kg/h for 6 – 12 h |
| 2 | Serum creatinine rise ≥2.0 – 2.9 × reference serum creatinine1 | <0.5 mL/kg/h for ≥12 h |
| 3 | Serum creatinine rise ≥3.0 × reference serum creatinine1 or serum creatinine ≥2.5 mg/dL2 or receipt of dialysis | <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
Differences between the proposed neonatal AKI definition and KDIGO include the following:
Reference serum creatinine will be defined as the lowest previous serum creatinine value.
Serum creatinine value of 2.5 mg/dL represents <10 mL/min/1.73 m2.
Identification of new biomarkers predicting nephrotoxicity and enabling the early detection of AKI could improve patient outcome [139, 147, 400, 401].
Most of the discussion around neonatal AKI occurs in high-resource countries where preterm babies have an increased chance of survival and access to ICU care. Little data exist from lower-income settings, where risks should be at least as high. One study from Kenya found 71% mortality by 7 days among asphyxiated term neonates with AKI, although specific therapies available were not described [402].
Data from pediatric ICU patients have shown that about 10% will develop CKD 1–3 years after AKI [163]. This burden may be higher in preterm infants, because of the reduced number of nephrons [137, 165], although this requires further study. A recent study from the US, where 40% of ICU neonates experienced AKI, found that AKI was only recorded in the discharge summary in 13.5% of infants, and none were referred for nephrology follow-up [159]. This study illustrated the lack of awareness of the potential long-term impact of neonatal AKI.
• Recommendations for action
Preterm birth, LBW, and SGA must be recognized as risk factors for AKI.
-
Every effort to prevent AKI should be made [142, 403]:
(i) Optimize fluid management in order to maintain circulating volume and to preserve blood pressure.
(ii) Minimize use of nephrotoxins (antibiotics, antifungals, antivirals, renin-angiotensin system inhibitors, NSAID, and radiocontrast agents).
(iii) Monitor aminoglycoside drug levels when prolonged treatment is necessary.
(iv) Administer nephrotoxic drugs (if necessary) at the lowest effective dose while monitoring drug levels, fluid balance, and renal function.
(v) With established nephrotoxicity, reassess the drug dose and avoid concomitant administration of more nephrotoxic drugs.
(vi) Pay close attention to nutrition to optimize renal recovery.
(vii) Consider renal ultrasound to assess congenital abnormalities and obstruction to enable timely correction.
AKI should be recognized early, and appropriate interventions should be instituted to minimize renal injury and optimize recovery.
Episodes of neonatal AKI, even if mild, should be reported to primary physicians upon discharge from hospitalization.
Infants who experienced AKI, especially if preterm, LBW, or SGA infants, require life-long follow up of blood pressure and renal function.
Recommendation 10: On Toxic Gestational Medication Exposure
• Rationale
The fetus and infant may be more vulnerable to toxic environmental exposure during the sensitive time of organ development. Exposure to nephrotoxic agents during kidney development can result in a reduction in nephron number, as well as disruption of nephron structure and/or function. Many medications that may impact kidney development, e.g., renin-angiotensin system inhibitors, are contraindicated in pregnancy; however, some medications, including gentamicin, penicillins, ceftriaxone, cyclosporine, long-term dexamethasone, nonsteroidal antibiotics, and COX-2 inhibitors, given during gestation have been found to reduce nephron number or result in abnormal nephron development in experimental animal offspring and, in some cases, to lead to renal dysfunction and higher blood pressures with age [149, 404, 405, 406, 407, 408, 409, 410, 411]. The impact of many of these medications on kidney development in humans is unknown. Very small studies in children of mothers with organ transplants who were exposed to cyclosporine in utero have not shown abnormalities in renal function or structure [157, 412]. Short-term antenatal steroid exposure has not been associated with an increase in blood pressure at age 2 or 30 years [413, 414]. Similarly, prenatal corticosteroids did not modify the association of blood pressure with renal function in preterm infants aged 12–36 months [415]. Short-term perinatal steroid use, therefore, may not have a programming effect on the developing kidney; however, the effects of long-term steroid use remain to be studied. Maternal vitamin supplementation is important to reduce the risk of LBW and preterm birth, but excessive vitamin A supplementation may arrest nephrogenesis, and folate intake alone may be associated with an increased risk of congenital renal anomalies [416, 417]. Maternal exposure to the tocolytic nimesulide and other NSAID shortly before delivery has been associated with severe neonatal AKI and even ESKD [156, 418, 419, 420]. The risks and benefits of medication use in pregnant women should be carefully considered. Screening and treatment of lower genital tract infections has been associated with reduced risks of preterm birth (RR 0.55; 95% CI, 0.41–0.75) and LBW (RR 0.48; 95% CI, 0.34–0.66), and treatment of asymptomatic bacteriuria was associated with a reduction in infant LBW (RR 0.66; 95% CI, 0.49–0.89) [Appendix in 107]. However, the choice of the antibiotic may affect nephrogenesis.
• Recommendations for action
Nephrotoxic drugs should be avoided or used only if there are no alternatives.
When used, nephrotoxic drugs should be administered at the lowest effective dose while monitoring drug levels, fluid balance, and renal function.
Long-term impact of maternal medication use on child and adult renal function requires further study.
Recommendation 11: On Infant and Child Nutrition
• Rationale
Childhood undernutrition remains a global problem, with 156 million children under age 5 years in 2015 being stunted and 50 million being wasted [421]. The majority of these children were from Asia and Africa. In contrast, during the same period, it was estimated that 42 million children under age 5 years were overweight, the majority of whom were from Asia and Africa [103, 421]. Stunting is associated with having been born LBW, SGA, or preterm [105]. Childhood stunting leads to short maternal stature and maternal underweight, which are risk factors for IUGR in pregnancy [103, 208], emphasizing the transgenerational impact of early nutrition. Most stunting occurs within the first 1,000 days after conception, and, therefore, optimizing nutrition during this period is crucial [103]. Weight gain during these first 1,000 days is also important in programming adult lean body mass [103]. Weight gain after 1,000 days is associated with later life obesity [103]. The timing and quality of postnatal and early childhood nutrition after LBW, SGA, or preterm birth are, therefore, important contributors to long-term risk of chronic diseases [422].
In preterm infants and infants with IUGR, both over- and undernutrition at critical stages of development may have undesirable consequences: while growing quickly increases the risk of obesity, dyslipidemia, hypertension, and type 2 diabetes, slower weight gain velocity may delay mental performance. This double detrimental-beneficial effect of early feeding in this high-risk population has become the neonatologist's dilemma [423]. Human milk is the optimal nutrient for term and preterm infants, but this may not meet the nutritional requirements of VLBW and ELBW infants [424]. Supplementation of human milk may be required, and, after hospital discharge, nutritional prescriptions may need to be adjusted to maintain optimal growth rates and avoid obesity.
In infancy, exclusive breastfeeding for 6 months has been recommended by the WHO because of its beneficial effects for preventing long-term NCD [425]. Both breastfeeding during the first months of life and avoiding rapid weight gain in childhood have been shown to prevent later risk of obesity [426, 427] as well as dyslipidemia, hypertension, and reduced glucose tolerance [428]. A higher protein content in infant formulas (than in breast milk) has been shown to increase weight gain velocity and kidney growth during the first few months of life [429], as well as the later risk of becoming overweight [31, 430]. In childhood and adolescence, the ingestion of a combination of sugars, salt, and fat and protein from red meat affects multiple metabolic functions and is associated with a higher incidence of the metabolic syndrome [431], which increases the risk of developing CKD. In contrast, a prudent dietary pattern (reduced sodium, carbohydrates, and saturated fat) is beneficial, especially when combined with increased physical activity [432]. Adherence to a Mediterranean diet (plant foods, fresh fruits, fish, poultry, dairy products, and olive oil as the main source of fat) is associated with lower blood pressure, blood glucose, and triglycerides. Obesity contributes significantly to the development and progression of CKD: hyperfiltration and hypertrophy occur in response to the increased metabolic needs of obesity [433, 434].
Poverty is not the only contributor to childhood undernutrition. A recent retrospective analysis of demographic and health surveys from 1990 to 2011 across 36 LMIC found no association between the change in the per-capita gross domestic product over time and the average prevalence of child undernutrition [435]. This study suggests that factors other than macro- or household economic growth can impact childhood nutrition, further emphasizing the importance of a multisectoral approach to child health outlined by the SDG. Others have suggested that investment in agriculture, social safety nets, child development, and parental education could all contribute to improved maternal and child nutrition [330].
• Recommendations for action
The recommended feeding for children is exclusive breastfeeding for the first 6 months of life and continued breastfeeding through the second year of life [103, 436].
Prudent introduction of other food sources should be done, enabling regular and balanced growth.
Quality of nutrition in preterm VLBW and ELBW infants must be closely monitored.
Child weight and height should be regularly monitored on appropriate growth charts, and any rapid upward crossing of weight centiles, especially when disproportionate to increases in height and even if still within the accepted range of centiles, must be noted, and appropriate nutrition and physical activity counseling given to prevent obesity.
In childhood and adolescence, a prudent dietary pattern (reduced sodium, carbohydrates, and saturated fat), possibly adhering to the Mediterranean diet, should be encouraged.
Increased physical activity must be encouraged to prevent hypertension and obesity.
Support population level programs to reduce sugar consumption, increase physical activity, and promote healthy diets to prevent and treat obesity [437].
Recommendation 12: On Follow-Up of LBW, SGA, and Preterm Infants, and Those Who Were Born in Pregnancies Complicated by Hypertension, Preeclampsia, or Gestational Diabetes
• Rationale
A recent systematic review (31 studies) showed a 70% risk increase in CKD in adulthood for LBW individuals or those who experienced IUGR, with combined OR of 1.81 (95% CI, 1.19–2.77) for albuminuria, 1.58 (95% CI, 1.33–1.88) for ESKD, and 1.79 (95% CI, 1.31–2.45) for low GFR [16]. Others have reported reduced GFR and greater albuminuria in individuals born preterm from childhood to young adulthood [31, 438]. Similarly, 2 meta-analyses found a significantly increased risk of higher blood pressures among those born <2.5 kg versus ≥2.5 kg (odds of hypertension 1.21; 95% CI, 1.13–1.30) or preterm or VLBW (pooled difference 2.55 mm Hg; 95% CI, 1.7–3.3 mm Hg) compared to term individuals [21, 22]. The mean increase in systolic blood pressure at age 9 years among offspring of pregnancies complicated by gestational hypertension was 2.04 mm Hg (95% CI, 1.42–2.67 mm Hg) and by preeclampsia was 2.05 mm Hg (95% CI, 0.72–3.38 mm Hg) [356]. Interestingly, the difference was attenuated after controlling for gestational age and birth weight in offspring who experienced preeclampsia but not gestational hypertension, suggesting possible diverse programming mechanisms. In addition, in women born preterm before 32 weeks of gestation, pregnancies have an increased risk of GDM (OR 2.34; 95% CI, 1.65–3.33), gestational hypertension (OR 1.56; 95% CI, 1.09–2.25), or preeclampsia/eclampsia (OR 1.79; 95% CI, 1.19–2.69) [124]. These odds were lower but still increased among those born between 32 and 36 gestational weeks. LBW and preterm birth should be recognized as risk factors (not causes) for hypertension, diabetes, CVD, and renal disease later in life, and as a risk factor for pregnancy-associated complications [21, 22, 23, 211, 262]. The risk of these conditions is augmented in the setting of overweight/obesity [439, 440].
Since there are no evidence-based guidelines for the follow up of individuals who had been LBW, SGA, preterm, or born in preeclamptic or diabetic pregnancies, we suggest the following recommendations, which may require local adaptation dependent on prevailing resources and practices.
• Recommendations for action
Health care workers should clearly communicate the need for follow-up in at-risk infants to parents and colleagues to ensure appropriate follow-up occurs.
Growth-restricted, preterm, or LBW infants as well as those exposed to preeclampsia or GDM should undergo annual blood pressure measurement at least from 3 years of age, as recommended by the American and European guidelines, and annual urinalysis [441, 442].
Very premature children (<32 weeks of gestation) or children with VLBW or AKI postnatally should be screened initially at not later than 1 year of age [88].
To detect small kidneys, asymmetry, or structural abnormalities, we suggest, if feasible, a baseline renal ultrasound, and follow-up should be performed as indicated [89].
If high blood pressure, previous AKI, proteinuria, associated CVD, renal anomalies, obesity, or diabetes are present, assessment of renal function, including also albuminuria and proteinuria, should be performed at least every 2 years until school entry [136].
Screening of children who were growth restricted, preterm, LBW, or exposed to preeclampsia or GDM should be performed at planned checks of child healthstatus, medical visits, or at 2-year intervals throughout school years.
In low-resource settings, such intense follow-up may not be possible; however, simplified screening could coincide with public health interventions such as vaccinations or mass drug administration campaigns or conducted by community health workers.
Under all circumstances, it is important that preterm or LBW children are not labeled “sick”, and, therefore, screening should be integrated with other health activities if possible.
Any abnormalities in kidney function or ultrasound should be followed up, with prompt referral, where possible, to a pediatrician or pediatric nephrologist.
Families of preterm or LBW children should be instructed about a healthy lifestyle and avoidance of nephrotoxic agents.
Rapid weight gain in infancy and early childhood should be avoided to reduce exacerbation of renal risk associated with obesity.
From childhood onwards, a careful dietary habit with low salt and reduced carbohydrates and saturated fat, as well as adequate physical activity should be adopted.
From age 18 years onwards, we recommend monitoring of blood pressure, BMI, and urinalysis 2 yearly until 40 years of age, and thereafter at yearly intervals.
Individuals with high blood pressure or abnormal proteinuria require long-term follow-up and timely institution of renoprotective therapy.
From 30 years of age, fasting blood glucose should be monitored in subjects with high BMI.
For pregnant women born preterm or with LBW, close monitoring for gestational weight gain, fetal growth, and preeclampsia is suggested.
Healthy lifestyle choices should be promoted throughout life.
Smoking should be avoided.
Recommendation 13: On Regular Monitoring of Mothers of LBW and Preterm Infants, and Those Who Experience Preeclampsia or GDM
• Rationale
Mothers of infants born SGA, of LBW, or preterm, or who experienced preeclampsia are at an increased risk of renal and CVD later in life [371, 443, 444]. Mothers who experience preeclampsia are at increased risk of renal disease, CVD, and ESKD in later life [58, 62]. Mothers experiencing GDM are at increased risk of developing overt diabetes over time (Table 11) [372, 445]. Although these long-term risks are well recognized, uptake of subsequent maternal screening is poor, even when offered free of charge [445]. New mothers must be educated about the need for follow-up immediately postpartum and at follow-up visits for the child, and health care workers must be aware of the risk and need for follow-up.
• Recommendations for action
The occurrence of GDM or preeclampsia must be prominently documented in a woman's medical record and communicated to health care providers.
Mothers should be followed up 3 monthly until blood pressure, proteinuria, and blood sugars normalize postpartum.
Mothers in whom these parameters do not normalize should be treated and followed appropriately.
The effectiveness of combined mother/baby clinics in improving rates of maternal follow-up within the first 5 years postpartum should be studied and if successful scaled up and rolled out.
At each follow-up visit, mothers should receive healthy lifestyle education and education about preventing risk of disease in themselves and their children.
Mothers should be screened lifelong for overweight/obesity, blood pressure, diabetes, hyperlipidemia, CVD, and renal diseases every 1–3 years as age-appropriate guidelines suggest and treated appropriately.
Recommendation 14: On Potential Living Kidney Donors Who Had Been Preterm, SGA, or LBW and Mothers Who Had Preeclampsia
• Rationale
Recent studies have suggested that living donation may not be as innocuous as once thought, making it imperative to better understand predictors of renal functional decline in living donors [193, 200, 446, 447]. Follow-up of living kidney donors for up to 5 years showed that GFR was lower, proteinuria was higher, and new-onset hypertension was more common in donors with lower birth weights [203]. The effects were more evident in donors aged over 50, suggesting that LBW, nephron loss, and age compound renal function worsening over time. Transplant donors for groups that are at higher risk of renal disease and LBW or HBW (African-Americans, Australian Aboriginals, and Aboriginal Canadians), all appear to have higher risks of proteinuria and renal dysfunction over time, meaning that potential donors from these groups must be thoroughly evaluated and followed up (Table 8). In addition, a recent study found an 86% increased risk of ESKD among obese compared to nonobese living kidney donors over 20 years [448].
Although, to our knowledge, no studies have explicitly examined the question, women who have experienced preeclampsia are themselves at increased risk of developing ESKD compared to those who did not experience preeclampsia (RR 4.7; 95% CI, 3.6–6.1 in a Norwegian registry study) [204]. Although the absolute risk remained small, the RR increased in women who experienced preeclampsia in more than 1 pregnancy (Table 11). It is possible that this risk may increase or accelerate after the donation of 1 kidney. In addition, women who donate a kidney are at increased risk of gestational hypertension and preeclampsia compared to nondonors (OR 2.4; 95% CI, 1.2–5.0) [449]. A cautious donor history in women must include obstetrical history.
• Recommendations for action
Caution should be exercised in screening potential kidney donors who were born growth restricted, LBW, or preterm, or who were offspring of diabetic pregnancies and LGA.
Questions about birth weight and birth circumstances, as well as prior history of preeclampsia or GDM, should be routine for all potential donors.
Renal function should be tested rigorously before donation is considered.
Any potential donor who was born SGA, LBW, preterm, or LGA should not be accepted for donation if there is any degree of proteinuria, elevation in blood pressure, diabetes, or BMI >25 given the fundamental obligation to “do no harm.”
Any donor who was born SGA, LBW, or preterm, or who experienced preeclampsia, should be monitored closely for the rest of their lives after donation, ideally by a nephrologist, and they should be strongly encouraged to avoid overweight/obesity, and to have hypertension and diabetes treated early.
Potential donors who had GDM, even if currently normoglycemic, must be counseled extensively about the risk of developing subsequent diabetes, the exacerbation of this risk with weight gain, and the risk of accelerated loss of renal function in a single kidney should overt diabetes develop. Such donors require long-term follow-up.
Global Health Implications of Renal Developmental Programming and Call to Action
Hypertension and CKD have a significant impact on global morbidity and mortality [9, 450]. LBW, prematurity, SGA, HBW, childhood obesity, maternal obesity, GDM, and preeclampsia are all highly prevalent across the globe. The proportion of the chronic disease burden contributed by developmental programming is difficult to quantify, but it is likely to be significant due to the high prevalence of programming risk factors in both high- and lower-income countries. Preventing risk factors for IUGR, LBW, and prematurity, and optimizing maternal health are likely to be the most comprehensive strategies to reduce this burden as outlined in the Minsk Declaration and the WHO publication on “Good Maternal Nutrition. The Best Start in Life” [1, 3]. Improving all health-related and structural factors that impact maternal health prior to conception, during pregnancy, and child nutrition, as well as development after birth, requires a multisectoral approach extending beyond the health system, encompassing governance and health policy, the allocation of appropriate funds for health care, education, infrastructure, medical technologies, access to medication, and appropriately trained health care professionals, reliable data capture and monitoring, and ongoing research to develop effective implementation strategies. Increased awareness of the risks of developmental programming is imperative. Infants should be weighed at birth, gestational age determined, and these facts documented. Neonatologists should be aware of the risk of AKI and the importance of documenting this in discharge communications, and they should try to minimize the exposure of preterm and SGA infants to nephrotoxins. Families should be educated about healthy lifestyle strategies to minimize obesity and malnutrition. Children should be monitored for hypertension or proteinuria, especially if they experience rapid catch-up growth. Mothers with LBW or preterm children, or those who had preeclamptic pregnancies or GDM, require long-term follow-up for cardiovascular risk factors and disease management, as well as close monitoring in future pregnancies. Potential kidney donors should be asked about their birth history and birth weight, and careful consideration should be given to potential risks in individual donors.
Understanding the potential long-term benefits of such interventions is crucial to inform policy decisions to interrupt the developmental programming cycle and stem the growing epidemics of hypertension and kidney disease worldwide.
Conclusions
The recommendations for action of our consensus workshop are the results of combined clinical experience, shared research expertise, and a review of the literature made by obstetricians, neonatologists, and nephrologists of the Low Birth Weight and Nephron Number Working Group (see Appendix).
They highlight the need to act early to prevent CKD and other related NCD later in life by reducing LBW, SGA, prematurity, and low nephron numbers at birth through coordinated intervention by obstetricians, neonatologists, nephrologists, and family physicians [15, 451]. This is particularly relevant for resource-poor countries that experience the burdens of maternal, fetal, and childhood undernutrition and poor health, which synergistically act to augment the effect of developmental programming of chronic diseases, which also disproportionally affects low- and middle-income regions. Thus, developing countries are the most vulnerable to the effects of the developmental programming life cycle. Our recommendations are consistent with and complement the Global Goals for Sustainable Development proposed by the United Nations [4], where ending poverty and hunger, enhancing food security, decreasing teenage pregnancy, empowering and educating girls and women, reducing maternal infections, and managing chronic diseases may reduce the risk of LBW, SGA, preterm birth, preeclampsia, GDM, and maternal and childhood obesity, and, therefore, hold the promise of a positive impact on the renal health of future generations.
The working group has also indicated that there are many remaining gaps that require further research studies in the field, including but not limited to the establishment of registries for documenting LBW, prematurity, and infants born SGA (especially in resource-poor regions), conducting intervention trials, and identifying new early biomarkers of risk [interesting readers may refer to the full list of suggestions for further research studies mentioned in 15, 451].
Meeting these unmet needs would help to define the most cost-effective strategies and to optimize interventions to limit or interrupt the developmental programming cycle of major NCD, including CKD later in life, especially in the poorest parts of the world.
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
We are extremely grateful for the support and generosity of the Menarini International Foundation and also want to thank Dr. Alessandro Casini (Rozzano, Milan, Italy) for his enthusiasm. Furthermore; we would like to acknowledge the additional contribution of the Chiesi Foundation (Parma, Italy). This meeting was organized with the generous support of the Italian Society of Nephrology (SIN), which also very kindly supported the publication of this consensus document.
Appendix
Contributors
The Low Birth Weight and Nephron Number Working Group
Writing Committee: Barry M. Brenner (Boston, MA, USA), Jennifer Charlton (Charlottesville, VA, USA), Valerie Luyckx (Zurich, Switzerland), Dario Manfellotto (Rome, Italy), Norberto Perico (Bergamo, Italy), Giuseppe Remuzzi (Bergamo, Italy), Marco Somaschini (Lugano, Switzerland), and Herbert Valensise (Rome, Italy).
Group Members: Dwomoa Adu (Accra, Ghana), Karel Allegaert (Leuven, Belgium), Chiara Benedetto (Turin, Italy), Irene Cetin (Milan, Italy), Robert Chevalier (Charlottesville, VA, USA), Monica Cortinovis (Bergamo, Italy), Rosario D'Anna (Messina, Italy), Johannes Duvekot (Rotterdam, The Netherlands), Joaquin Escribano (Reus, Spain), Vassilios Fanos (Cagliari, Italy), Enrico Ferrazzi (Milan, Italy), Tiziana Frusca (Parma, Italy), Richard J. Glassock (Laguna Niguel, CA, USA), Wilfried Gyselaers (Hasselt, Belgium), Federico Mecacci (Florence, Italy), Giovanni Montini (Milan, Italy), Clive Osmond (Southampton, UK), Luca Ramenghi (Genoa, Italy), Paola Romagnani (Florence, Italy), Antonio Santoro (Bologna, Italy), Umberto Simeoni (Lausanne, Switzerland), Eric A.P. Steegers (Rotterdam, The Netherlands), and Bjorn Egil Vikse (Bergen, Norway).
Affiliations
Renal Division, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA (Prof. B.M. Brenner, MD); Division of Nephrology, Department of Pediatrics, University of Virginia Children's Hospital, Charlottesville, VA, USA (Prof. J. Charlton, MD); Institute of Biomedical Ethics, University of Zurich, Zürich, Switzerland (Dr. V. Luyckx, MBBCh); Department of Internal Medicine, AFaR Division, Fatebenefratelli Foundation, San Giovanni Calibita Fatebenefratelli Hospital, Isola Tiberina, Rome, Italy (Prof. D. Manfellotto, MD); Clinical Research Center for Rare Diseases Aldo e Cele Daccò, IRCCS – Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy (Dr. N. Perico, MD); Clinical Research Center for Rare Diseases Aldo e Cele Daccò and Centro Anna Maria Astori, Science and Technology Park Kilometro Rosso, IRCCS – Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy (Prof. G. Remuzzi, MD); Unit of Nephrology, Dialysis, and Transplantation, Azienda Socio Sanitaria Territoriale (ASST) Papa Giovanni XXIII, Bergamo, Italy (Prof. G. Remuzzi); Department of Biomedical and Clinical Sciences, Hospital L. Sacco, University of Milan, Milan, Italy (Prof. G. Remuzzi); Unit of Neonatology, Sant'Anna Clinic, Lugano, Switzerland (Dr. M. Somaschini, MD); Department of Obstetrics and Gynecology, Tor Vergata University, Rome, Italy (Prof. H. Valensise, MD); School of Medicine and Dentistry, University of Ghana, Accra, Ghana (Dr. D. Adu, MD); Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center – Sophia Children's Hospital, Rotterdam, The Netherlands (Prof. K. Allegaert); Department of Development and Regeneration, KU Leuven, Leuven, Belgium (Prof. K. Allegaert); Unit of Gynecology and Obstetrics I, Department of Surgical Sciences, University of Turin, Turin, Italy (Prof. C. Benedetto, MD); Unit of Obstetrics and Gynecology, Department of Biomedical and Clinical Sciences, Hospital L. Sacco, and Center for Fetal Research Giorgio Pardi, University of Milan, Milan, Italy (Prof. I. Cetin, MD); University of Virginia, Charlottesville, VA, USA (Prof. R. Chevalier, MD); Clinical Research Center for Rare Diseases, Aldo e Cele Daccò, IRCCS – Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy (Dr. M. Cortinovis, Biotech. Dr.); Unit of Gynecology and Obstetrics, Department of Human Pathology in Adulthood and Childhood G. Barresi, University of Messina, Messina, Italy (Prof. R. D'Anna, MD); Division of Obstetrics and Prenatal Medicine, Department of Obstetrics and Gynecology, Erasmus Medical Center, Rotterdam, The Netherlands (Prof. J. Duvekot, MD); Pediatrics Research Unit, Universitat Rovira i Virgili, IISPV, Reus, Spain (Prof. J. Escribano, MD); Neonatal Intensive Care Unit, Neonatal Pathology, Puericulture Institute and Neonatal Section, Azienda Ospedaliera Universitaria, University of Cagliari, Cagliari, Italy (Prof. V. Fanos, MD); Department of Obstetrics, Gynecology, and Neonatology, ICP – Buzzi Children's Hospital, Biomedical and Clinical Sciences, School of Medicine, University of Milan, Milan, Italy (Prof. E. Ferrazzi, MD); Department of Obstetrics and Gynecology, Maggiore Hospital, University of Parma, Parma, Italy (Prof. T. Frusca, MD); Department of Medicine, David Geffen School of Medicine at UCLA, Laguna Niguel, CA, USA (Prof. R.J. Glassock, MD); Mobile Health Unit, Faculty of Medicine and Life Sciences, Department of Physiology, Hasselt University, Hasselt, Belgium (Prof. W. Gyselaers, MD); Department of Gynecology, Ziekenhuis Oost Limburg, Genk, Belgium (Prof. W. Gyselaers, MD); Department of Biomedical, Experimental, and Clinical Sciences, Division of Obstetrics and Gynecology, University of Florence, Careggi University Hospital, Florence, Italy (Prof. F. Mecacci, MD); Pediatric Nephrology and Dialysis Unit, Department of Clinical Sciences and Community Health, University of Milan, Fondazione IRCCS Ca' Granda – Ospedale Maggiore Policlinico, Milan, Italy (Prof. G. Montini, MD); MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, UK (Prof. C. Osmond, PhD); Neonatal Intensive Care Unit, Istituto Giannina Gaslini, Genoa, Italy (Prof. L. Ramenghi, MD); Excellence Center for Research, Transfer, and High Education for the Development of de novo Therapies, and Department of Biomedical, Experimental, and Clinical Sciences, University of Florence, Florence, Italy (Prof. P. Romagnani, MD); Nephrology Unit, Meyer Children's University Hospital, Florence, Italy (Prof. P. Romagnani, MD); Department of Nephrology and Dialysis, Policlinico S. Orsola-Malpighi, Bologna, Italy (Prof. A. Santoro, MD); Service de Pédiatrie, Université de Lausanne, Lausanne, Switzerland (Prof. U. Simeoni, MD); Division of Obstetrics and Prenatal Medicine, Department of Obstetrics and Gynecology, Erasmus University Medical Center Rotterdam, Rotterdam, The Netherlands (Prof. E.A.P, Steegers, PhD); Department of Medicine, Haugesund Hospital, Haugesund, Norway (Prof. B.E. Vikse, MD); Department of Clinical Medicine, University of Bergen, Bergen, Norway (Prof. B.E. Vikse, MD).
Participants of the Low Birth Weight and Nephron Number Working Group are listed in the Appendix.
References
- 1.Ketting E, Khomasuridze T. Towards a new WHO European Action Plan for human rights-based sexual and reproductive health (SRH). http://www.euro.who.int/_data/assets/pdf_file/0003/319305/3-Towards-new-WHO-EAP-human-rights-based-SRH.pdf?ua=1
- 2.WHO Global Action Plan for the Prevention and Control of NCDs, 2013–2020. http://www.who.int/nmh/events/ncd_action_plan/en/
- 3.WHO Good Maternal Nutrition – The Best Start in Life. http://www.euro.who.int/_data/assets/pdf_file/0008/313667/Good-maternal-nutrition-The-best-start-in-life.pdf?ua=1
- 4.UN Sustainable Development Goals. http://sustainabledevelopment.un.org.
- 5.Buse K, Hawkes S. Health in the sustainable development goals: ready for a paradigm shift? Global Health. 2015;11:13. doi: 10.1186/s12992-015-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global, regional, and national incidence and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation . IDF Diabetes Atlas. ed 7. Brussels: International Diabetes Federation; 2015. [Google Scholar]
- 8.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 9.Global, regional, and national incidence and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global, regional, and national life expectancy all-cause mortality and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 12.Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 14.Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4:e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 15.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes – a global concern. Nat Rev Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 16.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 17.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 18.Koyanagi A, Zhang J, Dagvadorj A, et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet. 2013;381:476–483. doi: 10.1016/S0140-6736(12)61605-5. [DOI] [PubMed] [Google Scholar]
- 19.Chatfield J. ACOG issues guidelines on fetal macrosomia. American College of Obstetricians and Gynecologists. Am Fam Physician. 2001;64:169–170. [PubMed] [Google Scholar]
- 20.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. 2012;8:265–274. doi: 10.1038/nrneph.2012.38. [DOI] [PubMed] [Google Scholar]
- 21.de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu M, Wang SF, Sheng J, et al. Birth weight and subsequent blood pressure: a meta-analysis. Arch Cardiovasc Dis. 2012;105:99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 24.Zetterstrom K, Lindeberg S, Haglund B, et al. Being born small for gestational age increases the risk of severe pre-eclampsia. BJOG. 2007;114:319–324. doi: 10.1111/j.1471-0528.2006.01231.x. [DOI] [PubMed] [Google Scholar]
- 25.Syddall HE, Sayer AA, Simmonds SJ, et al. Birth weight, infant weight gain, and cause-specific mortality: the Hertfordshire Cohort Study. Am J Epidemiol. 2005;161:1074–1080. doi: 10.1093/aje/kwi137. [DOI] [PubMed] [Google Scholar]
- 26.Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes. 1998;47:1489–1493. doi: 10.2337/diabetes.47.9.1489. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Li H, Liu SJ, et al. The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta-analysis. Hypertens Res. 2013;36:725–735. doi: 10.1038/hr.2013.33. [DOI] [PubMed] [Google Scholar]
- 28.Lee AC, Katz J, Blencowe H, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–e36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggajo P, Skrunes R, Svarstad E, et al. Familial factors, low birth weight, and development of ESRD: a nationwide registry study. Am J Kidney Dis. 2016;67:601–608. doi: 10.1053/j.ajkd.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Keijzer-Veen MG, Kleinveld HA, Lequin MH, et al. Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis. 2007;50:542–551. doi: 10.1053/j.ajkd.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Bacchetta J, Harambat J, Dubourg L, et al. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 2009;76:445–452. doi: 10.1038/ki.2009.201. [DOI] [PubMed] [Google Scholar]
- 32.Hallan S, Euser AM, Irgens LM, et al. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) Study. Am J Kidney Dis. 2008;51:10–20. doi: 10.1053/j.ajkd.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization WHA Global Nutritional Targets 2025. Low Birth Weight Policy Brief. http://www.who.int/nutrition/topics/globaltargets_lowbirthweight_policybrief.pdf
- 35.Khalsa DD, Beydoun HA, Carmody JB. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol. 2016;31:1509–1516. doi: 10.1007/s00467-016-3384-7. [DOI] [PubMed] [Google Scholar]
- 36.Hoy WE, Hughson MD, Bertram JF, et al. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16:2557–2564. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- 37.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CY, Lin F, Vittinghoff E, et al. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 39.Lackland DT, Bendall HE, Osmond C, et al. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 40.Hoy WE, Rees M, Kile E, et al. Low birthweight and renal disease in Australian aborigines. Lancet. 1998;352:1826–1827. doi: 10.1016/s0140-6736(05)79888-3. [DOI] [PubMed] [Google Scholar]
- 41.Lackland DT, Egan BM, Syddall HE, et al. Associations between birth weight and antihypertensive medication in black and white Medicaid recipients. Hypertension. 2002;39:179–183. doi: 10.1161/hy0102.100545. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Garcia G, Jha V. CKD in disadvantaged populations. Kidney Int. 2015;87:251–253. doi: 10.1038/ki.2014.369. [DOI] [PubMed] [Google Scholar]
- 43.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 44.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. discussion 121. [DOI] [PubMed] [Google Scholar]
- 45.Zeman FJ. Effects of maternal protein restriction on the kidney of the newborn young of rats. J Nutr. 1968;94:111–116. doi: 10.1093/jn/94.2.111. [DOI] [PubMed] [Google Scholar]
- 46.Cheong JN, Wlodek ME, Moritz KM, et al. Programming of maternal and offspring disease: impact of growth restriction, fetal sex and transmission across generations. J Physiol. 2016;594:4727–4740. doi: 10.1113/JP271745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kett MM, Denton KM. Renal programming: cause for concern? Am J Physiol Regul Integr Comp Physiol. 2011;300:R791–R803. doi: 10.1152/ajpregu.00791.2010. [DOI] [PubMed] [Google Scholar]
- 48.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 49.Hayman JM, Johnston SM. Experiments on the relation of creatinine and urea clearance tests of kidney function and the number of glomeruli in the human kidney obtained at autopsy. J Clin Invest. 1933;12:877–884. doi: 10.1172/JCI100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moritz AR, Hayman JM. The disappearance of glomeruli in chronic kidney disease. Am J Pathol. 1934;10:505–518. [PMC free article] [PubMed] [Google Scholar]
- 51.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 52.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 53.Bertram JF, Douglas-Denton RN, Diouf B, et al. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 54.Hughson MD. Low birth weight and kidney function: is there a relationship and is it determined by the intrauterine environment? Am J Kidney Dis. 2007;50:531–534. doi: 10.1053/j.ajkd.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Puelles VG, Hoy WE, Hughson MD, et al. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011;20:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- 56.Faa G, Gerosa C, Fanni D, et al. Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy. J Matern Fetal Neonatal Med. 2010;23(suppl 3):129–133. doi: 10.3109/14767058.2010.510646. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland MR, Gubhaju L, Moore L, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–1374. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez MM, Gomez AH, Abitbol CL, et al. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7:17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 59.Hinchliffe SA, Lynch MR, Sargent PH, et al. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- 60.Hughson M, Farris AB, 3rd, Douglas-Denton R, et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 61.Manalich R, Reyes L, Herrera M, et al. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–773. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert JS, Lang AL, Grant AR, et al. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughson MD, Johnson K, Young RJ, et al. Glomerular size and glomerulosclerosis: relationships to disease categories, glomerular solidification, and ischemic obsolescence. Am J Kidney Dis. 2002;39:679–688. doi: 10.1053/ajkd.2002.31980. [DOI] [PubMed] [Google Scholar]
- 64.Hoy WE, Douglas-Denton RN, Hughson MD, et al. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003;83:S31–S37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 65.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 66.Hoy WE, Bertram JF, Denton RD, et al. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 67.Luyckx VA, Mueller TF. Clinical importance of nephron mass. In: Schrier RW, Neilson E, Molitoris B, Coffman T, Falk R, editors. Schrier's Diseases of the Kidney and Urinary Tract. ed 9. Philadelphia, Lippincott: Williams & Wilkins; 2012. [Google Scholar]
- 68.Zhang Z, Quinlan J, Hoy W, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008;19:2027–2034. doi: 10.1681/ASN.2007101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bueters RR, van de Kar NC, Schreuder MF. Adult renal size is not a suitable marker for nephron numbers: an individual patient data meta-analysis. Kidney Blood Press Res. 2013;37:540–546. doi: 10.1159/000355734. [DOI] [PubMed] [Google Scholar]
- 70.Hoy WE, Hughson MD, Diouf B, et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with physical and clinical characteristics and with ethnic group. Am J Nephrol. 2011;33(suppl 1):15–20. doi: 10.1159/000327044. [DOI] [PubMed] [Google Scholar]
- 71.Baldelomar EJ, Charlton JR, Beeman SC, et al. Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction. Kidney Int. 2016;89:498–505. doi: 10.1038/ki.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beeman SC, Cullen-McEwen LA, Puelles VG, et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol. 2014;306:F1381–F1390. doi: 10.1152/ajprenal.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beeman SC, Mandarino LJ, Georges JF, et al. Cationized ferritin as a magnetic resonance imaging probe to detect microstructural changes in a rat model of non-alcoholic steatohepatitis. Magn Reson Med. 2013;70:1728–1738. doi: 10.1002/mrm.24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charlton JR, Beeman SC, Bennett KM. MRI-detectable nanoparticles: the potential role in the diagnosis of and therapy for chronic kidney disease. Adv Chronic Kidney Dis. 2013;20:479–487. doi: 10.1053/j.ackd.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grijalva-Eternod CS, Lawlor DA, Wells JC. Testing a capacity-load model for hypertension: disentangling early and late growth effects on childhood blood pressure in a prospective birth cohort. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056078. e56078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;55:3114–3127. doi: 10.1007/s00125-012-2689-8. [DOI] [PubMed] [Google Scholar]
- 77.Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 78.Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 79.Simonetti GD, Raio L, Surbek D, et al. Salt sensitivity of children with low birth weight. Hypertension. 2008;52:625–630. doi: 10.1161/HYPERTENSIONAHA.108.114983. [DOI] [PubMed] [Google Scholar]
- 80.de Boer MP, Ijzerman RG, de Jongh RT, et al. Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension. 2008;51:928–932. doi: 10.1161/HYPERTENSIONAHA.107.101881. [DOI] [PubMed] [Google Scholar]
- 81.Perala MM, Moltchanova E, Kaartinen NE, et al. The association between salt intake and adult systolic blood pressure is modified by birth weight. Am J Clin Nutr. 2011;93:422–426. doi: 10.3945/ajcn.2010.30022. [DOI] [PubMed] [Google Scholar]
- 82.Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- 83.Boubred F, Buffat C, Feuerstein JM, et al. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am J Physiol Renal Physiol. 2007;293:F1944–F1949. doi: 10.1152/ajprenal.00141.2007. [DOI] [PubMed] [Google Scholar]
- 84.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 2008;114:1–17. doi: 10.1042/CS20070113. [DOI] [PubMed] [Google Scholar]
- 86.Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schreuder MF, Wilhelm AJ, Bokenkamp A, et al. Impact of gestational age and birth weight on amikacin clearance on day 1 of life. Clin J Am Soc Nephrol. 2009;4:1774–1778. doi: 10.2215/CJN.02230409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frankfurt JA, Duncan AF, Heyne RJ, et al. Renal function and systolic blood pressure in very-low-birth-weight infants 1–3 years of age. Pediatr Nephrol. 2012;27:2285–2291. doi: 10.1007/s00467-012-2265-y. [DOI] [PubMed] [Google Scholar]
- 89.Starzec K, Klimek M, Grudzien A, et al. Longitudinal assessment of renal size and function in extremely low birth weight children at 7 and 11 years of age. Pediatr Nephrol. 2016;31:2119–2126. doi: 10.1007/s00467-016-3413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keijzer-Veen MG, Schrevel M, Finken MJ, et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol. 2005;16:2762–2768. doi: 10.1681/ASN.2004090783. [DOI] [PubMed] [Google Scholar]
- 91.Hsu CW, Yamamoto KT, Henry RK, et al. Prenatal risk factors for childhood CKD. J Am Soc Nephrol. 2014;25:2105–2111. doi: 10.1681/ASN.2013060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abi Khalil C, Travert F, Fetita S, et al. Fetal exposure to maternal type 1 diabetes is associated with renal dysfunction at adult age. Diabetes. 2010;59:2631–2636. doi: 10.2337/db10-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amri K, Freund N, Vilar J, et al. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48:2240–2245. doi: 10.2337/diabetes.48.11.2240. [DOI] [PubMed] [Google Scholar]
- 94.Hokke S, Arias N, Armitage JA, et al. Maternal glucose intolerance reduces offspring nephron endowment and increases glomerular volume in adult offspring. Diabetes Metab Res Rev. 2016;32:816–826. doi: 10.1002/dmrr.2805. [DOI] [PubMed] [Google Scholar]
- 95.Pavkov ME, Hanson RL, Knowler WC, et al. Effect of intrauterine diabetes exposure on the incidence of end-stage renal disease in young adults with type 2 diabetes. Diabetes Care. 2010;33:2396–2398. doi: 10.2337/dc10-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanguru L, Bezawada N, Hussein J, et al. The burden of diabetes mellitus during pregnancy in low- and middle-income countries: a systematic review. Glob Health Action. 2014;7:23987. doi: 10.3402/gha.v7.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cresswell JA, Campbell OM, De Silva MJ, et al. Effect of maternal obesity on neonatal death in sub-Saharan Africa: multivariable analysis of 27 national datasets. Lancet. 2012;380:1325–1330. doi: 10.1016/S0140-6736(12)60869-1. [DOI] [PubMed] [Google Scholar]
- 98.Goldenberg RL, McClure EM, Harrison MS, et al. Diabetes during pregnancy in low- and middle-income countries. Am J Perinatol. 2016;33:1227–1235. doi: 10.1055/s-0036-1584152. [DOI] [PubMed] [Google Scholar]
- 99.Parisi F, Laoreti A, Cetin I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann Nutr Metab. 2014;65:13–21. doi: 10.1159/000365794. [DOI] [PubMed] [Google Scholar]
- 100.Luyckx VA, Goodyer P, Bertram JF. Nephron endowment and developmental programming of blood pressure and renal function. In: Taal MW, Chertow GM, Marsden PA, et al., editors. Brenner and Rector's The Kidney. ed 10. Philadelphia: Elsevier; 2015. [Google Scholar]
- 101.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 102.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 104.Kozuki N, Katz J, Lee AC, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. 2015;145:2542–2550. doi: 10.3945/jn.115.216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42:1340–1355. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodyer P, Kurpad A, Rekha S, et al. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr Nephrol. 2007;22:209–214. doi: 10.1007/s00467-006-0213-4. [DOI] [PubMed] [Google Scholar]
- 107.Bhutta ZA, Das JK, Bahl R, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 108.Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med. 2013;11:42. doi: 10.1186/1741-7015-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nykjaer C, Alwan NA, Greenwood DC, et al. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J Epidemiol Community Health. 2014;68:542–549. doi: 10.1136/jech-2013-202934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cnattingius S, Granath F, Petersson G, et al. The influence of gestational age and smoking habits on the risk of subsequent preterm deliveries. N Engl J Med. 1999;341:943–948. doi: 10.1056/NEJM199909233411303. [DOI] [PubMed] [Google Scholar]
- 111.Al-Odat I, Chen H, Chan YL, et al. The impact of maternal cigarette smoke exposure in a rodent model on renal development in the offspring. PLoS One. 2014;9:e103443. doi: 10.1371/journal.pone.0103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hogberg L, Cnattingius S, Lundholm C, et al. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J Hypertens. 2012;30:693–699. doi: 10.1097/HJH.0b013e32835168f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caputo C, Wood E, Jabbour L. Impact of fetal alcohol exposure on body systems: a systematic review. Birth Defects Res C Embryo Today. 2016;108:174–180. doi: 10.1002/bdrc.21129. [DOI] [PubMed] [Google Scholar]
- 114.Kooijman MN, Bakker H, Franco OH, et al. Fetal smoke exposure and kidney outcomes in school-aged children. Am J Kidney Dis. 2015;66:412–420. doi: 10.1053/j.ajkd.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Med. 2014;12:174. doi: 10.1186/s12916-014-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fang S. Management of preterm infants with intrauterine growth restriction. Early Hum Dev. 2005;81:889–900. doi: 10.1016/j.earlhumdev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Sharma D, Shastri S, Farahbakhsh N, et al. Intrauterine growth restriction – part 1. J Matern Fetal Neonatal Med. 2016;29:3977–3987. doi: 10.3109/14767058.2016.1152249. [DOI] [PubMed] [Google Scholar]
- 118.Walker PG, ter Kuile FO, Garske T, et al. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2:e460–e467. doi: 10.1016/S2214-109X(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 119.Jolving LR, Nielsen J, Kesmodel US, et al. Prevalence of maternal chronic diseases during pregnancy – a nationwide population based study from 1989 to 2013. Acta Obstet Gynecol Scand. 2016;95:1295–1304. doi: 10.1111/aogs.13007. [DOI] [PubMed] [Google Scholar]
- 120.Kersten I, Lange AE, Haas JP, et al. Chronic diseases in pregnant women: prevalence and birth outcomes based on the SNiP-study. BMC Pregnancy Childbirth. 2014;14:75. doi: 10.1186/1471-2393-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hladunewich MA, Melamad N, Bramham K. Pregnancy across the spectrum of chronic kidney disease. Kidney Int. 2016;89:995–1007. doi: 10.1016/j.kint.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 122.Piccoli GB, Cabiddu G, Attini R, et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol. 2015;26:2011–2022. doi: 10.1681/ASN.2014050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bilano VL, Ota E, Ganchimeg T, et al. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS One. 2014;9:e91198. doi: 10.1371/journal.pone.0091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boivin A, Luo ZC, Audibert F, et al. Pregnancy complications among women born preterm. CMAJ. 2012;184:1777–1784. doi: 10.1503/cmaj.120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol. 2008;167:474–479. doi: 10.1093/aje/kwm319. [DOI] [PubMed] [Google Scholar]
- 126.De B, Lin S, Lohsoonthorn V, et al. Risk of preterm delivery in relation to maternal low birth weight. Acta Obstet Gynecol Scand. 2007;86:565–571. doi: 10.1080/00016340701223127. [DOI] [PubMed] [Google Scholar]
- 127.Innes KE, Byers TE, Marshall JA, et al. Association of a woman's own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287:2534–2541. doi: 10.1001/jama.287.19.2534. [DOI] [PubMed] [Google Scholar]
- 128.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 129.Gallo LA, Tran M, Moritz KM, et al. Developmental programming: variations in early growth and adult disease. Clin Exp Pharmacol Physiol. 2013;40:795–802. doi: 10.1111/1440-1681.12092. [DOI] [PubMed] [Google Scholar]
- 130.O'Sullivan L, Combes AN, Moritz KM. Epigenetics and developmental programming of adult onset diseases. Pediatr Nephrol. 2012;27:2175–2182. doi: 10.1007/s00467-012-2108-x. [DOI] [PubMed] [Google Scholar]
- 131.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Annual Transplant Report. 2014. https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf
- 132.Chevalier RL, Kim A, Thornhill BA, et al. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int. 1999;55:793–807. doi: 10.1046/j.1523-1755.1999.055003793.x. [DOI] [PubMed] [Google Scholar]
- 133.Chevalier RL, Thornhill BA, Chang AY. Unilateral ureteral obstruction in neonatal rats leads to renal insufficiency in adulthood. Kidney Int. 2000;58:1987–1995. doi: 10.1111/j.1523-1755.2000.00371.x. [DOI] [PubMed] [Google Scholar]
- 134.Chevalier RL, Thornhill BA, Chang AY, et al. Recovery from release of ureteral obstruction in the rat: relationship to nephrogenesis. Kidney Int. 2002;61:2033–2043. doi: 10.1046/j.1523-1755.2002.00359.x. [DOI] [PubMed] [Google Scholar]
- 135.Wuhl E, van Stralen KJ, Verrina E, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8:67–74. doi: 10.2215/CJN.03310412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney injury. Pediatrics. 2015;136:e463–e473. doi: 10.1542/peds.2014-3819. [DOI] [PubMed] [Google Scholar]
- 137.Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131:1168–1179. doi: 10.1542/peds.2013-0009. [DOI] [PubMed] [Google Scholar]
- 138.Askenazi DJ, Morgan C, Goldstein SL, et al. Strategies to improve the understanding of long-term renal consequences after neonatal acute kidney injury. Pediatr Res. 2016;79:502–508. doi: 10.1038/pr.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Askenazi DJ, Koralkar R, Patil N, et al. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin J Am Soc Nephrol. 2016;11:1527–1535. doi: 10.2215/CJN.13381215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCaffrey J, Dhakal AK, Milford DV, et al. Recent developments in the detection and management of acute kidney injury. Arch Dis Child. 2017;102:91–96. doi: 10.1136/archdischild-2015-309381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rhone ET, Carmody JB, Swanson JR, et al. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. 2014;27:1485–1490. doi: 10.3109/14767058.2013.860522. [DOI] [PubMed] [Google Scholar]
- 142.Jetton JG, Askenazi DJ. Acute kidney injury in the neonate. Clin Perinatol. 2014;41:487–502. doi: 10.1016/j.clp.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 143.Sutherland M, Ryan D, Black MJ, et al. Long-term renal consequences of preterm birth. Clin Perinatol. 2014;41:561–573. doi: 10.1016/j.clp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4:1275–1283. doi: 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
- 145.The Netherlands Perinatal Registry . Perinatal Care in The Netherlands 2005. Utrecht: The Netherlands Perinatal Registry; 2008. [Google Scholar]
- 146.Hanna MH, Askenazi DJ, Selewski DT. Drug-induced acute kidney injury in neonates. Curr Opin Pediatr. 2016;28:180–187. doi: 10.1097/MOP.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Girardi A, Raschi E, Galletti S, et al. Drug-induced renal damage in preterm neonates: state of the art and methods for early detection. Drug Saf. 2015;38:535–551. doi: 10.1007/s40264-015-0288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gilbert T, Gaonach S, Moreau E, et al. Defect of nephrogenesis induced by gentamicin in rat metanephric organ culture. Lab Invest. 1994;70:656–666. [PubMed] [Google Scholar]
- 149.Sutherland MR, Yoder BA, McCurnin D, et al. Effects of ibuprofen treatment on the developing preterm baboon kidney. Am J Physiol Renal Physiol. 2012;302:F1286–F1292. doi: 10.1152/ajprenal.00216.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kent AL, Maxwell LE, Koina ME, et al. Renal glomeruli and tubular injury following indomethacin, ibuprofen, and gentamicin exposure in a neonatal rat model. Pediatr Res. 2007;62:307–312. doi: 10.1203/PDR.0b013e318123f6e3. [DOI] [PubMed] [Google Scholar]
- 151.Vieux R, Fresson J, Guillemin F, et al. Perinatal drug exposure and renal function in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96:F290–F295. doi: 10.1136/adc.2009.197699. [DOI] [PubMed] [Google Scholar]
- 152.Giapros V, Papadimitriou P, Challa A, et al. The effect of intrauterine growth retardation on renal function in the first two months of life. Nephrol Dial Transplant. 2007;22:96–103. doi: 10.1093/ndt/gfl550. [DOI] [PubMed] [Google Scholar]
- 153.Carmody JB, Harer MW, Denotti AR, et al. Caffeine exposure and risk of acute kidney injury in a retrospective cohort of very low birth weight neonates. J Pediatr. 2016;172:63.e1–68.e1. doi: 10.1016/j.jpeds.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 154.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 155.Cuzzolin L, Fanos V, Pinna B, et al. Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol. 2006;21:931–938. doi: 10.1007/s00467-006-0118-2. [DOI] [PubMed] [Google Scholar]
- 156.Magnani C, Moretti S, Ammenti A. Neonatal chronic renal failure associated with maternal ingestion of nimesulide as analgesic. Eur J Obstet Gynecol Reprod Biol. 2004;116:244–245. doi: 10.1016/j.ejogrb.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 157.Cochat P, Decramer S, Robert-Gnansia E, et al. Renal outcome of children exposed to cyclosporine in utero. Transplant Proc. 2004;36:208S–210S. doi: 10.1016/j.transproceed.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 158.Vidal AC, Murphy SK, Murtha AP, et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes (Lond) 2013;37:907–913. doi: 10.1038/ijo.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carmody JB, Swanson JR, Rhone ET, et al. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–2043. doi: 10.2215/CJN.05190514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koralkar R, Ambalavanan N, Levitan EB, et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69:354–358. doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 161.Nishizaki N, Hirano D, Nishizaki Y, et al. Increased urinary angiotensinogen is an effective marker of chronic renal impairment in very low birth weight children. Clin Exp Nephrol. 2014;18:642–648. doi: 10.1007/s10157-013-0896-3. [DOI] [PubMed] [Google Scholar]
- 162.Kwinta P, Klimek M, Drozdz D, et al. Assessment of long-term renal complications in extremely low birth weight children. Pediatr Nephrol. 2011;26:1095–1103. doi: 10.1007/s00467-011-1840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 164.Bruel A, Roze JC, Quere MP, et al. Renal outcome in children born preterm with neonatal acute renal failure: IRENEO – a prospective controlled study. Pediatr Nephrol. 2016;31:2365–2373. doi: 10.1007/s00467-016-3444-z. [DOI] [PubMed] [Google Scholar]
- 165.Chaturvedi S, Ng KH, Mammen C. The path to chronic kidney disease following acute kidney injury: a neonatal perspective. Pediatr Nephrol. 2017;32:227–241. doi: 10.1007/s00467-015-3298-9. [DOI] [PubMed] [Google Scholar]
- 166.Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011;94:1754S–1758S. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- 167.Parkinson JR, Hyde MJ, Gale C, et al. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131:e1240–e1263. doi: 10.1542/peds.2012-2177. [DOI] [PubMed] [Google Scholar]
- 168.D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. QJ Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 169.Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–2403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- 170.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis. 1988;12:340–347. doi: 10.1016/s0272-6386(88)80021-0. [DOI] [PubMed] [Google Scholar]
- 171.Tsuboi N, Kawamura T, Koike K, et al. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:39–44. doi: 10.2215/CJN.04680709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zidar N, Cavic MA, Kenda RB, et al. Effect of intrauterine growth retardation on the clinical course and prognosis of IgA glomerulonephritis in children. Nephron. 1998;79:28–32. doi: 10.1159/000044987. [DOI] [PubMed] [Google Scholar]
- 173.Ruggajo P, Svarstad E, Leh S, et al. Low birth weight and risk of progression to end stage renal disease in IgA nephropathy – a retrospective registry-based cohort study. PLoS One. 2016;11:e0153819. doi: 10.1371/journal.pone.0153819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Teeninga N, Schreuder MF, Bokenkamp A, et al. Influence of low birth weight on minimal change nephrotic syndrome in children, including a meta-analysis. Nephrol Dial Transplant. 2008;23:1615–1620. doi: 10.1093/ndt/gfm829. [DOI] [PubMed] [Google Scholar]
- 175.Zidar N, Avgustin Cavic M, Kenda RB, et al. Unfavorable course of minimal change nephrotic syndrome in children with intrauterine growth retardation. Kidney Int. 1998;54:1320–1323. doi: 10.1046/j.1523-1755.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 176.Plank C, Ostreicher I, Hartner A, et al. Intrauterine growth retardation aggravates the course of acute mesangioproliferative glomerulonephritis in the rat. Kidney Int. 2006;70:1974–1982. doi: 10.1038/sj.ki.5001966. [DOI] [PubMed] [Google Scholar]
- 177.Sheu JN, Chen JH. Minimal change nephrotic syndrome in children with intrauterine growth retardation. Am J Kidney Dis. 2001;37:909–914. doi: 10.1016/s0272-6386(05)80005-8. [DOI] [PubMed] [Google Scholar]
- 178.Orskov B, Christensen KB, Feldt-Rasmussen B, et al. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney Int. 2012;81:919–924. doi: 10.1038/ki.2011.459. [DOI] [PubMed] [Google Scholar]
- 179.Garrett P, Sandeman D, Reza M, et al. Weight at birth and renal disease in adulthood. Nephrol Dial Transplant. 1993;8:920. [Google Scholar]
- 180.Hodgin JB, Rasoulpour M, Markowitz GS, et al. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:71–76. doi: 10.2215/CJN.01700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Duncan RC, Bass PS, Garrett PJ, et al. Weight at birth and other factors influencing progression of idiopathic membranous nephropathy. Nephrol Dial Transplant. 1994;9:875. [PubMed] [Google Scholar]
- 182.Rajan T, Barbour SJ, White CT, et al. Low birth weight and nephron mass and their role in the progression of chronic kidney disease: a case report on identical twins with Alport disease. Nephrol Dial Transplant. 2011;26:4136–4139. doi: 10.1093/ndt/gfr252. [DOI] [PubMed] [Google Scholar]
- 183.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 184.Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 185.Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75:1079–1087. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hollenberg NK, Rivera A, Meinking T, et al. Age, renal perfusion and function in island-dwelling indigenous Kuna Amerinds of Panama. Nephron. 1999;82:131–138. doi: 10.1159/000045389. [DOI] [PubMed] [Google Scholar]
- 187.Szabo AJ, Muller V, Chen GF, et al. Nephron number determines susceptibility to renal mass reduction-induced CKD in Lewis and Fisher 344 rats: implications for development of experimentally induced chronic allograft nephropathy. Nephrol Dial Transplant. 2008;23:2492–2495. doi: 10.1093/ndt/gfn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Veuthey T, Hoffmann D, Vaidya VS, et al. Impaired renal function and development in Belgrade rats. Am J Physiol Renal Physiol. 2014;306:F333–F343. doi: 10.1152/ajprenal.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luyckx VA, Compston CA, Simmen T, et al. Accelerated senescence in kidneys of low-birth-weight rats after catch-up growth. Am J Physiol Renal Physiol. 2009;297:F1697–F1705. doi: 10.1152/ajprenal.00462.2009. [DOI] [PubMed] [Google Scholar]
- 190.Tarry-Adkins JL, Ozanne SE, Norden A, et al. Lower antioxidant capacity and elevated p53 and p21 may be a link between gender disparity in renal telomere shortening, albuminuria, and longevity. Am J Physiol Renal Physiol. 2006;290:F509–F516. doi: 10.1152/ajprenal.00215.2005. [DOI] [PubMed] [Google Scholar]
- 191.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 192.Nagai K, Saito C, Yamagata K. Birth weight and end-stage diabetic nephropathy in later life: a Japanese multicenter study. Ther Apher Dial. 2014;18:111–112. doi: 10.1111/1744-9987.12159. [DOI] [PubMed] [Google Scholar]
- 193.Mueller TF, Luyckx VA. The natural history of residual renal function in transplant donors. J Am Soc Nephrol. 2012;23:1462–1466. doi: 10.1681/ASN.2011111080. [DOI] [PubMed] [Google Scholar]
- 194.Giral M, Foucher Y, Karam G, et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol. 2010;21:1022–1029. doi: 10.1681/ASN.2009121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Seun Kim Y, Soo Kim M, Suk Han D, et al. Evidence that the ratio of donor kidney weight to recipient body weight, donor age, and episodes of acute rejection correlate independently with live-donor graft function. Transplantation. 2002;74:280–283. doi: 10.1097/00007890-200207270-00021. [DOI] [PubMed] [Google Scholar]
- 196.Brenner BM, Cohen RA, Milford EL. In renal transplantation, one size may not fit all. J Am Soc Nephrol. 1992;3:162–169. doi: 10.1681/ASN.V32162. [DOI] [PubMed] [Google Scholar]
- 197.el-Agroudy AE, Hassan NA, Bakr MA, et al. Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am J Nephrol. 2003;23:294–299. doi: 10.1159/000072819. [DOI] [PubMed] [Google Scholar]
- 198.Gaston RS, Hudson SL, Julian BA, et al. Impact of donor/recipient size matching on outcomes in renal transplantation. Transplantation. 1996;61:383–388. doi: 10.1097/00007890-199602150-00010. [DOI] [PubMed] [Google Scholar]
- 199.Lee JH, Won JH, Oh CK. Impact of the ratio of graft kidney volume to recipient body surface area on graft function after live donor kidney transplantation. Clin Transplant. 2011;25:E647–E655. doi: 10.1111/j.1399-0012.2011.01502.x. [DOI] [PubMed] [Google Scholar]
- 200.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rogers NM, Lawton PD, Jose MD. Indigenous Australians and living kidney donation. N Engl J Med. 2009;361:1513–1516. doi: 10.1056/NEJMc0905777. [DOI] [PubMed] [Google Scholar]
- 202.Storsley LJ, Young A, Rush DN, et al. Long-term medical outcomes among Aboriginal living kidney donors. Transplantation. 2010;90:401–406. doi: 10.1097/TP.0b013e3181e6e79b. [DOI] [PubMed] [Google Scholar]
- 203.Schachtner T, Reinke P. Estimated nephron number of the remaining donor kidney: impact on living kidney donor outcomes. Nephrol Dial Transplant. 2016;31:1523–1530. doi: 10.1093/ndt/gfv458. [DOI] [PubMed] [Google Scholar]
- 204.Vikse BE, Irgens LM, Leivestad T, et al. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 205.Wang IK, Muo CH, Chang YC, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;185:207–213. doi: 10.1503/cmaj.120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lackland DT. Mechanisms and fetal origins of kidney disease. J Am Soc Nephrol. 2005;16:2531–2532. doi: 10.1681/ASN.2005070740. [DOI] [PubMed] [Google Scholar]
- 207.Uauy R, Kain J, Corvalan C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries? Am J Clin Nutr. 2011;94:1759S–1764S. doi: 10.3945/ajcn.110.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. 2012;61(suppl 1):8–17. doi: 10.1159/000345165. [DOI] [PubMed] [Google Scholar]
- 209.Chang HH, Larson J, Blencowe H, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–445. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 211.Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 1. Changes in albuminuria and glomerular filtration rate over time. Kidney Int. 2001;60:243–248. doi: 10.1046/j.1523-1755.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- 212.Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 2. Albuminuria predicts natural death and renal failure. Kidney Int. 2001;60:249–256. doi: 10.1046/j.1523-1755.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 213.Fall CH. Fetal programming and the risk of noncommunicable disease. Indian J Pediatr. 2013;80(suppl 1):S13–S20. doi: 10.1007/s12098-012-0834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hemachandra AH, Klebanoff MA, Furth SL. Racial disparities in the association between birth weight in the term infant and blood pressure at age 7 years: results from the collaborative perinatal project. J Am Soc Nephrol. 2006;17:2576–2581. doi: 10.1681/ASN.2005090898. [DOI] [PubMed] [Google Scholar]
- 215.Cassidy-Bushrow AE, Wegienka G, Barone CJ, 2nd, et al. Race-specific relationship of birth weight and renal function among healthy young children. Pediatr Nephrol. 2012;27:1317–1323. doi: 10.1007/s00467-012-2136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hult M, Tornhammar P, Ueda P, et al. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS One. 2010;5:e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stein AD, Zybert PA, van der Pal-de Bruin K, et al. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur J Epidemiol. 2006;21:759–765. doi: 10.1007/s10654-006-9065-2. [DOI] [PubMed] [Google Scholar]
- 218.Painter RC, Roseboom TJ, van Montfrans GA, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol. 2005;16:189–194. doi: 10.1681/ASN.2004060474. [DOI] [PubMed] [Google Scholar]
- 219.Tennant IA, Barnett AT, Thompson DS, et al. Impaired cardiovascular structure and function in adult survivors of severe acute malnutrition. Hypertension. 2014;64:664–671. doi: 10.1161/HYPERTENSIONAHA.114.03230. [DOI] [PubMed] [Google Scholar]
- 220.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ulasi, Tzur S, Wasser WG, et al. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract. 2013;123:123–128. doi: 10.1159/000353223. [DOI] [PubMed] [Google Scholar]
- 223.Martin JA, Osterman MJ, Kirmeyer SE, et al. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep. 2015;64:1–20. [PubMed] [Google Scholar]
- 224.McNamara BJ, Diouf B, Douglas-Denton RN, et al. A comparison of nephron number, glomerular volume and kidney weight in Senegalese Africans and African Americans. Nephrol Dial Transplant. 2010;25:1514–1520. doi: 10.1093/ndt/gfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Douglas-Denton RN, McNamara BJ, Hoy WE, et al. Does nephron number matter in the development of kidney disease? Ethn Dis. 2006;16((2 suppl 2)):S2-40–S2-45. [PubMed] [Google Scholar]
- 226.Hoy WE, Hughson MD, Kopp JB, et al. APOL1 risk alleles are associated with exaggerated age-related changes in glomerular number and volume in African-American adults: an autopsy study. J Am Soc Nephrol. 2015;26:3179–3189. doi: 10.1681/ASN.2014080768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jain V, Singhal A. Catch up growth in low birth weight infants: striking a healthy balance. Rev Endocr Metab Disord. 2012;13:141–147. doi: 10.1007/s11154-012-9216-6. [DOI] [PubMed] [Google Scholar]
- 228.Wlodek ME, Mibus A, Tan A, et al. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol. 2007;18:1688–1696. doi: 10.1681/ASN.2007010015. [DOI] [PubMed] [Google Scholar]
- 229.Drake KA, Sauerbry MJ, Blohowiak SE, et al. Iron deficiency and renal development in the newborn rat. Pediatr Res. 2009;66:619–624. doi: 10.1203/PDR.0b013e3181be79c2. [DOI] [PubMed] [Google Scholar]
- 230.Merlet-Benichou C, Vilar J, Lelievre-Pegorier M, et al. Role of retinoids in renal development: pathophysiological implication. Curr Opin Nephrol Hypertens. 1999;8:39–43. doi: 10.1097/00041552-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 231.Andersen LG, Angquist L, Eriksson JG, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5:e14126. doi: 10.1371/journal.pone.0014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Barker DJ, Osmond C, Forsen TJ, et al. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 233.Fall CH, Sachdev HS, Osmond C, et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: data from the New Delhi Birth Cohort. Diabetes Care. 2008;31:2349–2356. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bansal N, Ayoola OO, Gemmell I, et al. Effects of early growth on blood pressure of infants of British European and South Asian origin at one year of age: the Manchester children's growth and vascular health study. J Hypertens. 2008;26:412–418. doi: 10.1097/HJH.0b013e3282f3168e. [DOI] [PubMed] [Google Scholar]
- 235.Vohr BR, Allan W, Katz KH, et al. Early predictors of hypertension in prematurely born adolescents. Acta Paediatr. 2010;99:1812–1818. doi: 10.1111/j.1651-2227.2010.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern Child Nutr. 2013;9(suppl 1):105–119. doi: 10.1111/mcn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Skelton JA, Irby MB, Grzywacz JG, et al. Etiologies of obesity in children: nature and nurture. Pediatr Clin North Am. 2011;58:1333–1354. doi: 10.1016/j.pcl.2011.09.006. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Abitbol CL, Chandar J, Rodriguez MM, et al. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr Nephrol. 2009;24:1363–1370. doi: 10.1007/s00467-009-1120-2. [DOI] [PubMed] [Google Scholar]
- 241.Abitbol CL, Bauer CR, Montane B, et al. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol. 2003;18:887–893. doi: 10.1007/s00467-003-1186-1. [DOI] [PubMed] [Google Scholar]
- 242.Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172:1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Laaksonen DE, Lakka HM, Lynch J, et al. Cardiorespiratory fitness and vigorous leisure-time physical activity modify the association of small size at birth with the metabolic syndrome. Diabetes Care. 2003;26:2156–2164. doi: 10.2337/diacare.26.7.2156. [DOI] [PubMed] [Google Scholar]
- 244.Siebel AL, Carey AL, Kingwell BA. Can exercise training rescue the adverse cardiometabolic effects of low birth weight and prematurity? Clin Exp Pharmacol Physiol. 2012;39:944–957. doi: 10.1111/j.1440-1681.2012.05732.x. [DOI] [PubMed] [Google Scholar]
- 245.Bergvall N, Iliadou A, Johansson S, et al. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: a study among Swedish twins. Circulation. 2007;115:2931–2938. doi: 10.1161/CIRCULATIONAHA.106.674812. [DOI] [PubMed] [Google Scholar]
- 246.Hubinette A, Cnattingius S, Ekbom A, et al. Birthweight, early environment, and genetics: a study of twins discordant for acute myocardial infarction. Lancet. 2001;357:1997–2001. doi: 10.1016/S0140-6736(00)05111-4. [DOI] [PubMed] [Google Scholar]
- 247.Lei HH, Perneger TV, Klag MJ, et al. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9:1270–1276. doi: 10.1681/ASN.V971270. [DOI] [PubMed] [Google Scholar]
- 248.Giapros V, Drougia A, Hotoura E, et al. Kidney growth in twin children born small for gestational age. Nephrol Dial Transplant. 2010;25:3548–3554. doi: 10.1093/ndt/gfq261. [DOI] [PubMed] [Google Scholar]
- 249.Roseboom TJ, van der Meulen JH, Ravelli AC, et al. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17:325–330. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
- 250.Kwong WY, Wild AE, Roberts P, et al. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 251.La Batide-Alanore A, Tregouet DA, Jaquet D, et al. Familial aggregation of fetal growth restriction in a French cohort of 7,822 term births between 1971 and 1985. Am J Epidemiol. 2002;156:180–187. doi: 10.1093/aje/kwf002. [DOI] [PubMed] [Google Scholar]
- 252.Wang X, Zuckerman B, Coffman GA, et al. Familial aggregation of low birth weight among whites and blacks in the United States. N Engl J Med. 1995;333:1744–1749. doi: 10.1056/NEJM199512283332606. [DOI] [PubMed] [Google Scholar]
- 253.ten Kate LP, Boman H, Daiger SP, et al. Familial aggregation of coronary heart disease and its relation to known genetic risk factors. Am J Cardiol. 1982;50:945–953. doi: 10.1016/0002-9149(82)90400-3. [DOI] [PubMed] [Google Scholar]
- 254.Knuiman MW, Divitini ML, Welborn TA, et al. Familial correlations, cohabitation effects, and heritability for cardiovascular risk factors. Ann Epidemiol. 1996;6:188–194. doi: 10.1016/1047-2797(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 255.Satko SG, Sedor JR, Iyengar SK, et al. Familial clustering of chronic kidney disease. Semin Dial. 2007;20:229–236. doi: 10.1111/j.1525-139X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 256.McNeill G, Tuya C, Smith WC. The role of genetic and environmental factors in the association between birthweight and blood pressure: evidence from meta-analysis of twin studies. Int J Epidemiol. 2004;33:995–1001. doi: 10.1093/ije/dyh260. [DOI] [PubMed] [Google Scholar]
- 257.Kramer MS. Invited commentary: association between restricted fetal growth and adult chronic disease: is it causal? Is it important? Am J Epidemiol. 2000;152:605–608. doi: 10.1093/aje/152.7.605. [DOI] [PubMed] [Google Scholar]
- 258.Myklestad K, Vatten LJ, Magnussen EB, et al. Offspring birth weight and cardiovascular risk in parents: a population-based HUNT 2 study. Am J Epidemiol. 2012;175:546–555. doi: 10.1093/aje/kwr347. [DOI] [PubMed] [Google Scholar]
- 259.Gielen M, Pinto-Sietsma SJ, Zeegers MP, et al. Birth weight and creatinine clearance in young adult twins: influence of genetic, prenatal, and maternal factors. J Am Soc Nephrol. 2005;16:2471–2476. doi: 10.1681/ASN.2004030210. [DOI] [PubMed] [Google Scholar]
- 260.Mumford SL, Michels KA, Salaria N, et al. Preconception care: it's never too early. Reprod Health. 2014;11:73. doi: 10.1186/1742-4755-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Steegers-Theunissen RP, Steegers EA. Embryonic health: new insights, mHealth and personalised patient care. Reprod Fertil Dev. 2015;27:712–715. doi: 10.1071/RD14386. [DOI] [PubMed] [Google Scholar]
- 262.Jaddoe VW, de Jonge LL, Hofman A, et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dean SV, Lassi ZS, Imam AM, et al. Preconception care: nutritional risks and interventions. Reprod Health. 2014;11(suppl 3):S3. doi: 10.1186/1742-4755-11-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dean SV, Lassi ZS, Imam AM, et al. Preconception care: closing the gap in the continuum of care to accelerate improvements in maternal, newborn and child health. Reprod Health. 2014;11(suppl 3):S1. doi: 10.1186/1742-4755-11-S3-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lassi ZS, Dean SV, Mallick D, et al. Preconception care: delivery strategies and packages for care. Reprod Health. 2014;11(suppl 3):S7. doi: 10.1186/1742-4755-11-S3-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ferrero DM, Larson J, Jacobsson B, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS One. 2016;11:e0162506. doi: 10.1371/journal.pone.0162506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ota E, Ganchimeg T, Morisaki N, et al. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: secondary analyses of the WHO Multi-Country Survey on Maternal and Newborn Health. PLoS One. 2014;9:e105155. doi: 10.1371/journal.pone.0105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lassi ZS, Imam AM, Dean SV, et al. Preconception care: screening and management of chronic disease and promoting psychological health. Reprod Health. 2014;11(suppl 3):S5. doi: 10.1186/1742-4755-11-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Boafor TK, Olayemi E, Galadanci N, et al. Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis. BJOG. 2016;123:691–698. doi: 10.1111/1471-0528.13786. [DOI] [PubMed] [Google Scholar]
- 271.von Ehr J, von Versen-Hoynck F. Implications of maternal conditions and pregnancy course on offspring's medical problems in adult life. Arch Gynecol Obstet. 2016;294:673–679. doi: 10.1007/s00404-016-4178-7. [DOI] [PubMed] [Google Scholar]
- 272.Dean SV, Lassi ZS, Imam AM, et al. Preconception care: promoting reproductive planning. Reprod Health. 2014;11(suppl 3):S2. doi: 10.1186/1742-4755-11-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ganchimeg T, Ota E, Morisaki N, et al. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG. 2014;121((suppl 1)):40–48. doi: 10.1111/1471-0528.12630. [DOI] [PubMed] [Google Scholar]
- 274.Fall CH, Sachdev HS, Osmond C, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low- income and middle-income countries (COHORTS collaboration) Lancet Glob Health. 2015;3:e366–e377. doi: 10.1016/S2214-109X(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Barker D, Barker M, Fleming T, et al. Developmental biology: support mothers to secure future public health. Nature. 2013;504:209–211. doi: 10.1038/504209a. [DOI] [PubMed] [Google Scholar]
- 276.Hanson M, Barker M, Dodd JM, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5:65–76. doi: 10.1016/S2213-8587(16)30108-5. [DOI] [PubMed] [Google Scholar]
- 277.Feldman AZ, Brown FM. Management of type 1 diabetes in pregnancy. Curr Diab Rep. 2016;16:76. doi: 10.1007/s11892-016-0765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 279.American Diabetes Association 12. Management of diabetes in pregnancy. Diabetes Care. 2015;38(suppl):S77–S79. doi: 10.2337/dc15-S015. [DOI] [PubMed] [Google Scholar]
- 280.Gillon TE, Pels A, von Dadelszen P, et al. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One. 2014;9:e113715. doi: 10.1371/journal.pone.0113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 282.Sijpkens MK, Steegers EA, Rosman AN. Facilitators and barriers for successful implementation of interconception care in preventive child health care services in the Netherlands. Matern Child Health J. 2016;20:117–124. doi: 10.1007/s10995-016-2046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bogaerts A, Van den Bergh BR, Ameye L, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol. 2013;122:999–1009. doi: 10.1097/AOG.0b013e3182a7f63e. [DOI] [PubMed] [Google Scholar]
- 284.Steegers EA, Barker ME, Steegers-Theunissen RP, et al. Societal valorisation of new knowledge to improve perinatal health: time to act. Paediatr Perinat Epidemiol. 2016;30:201–204. doi: 10.1111/ppe.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Okun N, Sierra S. Pregnancy outcomes after assisted human reproduction. J Obstet Gynaecol Can. 2014;36:64–83. doi: 10.1016/S1701-2163(15)30685-X. [DOI] [PubMed] [Google Scholar]
- 286.Thomson F, Shanbhag S, Templeton A, et al. Obstetric outcome in women with subfertility. BJOG. 2005;112:632–637. doi: 10.1111/j.1471-0528.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 287.Declercq E, Luke B, Belanoff C, et al. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART) Fertil Steril. 2015;103:888–895. doi: 10.1016/j.fertnstert.2014.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Newnham JP, Dickinson JE, Hart RJ, et al. Strategies to prevent preterm birth. Front Immunol. 2014;5:584. doi: 10.3389/fimmu.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Avnon T, Haham A, Many A. Twin pregnancy in women above the age of 45 years: maternal and neonatal outcomes. J Perinat Med. doi: 10.1515/jpm-2016-0196. DOI: 10.1515/jpm-2016-0196. [DOI] [PubMed] [Google Scholar]
- 290.Turkgeldi E, Yagmur H, Seyhan A, et al. Short and long term outcomes of children conceived with assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol. 2016;207:129–136. doi: 10.1016/j.ejogrb.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 291.Pereira N, Cozzubbo T, Cheung S, et al. Identifying maternal constraints on fetal growth and subsequent perinatal outcomes using a multiple embryo implantation model. PLoS One. 2016;11:e0166222. doi: 10.1371/journal.pone.0166222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Calhoun KC, Barnhart KT, Elovitz MA, et al. Evaluating the association between assisted conception and the severity of preeclampsia. ISRN Obstet Gynecol. 2011;2011:928592. doi: 10.5402/2011/928592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Silberstein T, Levy A, Harlev A, et al. Perinatal outcome of pregnancies following in vitro fertilization and ovulation induction. J Matern Fetal Neonatal Med. 2014;27:1316–1319. doi: 10.3109/14767058.2013.856415. [DOI] [PubMed] [Google Scholar]
- 294.Watanabe N, Fujiwara T, Suzuki T, et al. Is in vitro fertilization associated with preeclampsia? A propensity score matched study. BMC Pregnancy Childbirth. 2014;14:69. doi: 10.1186/1471-2393-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Blazquez A, Garcia D, Rodriguez A, et al. Is oocyte donation a risk factor for preeclampsia? A systematic review and meta-analysis. J Assist Reprod Genet. 2016;33:855–863. doi: 10.1007/s10815-016-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103:1136–1143. doi: 10.1016/j.fertnstert.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 297.Schoen C, Rosen T. Maternal and perinatal risks for women over 44 – a review. Maturitas. 2009;64:109–113. doi: 10.1016/j.maturitas.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 298.Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, et al. Pregnancy at very advanced maternal age: a UK population-based cohort study. BJOG. doi: 10.1111/1471-0528.14269. DOI: 10.1111/1471-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 300.Mitanchez D, Yzydorczyk C, Siddeek B, et al. The offspring of the diabetic mother – short- and long-term implications. Best Pract Res Clin Obstet Gynaecol. 2015;29:256–269. doi: 10.1016/j.bpobgyn.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 301.Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173–S211. doi: 10.1016/S0020-7292(15)30033-3. [DOI] [PubMed] [Google Scholar]
- 302.Linne Y, Rossner S. Interrelationships between weight development and weight retention in subsequent pregnancies: the SPAWN study. Acta Obstet Gynecol Scand. 2003;82:318–325. doi: 10.1080/j.1600-0412.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 303.Nehring I, Schmoll S, Beyerlein A, et al. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94:1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 304.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 305.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Martens PJ, Shafer LA, Dean HJ, et al. Breastfeeding initiation associated with reduced incidence of diabetes in mothers and offspring. Obstet Gynecol. 2016;128:1095–1104. doi: 10.1097/AOG.0000000000001689. [DOI] [PubMed] [Google Scholar]
- 307.Committee on Practice Bulletins – Obstetrics Practice Bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122:406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 308.Tita AT, Landon MB, Spong CY, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111–120. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Coustan DR, Lowe LP, Metzger BE, et al. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202:654.e1–e6. doi: 10.1016/j.ajog.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.World Health Organization WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. apps.who.int/iris/bitstream/10665/250796/1/9789241549912-eng.pdf [PubMed]
- 311.Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: re-examining the guidelines. Washington: The National Academies Press; 2009. Institute of Medicine and National Research Council. http://www.nationalacademies.org/hmd/Reports/2009/Weight-Gain-During-Pregnancy-Reexaminingthe-Guidelines.aspx (accessed September 29, 2016). [PubMed] [Google Scholar]
- 312.Cetin I, Berti C, Calabrese S. Role of micronutrients in the periconceptional period. Hum Reprod Update. 2010;16:80–95. doi: 10.1093/humupd/dmp025. [DOI] [PubMed] [Google Scholar]
- 313.Yousafzai AK, Rasheed MA, Rizvi A, et al. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. Lancet. 2014;384:1282–1293. doi: 10.1016/S0140-6736(14)60455-4. [DOI] [PubMed] [Google Scholar]
- 314.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD004905.pub4. CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hillesund ER, Bere E, Haugen M, et al. Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth – a study performed in the Norwegian Mother and Child Cohort Study (MoBa) Public Health Nutr. 2014;17:1909–1918. doi: 10.1017/S1368980014000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Timmermans S, Steegers-Theunissen RP, Vujkovic M, et al. The Mediterranean diet and fetal size parameters: the Generation R Study. Br J Nutr. 2012;108:1399–1409. doi: 10.1017/S000711451100691X. [DOI] [PubMed] [Google Scholar]
- 318.Wen LM, Simpson JM, Rissel C, et al. Maternal “junk food” diet during pregnancy as a predictor of high birthweight: findings from the healthy beginnings trial. Birth. 2013;40:46–51. doi: 10.1111/birt.12028. [DOI] [PubMed] [Google Scholar]
- 319.Mikkelsen TB, Osterdal ML, Knudsen VK, et al. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87:325–330. doi: 10.1080/00016340801899347. [DOI] [PubMed] [Google Scholar]
- 320.Haugen M, Meltzer HM, Brantsaeter AL, et al. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87:319–324. doi: 10.1080/00016340801899123. [DOI] [PubMed] [Google Scholar]
- 321.Leventakou V, Roumeliotaki T, Martinez D, et al. Fish intake during pregnancy, fetal growth, and gestational length in 19 European birth cohort studies. Am J Clin Nutr. 2014;99:506–516. doi: 10.3945/ajcn.113.067421. [DOI] [PubMed] [Google Scholar]
- 322.Kinra S, Rameshwar Sarma KV, Ghafoorunissa, et al. Effect of integration of supplemental nutrition with public health programmes in pregnancy and early childhood on cardiovascular risk in rural Indian adolescents: long term follow-up of Hyderabad nutrition trial. BMJ. 2008;337:a605. doi: 10.1136/bmj.a605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hawkesworth S. Conference on “Multidisciplinary approaches to nutritional problems”. Postgraduate Symposium. Exploiting dietary supplementation trials to assess the impact of the prenatal environment on CVD risk. Proc Nutr Soc. 2009;68:78–88. doi: 10.1017/S0029665108008781. [DOI] [PubMed] [Google Scholar]
- 324.Hawkesworth S, Sawo Y, Fulford AJ, et al. Effect of maternal calcium supplementation on offspring blood pressure in 5- to 10-y-old rural Gambian children. Am J Clin Nutr. 2010;92:741–747. doi: 10.3945/ajcn.2010.29475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Stewart MJ, Kushner KE, Greaves L, et al. Impacts of a support intervention for low-income women who smoke. Soc Sci Med. 2010;71:1901–1909. doi: 10.1016/j.socscimed.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 326.Bergel E, Barros AJ. Effect of maternal calcium intake during pregnancy on children's blood pressure: a systematic review of the literature. BMC Pediatr. 2007;7:15. doi: 10.1186/1471-2431-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Jamshidi F, Kelishadi R. A systematic review on the effects of maternal calcium supplementation on offspring's blood pressure. J Res Med Sci. 2015;20:994–999. doi: 10.4103/1735-1995.172794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Devakumar D, Fall CH, Sachdev HS, et al. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. BMC Med. 2016;14:90. doi: 10.1186/s12916-016-0633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mispireta ML, Caulfield LE, Zavaleta N, et al. Effect of maternal zinc supplementation on the cardiometabolic profile of Peruvian children: results from a randomized clinical trial. J Dev Orig Health Dis. 2017;8:56–64. doi: 10.1017/S2040174416000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ruel MT, Alderman H. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382:536–551. doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- 331.Van Dijk MR, Huijgen NA, Willemsen SP, et al. Impact of an mHealth platform for pregnancy on nutrition and lifestyle of the reproductive population: a survey. JMIR Mhealth Uhealth. 2016;4:e53. doi: 10.2196/mhealth.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cetin I, Mando C, Calabrese S. Maternal predictors of intrauterine growth restriction. Curr Opin Clin Nutr Metab Care. 2013;16:310–319. doi: 10.1097/MCO.0b013e32835e8d9c. [DOI] [PubMed] [Google Scholar]
- 333.Stangenberg S, Chen H, Wong MG, et al. Fetal programming of chronic kidney disease: the role of maternal smoking, mitochondrial dysfunction, and epigenetic modification. Am J Physiol Renal Physiol. 2015;308:F1189–F1196. doi: 10.1152/ajprenal.00638.2014. [DOI] [PubMed] [Google Scholar]
- 334.Bay B, Kesmodel US. Prenatal alcohol exposure – a systematic review of the effects on child motor function. Acta Obstet Gynecol Scand. 2011;90:210–226. doi: 10.1111/j.1600-0412.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 335.Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- 336.Henderson J, Kesmodel U, Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J Epidemiol Community Health. 2007;61:1069–1073. doi: 10.1136/jech.2006.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kleiber ML, Diehl EJ, Laufer BI, et al. Long-term genomic and epigenomic dysregulation as a consequence of prenatal alcohol exposure: a model for fetal alcohol spectrum disorders. Front Genet. 2014;5:161. doi: 10.3389/fgene.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Mandal C, Halder D, Chai JC, et al. Profiling ethanol-targeted transcription factors in human carcinoma cell-derived embryoid bodies. Gene. 2016;576:119–125. doi: 10.1016/j.gene.2015.09.085. [DOI] [PubMed] [Google Scholar]
- 339.Gray R. Low-to-moderate alcohol consumption during pregnancy and child development – moving beyond observational studies. BJOG. 2013;120:1039–1041. doi: 10.1111/1471-0528.12211. [DOI] [PubMed] [Google Scholar]
- 340.Lundsberg LS, Bracken MB, Saftlas AF. Low-to-moderate gestational alcohol use and intrauterine growth retardation, low birthweight, and preterm delivery. Ann Epidemiol. 1997;7:498–508. doi: 10.1016/s1047-2797(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 341.Ornoy A, Ergaz Z. Alcohol abuse in pregnant women: effects on the fetus and newborn, mode of action and maternal treatment. Int J Environ Res Public Health. 2010;7:364–379. doi: 10.3390/ijerph7020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Patra J, Bakker R, Irving H, et al. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA) – a systematic review and meta-analyses. BJOG. 2011;118:1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gray SP, Denton KM, Cullen-McEwen L, et al. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Nephrol. 2010;21:1891–1902. doi: 10.1681/ASN.2010040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Blanco-Munoz J, Torres-Sanchez L, Lopez-Carrillo L. Exposure to maternal and paternal tobacco consumption and risk of spontaneous abortion. Public Health Rep. 2009;124:317–322. doi: 10.1177/003335490912400220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Secker-Walker RH, Vacek PM, Flynn BS, et al. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet Gynecol. 1997;89:648–653. doi: 10.1016/s0029-7844(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 346.Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83:699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 347.Ion R, Bernal AL. Smoking and preterm birth. Reprod Sci. 2015;22:918–926. doi: 10.1177/1933719114556486. [DOI] [PubMed] [Google Scholar]
- 348.Caleyachetty R, Tait CA, Kengne AP, et al. Tobacco use in pregnant women: analysis of data from demographic and health surveys from 54 low-income and middle-income countries. Lancet Glob Health. 2014;2:e513–e520. doi: 10.1016/S2214-109X(14)70283-9. [DOI] [PubMed] [Google Scholar]
- 349.Levy D, Mohlman MK, Zhang Y. Estimating the potential impact of tobacco control policies on adverse maternal and child health outcomes in the United States using the SimSmoke Tobacco Control Policy Simulation Model. Nicotine Tob Res. 2016;18:1240–1249. doi: 10.1093/ntr/ntv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Stangenberg S, Nguyen LT, Chen H, et al. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int J Biochem Cell Biol. 2015;64:81–90. doi: 10.1016/j.biocel.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 351.Block DB, Mesquita FF, de Lima IP, et al. Fetal kidney programming by maternal smoking exposure: effects on kidney structure, blood pressure and urinary sodium excretion in adult offspring. Nephron. 2015;129:283–292. doi: 10.1159/000377634. [DOI] [PubMed] [Google Scholar]
- 352.Taal HR, Geelhoed JJ, Steegers EA, et al. Maternal smoking during pregnancy and kidney volume in the offspring: the Generation R Study. Pediatr Nephrol. 2011;26:1275–1283. doi: 10.1007/s00467-011-1848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Bao W, Michels KB, Tobias DK, et al. Parental smoking during pregnancy and the risk of gestational diabetes in the daughter. Int J Epidemiol. 2016;45:160–169. doi: 10.1093/ije/dyv334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 355.Abalos E, Cuesta C, Carroli G, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(suppl 1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 356.Geelhoed JJ, Fraser A, Tilling K, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 358.Innes KE, Byers TE, Marshall JA, et al. Association of a woman's own birth weight with her subsequent risk for pregnancy-induced hypertension. Am J Epidemiol. 2003;158:861–870. doi: 10.1093/aje/kwg211. [DOI] [PubMed] [Google Scholar]
- 359.Rasmussen S, Irgens LM. Pregnancy-induced hypertension in women who were born small. Hypertension. 2007;49:806–812. doi: 10.1161/01.HYP.0000259924.74947.aa. [DOI] [PubMed] [Google Scholar]
- 360.Magnussen EB, Vatten LJ, Lund-Nilsen TI, et al. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 362.Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112:359–372. doi: 10.1097/AOG.0b013e3181801d56. [DOI] [PubMed] [Google Scholar]
- 363.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.McDonald SD, Han Z, Walsh MW, et al. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55:1026–1039. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 366.Vikse BE. Pre-eclampsia and the risk of kidney disease. Lancet. 2013;382:104–106. doi: 10.1016/S0140-6736(13)60741-2. [DOI] [PubMed] [Google Scholar]
- 367.Andersgaard AB, Acharya G, Mathiesen EB, et al. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: a population-based study. Am J Obstet Gynecol. 2012;206:143. doi: 10.1016/j.ajog.2011.09.032. e1-e8. [DOI] [PubMed] [Google Scholar]
- 368.Chambers JC, Fusi L, Malik IS, et al. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 369.Lampinen KH, Ronnback M, Groop PH, et al. Renal and vascular function in women with previous preeclampsia: a comparison of low- and high-degree proteinuria. Kidney Int. 2006;70:1818–1822. doi: 10.1038/sj.ki.5001902. [DOI] [PubMed] [Google Scholar]
- 370.McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 371.Vikse BE, Irgens LM, Bostad L, et al. Adverse perinatal outcome and later kidney biopsy in the mother. J Am Soc Nephrol. 2006;17:837–845. doi: 10.1681/ASN.2005050492. [DOI] [PubMed] [Google Scholar]
- 372.Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 373.Nisell H, Lintu H, Lunell NO, et al. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol. 1995;102:876–881. doi: 10.1111/j.1471-0528.1995.tb10874.x. [DOI] [PubMed] [Google Scholar]
- 374.Bar J, Kaplan B, Wittenberg C, et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant. 1999;14:1129–1132. doi: 10.1093/ndt/14.5.1129. [DOI] [PubMed] [Google Scholar]
- 375.Sandvik MK, Hallan S, Svarstad E, et al. Preeclampsia and prevalence of microalbuminuria 10 years later. Clin J Am Soc Nephrol. 2013;8:1126–1134. doi: 10.2215/CJN.10641012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kattah AG, Scantlebury DC, Agarwal S, et al. Preeclampsia and ESRD: the role of shared risk factors. Am J Kidney Dis. doi: 10.1053/j.ajkd.2016.07.034. DOI: 10.1053/ j.ajkd.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.World Health Organization WHO Recommendations for Prevention and Treatment of Pre-Eclampsia. whqlibdoc.who.int/publications/2011/9789241548335_eng.pdf [PubMed]
- 378.Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131–138. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 379.Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 380.Valensise H, Bezzeccheri V, Rizzo G, et al. Doppler velocimetry of the uterine artery as a screening test for gestational hypertension. Ultrasound Obstet Gynecol. 1993;3:18–22. doi: 10.1046/j.1469-0705.1993.03010018.x. [DOI] [PubMed] [Google Scholar]
- 381.Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384:869–879. doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- 382.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 383.McCarthy EA, Walker SP. International fetal growth standards: one size fits all. Lancet. 2014;384:835–836. doi: 10.1016/S0140-6736(14)61416-1. [DOI] [PubMed] [Google Scholar]
- 384.Gardosi J. Fetal growth and ethnic variation. Lancet Diabetes Endocrinol. 2014;2:773–774. doi: 10.1016/S2213-8587(14)70188-3. [DOI] [PubMed] [Google Scholar]
- 385.Clausson B, Gardosi J, Francis A, et al. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001;108:830–834. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 386.Romero R, Deter R. Should serial fetal biometry be used in all pregnancies? Lancet. 2015;386:2038–2040. doi: 10.1016/S0140-6736(15)00148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bricker L, Medley N, Pratt JJ. Routine ultrasound in late pregnancy (after 24 weeks' gestation) Cochrane Database Syst Rev. 2015;6:CD001451. doi: 10.1002/14651858.CD001451.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Bakalis S, Silva M, Akolekar R, et al. Prediction of small-for-gestational-age neonates: screening by fetal biometry at 30–34 weeks. Ultrasound Obstet Gynecol. 2015;45:551–558. doi: 10.1002/uog.14771. [DOI] [PubMed] [Google Scholar]
- 389.Lees C, Marlow N, Arabin B, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the Trial of Randomized Umbilical and Fetal Flow in Europe (TRUFFLE) Ultrasound Obstet Gynecol. 2013;42:400–408. doi: 10.1002/uog.13190. [DOI] [PubMed] [Google Scholar]
- 390.Mahmoodi Z, Karimlou M, Sajjadi H, et al. Association of maternal working condition with low birth weight: the social determinants of health approach. Ann Med Health Sci Res. 2015;5:385–391. doi: 10.4103/2141-9248.177982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Vasapollo B, Novelli GP, Gagliardi G, et al. Medical treatment of early-onset mild gestational hypertension reduces total peripheral vascular resistance and influences maternal and fetal complications. Ultrasound Obstet Gynecol. 2012;40:325–331. doi: 10.1002/uog.11103. [DOI] [PubMed] [Google Scholar]
- 392.Lees CC, Marlow N, van Wassenaer-Leemhuis A, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet. 2015;385:2162–2172. doi: 10.1016/S0140-6736(14)62049-3. [DOI] [PubMed] [Google Scholar]
- 393.Royal College of Obstetricians and Gynaecologists Small-for-Gestational-Age Fetus, Investigation and Management. Green-Top Guideline No. 31, 2013. [DOI] [PubMed]
- 394.WHO . Low Birth Weight: A Tabulation of Available Information, WHO/MCH92.2. Geneva: WHO; 1992. [Google Scholar]
- 395.Rodriguez MM, Gomez A, Abitbol C, et al. Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr Nephrol. 2005;20:945–949. doi: 10.1007/s00467-004-1800-x. [DOI] [PubMed] [Google Scholar]
- 396.Hinchliffe SA, Sargent PH, Howard CV, et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–784. [PubMed] [Google Scholar]
- 397.Selewski DT, Jordan BK, Askenazi DJ, et al. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr. 2013;162:725.e1–729.e1. doi: 10.1016/j.jpeds.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 398.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 399.Bruel A, Roze JC, Flamant C, et al. Critical serum creatinine values in very preterm newborns. PLoS One. 2013;8:e84892. doi: 10.1371/journal.pone.0084892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Elmas AT, Tabel Y, Elmas ON. Serum cystatin C predicts acute kidney injury in preterm neonates with respiratory distress syndrome. Pediatr Nephrol. 2013;28:477–484. doi: 10.1007/s00467-012-2331-5. [DOI] [PubMed] [Google Scholar]
- 401.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) and Paediatric Committee (PDCO) Guideline on the Investigation of Medicinal Products in the Term and Preterm Neonate. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003750.pdf
- 402.Alaro D, Bashir A, Musoke R, et al. Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. Afr Health Sci. 2014;14:682–688. doi: 10.4314/ahs.v14i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.O'Leary MJ, Bihari DJ. Preventing renal failure in the critically ill. There are no magic bullets – just high quality intensive care. BMJ. 2001;322:1437–1439. doi: 10.1136/bmj.322.7300.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Gilbert T, Lelievre-Pegorier M, Merlet-Benichou C. Immediate and long-term renal effects of fetal exposure to gentamicin. Pediatr Nephrol. 1990;4:445–450. doi: 10.1007/BF00862534. [DOI] [PubMed] [Google Scholar]
- 405.Gilbert T, Cibert C, Moreau E, et al. Early defect in branching morphogenesis of the ureteric bud in induced nephron deficit. Kidney Int. 1996;50:783–795. doi: 10.1038/ki.1996.377. [DOI] [PubMed] [Google Scholar]
- 406.Nathanson S, Moreau E, Merlet-Benichou C, et al. In utero and in vitro exposure to beta-lactams impair kidney development in the rat. J Am Soc Nephrol. 2000;11:874–884. doi: 10.1681/ASN.V115874. [DOI] [PubMed] [Google Scholar]
- 407.Tendron-Franzin A, Gouyon JB, Guignard JP, et al. Long-term effects of in utero exposure to cyclosporin A on renal function in the rabbit. J Am Soc Nephrol. 2004;15:2687–2693. doi: 10.1097/01.ASN.0000139069.59466.D8. [DOI] [PubMed] [Google Scholar]
- 408.Celsi G, Kistner A, Aizman R, et al. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 409.Ortiz LA, Quan A, Zarzar F, et al. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wintour EM, Moritz KM, Johnson K, et al. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Akinola O, Noronha C, Oremosu A, et al. The effect of the cyclooxygenase blockers, ibuprofen on the development of glomeruli in Sprague-Dawley rats. Niger Postgrad Med J. 2003;10:46–50. [PubMed] [Google Scholar]
- 412.Kociszewska-Najman B, Pietrzak B, Schreiber-Zamora J, et al. Ultrasonography of the brain, abdomen, and heart in neonates born to liver or renal transplant recipient mothers. Ann Transplant. 2012;17:113–119. doi: 10.12659/aot.883701. [DOI] [PubMed] [Google Scholar]
- 413.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 414.Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 415.Carballo-Magdaleno D, Guizar-Mendoza JM, Amador-Licona N, et al. Renal function, renal volume, and blood pressure in infants with antecedent of antenatal steroids. Pediatr Nephrol. 2011;26:1851–1856. doi: 10.1007/s00467-011-1860-7. [DOI] [PubMed] [Google Scholar]
- 416.Lee LM, Leung CY, Tang WW, et al. A paradoxical teratogenic mechanism for retinoic acid. Proc Natl Acad Sci USA. 2012;109:13668–13673. doi: 10.1073/pnas.1200872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Groen In ‘t Woud S, Renkema KY, Schreuder MF, et al. Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: a case-control study. Birth Defects Res A Clin Mol Teratol. 2016;106:596–603. doi: 10.1002/bdra.23500. [DOI] [PubMed] [Google Scholar]
- 418.Benini D, Fanos V, Cuzzolin L, et al. In utero exposure to nonsteroidal anti-inflammatory drugs: neonatal renal failure. Pediatr Nephrol. 2004;19:232–234. doi: 10.1007/s00467-003-1338-3. [DOI] [PubMed] [Google Scholar]
- 419.Phadke V, Bhardwaj S, Sahoo B, et al. Maternal ingestion of diclofenac leading to renal failure in newborns. Pediatr Nephrol. 2012;27:1033–1036. doi: 10.1007/s00467-012-2114-z. [DOI] [PubMed] [Google Scholar]
- 420.Peruzzi L, Gianoglio B, Porcellini MG, et al. Neonatal end-stage renal failure associated with maternal ingestion of cyclo-oxygenase-type-1 selective inhibitor nimesulide as tocolytic. Lancet. 1999;354:1615. doi: 10.1016/S0140-6736(99)03105-0. [DOI] [PubMed] [Google Scholar]
- 421.UNICEF/WHO/World Bank Joint Child Malnutrition Estimates Levels and Trends in Child Malnutrition. http://www.who.int/nutrition/publications/jointchildmalnutrition_2016_estimates.pdf
- 422.Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr. 2013;162:S7–S16. doi: 10.1016/j.jpeds.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 423.Thureen P. The neonatologist's dilemma: catch-up growth or beneficial undernutrition in very low birth weight infants – what are optimal growth rates? J Pediatr Gastroenterol Nutr. 2009;48:121–122. doi: 10.1097/01.mpg.0000302962.08794.62. [DOI] [PubMed] [Google Scholar]
- 424.Nzegwu NI, Ehrenkranz RA. Post-discharge nutrition and the VLBW infant: to supplement or not supplement?: a review of the current evidence. Clin Perinatol. 2014;41:463–474. doi: 10.1016/j.clp.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 425.Kramer MS. Breastfeeding, complementary (solid) foods, and long-term risk of obesity. Am J Clin Nutr. 2010;91:500–501. doi: 10.3945/ajcn.2010.29199. [DOI] [PubMed] [Google Scholar]
- 426.Weng SF, Redsell SA, Nathan D, et al. Estimating overweight risk in childhood from predictors during infancy. Pediatrics. 2013;132:e414–e421. doi: 10.1542/peds.2012-3858. [DOI] [PubMed] [Google Scholar]
- 427.Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 429.Escribano J, Luque V, Ferre N, et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU Childhood Obesity Programme. Int J Obes (Lond) 2012;36:548–553. doi: 10.1038/ijo.2011.276. [DOI] [PubMed] [Google Scholar]
- 430.Weber M, Grote V, Closa-Monasterolo R, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–1051. doi: 10.3945/ajcn.113.064071. [DOI] [PubMed] [Google Scholar]
- 431.Ambrosini GL, Huang RC, Mori TA, et al. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr Metab Cardiovasc Dis. 2010;20:274–283. doi: 10.1016/j.numecd.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 432.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 433.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 434.Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301:F919–F931. doi: 10.1152/ajprenal.00068.2011. [DOI] [PubMed] [Google Scholar]
- 435.Vollmer S, Harttgen K, Subramanyam MA, et al. Association between economic growth and early childhood undernutrition: evidence from 121 demographic and health surveys from 36 low-income and middle-income countries. Lancet Glob Health. 2014;2:e225–e234. doi: 10.1016/S2214-109X(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 436.World Health Organization, UNICEF . Global Strategy for Infant and Young Child Feeding. Geneva: WHO; 2003. http://whqlibdoc.who.int/publications/2003/9241562218.pdf. [Google Scholar]
- 437.World Health Organization Report of the Commission on Ending Childhood Obesity. Geneva: WHO; 2016. http://apps.who.int/iris/bitstream/10665/204176/1/9789241510066_eng.pdf?ua=1. [Google Scholar]
- 438.Keijzer-Veen MG, Devos AS, Meradji M, et al. Reduced renal length and volume 20 years after very preterm birth. Pediatr Nephrol. 2010;25:499–507. doi: 10.1007/s00467-009-1371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 440.Silverwood RJ, Pierce M, Hardy R, et al. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 2013;84:1262–1270. doi: 10.1038/ki.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lurbe E, Cifkova R, Cruickshank JK, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 442.US Department of Health and Human Services National Institutes of Health National Heart and Blood Institute The Fourth Report on the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. NIH Publ No 05–5267. https://www.nhlbi.nih.gov/files/docs/resources/heart/hbp_ped.pdf
- 443.Bukowski R, Davis KE, Wilson PW. Delivery of a small for gestational age infant and greater maternal risk of ischemic heart disease. PLoS One. 2012;7:e33047. doi: 10.1371/journal.pone.0033047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Hastie CE, Smith GC, Mackay DF, et al. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol. 2011;40:914–919. doi: 10.1093/ije/dyq270. [DOI] [PubMed] [Google Scholar]
- 445.Damm P, Houshmand-Oeregaard A, Kelstrup L, et al. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59:1396–1399. doi: 10.1007/s00125-016-3985-5. [DOI] [PubMed] [Google Scholar]
- 446.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–167. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 447.Berglund D, MacDonald D, Jackson S, et al. Low birthweight and risk of albuminuria in living kidney donors. Clin Transplant. 2014;28:361–367. doi: 10.1111/ctr.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Locke JE, Reed RD, Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. doi: 10.1016/j.kint.2016.10.014. DOI: 10.1016/j.kint.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Garg AX, Nevis IF, McArthur E, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124–133. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Luyckx VA, Perico N, Somaschini M, et al. A developmental approach to the prevention of hypertension and kidney disease – a report from the Birth Weight and Nephron Number Working Group. Lancet. 2017 doi: 10.1016/S0140-6736(17)30576-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]